Trace Metals and Metalloids Present in Springwater of a Mining Area: Assessment Based on Chemical and Isotopic Data (δ2H, δ18O, 3H and 87Sr/86Sr)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
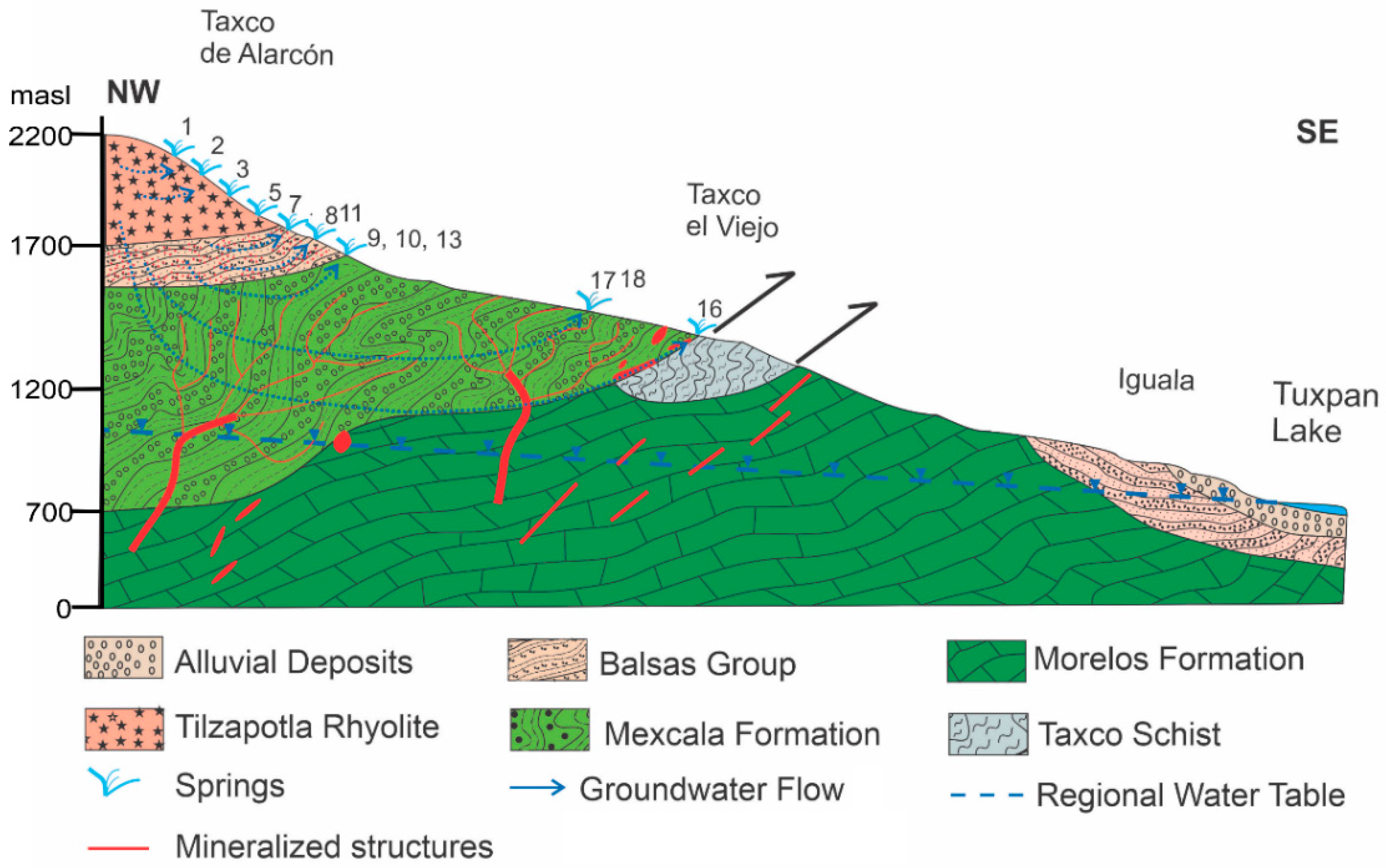
2.2. Geological and Hydrogeological Setting
2.3. Sample Collection and Analyses
3. Results and Discussion
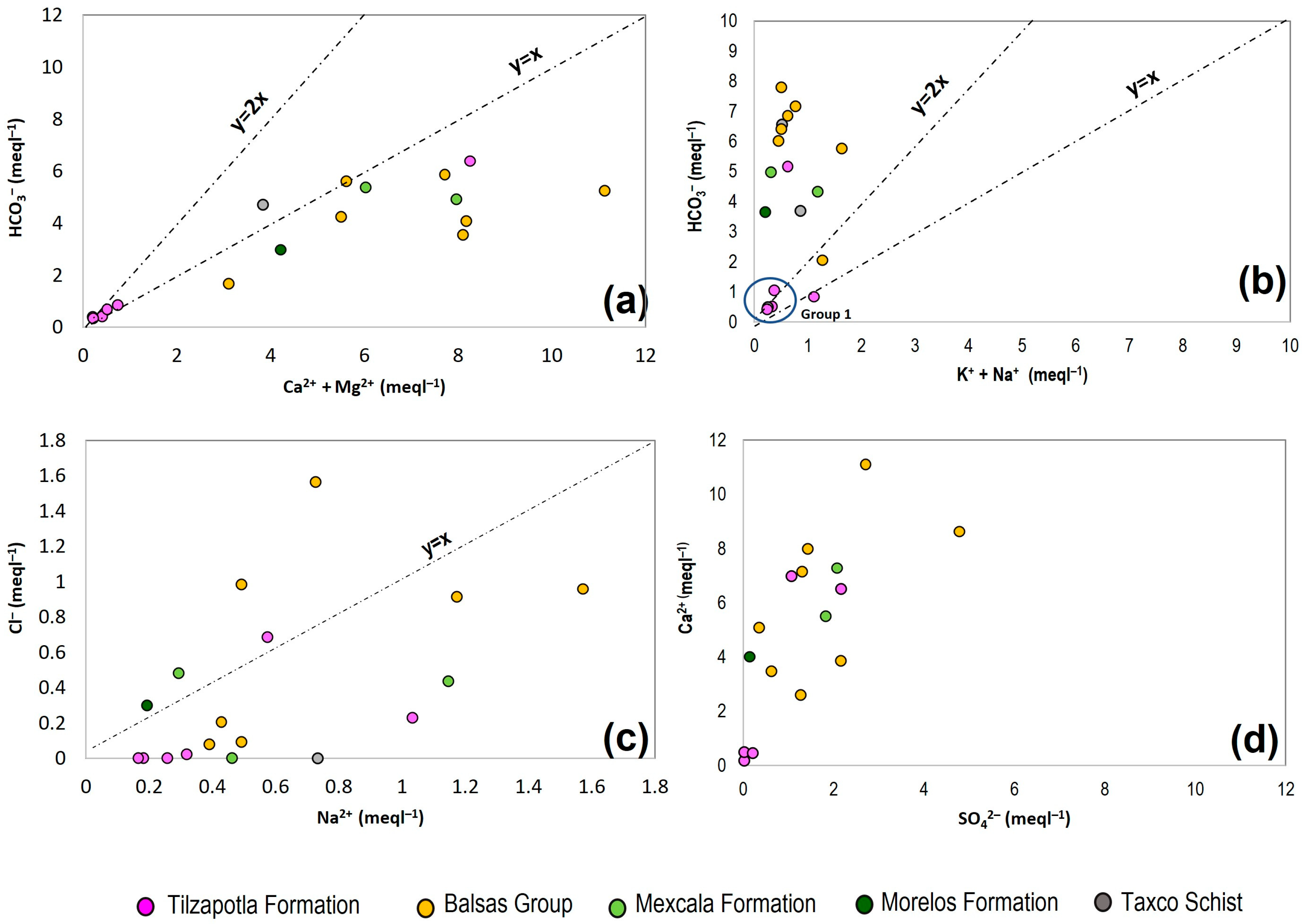
3.1. Main Hydrochemical Features
3.2. Water Quality of the Springwater
3.3. Chemical Composition of Springwater
3.4. Environmental Isotopes
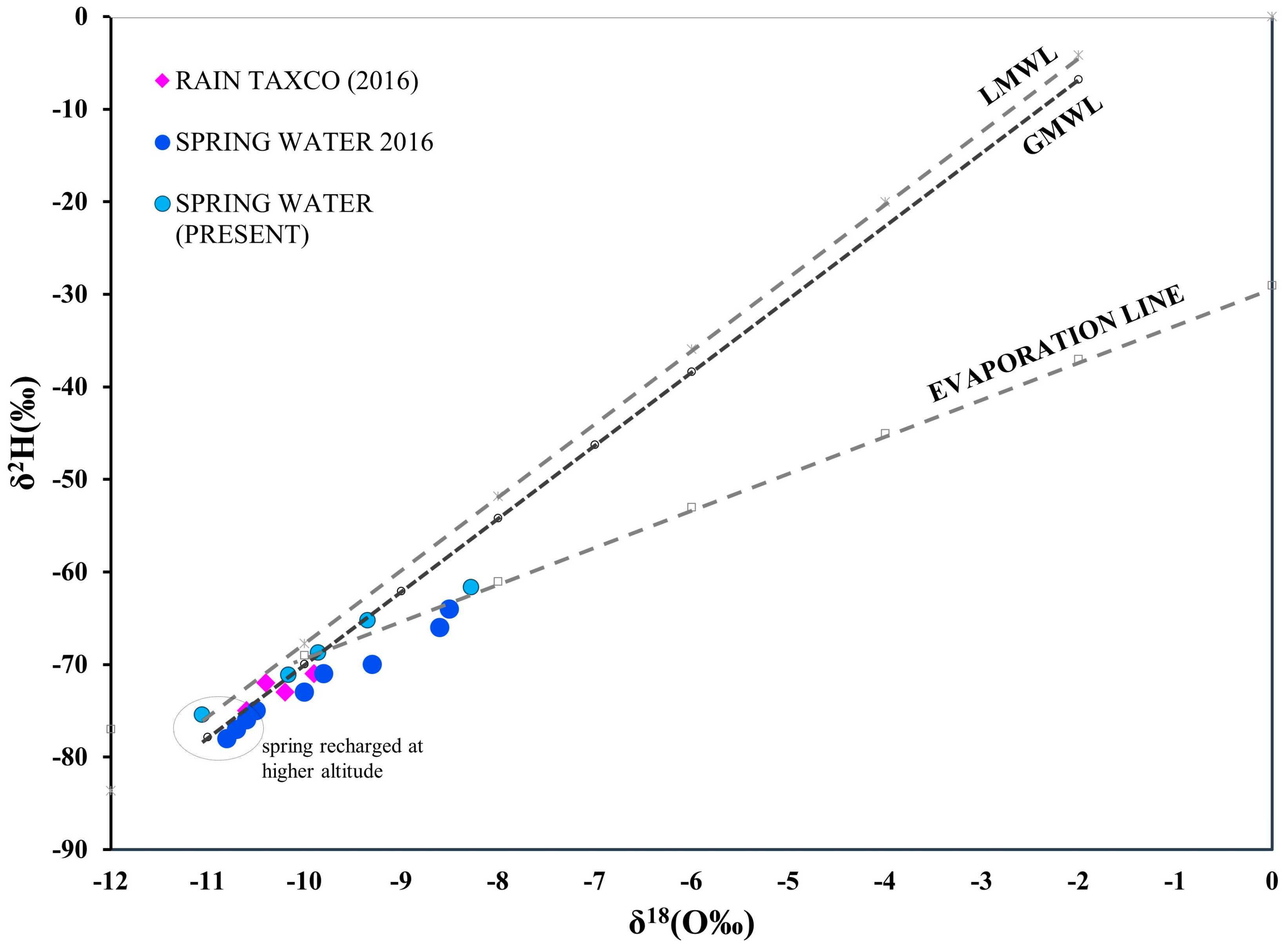
3.4.1. Deuterium and Oxygen Isotopes and Recharge Altitudes
3.4.2. Tritium Isotopes
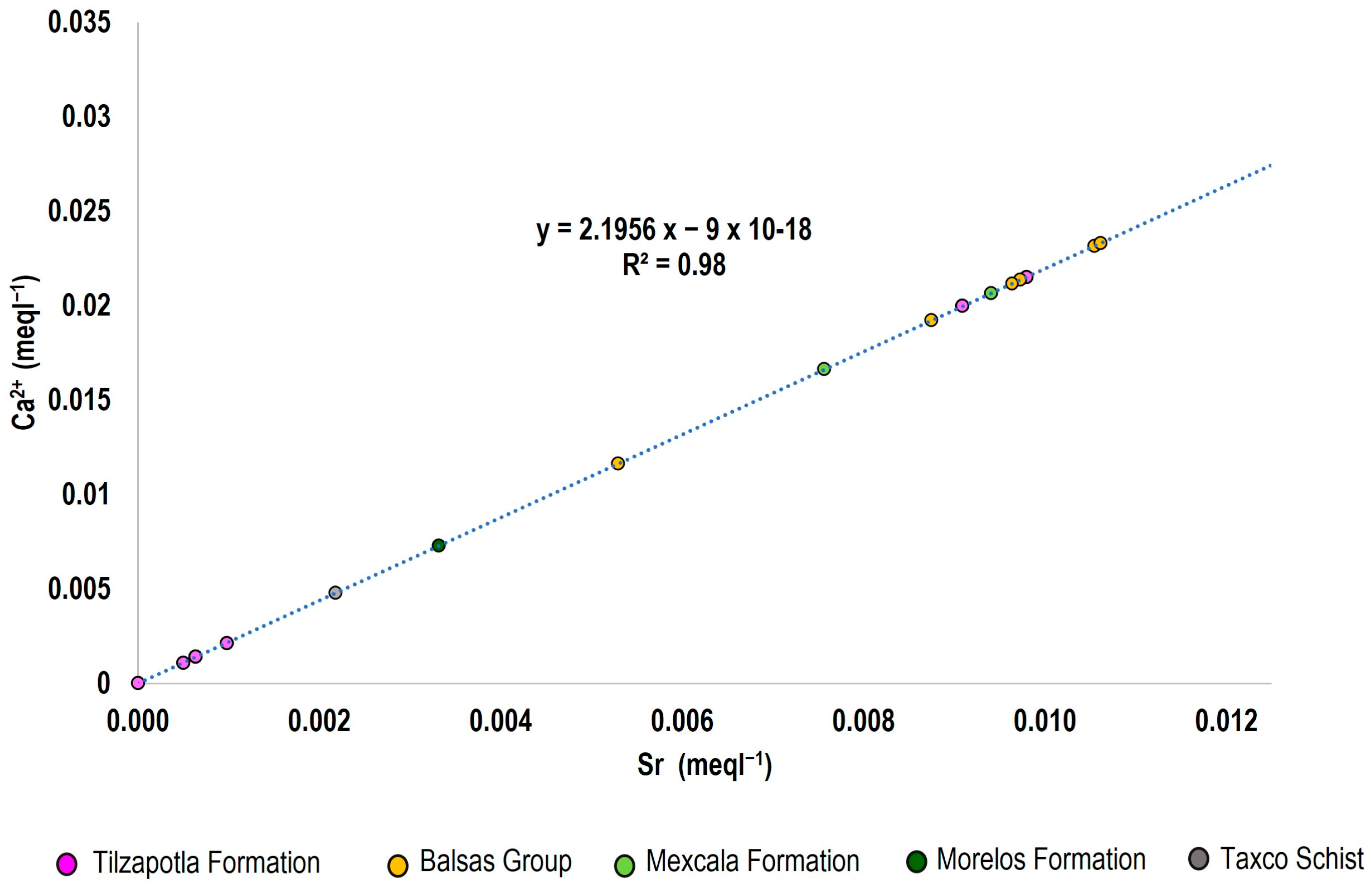
3.4.3. Strontium Isotopes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Feng, Q.; Gong, M. Contamination Characteristics and Source Identification of Groundwater in Xishan Coal Mining Area of Taiyuan Based on Hydrochemistry and Sulfur–Oxygen Isotopes. Water 2023, 15, 1169. [Google Scholar] [CrossRef]
- Doveri, M.; Natali, S.; Franceschi, L.; Menichini, M.; Trifiró, S.; Giannecchini, R. Carbonate aquifers threatened by legacy mining: Hydrodynamics, hydrochemistry, and water isotopes integrated approach for spring water management. J. Hydrol. 2021, 593, 125850. [Google Scholar] [CrossRef]
- Zamora, H.A.; Eastoe, C.J.; Wilder, B.T.; McIntosh, J.C.; Meixner, T.; Flessa, K.W. Groundwater Isotopes in the Sonoyta River Watershed, USA-Mexico: Implications for Recharge Sources and Management of the Quitobaquito Springs. Water 2020, 12, 3307. [Google Scholar] [CrossRef]
- Pantoja-Irys, J.R.; Mujica-Sánchez, H.; Arista-Cázares, L.E.; Hernández-García, C.M.; Wagner, M. Environmental geology, and isotopic evaluation of springs within the central part of the Sierra Cerro de La Silla, northeastern México. J. S. Am. Earth Sci. 2022, 119, 104017. [Google Scholar] [CrossRef]
- Khayat, S.; Marei, A.; Hippler, D.; Barghouthi, Z.; Dietzel, M. Using environmental isotopes to investigate the groundwater recharge mechanisms and dynamics in the North-eastern Basin, Palestine. Hydrol. Sci. J. 2020, 65, 583–596. [Google Scholar] [CrossRef]
- Madrigal-Solís, H.; Jiménez-Gavilán, P.; Vadillo-Pérez, I.; Fonseca-Sánchez, A.; Quesada-Hernández, L.; Sánchez-Gutiérrez, R.; Calderón-Sánchez, H.; Pardo-Vargas, C. Application of hydrogeochemistry and isotopic characterization for the assessment of recharge in a volcanic aquifer in the eastern region of central Costa Rica. Isot. Environ. Health Stud. 2021, 56, 446–464. [Google Scholar] [CrossRef]
- López, S.; Expósito, J.L.; Esteller, M.V.; Gómez, M.A.; Franco, R.; Morales, G.P. Prioritization to protect springs for public urban water supplies, based on multi-criteria evaluation and GIS (State of Mexico, Mexico). Appl. Geogr. 2019, 107, 26–37. [Google Scholar] [CrossRef]
- Ranjan, P.; Pandey, P.K. Spring Protection: Step Towards Water Security and Sustainable Rural Water Supply. Ann. Rom. Soc. Cell Biol. 2021, 25, 1216–1222. [Google Scholar]
- Zhou, J.; Zhang, Y.; Zhang, Q.; Kang, F.; Yuan, L.; Wei, D.; Lin, S. Using multi-isotopes (34S, 18O, 2H) to track local contamination of the groundwater from Hongshan-Zhaili abandoned coal mine, Zibo city, Shandong Province. Int. Biodeterior. Biodegrad. 2018, 128, 48–55. [Google Scholar] [CrossRef]
- Page, D.; Bekele, E.; Vanderzalm, J.; Sidhu, J. Managed Aquifer Recharge (MAR) in Sustainable Urban Water Management. Water 2018, 10, 239. [Google Scholar] [CrossRef]
- Qu, S.; Wang, G.; Shi, Z.; Xu, Q.; Guo, Y.; Ma, L.; Sheng, Y. Using stable isotopes (δD, δ 18O, δ 34S and 87Sr/86Sr) to identify sources of water in abandoned mines in the Feng coal mining district, Northern China. Hydrogeol. J. 2018, 26, 1443–1453. [Google Scholar] [CrossRef]
- Barbieri, M.; Morotti, M. Hydrogeochemistry and strontium isotopes of spring and mineral waters from monte Vulture Volcano, Italy. Appl. Geochem. 2003, 18, 117–125. [Google Scholar] [CrossRef]
- Anuard, P.-G.; Julián, G.-T.; Hugo, J.-F.; Carlos, B.-C.; Arturo, H.-A.; Edith, O.-T.; Claudia, Á.-S. Integration of Isotopic (2H and 18O) and Geophysical Applications to Define a Groundwater Conceptual Model in Semiarid Regions. Water 2019, 11, 488. [Google Scholar] [CrossRef]
- Tian, X.; Gong, Z.; Fu, L.; You, D.; Li, F.; Wang, Y.; Chen, Z.; Zhou, Y. Determination of Groundwater Recharge Mechanism Based on Environmental Isotopes in Chahannur Basin. Water 2023, 15, 180. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, Y.; Talma, A.S. Hydrochemical and isotopic approach to dynamic recharge of a dolomite aquifer in South Africa. Hydrogeol. J. 2019, 27, 945–964. [Google Scholar] [CrossRef]
- Nigro, A.; Sappa, G.; Barbieri, M. Strontium Isotope as Tracers of Groundwater Contamination. Procedia Earth Planet. Sci. 2017, 17, 352–355. [Google Scholar] [CrossRef]
- Stewart, M.K.; Taylor, C.B. Environmental isotopes in New Zealand hydrology. 1. Introduction. The role of oxygen-18, deuterium, and tritium in hydrology. N. Z. J. Sci. 1981, 24, 295–311. [Google Scholar]
- Ayadi, R.; Trabelsi, R.; Zouari, K.; Saibi, H.; Itoi, R.; Khanfir, H. Hydrogeological and hydrochemical investigation of groundwater using environmental isotopes (18O, 2H, 3H, 14C) and chemical tracers: A case study of the intermediate aquifer, Sfax, Southeastern Tunisia. Hydrogeol. J. 2018, 26, 983–1007. [Google Scholar] [CrossRef]
- Moya, C.; Raiber, M.; Taulis, M.; Cox, M. Using environmental isotopes and dissolved methane concentrations to constrain hydrochemical processes and inter-aquifer mixing in the galilee and eromanga basins, great artesian basin, Australia. J. Hydrol. 2016, 539, 304–318. [Google Scholar] [CrossRef]
- Salcedo Sánchez, E.R.; Martínez, J.M.E.; Morales, M.M.; Talavera Mendoza, O.; Alberich, M.V.E. Ecological and Health Risk Assessment of Potential Toxic Elements from a Mining Area (Water and Sediments): The San Juan-Taxco River System, Guerrero, Mexico. Water 2022, 14, 518. [Google Scholar] [CrossRef]
- Talavera, M.O.; Ruiz, J.; Díaz, V.E.; Ramírez, G.A.; Cortés, A.; Salgado, S.S.A.; Rivera, B.R. Water-rock-tailings interactions and sources of sulfur and metals in the subtropical mining region of Taxco, Guerrero (Southern Mexico): A multi-isotopic approach. Appl. Geochem. 2016, 66, 73–81. [Google Scholar] [CrossRef]
- DOF. CONAGUA, Actualización de la Disponibilidad Media Anual de Agua en el Acuífero Buena vista de Cuellar (1204), Estado de Guerrero; Comisión Nacional del Agua: Mexico City, México, 2015; Available online: https://www.gob.mx/cms/uploads/attachment/file/103667/DR_1204.pdf (accessed on 20 April 2015).
- DOF. CONAGUA Actualización de la Disponibilidad Media Anual de Agua en el Acuífero de Iguala (1205), Estado de Guerrero; Comisión Nacional del Agua: Mexico City, México, 2015; Available online: https://www.gob.mx/cms/uploads/attachment/file/103668/DR_1205.pdf (accessed on 20 April 2015).
- Dótor-Almazán, A.; Armienta-Hernández, M.A.; Talavera-Mendoza, O.; Ruiz, J. Geochemical behavior of cu and sulfur isotopes in the tropical mining region of Taxco, Guerrero (southern Mexico). Chem. Geol. 2017, 471, 1–12. [Google Scholar] [CrossRef]
- Dotor, A.A.; Armienta, M.A.; Arcega, F.; Talavera, M.O. Procesos de transporte de arsénico y metales en aguas superficiales del distrito minero de Taxco, Mexico: Aplicación de isótopos estables (Transport processes of arsenic and metals in surface waters in the mining district of Taxco, Mexico). Hidrobiológica 2014, 24, 256. [Google Scholar]
- Norwegian Meteorological Institute. 2019. Available online: http://www.weatherbase.com/weather/weather.php3?s=912122&cityname=Taxco%2C+Guerrero%2C+Mexico&units=&set=metric (accessed on 30 March 2023).
- Armienta, M.A.; Talavera, O.; Villaseñor, G.; Espinosa, E.; Pérez-Martínez, I.; Cruz, O.; Ceniceros, N.; Aguayo, A. Environmental behaviour of metals from tailings in shallow rivers: Taxco, central Mexico. Appl. Earth Sci. 2004, 113, 76–82. [Google Scholar] [CrossRef]
- Romero, F.M.; Núñez, L.; Gutiérrez, M.E.; Armienta, M.A.; Ceniceros-Gómez, A.E. Evaluation of the potential of indigenous calcareous shale for neutralization and removal of arsenic and heavy metals from acid mine drainage in the Taxco mining area, Mexico. Arch. Environ. Contam. Toxicol. 2011, 60, 191–203. [Google Scholar] [CrossRef]
- Talavera, M.O.; Moreno, T.R.; Dotor-Almazán, A.; Flores-Mundo, N.; Duarte Gutiérrez, C. Mineralogy and geochemistry of sulfide-bearing tailings from silver mines in the Taxco, Mexico Area to evaluate their potential environmental impact. Geofísica Int. 2005, 44, 49–64. [Google Scholar] [CrossRef]
- Ruiz, H.E.; Armienta, H.M.A. Acumulación de arsénico y metales pesados en maíz en suelos cercanos a jales o residuos mineros. Rev. Int. Contam. Ambient 2012, 28, 103–117. [Google Scholar]
- Gómez, B.J.M.; Santana, C.J.; Romero, M.F.; Armienta, H.M.A.; Morton, B.O.; Ruiz, H.E.A. Plantas de sitios contaminados con desechos mineros en Taxco, Guerrero. México. Bol. Soc. Bot. Mex. 2010, 87, 131–133. [Google Scholar]
- Vázquez, B.A.; Talavera, M.O.; Moreno, G.M.; Salgado, S.S.; Ruiz, J.; Huerta, B.G. Source apportionment of lead in the blood of women of reproductive age living near tailings in Taxco, Guerrero, Mexico: An isotopic study. Sci. Total Environ. 2017, 583, 104–114. [Google Scholar] [CrossRef]
- Campa, U.M.F.; Ramírez, E.J. La evolución geológica y la metalogenesis del noroccidente de Guerrero (The Geological Evolution and Metallogeny of Northwest Guerrero). In Serie Técnico-Científica; Universidad Autónoma de Guerrero: Chilpancingo, Mexico, 1979; Volume 1, p. 101. [Google Scholar]
- DOF. NOM-230-SSA1-2002, Salud Ambiental. Agua Para uso y Consumo Humano, Requisitos Sanitarios que se Deben Cumplir en los Sistemas de Abastecimiento Públicos y Privados Durante el Manejo del Agua. Procedimientos Sanitarios Para el Muestreo. Diario Oficial de la Federación (DOF). 4 November 2003. Available online: https://dof.gob.mx/nota_detalle.php?codigo=2081772&fecha=12/07/2005 (accessed on 30 March 2023).
- APHA; AWWA; WEF. Standard Methods for the Examination Water and Wastewater, 21st ed.; APHA; AWWA; WEF: Washington, DC, USA, 2004. [Google Scholar]
- Deutsch, W. Groundwater Geochemistry. Fundamentals and Applications to Contamination; Lewis Publishers: New York, NY, USA, 1997. [Google Scholar]
- Thibodeau, A.M.; Habicht-Mauche, J.; Huntley, D.L.; Chesley, J.T.; Ruiz, J. High precision isotopic analyses of lead ores from New Mexico by MC-ICP-MS: Implications for tracing the production and exchange of Pueblo IV glaze-decorated pottery. J. Archaeol. Sci. 2013, 40, 3067–3075. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Guidelines for Drinking-Water Quality [Electronic Resource]: Incorporating 1st and 2nd Addenda, 3rd ed.; WHO: Geneva, Switzerland, 2008; Volume 1. [Google Scholar]
- DOF. Norma Oficial Mexicana NOM-127-SSA1-2021 Agua Para uso y Consumo Humano. Límites Permisibles de la Calidad del agua. Secretaria de Salud. Diario Oficial de la Federación (DOF). 5 May 2022. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5650705&fecha=02/05/2022 (accessed on 30 March 2023).
- Aqueous Solutions LCC. Geochemist’s Workbench, Version 11; Department of Geology at the University of Illinois Urbana Champaign: Urbana, IL, USA, 2016. Available online: https://www.gwb.com/(accessed on 30 March 2023).
- Appelo, C.; Postma, D. Geochemistry, Groundwater and Pollution, 2nd ed.; Routledge: New York, NY, USA, 2005. [Google Scholar]
- Esteller, M.V.; Kondratenko, N.; Expósito, J.L.; Medina, M.; Martin del Campo, M.A. Hydrogeochemical characteristics of a volcanic-sedimentary aquifer with special emphasis on Fe and Mn content: A case study in Mexico. J. Geochem. Explor. 2017, 180, 113–126. [Google Scholar] [CrossRef]
- Biswas, A.; Nath, B.; Bhattacharya, P.; Halder, D.; Kundu, A.K.; Mandal, U.; Mukherjee, A.; Chatterjee, D.; Mörth, C.M.; Jacks, G. Hydrogeochemical contrast between brown and grey sand aquifers in shallow depth of Bengal Basin: Consequences for sustainable drinking water supply. Sci. Total Environ. 2012, 431, 402–412. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, D.; Zhou, P.; Qu, S.; Liao, F.; Wang, G. Hydrochemical Characteristics of Groundwater and Dominant Water–Rock Interactions in the Delingha Area, Qaidam Basin, Northwest China. Water 2020, 12, 836. [Google Scholar] [CrossRef]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- Cortes, A.; Farvolden, R.N. Isotope studies of precipitation and groundwater in the Sierra de las Cruces, Mexico. J. Hydrol. 1988, 107, 147–153. [Google Scholar] [CrossRef]
- Horst, A.; Mahlknecht, J.; Merkel, B.J.; Aravena, R.; Ramos-Arroyo, Y.R. Evaluation of the recharge processes and impacts of irrigation on groundwater using CFCs and radiogenic isotopes in the Silao-Romita basin, Mexico. Hydrogeol. J. 2008, 16, 1601–1614. [Google Scholar] [CrossRef]
- Mahlknecht, J.; Daessle, L.W.; Esteller, M.V.; Torres-Martinez, J.A.; Mora, A. Groundwater flow processes and human impact along the arid US-mexican border, evidenced by environmental tracers: The case of tecate, baja california. Int. J. Environ. Res. Public Health 2018, 15, 887. [Google Scholar] [CrossRef]
- Arroyo-Díaz, F.; Salgado-Souto, S.A.; Del Rio-Salas, R.; Talavera-Mendoza, O.; Ramírez-Guzmán, A.; Ruíz, J.; Sarmiento-Villagrana, A.; Guzmán-Martínez, M. PTE and Multi-Isotope Assessment of Spring Water Used for Human Consumption in the Historical Mining Region of Taxco de Alarcón in Southern Mexico. J. S. Am. Earth Sci. 2022, 116, 103811. [Google Scholar] [CrossRef]
- Morales-Arredondo, J.I.; Armienta Hernández, M.A.; Cuellar-Ramírez, E.; Morton-Bermea, O.; Ortega-Gutiérrez, J.E. Hydrogeochemical behavior of Ba, B, Rb, and Sr in an urban aquifer located in central Mexico and its environmental implications. J. S. Am. Earth Sci. 2022, 116, 103870. [Google Scholar] [CrossRef]



| Limits/Spring ID | pH | EC | Eh | TDS | Total Hardness CaCO3 | HCO3− | NO3− | SO42− | Cl− | Na2+ | Mg2+ | Ca2+ | K+ | Fe2+ | Mn2+ | Ba | Cu2+ | Zn2+ | As | SiO2 | F− | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Units | (µS/cm) | (mV) | (mgL−1) | |||||||||||||||||||
| NOM-SSA1-127-2021 | 6.5–8.5 | - | - | 1000 | 500 | 11 | 400 | - | - | 0.3 | 0.15 | 1.3 | 2 | 5 | 0.025 | - | 1.5 | 0.01 | ||||
| WHO, 2017 | - | - | 1000 | 100 | 40 | 250 | 0.1 | 0.4 | 0.7 | 1 | 5 | 0.01 | - | 1.5 | ||||||||
| Horconcito 1 | 6.56 | 44.6 | 201.72 | 41.5 | 11.2 | 24.8 | 1.35 | 1 | <dl | 5.91 | 2.91 | 3.46 | 2.91 | 0.05 | <dl | 0.05 | <ql | <ql | <ql | 20.25 | <ql | <ql |
| Horconcito 2 | 6.76 | 41.7 | 224.23 | 38.6 | 12.7 | 24.0 | 2.01 | <dl | <dl | 4.16 | 0.38 | 3.52 | 2.86 | 0.04 | <ql | <ql | <ql | <ql | <ql | 24.1 | <ql | <ql |
| Cascada | 6.9 | 35.9 | 171.42 | 32.3 | 12.9 | 20.0 | 1.64 | <dl | <dl | 3.78 | 0.44 | 3.62 | 3.12 | 0.26 | <ql | 0.03 | <ql | <ql | <ql | 14.8 | 0.04 | <ql |
| Chacoalco | 6.83 | 89.6 | 263.95 | 83.6 | 27.7 | 52.0 | 0.94 | 1 | 0.83 | 7.3 | 3 | 9.84 | 2.18 | <ql | <ql | <ql | <ql | <ql | <ql | 34.87 | 0.08 | <ql |
| Mulata | 5.61 | 113.6 | 277.3 | g | 32.5 | 42.0 | 14.78 | 10 | 8.33 | 23.67 | 0.73 | 9 | 3.2 | 0.08 | <ql | 0.03 | <ql | <ql | <ql | 29.25 | 0.08 | <ql |
| Pozitos | 7.46 | 730.7 | 175.19 | 609.1 | 404.9 | 258.0 | 6.16 | 104 | 25 | 13.17 | 20.88 | 130.5 | 1.8 | 0.07 | 0.291 | 0.2 | <ql | 0.041 | <ql | 19.64 | 0.2 | <ql |
| Landa | 6.7 | 681.4 | 181.98 | 694.4 | 297.1 | 389.7 | 13.19 | 104 | 35.83 | 11.29 | 20 | 77 | 0.52 | 0.03 | <ql | 0.11 | <ql | <ql | <ql | 12.22 | 0.44 | <ql |
| Pedro Martin | 7.28 | 404.9 | 193.81 | 295.9 | 155.1 | 102.0 | 6.3 | 61 | 33.33 | 26.9 | 6.13 | 52.04 | 3.77 | 0.16 | <ql | 0.05 | <ql | <ql | 0.015 | 14.99 | 0.3 | <ql |
| Cacalotenango | 7.1 | 529.7 | 227.16 | 498 | 288 | 300.0 | 4.04 | 17 | 7.5 | 9.8 | 6.57 | 101.6 | 0.98 | <ql | <ql | 0.09 | <ql | <ql | <ql | 19.29 | 0.41 | <ql |
| Ejido Arroyo 1 | 6.72 | 609.4 | 197.14 | 589.2 | 377.9 | 328.0 | 11.97 | 63 | 3.3 | 11.27 | 12.27 | 143.2 | 0.81 | 0.16 | <ql | 0.04 | <ql | <ql | <ql | 11.03 | 0.34 | <ql |
| Ejido Arroyo 2 | 6.83 | 645.8 | 118.35 | 622.5 | 391.8 | 342.0 | 8.59 | 69 | 2.83 | 8.96 | 1.38 | 160 | 8.91 | 0.3 | <ql | <ql | <ql | <ql | <ql | 10.92 | 0.27 | 0.018 |
| Arroyo 1 | 7.04 | 661.1 | 197.02 | 565.9 | 377.5 | 248.0 | 14.14 | 100 | 17.5 | 6.73 | 8.04 | 146 | 0.64 | 0.08 | 0.041 | <ql | <ql | <ql | <ql | 9.71 | 0.44 | <ql |
| Arroyo 2 | 7.2 | 563.3 | 149.4 | 483 | 270.4 | 216.0 | 1.5 | 88 | 15.83 | 26.27 | 6.46 | 110 | 1.68 | 0.09 | 0.042 | <ql | <ql | <ql | <ql | 10.12 | 0.34 | <ql |
| Atzala | 7.87 | 379.6 | 208.88 | 322.9 | 203.9 | 182.0 | 1.6 | 7 | 10.83 | 4.45 | 2.47 | 80.21 | 0.67 | <ql | <ql | 0.04 | <ql | <ql | <ql | 12.02 | 0.03 | <ql |
| Taxco el Viejo 1 | 6.68 | 953.9 | 240.77 | 839 | 583.4 | 358.0 | 47.3 | 130 | 57 | 16.67 | 14.25 | 222.3 | 1.61 | 0.05 | <ql | 0.12 | <ql | <ql | <ql | 12.02 | 0.4 | 0.012 |
| Taxco el Viejo 2 | 6.85 | 941.3 | 210.53 | 814.5 | 516.2 | 288.0 | 5.25 | 230 | 35 | 36.02 | 30.01 | 172.7 | 2.54 | 0.05 | 0.133 | 0.11 | <ql | <ql | <ql | 9.84 | 0.63 | 0.012 |
| Huamuchilito | 7.06 | 361.5 | 119.51 | 328.7 | 164.7 | 184.0 | 1.48 | 30 | <dl | 16.8 | 4.37 | 69.44 | 5.03 | 0.7 | 1.1 | 1.12 | <ql | <ql | <ql | 21.06 | 0.36 | <ql |
| Hueymatla | 7.01 | 593.1 | 99.44 | 564.8 | 340.9 | 320.0 | 1.79 | 51 | <dl | 10.59 | 8.59 | 140 | 1.77 | 0.13 | 0.036 | 0.07 | <ql | <ql | <ql | 9.91 | 0.33 | <ql |
| δ18 O (‰) | δ2H (‰) | Recharge Altitude | |
|---|---|---|---|
| Springwater | |||
| Horconcito 1 | −11.06 | −75.4 | 2761.9 |
| Mulata | −10.17 | −71.1 | 2126.2 |
| Landa | −9.86 | −68.7 | 1904.8 |
| Pedro Martin | ─ | ─ | 1861.9 |
| Arroyo 1 | −9.35 | −65.2 | 1540.5 |
| Hueymatla | −8.28 | −61.6 | 776.2 |
| Chacualco a | −10.7 | −77 | 2504.8 |
| Estacas a | −9.3 | −70 | 1504.8 |
| La Molanca a | −10.6 | −76 | 2433.4 |
| Equipada a | −10.8 | −78 | 2576.2 |
| El Copal a | −8.5 | −64 | 933.4 |
| La Bomba a | −8.6 | −66 | 1004.8 |
| El Jumil a | −10.0 | −73 | 2004.8 |
| Pedro Martin a | −9.8 | −71 | |
| Agua Zarca a | −10.5 | −75 | 2361.9 |
| Rainwater | |||
| Atzala a | −9.9 | −71 | |
| Minas Viejas a | −10.6 | −75 | |
| Taxco el Viejo a | −10.2 | −73 | |
| Taxco a | −10.4 | −72 |
| Geological Formation | Lithology a | Rock | Spring | Water Type | 3H | Sr | Water | Signature | Emerge |
|---|---|---|---|---|---|---|---|---|---|
| 86Sr/87Sr | (TU) | (mgL−1) | 86Sr/87Sr | ||||||
| Tilzapotla Formation | Ignimbrites, vitrofides, and volcanic rocks (rhyolites) | 0.70533 | Horconcito 1 | HCO3-Na | 2.49 | 0.028 | 0.70601 | Tilzapotla | Tilzapotla |
| Horconcito 2 | HCO3-Na | - | 0.028 | 0.70619 | Tilzapotla | Tilzapotla | |||
| Cascada | HCO3-Na | - | <ql. | 0.7062 | Tilzapotla | Tilzapotla | |||
| Chacualco b | HCO3-Na | - | 0.022 | 0.70594 b | Tilzapotla | Tilzapotla | |||
| Mulata | HCO3-Na | 1.42 | 0.043 | 0.70591 | Tilzapotla | Tilzapotla | |||
| Pozitos | HCO3-Ca-SO4-Mg | 1.1 | 0.2 | 0.70572 | Tilzapotla | Balsas | |||
| Balsas Group | Conglomerates, breccias, and siltstones | 0.70692 | Landa | HCO3-Ca-SO4-Mg | 1.83 | 0.44 | 0.7066 b | Balsas | Balsas |
| Pedro Martin | HCO3-Ca-SO4-Mg | 1.13 | 0.3 | 0.70682 | Balsas | Balsas | |||
| Cacalotenango | HCO3-Ca | - | 0.41 | 0.70622 | Balsas | Balsas | |||
| Ejido de Arroyo 2 | HCO3-Ca | 0.56 | 0.467 | 0.70708 | Balsas | Mexcala | |||
| Taxco el Viejo 1 | HCO3-Ca-SO4-Mg | 1.1 | 0.603 | 0.70658 | Balsas | Mexcala | |||
| Taxco el Viejo 2 | HCO3-Ca-SO4-Mg | 1.69 | 0.431 | 0.70705 | Balsas | Mexcala | |||
| Mexcala | Sandstones, shales, marls, and limestones | 0.70726 | Arroyo 1 | HCO3-Ca-SO4-Mg | 2.49 | 0.414 | 0.70714 | Mexcala | Mexcala |
| Arroyo 2 | HCO3-Ca-SO4-Mg | - | 0.333 | 0.70714 | Mexcala | Mexcala | |||
| Morelos | Reef and sub-reef limestones, dolomites, loams, and flint | 0.70746 | Atzala | HCO3-Ca | - | 0.146 | 0.70709 | Morelos | Morelos |
| Taxco Schist | Milonites, sericite schists, meta-andesite, meta-rhyolite, quartzite | 0.71528 | Huamuchilito | HCO3-Ca | 0 | 0.096 | 0.70848 | E. Taxco | E. Taxco |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores Ronces, J.A.; Sánchez, E.R.S.; Martínez Morales, M.; Esquivel Martínez, J.M.; Talavera Mendoza, O.; Esteller Alberich, M.V. Trace Metals and Metalloids Present in Springwater of a Mining Area: Assessment Based on Chemical and Isotopic Data (δ2H, δ18O, 3H and 87Sr/86Sr). Water 2023, 15, 1917. https://doi.org/10.3390/w15101917
Flores Ronces JA, Sánchez ERS, Martínez Morales M, Esquivel Martínez JM, Talavera Mendoza O, Esteller Alberich MV. Trace Metals and Metalloids Present in Springwater of a Mining Area: Assessment Based on Chemical and Isotopic Data (δ2H, δ18O, 3H and 87Sr/86Sr). Water. 2023; 15(10):1917. https://doi.org/10.3390/w15101917
Chicago/Turabian StyleFlores Ronces, José Alfredo, Edith R. Salcedo Sánchez, Manuel Martínez Morales, Juan Manuel Esquivel Martínez, Oscar Talavera Mendoza, and María Vicenta Esteller Alberich. 2023. "Trace Metals and Metalloids Present in Springwater of a Mining Area: Assessment Based on Chemical and Isotopic Data (δ2H, δ18O, 3H and 87Sr/86Sr)" Water 15, no. 10: 1917. https://doi.org/10.3390/w15101917
APA StyleFlores Ronces, J. A., Sánchez, E. R. S., Martínez Morales, M., Esquivel Martínez, J. M., Talavera Mendoza, O., & Esteller Alberich, M. V. (2023). Trace Metals and Metalloids Present in Springwater of a Mining Area: Assessment Based on Chemical and Isotopic Data (δ2H, δ18O, 3H and 87Sr/86Sr). Water, 15(10), 1917. https://doi.org/10.3390/w15101917








