Rapid Recovery of Buoyancy in Eutrophic Environments Indicates That Cyanobacterial Blooms Cannot Be Effectively Controlled by Simply Collapsing Gas Vesicles Alone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phytoplankton and Experiment Conditions
2.2. Measurement of Gas Vesicle Content
2.3. Measurement of Migration Speed
2.4. Percentage of Floating Cells Calculation
2.5. Statistical Analysis
3. Results
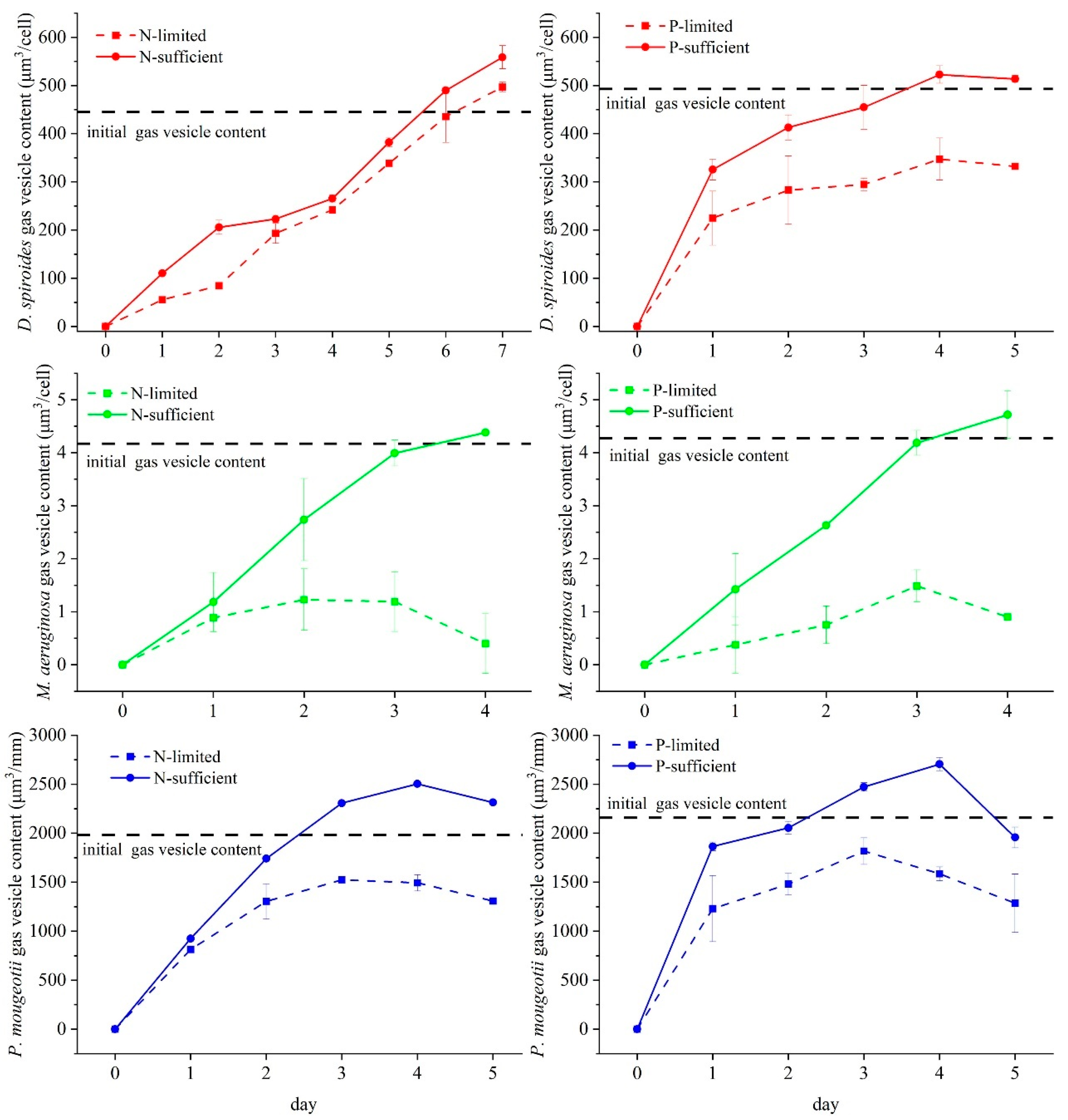
3.1. Recovery of Gas Vesicle in Different Trophic Levels
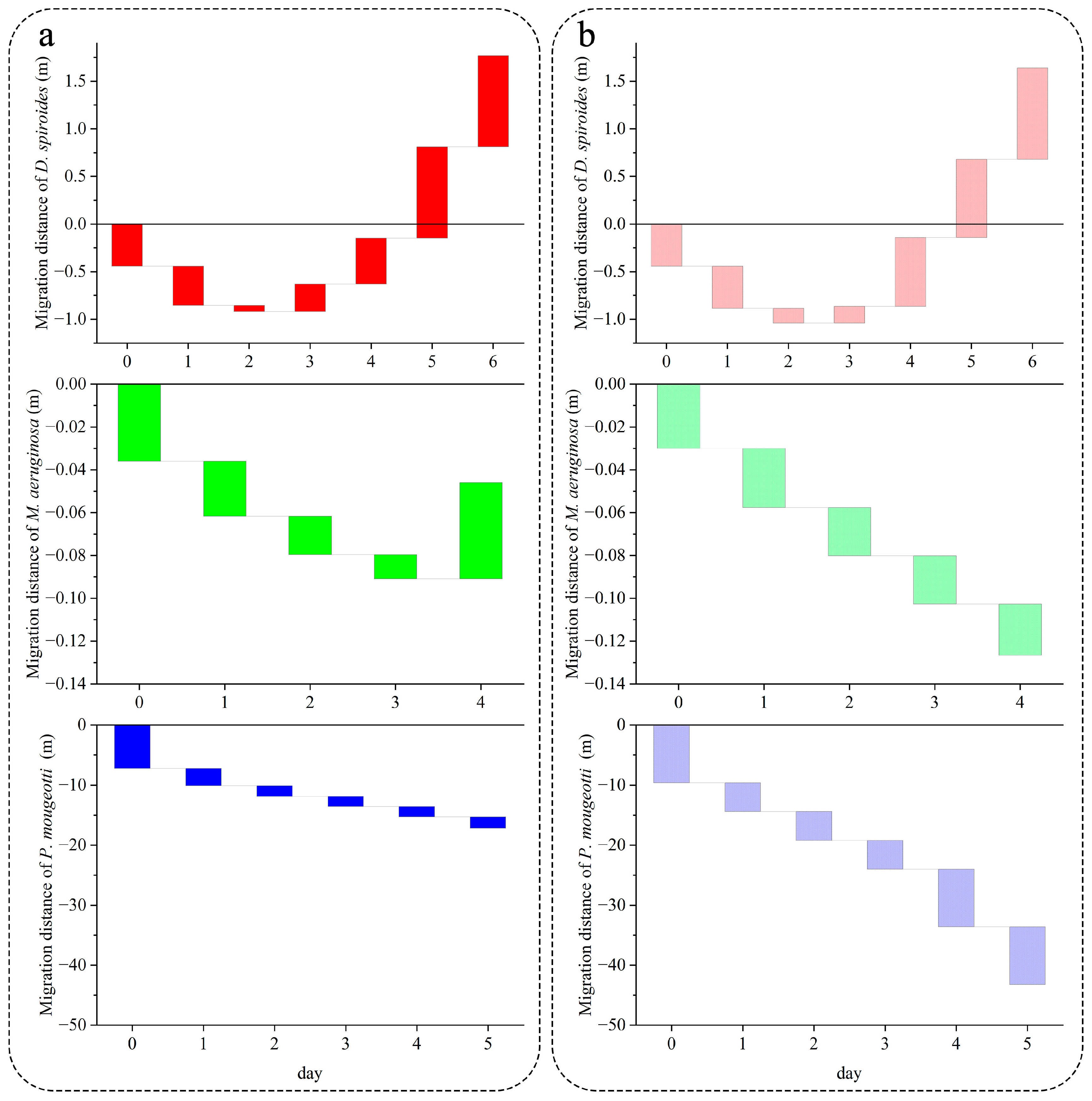
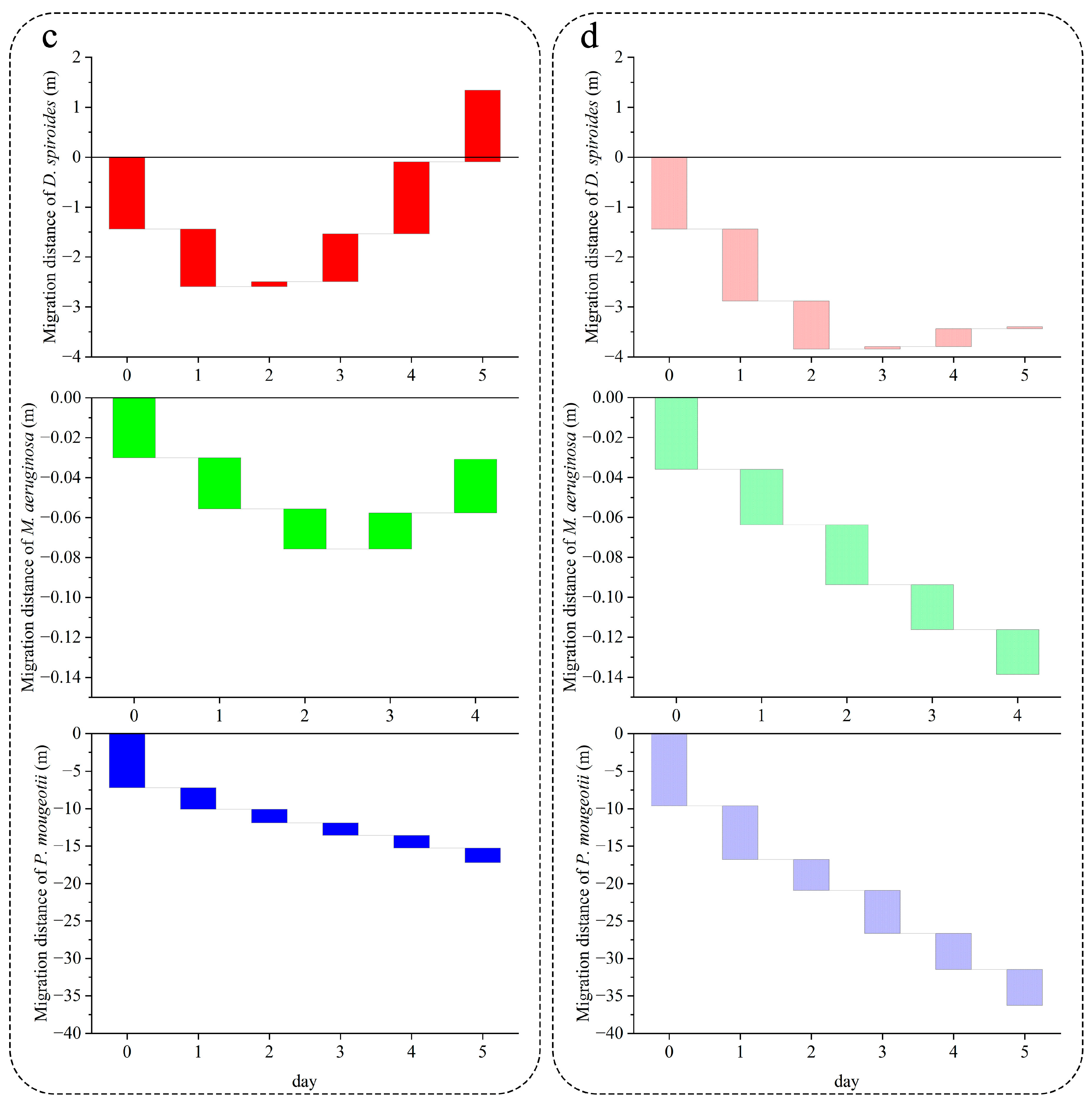
3.2. Recovery of Migration Speed in Different Trophic Levels
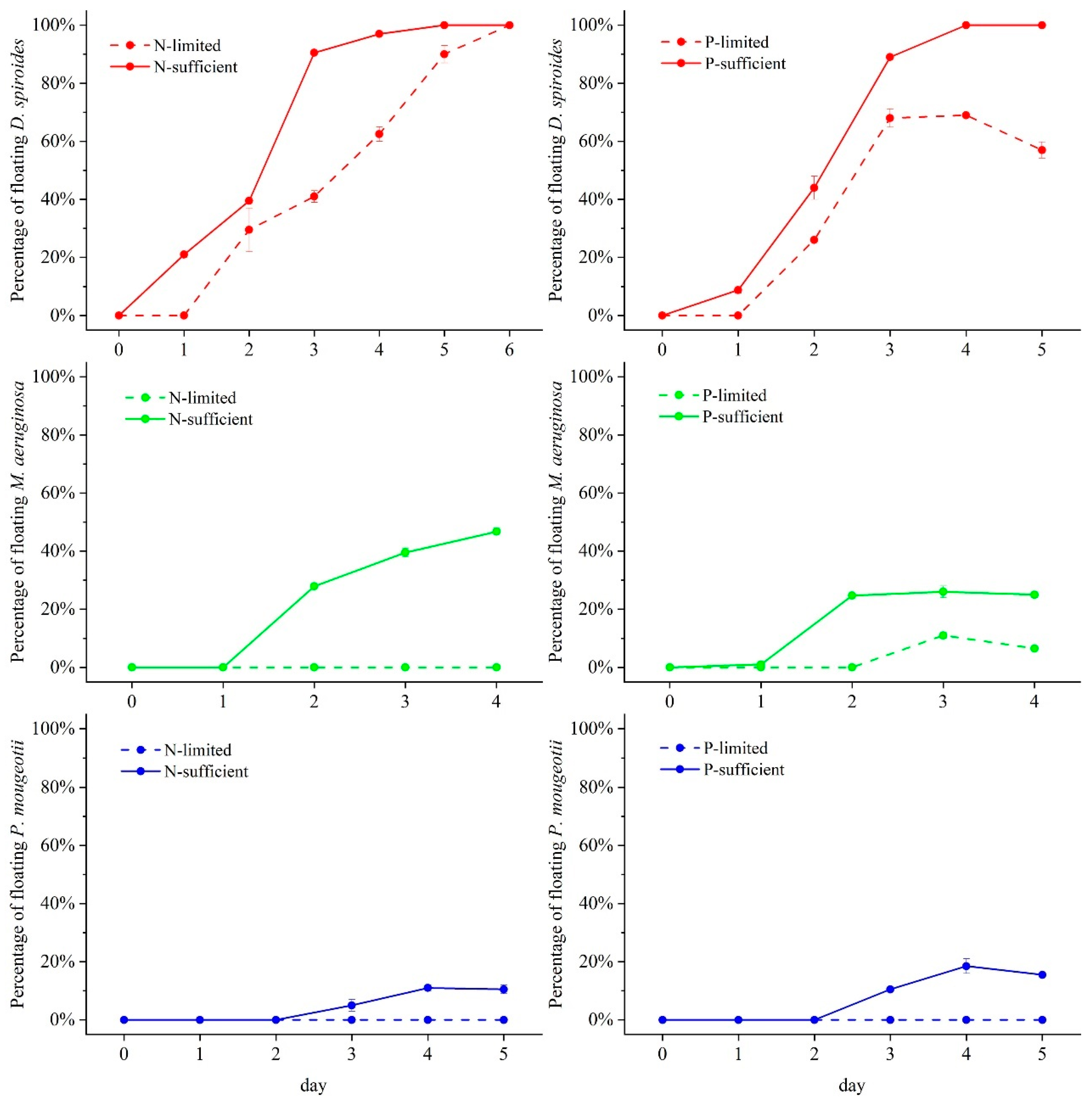
3.3. Floating Percentage in Different Trophic Levels
4. Discussion
4.1. Effect of Nutrient Limitation on the Recovery of Cyanobacterial Floating Capacity
4.2. Characteristics of Different Cyanobacteria for Restoring Buoyancy
4.2.1. D. spiroides
4.2.2. M. aeruginosa
4.2.3. P. mougeotii
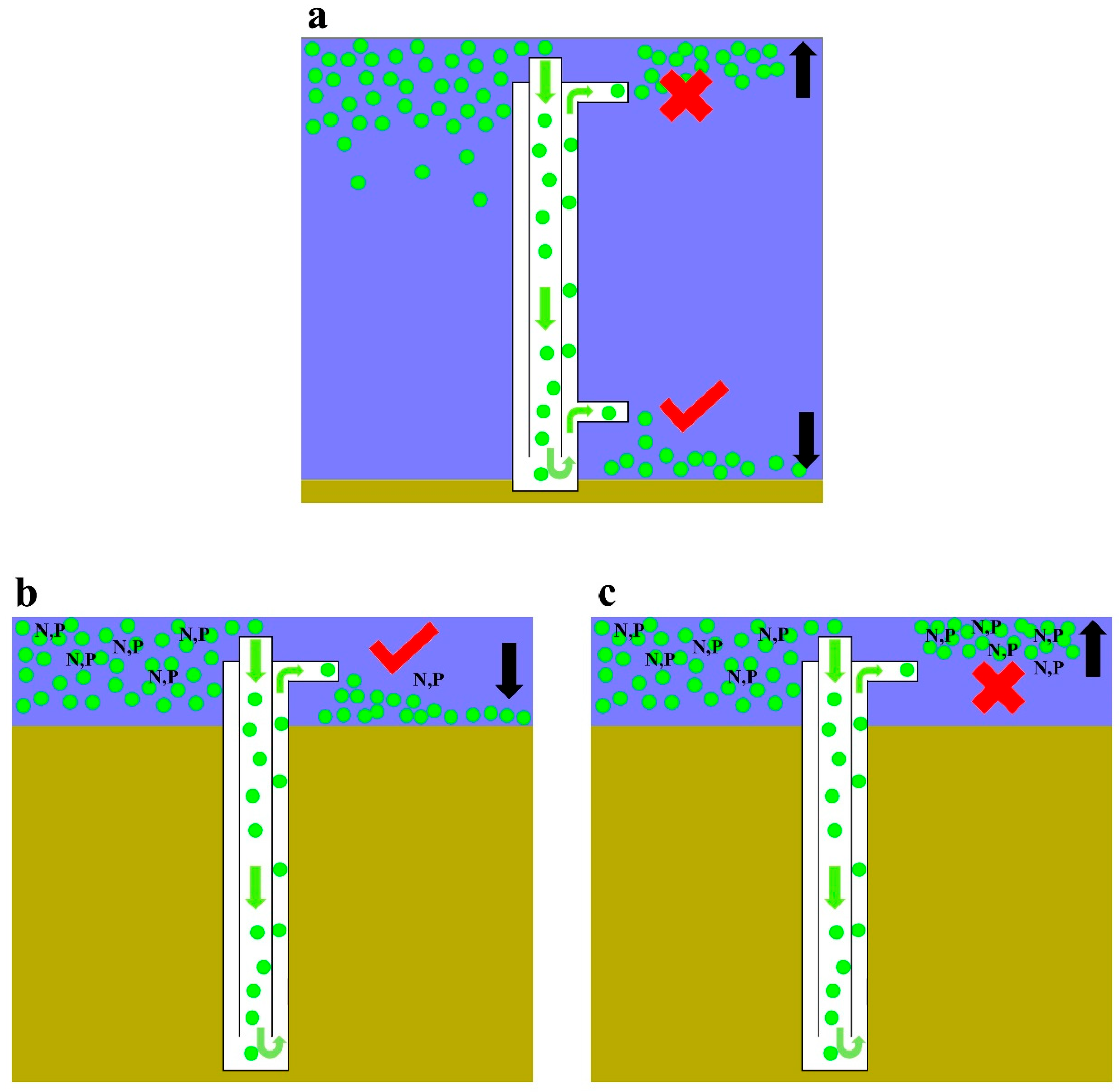
4.3. Implications for Lake Management
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qin, B.; Gao, G.; Zhu, G.; Zhang, Y.; Song, Y.; Tang, X.; Xu, H.; Deng, J. Lake eutrophication and its ecosystem response. Chin. Sci. Bull. 2013, 58, 961–970. [Google Scholar] [CrossRef]
- Wang, K.; Mou, X.Z.; Cao, H.S.; Struewing, I.; Allen, J.; Lu, J.R. Co-occurring microorganisms regulate the succession of cyanobacterial harmful algal blooms. Environ. Pollut. 2021, 288, 117682. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.; Paul, V. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef]
- Chen, J.; Xie, P.; Li, L.; Xu, J. First Identification of the Hepatotoxic Microcystins in the Serum of a Chronically Exposed Human Population Together with Indication of Hepatocellular Damage. Toxicol. Sci. 2009, 108, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.; Monis, P.; Baker, P.; Giglio, S. Biochemistry and genetics of taste- and odor-producing cyanobacteria. Harmful Algae 2016, 54, 112–127. [Google Scholar] [CrossRef]
- Yang, M.; Yu, J.; Li, Z.; Guo, Z.; Burch, M.; Lin, T.-F. Taihu Lake Not to Blame for Wuxi’s Woes. Science 2008, 319, 158. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Razzano, M.; Mou, X.Z. Cyanobacterial blooms alter the relative importance of neutral and selective processes in assembling freshwater bacterioplankton community. Sci. Total Environ. 2020, 706, 135724. [Google Scholar] [CrossRef]
- Ho, J.; Michalak, A.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef]
- Paerl, H.; Huisman, J. Blooms Like It Hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef]
- Chang, H.; Feng, H.; Wang, R.; Zhang, X.; Wang, J.; Li, C.; Zhang, Y.; Li, L.; Ho, S.-H. Enhanced energy recovery from landfill leachate by linking light and dark bio-reactions: Underlying synergistic effects of dual microalgal interaction. Water Res. 2023, 231, 119578. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.X.; Wu, H.H.; Zhang, L.; Wu, W.B.; Zhang, C.F.; Zhong, N.B.; Zhong, D.J.; Xu, Y.L.; He, X.F.; Yang, J.; et al. Gradient electro-processing strategy for efficient conversion of harmful algal blooms to biohythane with mechanisms insight. Water Res. 2022, 222, 118929. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.; Otten, T. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Gao, G. Hypothesis on cyanobacteria bloom-forming mechanism in large shallow eutrophic lakes. Acta Ecol. Sin. 2005, 25, 589–595. [Google Scholar]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Pfeifer, F. Distribution, formation and regulation of gas vesicles. Nat. Rev. Microbiol. 2012, 10, 705–715. [Google Scholar] [CrossRef]
- Serizawa, H.; Amemiya, T.; Itoh, K. Effects of buoyancy, transparency and zooplankton feeding on surface maxima and deep maxima: Comprehensive mathematical model for vertical distribution in cyanobacterial biomass. Ecol. Model. 2010, 221, 2028–2037. [Google Scholar] [CrossRef]
- Filstrup, C.T.; Heathcote, A.J.; Kendall, D.L.; Downing, J.A. Phytoplankton taxonomic compositional shifts across nutrient and light gradients in temperate lakes. Inland Waters 2016, 6, 234–249. [Google Scholar] [CrossRef]
- Clarke, K.B.; Walsby, A.E. Treatment of Water to Remove Gas Vacoulate Cyanobacteria; United States Patent and Trademark Office: Alexandria, VA, USA, 1996. [Google Scholar]
- Abeynayaka, H.D.L.; Asaeda, T.; Kaneko, Y. Buoyancy Limitation of Filamentous Cyanobacteria under Prolonged Pressure due to the Gas Vesicles Collapse. Environ. Manag. 2017, 60, 293–303. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, P.; Wang, B.; Liu, H. Ultrasonic frequency effects on the removal of Microcystis aeruginosa. Ultrason. Sonochem. 2006, 13, 446–450. [Google Scholar] [CrossRef]
- Rodriguez-Molares, A.; Dickson, S.; Hobson, P.; Howard, C.; Zander, A.; Burch, M. Quantification of the ultrasound induced sedimentation of Microcystis aeruginosa. Ultrason. Sonochem. 2014, 21, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Piepho, M.; Martin-Creuzburg, D.; Wacker, A. Simultaneous effects of light intensity and phosphorus supply on the sterol content of phytoplankton. PLoS ONE 2010, 5, e15828. [Google Scholar] [CrossRef] [PubMed]
- Walsby, A.E.; Kinsman, R.; George, K.I. The measurement of gas vesicle volume and buoyant density in planktonic bacteria. J. Microbiol. Methods 1992, 15, 293–309. [Google Scholar] [CrossRef]
- Chu, Z.; Jin, X.; Yang, B.; Zeng, Q. Buoyancy regulation of Microcystis flos-aquae during phosphorus-limited and nitrogen-limited growth. J. Plankton Res. 2007, 29, 739–745. [Google Scholar] [CrossRef]
- Tashiro, Y.; Monson, R.E.; Ramsay, J.P.; Salmond, G.P.C. Molecular genetic and physical analysis of gas vesicles in buoyant enterobacteria. Environ. Microbiol. 2016, 18, 1264–1276. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Miao, R.; Huang, L.; Jiang, H.; Cheng, Y. Vegetative cells may perform nitrogen fixation function under nitrogen deprivation in Anabaena sp. strain PCC 7120 based on genome-wide differential expression analysis. PLoS ONE 2021, 16, e0248155. [Google Scholar] [CrossRef]
- Walsby, A.E. Gas vesicles. Microbiol. Rev. 1994, 58, 94–144. [Google Scholar] [CrossRef]
- Wacker, A.; Piepho, M.; Spijkerman, E. Photosynthetic and fatty acid acclimation of four phytoplankton species in response to light intensity and phosphorus availability. Eur. J. Phycol. 2015, 50, 288–300. [Google Scholar] [CrossRef]
- Maat, D.S.; de Blok, R.; Brussaard, C.P.D. Combined Phosphorus Limitation and Light Stress Prevent Viral Proliferation in the Phytoplankton Species Phaeocystis globosa, but Not in Micromonas pusilla. Front. Mar. Sci. 2016, 3, 160. [Google Scholar] [CrossRef]
- Cook, K.V.; Li, C.; Cai, H.; Krumholz, L.R.; Hambright, K.D.; Paerl, H.W.; Steffen, M.M.; Wilson, A.E.; Burford, M.A.; Grossart, H.P.; et al. The global Microcystis interactome. Limnol. Oceanogr. 2020, 65, S194–S207. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Cao, X.; Zhou, Z.; Wang, S.; Xiao, J.; Song, C.; Zhou, Y. Community composition specificity and potential role of phosphorus solubilizing bacteria attached on the different bloom-forming cyanobacteria. Microbiol. Res. 2017, 205, 59–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, B.; Shi, K.; Zhang, Y.; Deng, J.; Wild, M.; Li, L.; Zhou, Y.; Yao, X.; Liu, M.; et al. Radiation dimming and decreasing water clarity fuel underwater darkening in lakes. Sci. Bull. 2020, 65, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Schelske, C.L.; Lowe, E.F.; Kenney, W.F.; Battoe, L.E.; Brenner, M.; Coveney, M.F. How anthropogenic darkening of Lake Apopka induced benthic light limitation and forced the shift from macrophyte to phytoplankton dominance. Limnol. Oceanogr. 2010, 55, 1201–1212. [Google Scholar] [CrossRef]
- Wang, C.; Hou, Z.; Chu, Z.; Wang, P.; Cao, J.; Yang, Y. Vertical Distribution of Microcystis and Its Influence Factors in Lake Erhai during High Risk Period for Algal Bloom. Res. Environ. Sci. 2018, 31, 1250–1257. [Google Scholar]
- Li, M.; Zhu, W.; Guo, L.; Hu, J.; Chen, H.; Xiao, M. To increase size or decrease density? Different Microcystis species has different choice to form blooms. Sci. Rep. 2016, 6, 37056. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, M.; Luo, Y.; Dai, X.; Guo, L.; Xiao, M.; Huang, J.; Tan, X. Vertical distribution of Microcystis colony size in Lake Taihu: Its role in algal blooms. J. Great Lakes Res. 2014, 40, 949–955. [Google Scholar] [CrossRef]
- Su, C. Investigation on the Exopolysaccharide from Marine Cyanothece sp. 133. Ph.D. Thesis, Ocean University of China, Qingdao, China, 2005. [Google Scholar]
- Jacquet, S.; Briand, J.F.; Leboulanger, C.; Avois-Jacquet, C.; Oberhaus, L.; Tassin, B.; Vincon-Leite, B.; Paolini, G.; Druart, J.C.; Anneville, O.; et al. The proliferation of the toxic cyanobacterium Planktothrix rubescens following restoration of the largest natural French lake (Lac du Bourget). Harmful Algae 2005, 4, 651–672. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S. Towards a functional classification of the freshwater phytoplankton. J. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Hong, J.; Huang, Y.; Liang, C.; Shen, L.; Li, G.; Chen, X. The effect of light on the specific density of Microcystis and its regulation mechanism. Environ. Sci. Technol. 2019, 42, 1–7. [Google Scholar] [CrossRef]
- Walsby, A.E. Homeostasis in Buoyancy Regulation by Planktonic Cyanobacteria. FEMS Symposium; Bath University Press: Bath, UK, 1988. [Google Scholar]
- Kinsman, R.; Ibelings, B.W.; Walsby, A.E. Gas vesicle collapse by turgor pressure and its role in buoyancy regulation by Anabaena flos-aquae. J. Gen. Microbiol. 1991, 137, 1171–1178. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Dai, R.; Chu, Z.; Cao, J. Rapid Recovery of Buoyancy in Eutrophic Environments Indicates That Cyanobacterial Blooms Cannot Be Effectively Controlled by Simply Collapsing Gas Vesicles Alone. Water 2023, 15, 1898. https://doi.org/10.3390/w15101898
Wu T, Dai R, Chu Z, Cao J. Rapid Recovery of Buoyancy in Eutrophic Environments Indicates That Cyanobacterial Blooms Cannot Be Effectively Controlled by Simply Collapsing Gas Vesicles Alone. Water. 2023; 15(10):1898. https://doi.org/10.3390/w15101898
Chicago/Turabian StyleWu, Tianhao, Ran Dai, Zhaosheng Chu, and Jing Cao. 2023. "Rapid Recovery of Buoyancy in Eutrophic Environments Indicates That Cyanobacterial Blooms Cannot Be Effectively Controlled by Simply Collapsing Gas Vesicles Alone" Water 15, no. 10: 1898. https://doi.org/10.3390/w15101898



