Comparison of Phenol Adsorption Property and Mechanism onto Different Moroccan Clays
Abstract
:1. Introduction
2. Materials and Methods
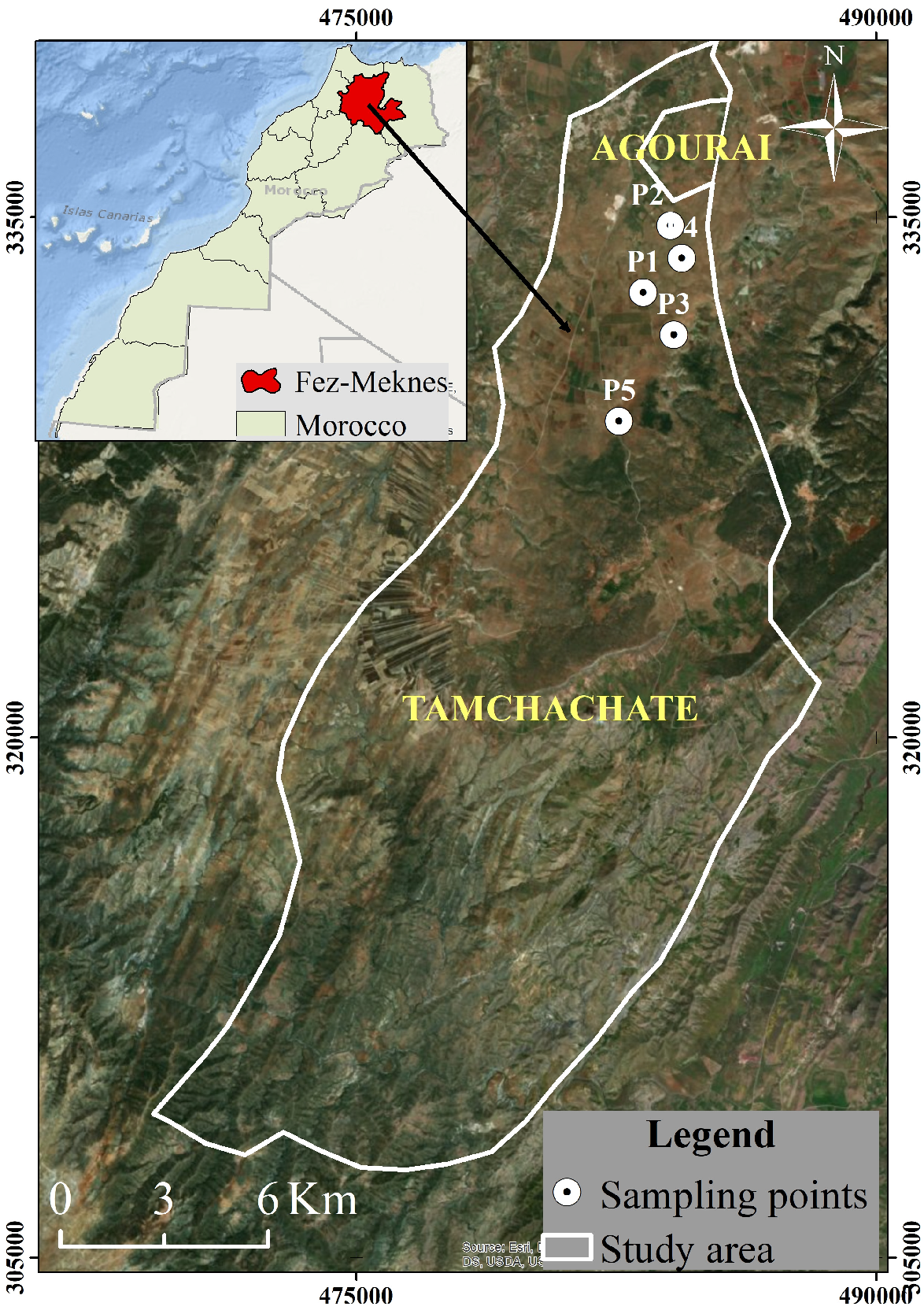
2.1. Sampling Area
2.2. Adsorption Batch
2.3. Kinetic Modelling
2.4. Isotherm Modeling through the Physic-Statistics Approach
2.4.1. Monolayer Model with Single Energy Site (MLO)
2.4.2. Monolayer Model with Two Energy Sites (MLT)
2.4.3. Double-Layer Model with One Energy Site (DLO)
2.4.4. Double-Layer Model with Two Energy Sites (DLT)
2.4.5. Multilayer Model (MM)
2.5. Parameter Estimation and Model Evaluation
2.6. Structure Characterization
3. Results
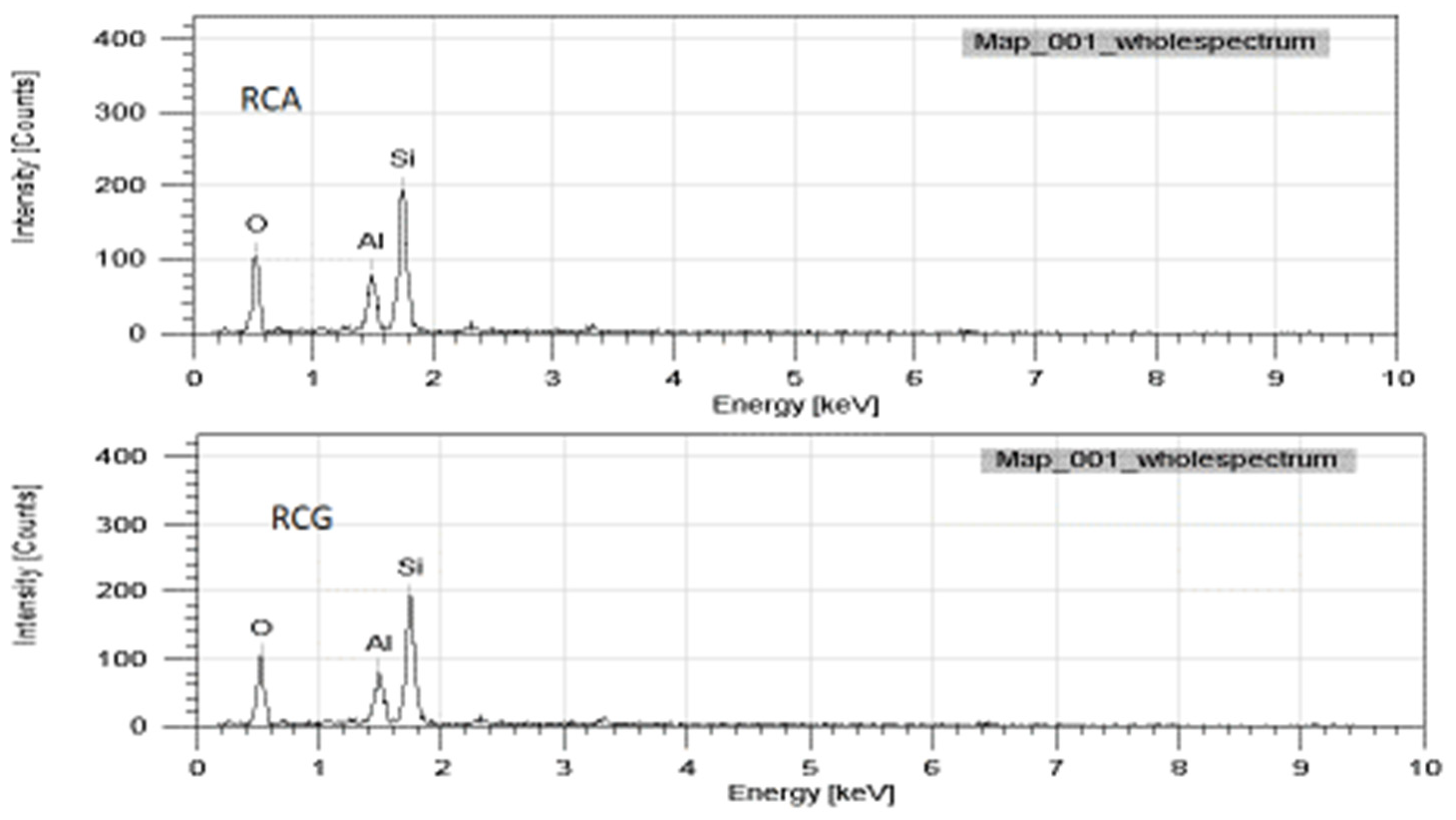
3.1. X-ray Fluorescence
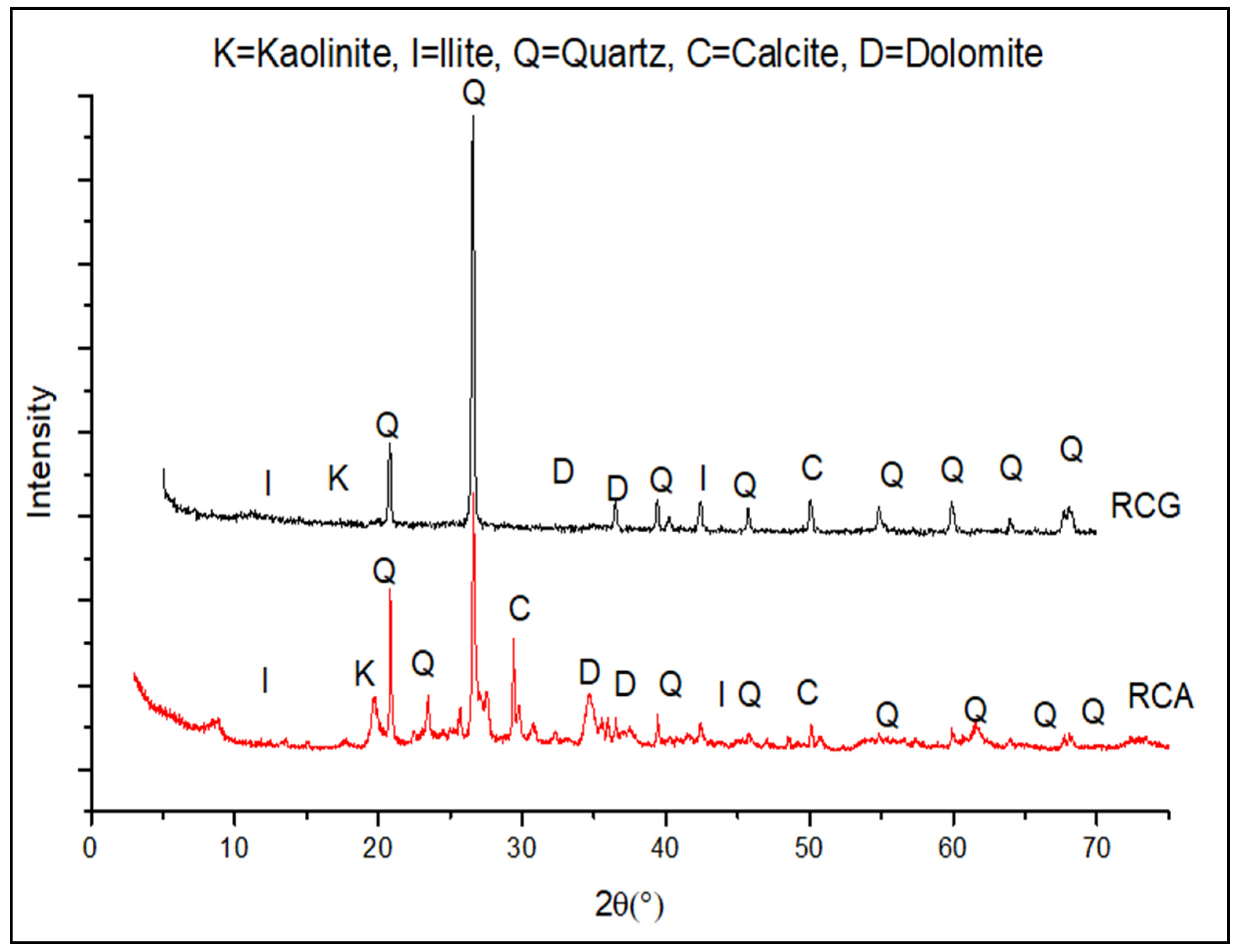
3.2. X-ray Patterns of RCA and RCG
3.3. FTIR Spectra of RCA and RCG
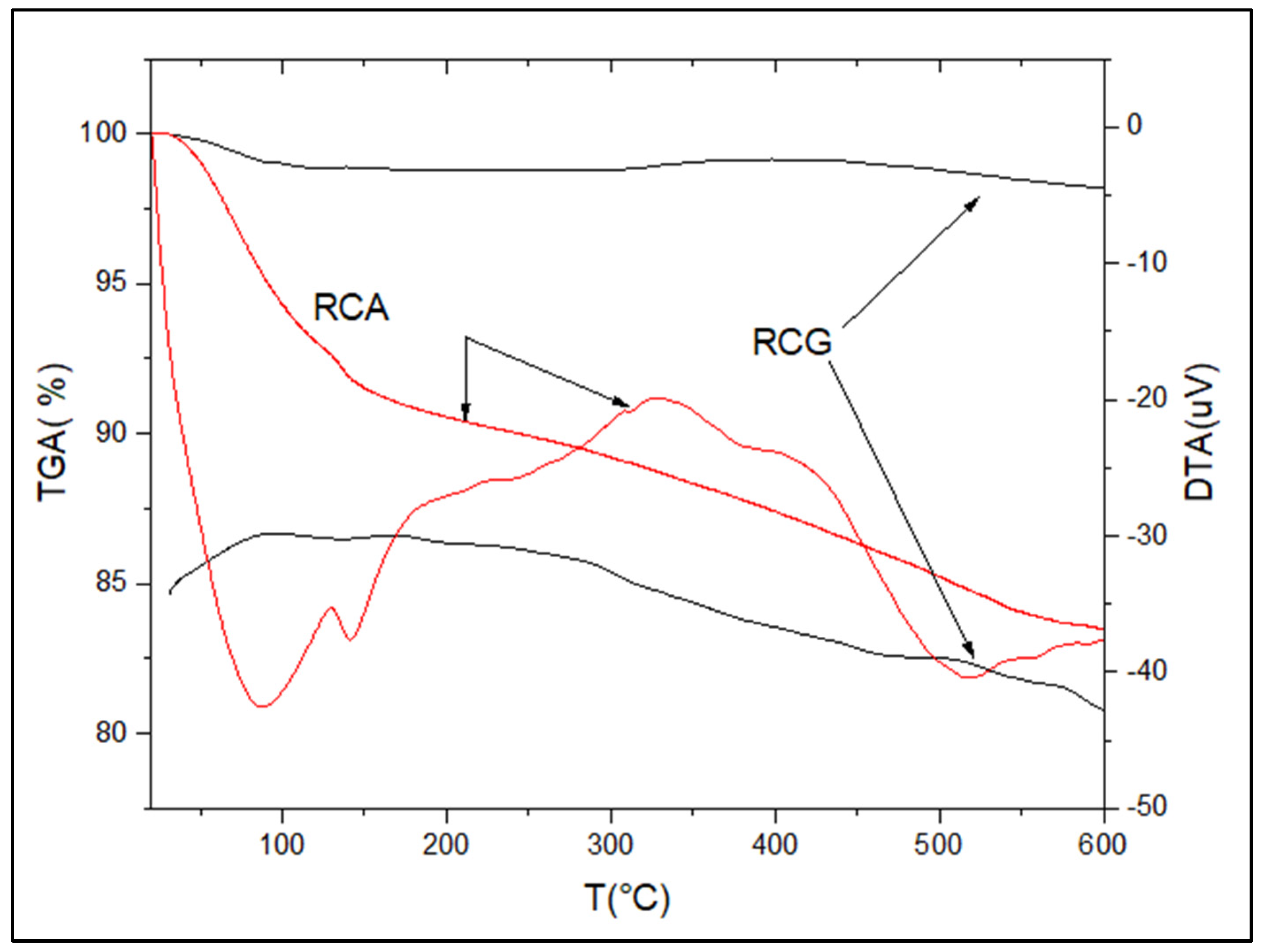
3.4. TGA/TDA
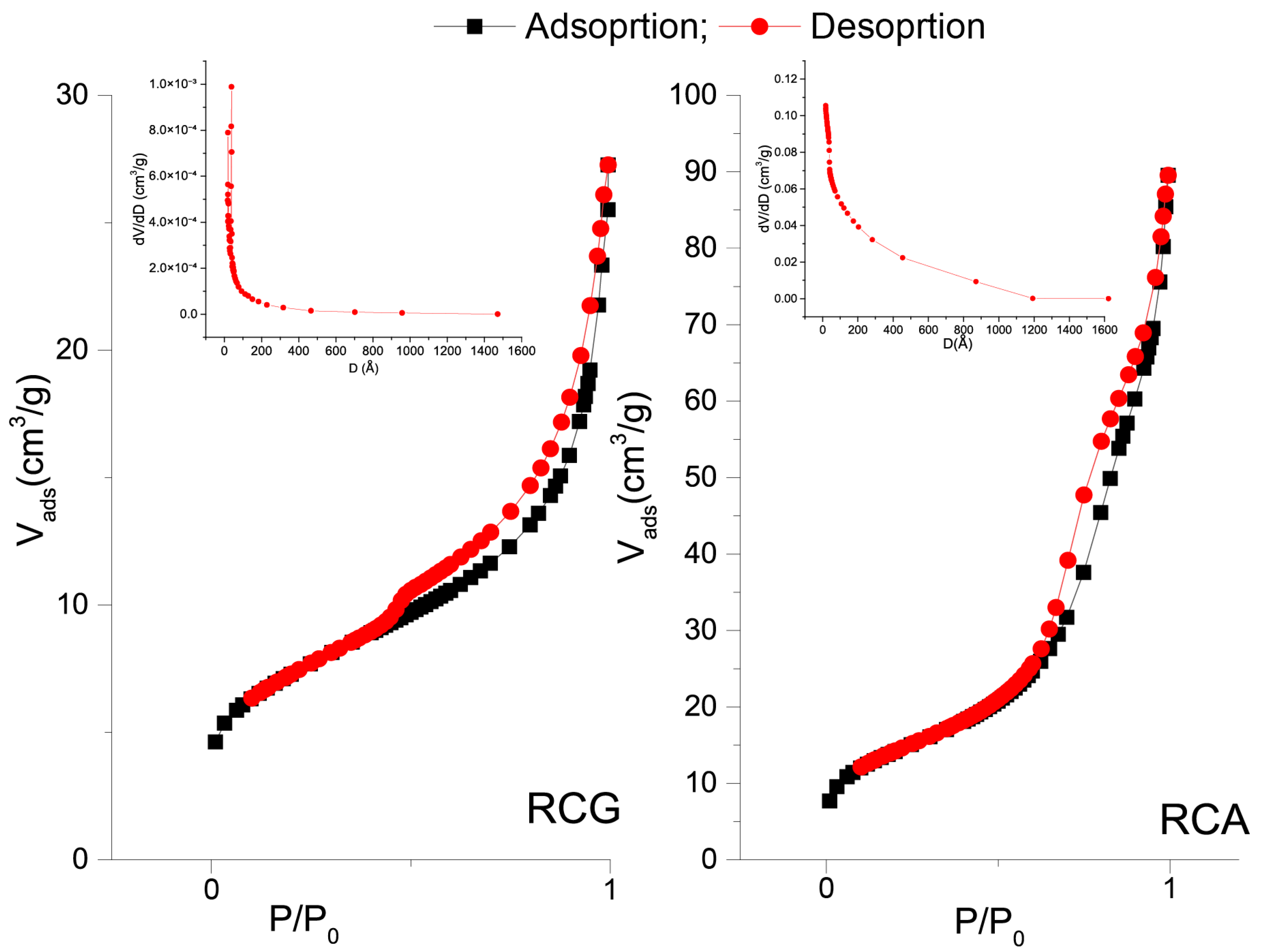
3.5. N2 Adsorption/Desorption Isotherm of RCA and RCG
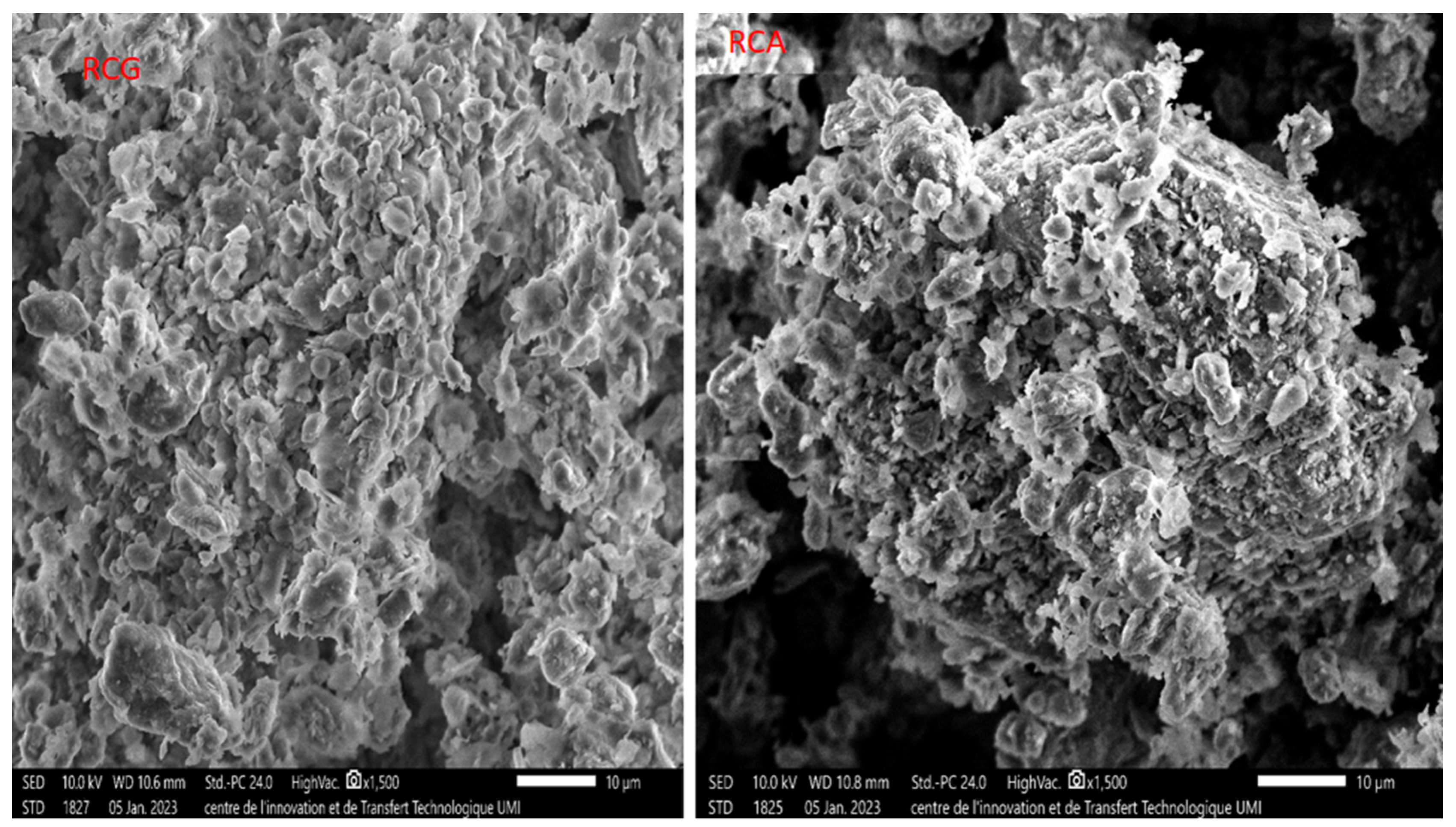
3.6. SEM
3.7. Point of Zero Charge
3.8. Determination of CEC
3.9. pH Effect
3.10. Adsorption of Phenol
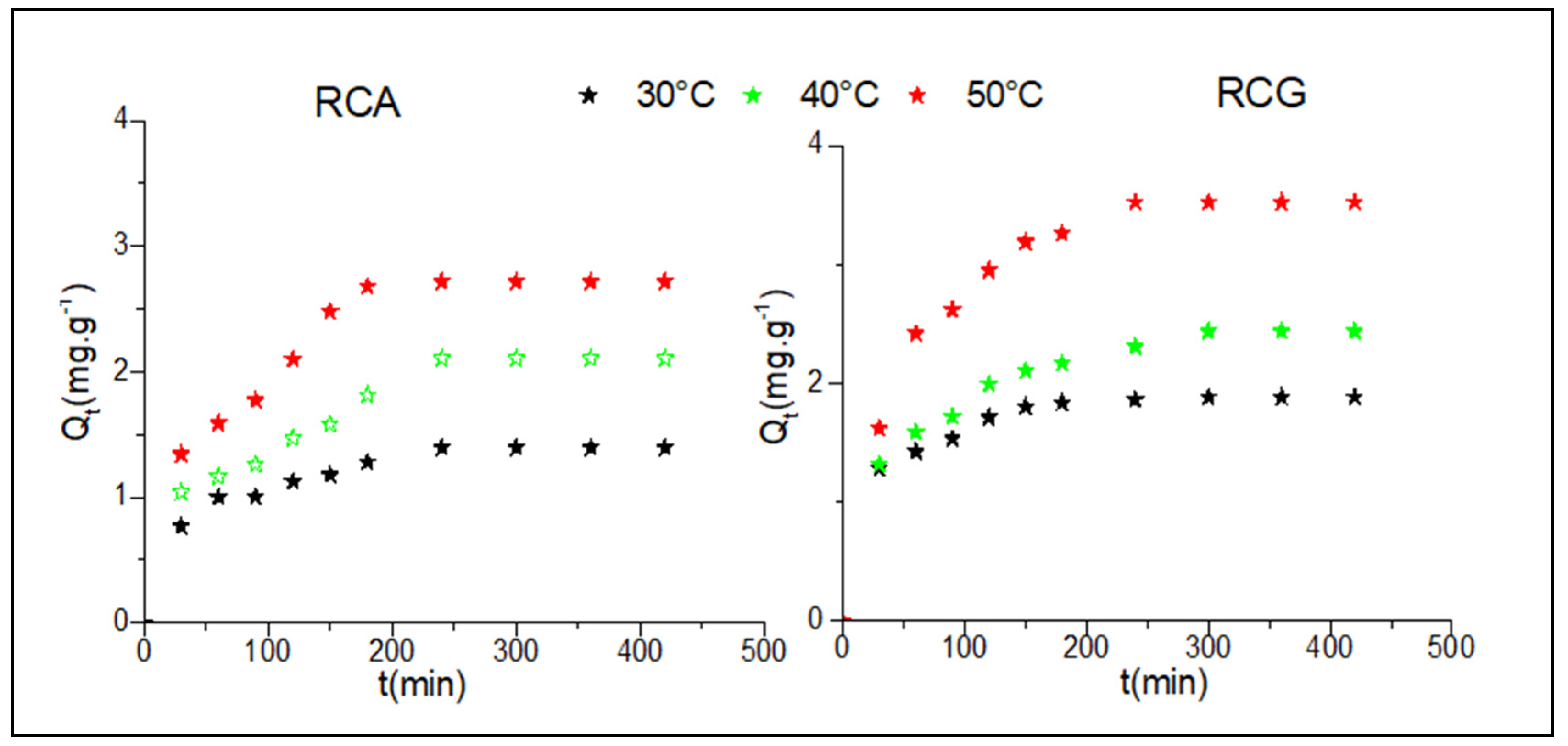
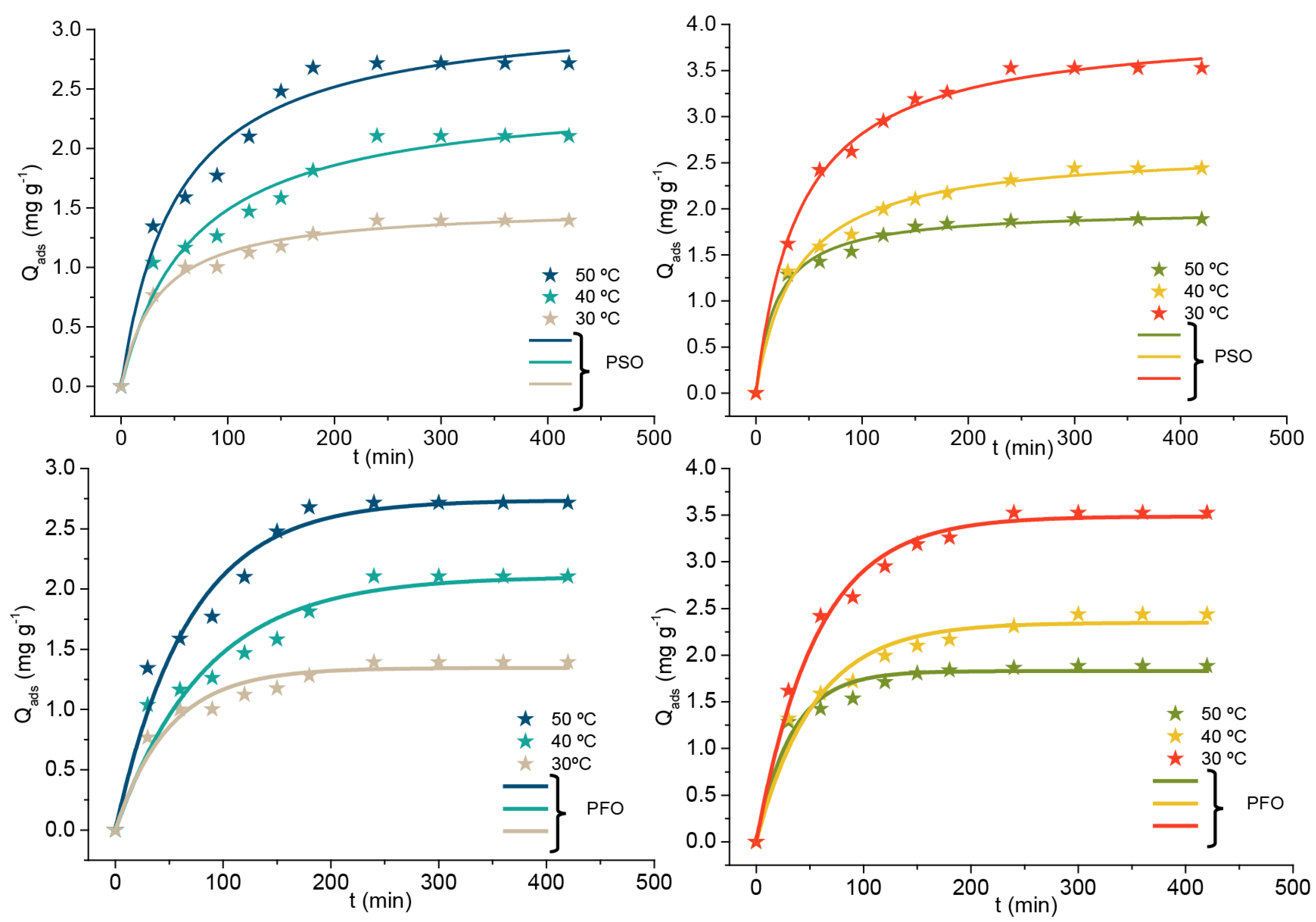
3.10.1. Adsorption Kinetics
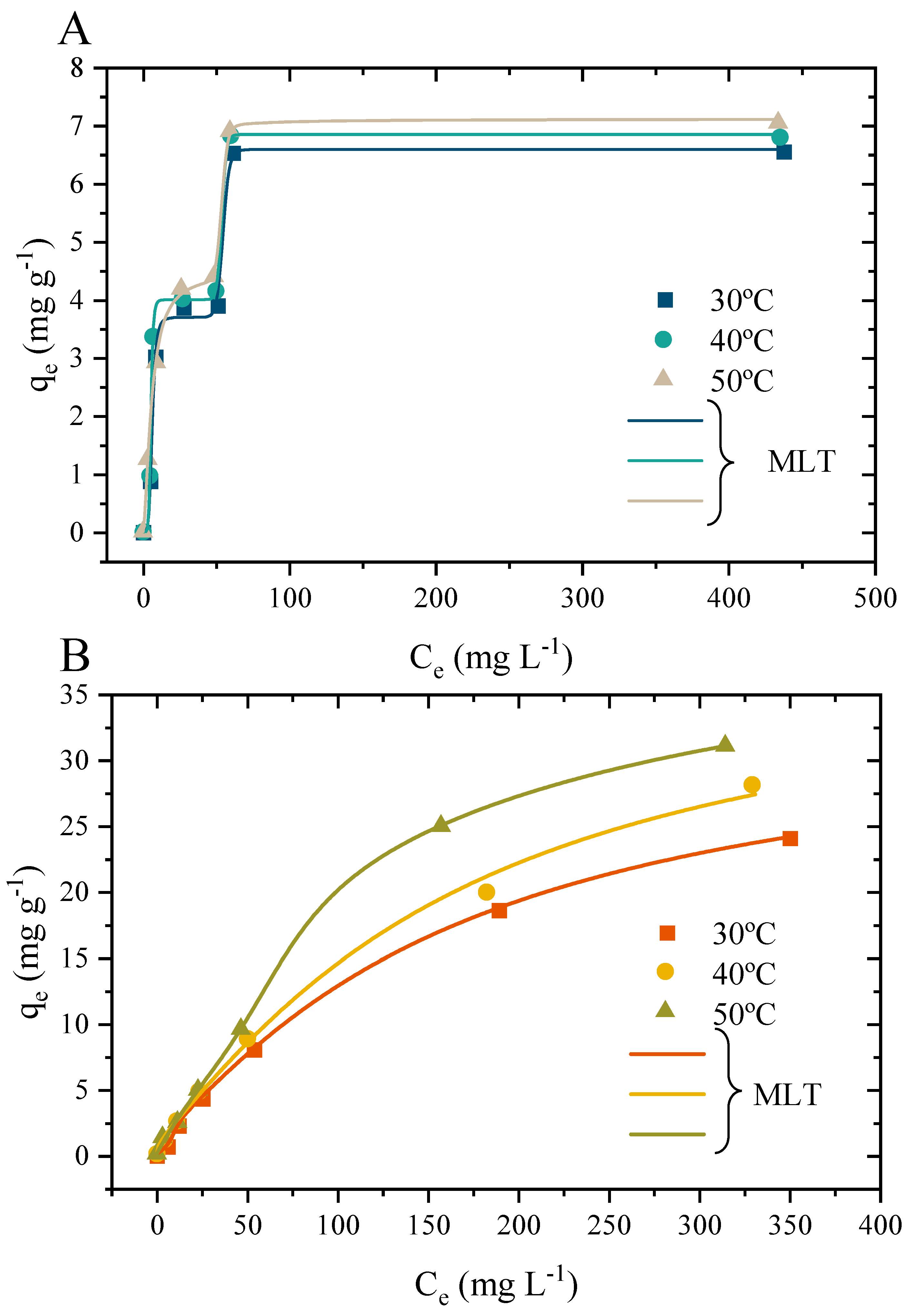
3.10.2. Adsorption Isotherms and Physical Statistical Interpretations
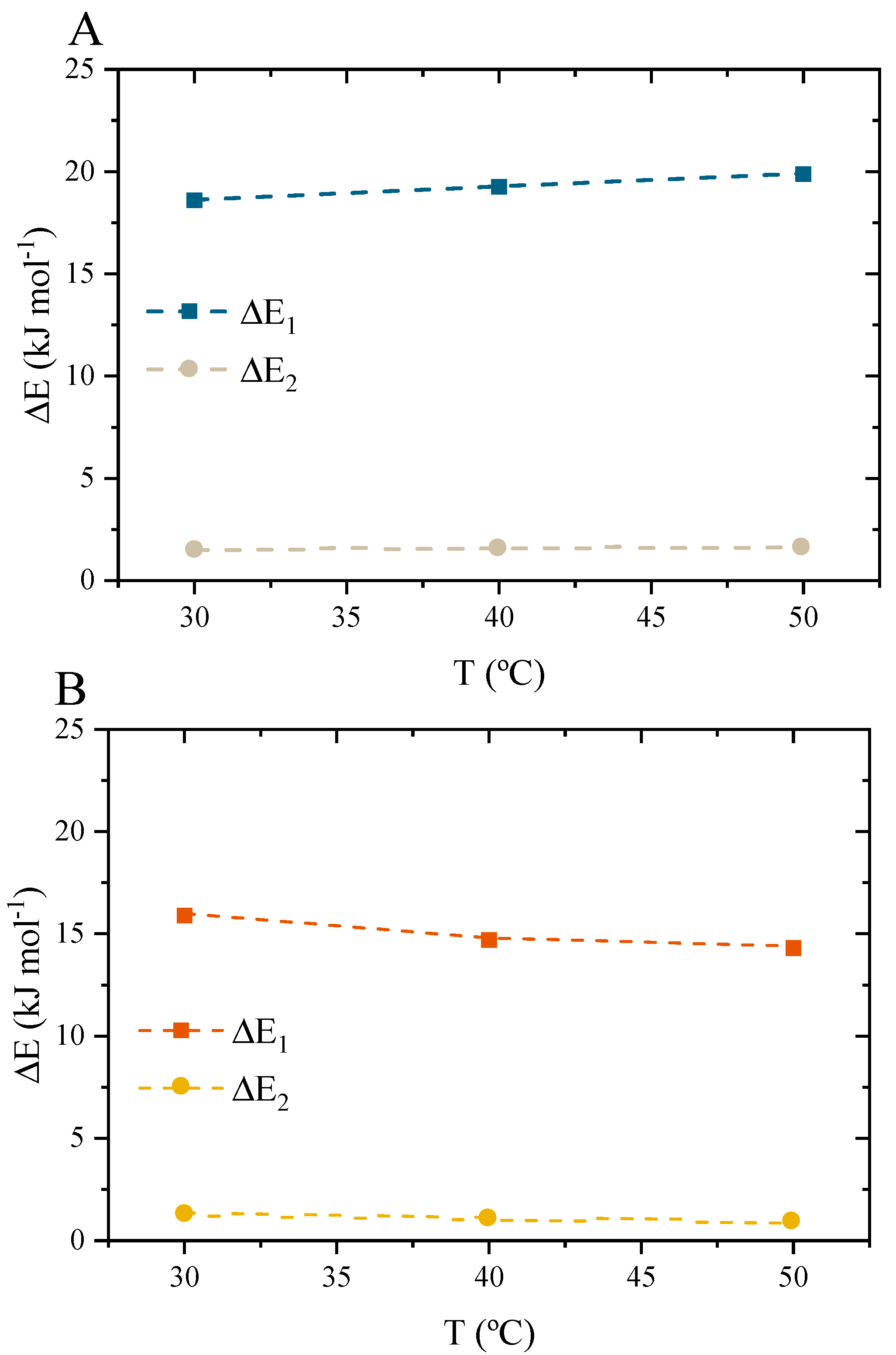
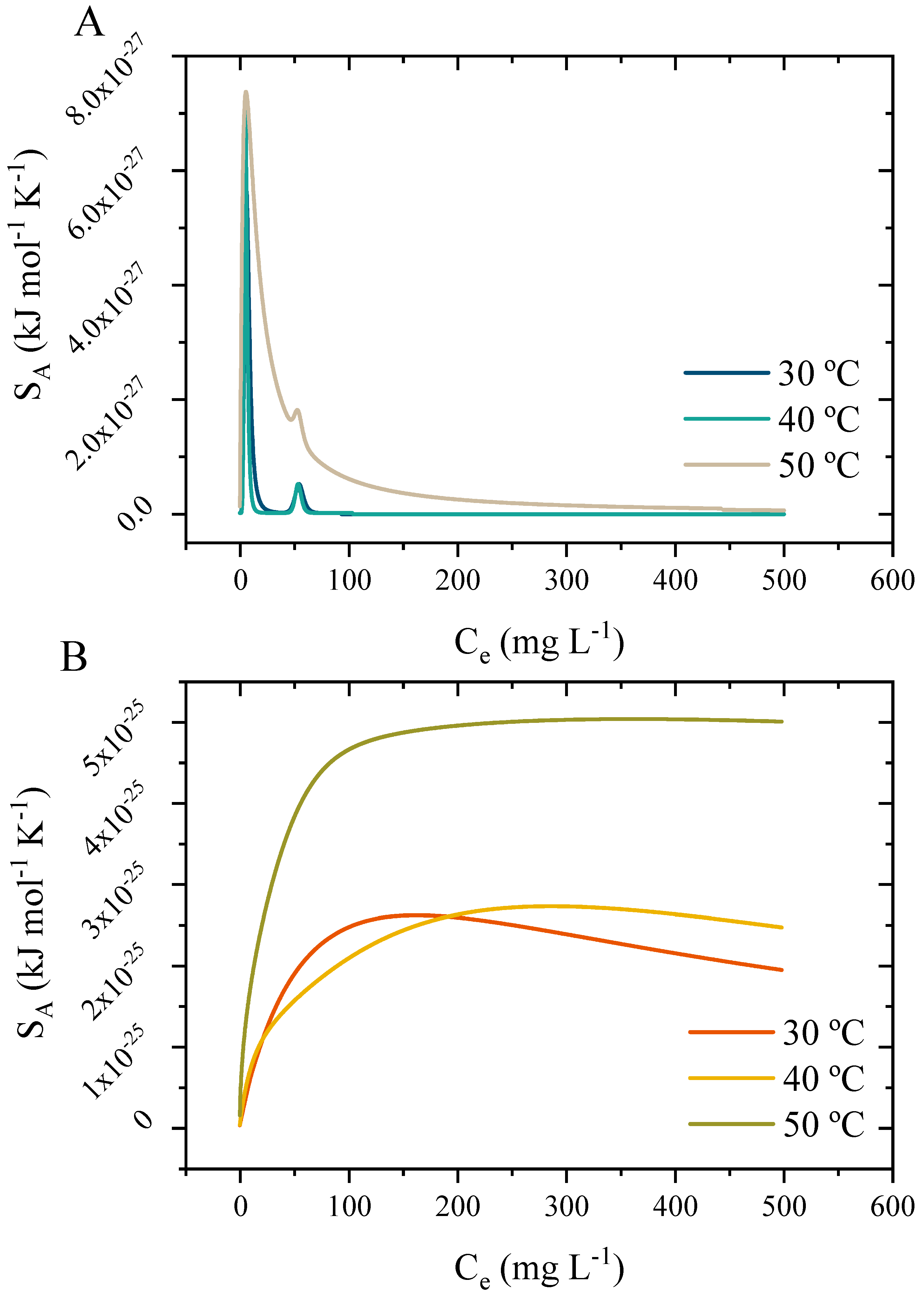
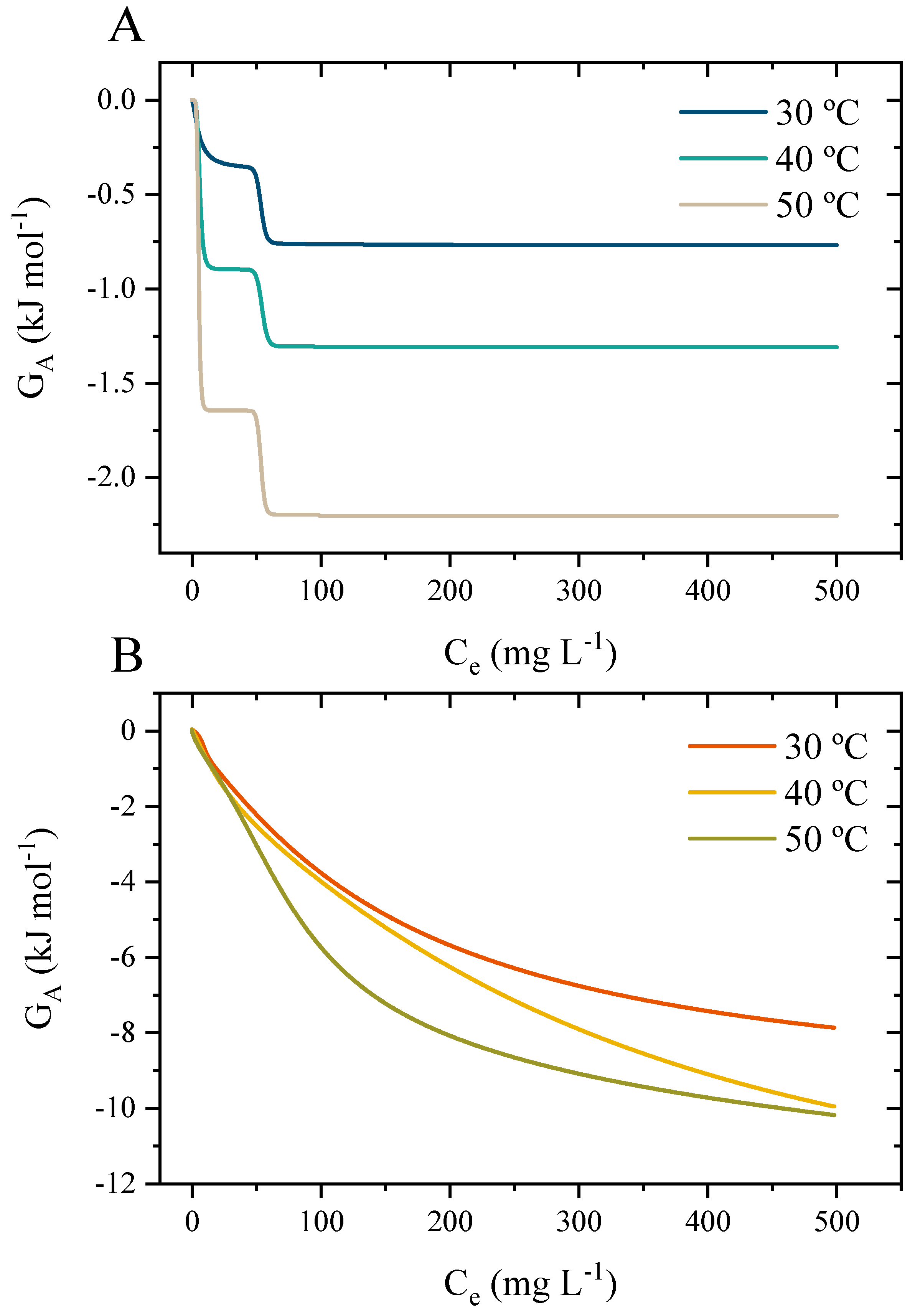
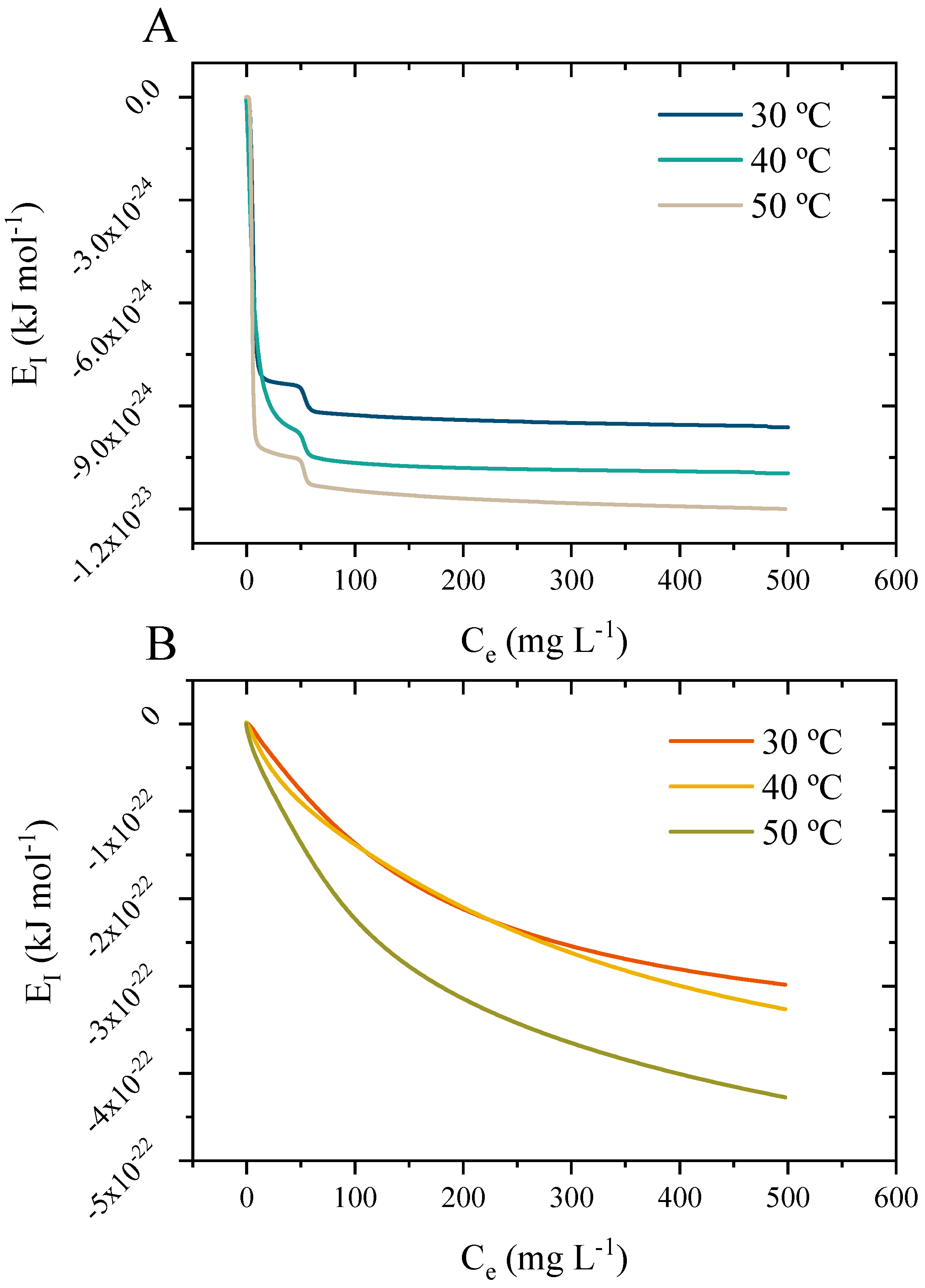
3.10.3. Application of the Stat-Phys Model with the Thermodynamic Potential Functions
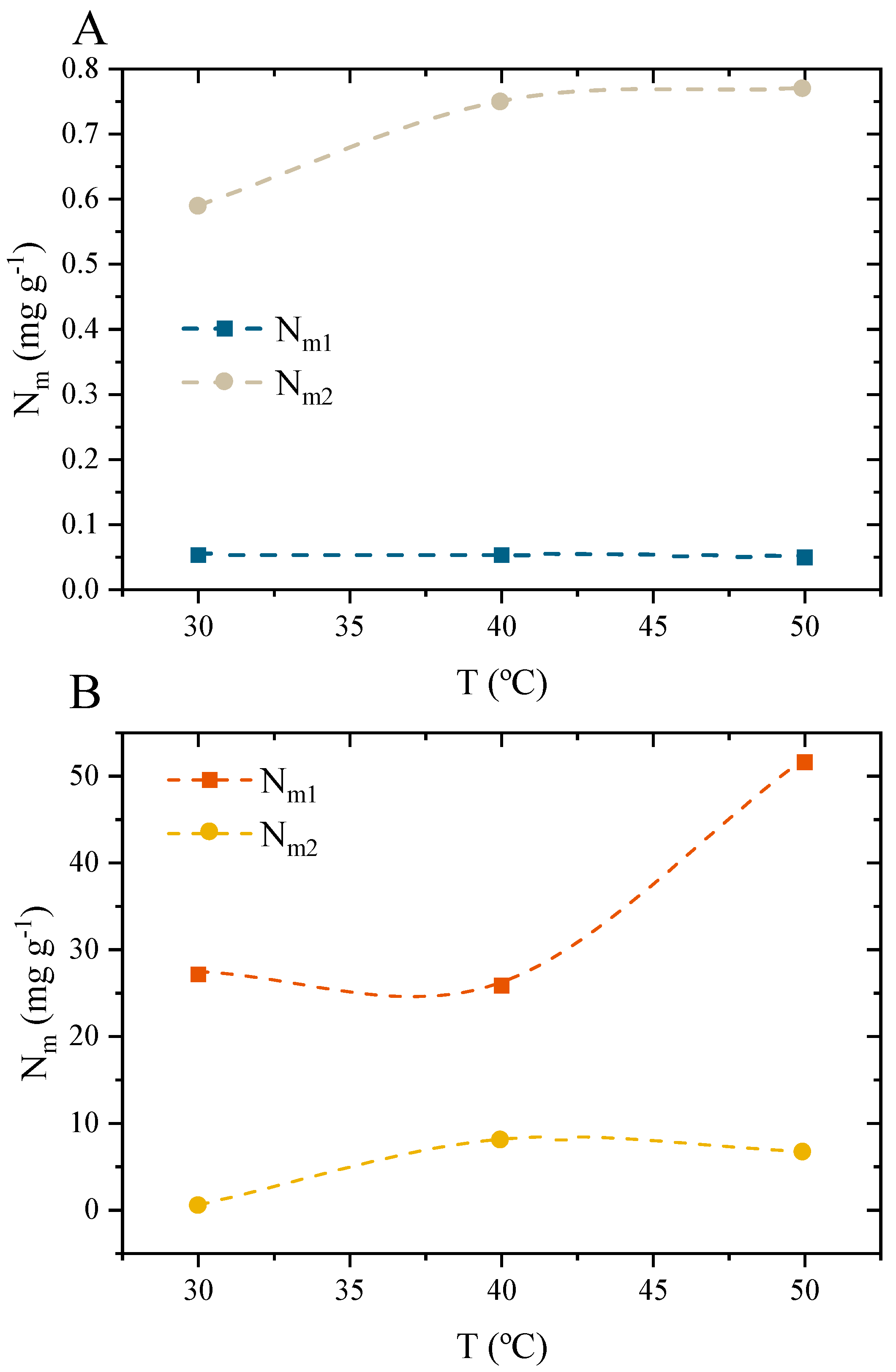
3.10.4. Interpretations of the Potential Function
3.10.5. Mechanism of Phenol Adsorption
- ➣
- Van der Waals force: Dipole-dipole attraction between atoms or molecules through low-level electrical interference. This attractive force is very important for the adsorption of organic substances such as phenol. We are well aware of the inhomogeneity of clay solids.
- ➣
- Coulomb force: The electrostatic force developed between a charged surface and an opposite charge. Surface charges can result from isostructural substitution or protonation or deprotonation of surface functional groups. Surface charge is determined by changes in the pH of the medium, and the pH at the point of zero charges can help us identify and assign the dominant charge on a clay surface.
- ➣
- The hydrogen bonds, or the role of H2O in the adsorption, have intermolecular interactions that occur between hydrogen atoms and electronegative atoms (O, F, S, Cl).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasan, M.K.; Shahriar, A.; Jim, K.U. Water pollution in Bangladesh and its impact on public health. Heliyon 2019, 5, e02145. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.N.; El-Shafey, E.; Al-Busafi, S.; Al-Lawati, H.A. Adsorption of chlorpheniramine and ibuprofen on surface functionalized activated carbons from deionized water and spiked hospital wastewater. J. Environ. Chem. Eng. 2018, 7, 102860. [Google Scholar] [CrossRef]
- Mohammed, B.B.; Yamni, K.; Tijani, N.; Alrashdi, A.A.; Zouihri, H.; Dehmani, Y.; Chung, I.-M.; Kim, S.-H.; Lgaz, H. Adsorptive removal of phenol using faujasite-type Y zeolite: Adsorption isotherms, kinetics and grand canonical Monte Carlo simulation studies. J. Mol. Liq. 2019, 296, 111997. [Google Scholar] [CrossRef]
- Dehbi, A.; Dehmani, Y.; Omari, H.; Lammini, A.; Elazhari, K.; Abdallaoui, A. Hematite iron oxide nanoparticles (α-Fe2O3): Synthesis and modelling adsorption of malachite green. J. Environ. Chem. Eng. 2019, 8, 103394. [Google Scholar] [CrossRef]
- Dehbi, A.; Dehmani, Y.; Omari, H.; Lammini, A.; Elazhari, K.; Abouarnadasse, S.; Abdallaoui, A. Comparative study of malachite green and phenol adsorption on synthetic hematite iron oxide nanoparticles (α-Fe2O3). Surf. Interfaces 2020, 21, 100637. [Google Scholar] [CrossRef]
- Dehmani, Y.; Dridi, D.; Lamhasni, T.; Abouarnadasse, S.; Chtourou, R.; Lima, E.C. Review of phenol adsorption on transition metal oxides and other adsorbents. J. Water Process. Eng. 2022, 49, 102965. [Google Scholar] [CrossRef]
- Awad, A.M.; Shaikh, S.M.; Jalab, R.; Gulied, M.H.; Nasser, M.S.; Benamor, A.; Adham, S. Adsorption of organic pollutants by natural and modified clays: A comprehensive review. Sep. Purif. Technol. 2019, 228, 115719. [Google Scholar] [CrossRef]
- Tariq, R.; Abatal, M.; Bassam, A. Computational intelligence for empirical modeling and optimization of methylene blue adsorption phenomena using available local zeolites and clay of Morocco. J. Clean. Prod. 2022, 370, 133517. [Google Scholar] [CrossRef]
- Es-Sahbany, H.; El Hachimi, M.; Hsissou, R.; Belfaquir, M.; Nkhili, S.; Loutfi, M.; Elyoubi, M. Adsorption of heavy metal (Cadmium) in synthetic wastewater by the natural clay as a potential adsorbent (Tangier-Tetouan-Al Hoceima—Morocco region). Mater. Today Proc. 2021, 45, 7299–7305. [Google Scholar] [CrossRef]
- Azarkan, S.; Peña, A.; Draoui, K.; Sainz-Díaz, C.I. Adsorption of two fungicides on natural clays of Morocco. Appl. Clay Sci. 2016, 123, 37–46. [Google Scholar] [CrossRef]
- Gładysz-Płaska, A.; Majdan, M.; Pikus, S.; Sternik, D. Simultaneous adsorption of chromium(VI) and phenol on natural red clay modified by HDTMA. Chem. Eng. J. 2012, 179, 140–150. [Google Scholar] [CrossRef]
- Chaari, I.; Fakhfakh, E.; Medhioub, M.; Jamoussi, F. Comparative study on adsorption of cationic and anionic dyes by smectite rich natural clays. J. Mol. Struct. 2018, 1179, 672–677. [Google Scholar] [CrossRef]
- Wang, M.; Hearon, S.E.; Johnson, N.M.; Phillips, T.D. Development of broad-acting clays for the tight adsorption of benzo[a]pyrene and aldicarb. Appl. Clay Sci. 2018, 168, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Nazli, Z.; Nawaz, H.; Bhatti, H.N.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Bentahar, Y.; Hurel, C.; Draoui, K.; Khairoun, S.; Marmier, N. Adsorptive properties of Moroccan clays for the removal of arsenic(V) from aqueous solution. Appl. Clay Sci. 2016, 119, 385–392. [Google Scholar] [CrossRef]
- Nabbou, N.; Belhachemi, M.; Boumelik, M.; Merzougui, T.; Lahcene, D.; Harek, Y.; Zorpas, A.A.; Jeguirim, M. Removal of fluoride from groundwater using natural clay (kaolinite): Optimization of adsorption conditions. Comptes Rendus Chim. 2018, 22, 105–112. [Google Scholar] [CrossRef]
- Ouallal, H.; Dehmani, Y.; Moussout, H.; Messaoudi, L.; Azrour, M. Kinetic, isotherm and mechanism investigations of the removal of phenols from water by raw and calcined clays. Heliyon 2019, 5, e01616. [Google Scholar] [CrossRef]
- Khalfaoui, M.; Baouab, M.; Gauthier, R.; Ben Lamine, A. Statistical Physics Modelling of Dye Adsorption on Modified Cotton. Adsorpt. Sci. Technol. 2002, 20, 17–31. [Google Scholar] [CrossRef]
- Knani, S.; Mathlouthi, M.; Ben Lamine, A. Modeling of the Psychophysical Response Curves Using the Grand Canonical Ensemble in Statistical Physics. Food Biophys. 2007, 2, 183–192. [Google Scholar] [CrossRef]
- Khalfaoui, M.; Baouab, M.; Gauthier, R.; Ben Lamine, A. Dye Adsorption by Modified Cotton. Steric and Energetic Interpretations of Model Parameter Behaviours. Adsorpt. Sci. Technol. 2002, 20, 33–47. [Google Scholar] [CrossRef]
- Hua, P.; Sellaoui, L.; Franco, D.; Netto, M.S.; Dotto, G.L.; Bajahzar, A.; Belmabrouk, H.; Bonilla-Petriciolet, A.; Li, Z. Adsorption of acid green and procion red on a magnetic geopolymer based adsorbent: Experiments, characterization and theoretical treatment. Chem. Eng. J. 2019, 383, 123113. [Google Scholar] [CrossRef]
- Zhang, L.; Sellaoui, L.; Franco, D.; Dotto, G.L.; Bajahzar, A.; Belmabrouk, H.; Bonilla-Petriciolet, A.; Oliveira, M.L.; Li, Z. Adsorption of dyes brilliant blue, sunset yellow and tartrazine from aqueous solution on chitosan: Analytical interpretation via multilayer statistical physics model. Chem. Eng. J. 2019, 382, 122952. [Google Scholar] [CrossRef]
- Manni, A.; El, A.; El Amrani, I.; Hassani, E.; El, A.; Sadik, C. Valorization of coffee waste with Moroccan clay to produce a porous red ceramics (class BIII), Boletín La Soc. Española Cerámica Y Vidr. 2019, 58, 211–220. [Google Scholar] [CrossRef]
- Ba Mohammed, B.; Yamni, K.; Tijani, N.; Lee, H.-S.; Dehmani, Y.; El Hamdani, H.; Alrashdi, A.A.; Ramola, S.; Belwal, T.; Lgaz, H. Enhanced removal efficiency of NaY zeolite toward phenol from aqueous solution by modification with nickel (Ni-NaY). J. Saudi Chem. Soc. 2021, 25, 101224. [Google Scholar] [CrossRef]
- Jedli, H.; Brahmi, J.; Hedfi, H.; Mbarek, M.; Bouzgarrou, S.; Slimi, K. Adsorption kinetics and thermodynamics properties of Supercritical CO2 on activated clay. J. Pet. Sci. Eng. 2018, 166, 476–481. [Google Scholar] [CrossRef]
- Dehmani, Y.; Ed-Dra, A.; Zennouhi, O.; Bouymajane, A.; Filali, F.R.; Nassiri, L.; Abouarnadasse, S. Chemical characterization and adsorption of oil mill wastewater on Moroccan clay in order to be used in the agricultural field. Heliyon 2020, 6, e03164. [Google Scholar] [CrossRef]
- Hadjltaief, H.B.; Sdiri, A.; Ltaief, W.; Da Costa, P.; Gálvez, M.E.; Ben Zina, M. Efficient removal of cadmium and 2-chlorophenol in aqueous systems by natural clay: Adsorption and photo-Fenton degradation processes. Comptes Rendus Chim. 2018, 21, 253–262. [Google Scholar] [CrossRef]
- Ouaddari, H.; Beqqour, D.; Bennazha, J.; El Amrani, I.-E.; Albizane, A.; Solhy, A.; Varma, R.S. Natural Moroccan clays: Comparative study of their application as recyclable catalysts in Knoevenagel condensation. Sustain. Chem. Pharm. 2018, 10, 1–8. [Google Scholar] [CrossRef]
- Bentahar, S.; Dbik, A.; El Khomri, M.; El Messaoudi, N.; Lacherai, A. Adsorption of methylene blue, crystal violet and congo red from binary and ternary systems with natural clay: Kinetic, isotherm, and thermodynamic. J. Environ. Chem. Eng. 2017, 5, 5921–5932. [Google Scholar] [CrossRef]
- Bouna, L.; El Fakir, A.A.; Benlhachemi, A.; Draoui, K.; Villain, S.; Guinneton, F. Physico-chemical characterization of clays from Assa-Zag for valorization in cationic dye methylene blue adsorption. Mater. Today Proc. 2019, 22, 8–13. [Google Scholar] [CrossRef]
- Richards, S.; Bouazza, A. Phenol adsorption in organo-modified basaltic clay and bentonite. Appl. Clay Sci. 2007, 37, 133–142. [Google Scholar] [CrossRef]
- Pawar, R.R.; Lalhmunsiama; Gupta, P.; Sawant, S.Y.; Shahmoradi, B.; Lee, S.-M. Porous synthetic hectorite clay-alginate composite beads for effective adsorption of methylene blue dye from aqueous solution. Int. J. Biol. Macromol. 2018, 114, 1315–1324. [Google Scholar] [CrossRef]
- Gamoudi, S.; Srasra, E. Characterization of Tunisian clay suitable for pharmaceutical and cosmetic applications. Appl. Clay Sci. 2017, 146, 162–166. [Google Scholar] [CrossRef]
- Kragović, M.; Stojmenović, M.; Petrović, J.; Loredo, J.; Pašalić, S.; Nedeljković, A.; Ristović, I. Influence of Alginate Encapsulation on Point of Zero Charge (pHpzc) and Thermodynamic Properties of the Natural and Fe(III)—Modified Zeolite. Procedia Manuf. 2019, 32, 286–293. [Google Scholar] [CrossRef]
- Asuha, S.; Fei, F.; Wurendaodi, W.; Zhao, S.; Wu, H.; Zhuang, X. Activation of kaolinite by a low-temperature chemical method and its effect on methylene blue adsorption. Powder Technol. 2019, 361, 624–632. [Google Scholar] [CrossRef]
- Aran, D.; Maul, A.; Masfaraud, J.-F. A spectrophotometric measurement of soil cation exchange capacity based on cobaltihexamine chloride absorbance. Comptes Rendus Geosci. 2008, 340, 865–871. [Google Scholar] [CrossRef]
- Cheng, W.; Gao, W.; Cui, X.; Hong, J.; Feng, R. Phenol adsorption equilibrium and kinetics on zeolite X/activated. J. Taiwan Inst. Chem. Eng. 2016, 62, 192–198. [Google Scholar] [CrossRef]
- Selim, A.Q.; Sellaoui, L.; Mobarak, M. Statistical physics modeling of phosphate adsorption onto chemically modified carbonaceous clay. J. Mol. Liq. 2019, 279, 94–107. [Google Scholar] [CrossRef]
- Kong, X.; Gao, H.; Song, X.; Deng, Y.; Zhang, Y. Adsorption of phenol on porous carbon from Toona sinensis leaves and its mechanism. Chem. Phys. Lett. 2019, 739, 137046. [Google Scholar] [CrossRef]
- Franco, D.; Piccin, J.S.; Lima, E.C.; Dotto, G.L. Interpretations about methylene blue adsorption by surface modified chitin using the statistical physics treatment. Adsorption 2015, 21, 557–564. [Google Scholar] [CrossRef]
- Oueslati, K.; Naifar, A.; Sakly, A.; Kyzas, G.Z.; Ben Lamine, A. Statistical and physical interpretation of dye adsorption onto low-cost biomass by using simulation methods. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 646, 128969. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, E.; Kim, T.; Wang, J.; Hableel, G.; Reardon, P.J.T.; Ananthakrishna, S.J.; Wang, T.; Arconada-Alvarez, S.; Knowles, J.C.; et al. Organosilica Nanoparticles with an Intrinsic Secondary Amine: An Efficient and Reusable Adsorbent for Dyes. ACS Appl. Mater. Interfaces 2017, 9, 15566–15576. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gómez-Avilés, A.; Sellaoui, L.; Bedia, J.; Bonilla-Petriciolet, A.; Belver, C. Adsorption of ibuprofen on organo-sepiolite and on zeolite/sepiolite heterostructure: Synthesis, characterization and statistical physics modeling. Chem. Eng. J. 2019, 371, 868–875. [Google Scholar] [CrossRef]
- Hank, D.; Azi, Z.; Hocine, S.A.; Chaalal, O.; Hellal, A. Optimization of phenol adsorption onto bentonite by factorial design methodology. J. Ind. Eng. Chem. 2014, 20, 2256–2263. [Google Scholar] [CrossRef]
- Dehmani, Y.; Sellaoui, L.; Alghamdi, Y.; Lainé, J.; Badawi, M.; Amhoud, A.; Bonilla-Petriciolet, A.; Lamhasni, T.; Abouarnadasse, S. Kinetic, thermodynamic and mechanism study of the adsorption of phenol on Moroccan clay. J. Mol. Liq. 2020, 312, 113383. [Google Scholar] [CrossRef]
- Knani, S.; Khalfaoui, M.; Hachicha, M.; Mathlouthi, M.; Ben Lamine, A. Interpretation of psychophysics response curves using statistical physics. Food Chem. 2014, 151, 487–499. [Google Scholar] [CrossRef]
- Franco, D.S.P.; Georgin, J.; Netto, M.S.; Martinello, K.D.B.; Silva, L.F. Preparation of activated carbons from fruit residues for the removal of naproxen (NPX): Analytical interpretation via statistical physical model. J. Mol. Liq. 2022, 356, 119021. [Google Scholar] [CrossRef]
- Sellaoui, L.; Depci, T.; Kul, A.R.; Knani, S.; Ben Lamine, A. A new statistical physics model to interpret the binary adsorption isotherms of lead and zinc on activated carbon. J. Mol. Liq. 2016, 214, 220–230. [Google Scholar] [CrossRef]
- Jedli, H.; Briki, C.; Chrouda, A.; Brahmi, J.; Abassi, A.; Jbara, A.; Slimi, K.; Jemni, A. Experimental and theoretical study of CO2 adsorption by activated clay using statistical physics modeling. RSC Adv. 2019, 9, 38454–38463. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Lee, S.G.; Choi, J.I.; Koh, W.; Jang, S.S. Adsorption of β-d-glucose and cellobiose on kaolinite surfaces: Density functional theory (DFT) approach. Appl. Clay Sci. 2013, 71, 73–81. [Google Scholar] [CrossRef]
- Asnaoui, H.; Dehmani, Y.; Khalis, M.; Hachem, E.-K. Adsorption of phenol from aqueous solutions by Na–bentonite: Kinetic, equilibrium and thermodynamic studies. Int. J. Environ. Anal. Chem. 2022, 102, 3043–3057. [Google Scholar] [CrossRef]
- Luo, Z.; Gao, M.; Yang, S.; Yang, Q. Adsorption of phenols on reduced-charge montmorillonites modified by bispyridinium dibromides: Mechanism, kinetics and thermodynamics studies. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 222–230. [Google Scholar] [CrossRef]



| Adsorbent | Oxide (Weight %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | MgO | Fe2O3 | S | BaO | P2O5 | L.O.I | |
| RCA | 38.74 | 10.60 | 16.80 | 0.92 | 6.00 | 0.19 | 0.02 | 0.10 | 16.61 |
| RCG | 48.39 | 16.16 | 2.17 | 1.75 | 15.44 | 3.55 | 0.26 | 1.39 | 10.09 |
| Adsorbent | Proprieties | ||
|---|---|---|---|
| Pore Volume (cm3 g−1) | Specific Surface Area (m2 g−1) | Pore Diameter (Å) | |
| RCA | 0.13 | 51.41 | 91.35 |
| RCG | 0.03 | 25.31 | 38.36 |
| Parameters | RCA | RCG | |||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Temperature (°C) | ||||||
| 30 | 40 | 50 | 30 | 40 | 50 | ||
| Models | qtexp (mg g−1) | 1.39 | 2.10 | 2.71 | 1.86 | 2.43 | 3.52 |
| Pseudo-first-order | k1 (min−1) | 0.02 | 0.01 | 0.14 | 0.03 | 0.01 | 0.01 |
| qe (mg g−1) | 1.34 | 2.10 | 3.73 | 1.83 | 2.34 | 3.48 | |
| R2 | 0.95 | 0.93 | 0.96 | 0.96 | 0.96 | 0.99 | |
| Pseudo-second-order | k2 (g mg−1.min−1) | 0.101 | 0.2278 | 0.6124 | 0.4033 | 0.4918 | 1.521 |
| qe (mg g−1) | 1.515 | 2.481 | 3.17 | 1.992 | 2.663 | 3.99 | |
| R2 | 0.9848 | 0.96018 | 0.9712 | 0.9911 | 0.9895 | 0.9955 | |
| Adsorbent | Model | T (°C) | Statistical Indicators | |||
|---|---|---|---|---|---|---|
| R2 | ARE (%) | MSE (mg g−1)2 | BIC | |||
| RCA | MLO | 20 | 0.8946 | 25.86 | 1.005 | 1.953 |
| 30 | 0.8766 | 29.38 | 1.2663 | 3.573 | ||
| 40 | 0.9371 | 10.50 | 0.6686 | −0.8976 | ||
| RCG | 20 | 0.9942 | 11.74 | 0.7757 | 0.1421 | |
| 30 | 0.9859 | 20.46 | 2.420 | 8.106 | ||
| 40 | 0.9967 | 22.55 | 0.7605 | 0.003962 | ||
| RCA | MLT | 20 | 0.9979 | 1.957 | 0.08037 | −19.59 |
| 30 | 0.9999 | 0.3973 | 0.00562 | −38.22 | ||
| 40 | 0.9995 | 1.613 | 0.02302 | −28.35 | ||
| RCG | 20 | 0.9999 | 0.4556 | 0.008035 | −35.71 | |
| 30 | 0.9999 | 3.943 | 0.09776 | −18.22 | ||
| 40 | 0.9998 | 5.033 | 0.1411 | −15.65 | ||
| RCA | DLO | 20 | 0.8939 | 26.19 | 1.011 | 2.000 |
| 30 | 0.8761 | 29.59 | 1.271 | 3.601 | ||
| 40 | 0.9369 | 10.90 | 0.6703 | −0.880 | ||
| RCG | 20 | 0.9995 | 13.23 | 0.06972 | −16.72 | |
| 30 | 0.9966 | 5.001 | 0.5792 | −1.902 | ||
| 40 | 0.9991 | 12.03 | 0.2102 | −8.998 | ||
| RCA | DLT | 20 | 0.8939 | 26.19 | 1.349 | 3.946 |
| 30 | 0.8761 | 29.59 | 1.695 | 5.547 | ||
| 40 | 0.9369 | 10.90 | 0.8937 | 1.066 | ||
| RCG | 20 | 0.9995 | 13.23 | 0.09296 | −14.78 | |
| 30 | 0.9966 | 5.001 | 0.7723 | 0.0442 | ||
| 40 | 0.9991 | 12.03 | 0.2802 | −7.052 | ||
| RCA | MM | 20 | 0.7985 | 28.92 | 3.840 | 10.38 |
| 30 | −0.2504 | 44.44 | 25.66 | 23.67 | ||
| 40 | −0.2296 | 28.12 | 26.14 | 23.80 | ||
| RCG | 20 | −0.2536 | 83.57 | 334.9 | 41.66 | |
| 30 | −0.3040 | 83.35 | 446.7 | 43.67 | ||
| 40 | −0.1736 | 83.41 | 541.9 | 45.03 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehmani, Y.; Franco, D.S.P.; Georgin, J.; Lamhasni, T.; Brahmi, Y.; Oukhrib, R.; Mustapha, B.; Moussout, H.; Ouallal, H.; Sadik, A. Comparison of Phenol Adsorption Property and Mechanism onto Different Moroccan Clays. Water 2023, 15, 1881. https://doi.org/10.3390/w15101881
Dehmani Y, Franco DSP, Georgin J, Lamhasni T, Brahmi Y, Oukhrib R, Mustapha B, Moussout H, Ouallal H, Sadik A. Comparison of Phenol Adsorption Property and Mechanism onto Different Moroccan Clays. Water. 2023; 15(10):1881. https://doi.org/10.3390/w15101881
Chicago/Turabian StyleDehmani, Younes, Dison S. P. Franco, Jordana Georgin, Taibi Lamhasni, Younes Brahmi, Rachid Oukhrib, Belfaquir Mustapha, Hamou Moussout, Hassan Ouallal, and Abouarnadasse Sadik. 2023. "Comparison of Phenol Adsorption Property and Mechanism onto Different Moroccan Clays" Water 15, no. 10: 1881. https://doi.org/10.3390/w15101881






