Isotope Composition of Natural Water in Lake Onega Basin
Abstract
:1. Introduction
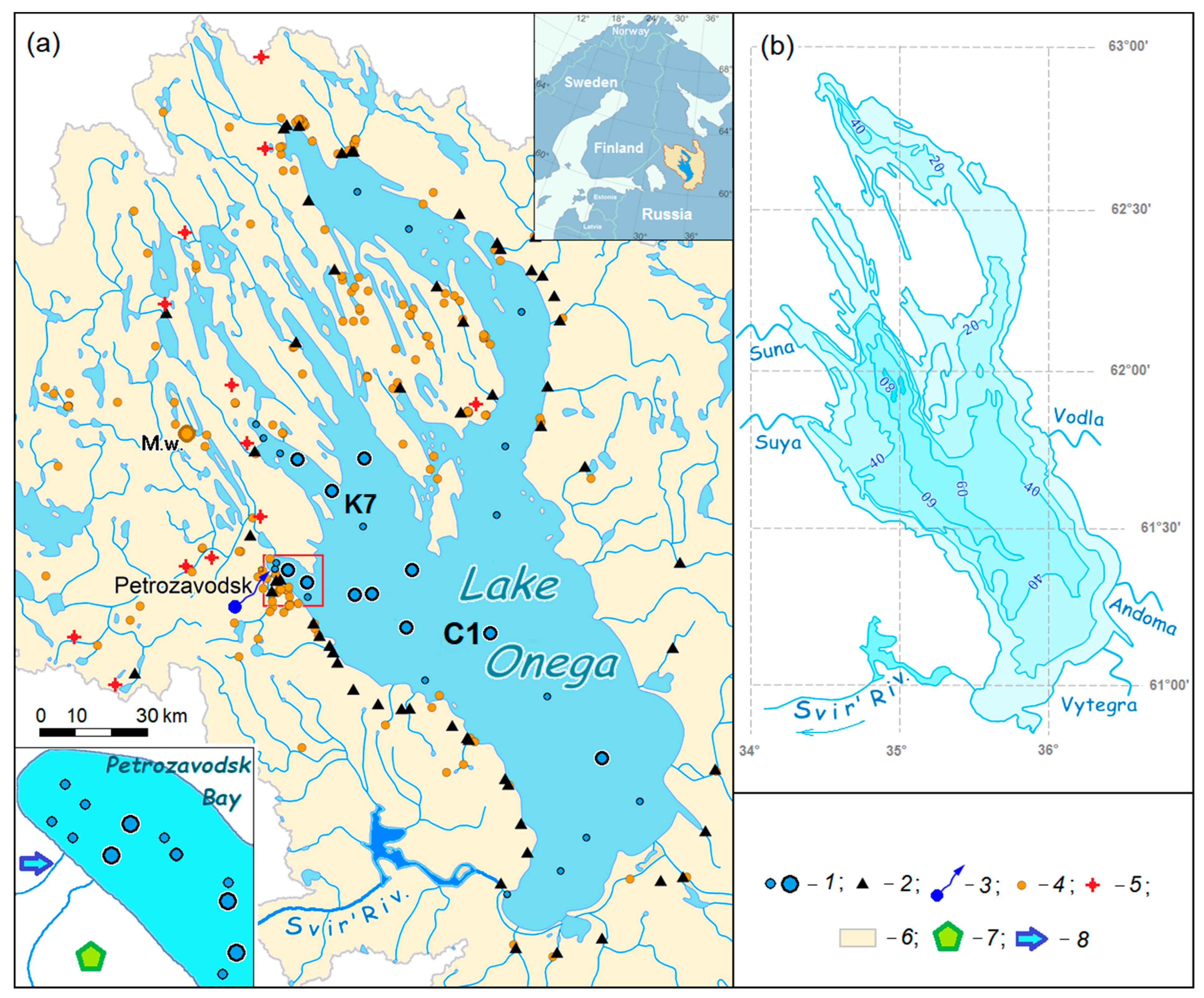
2. Site Description
3. Materials and Methods
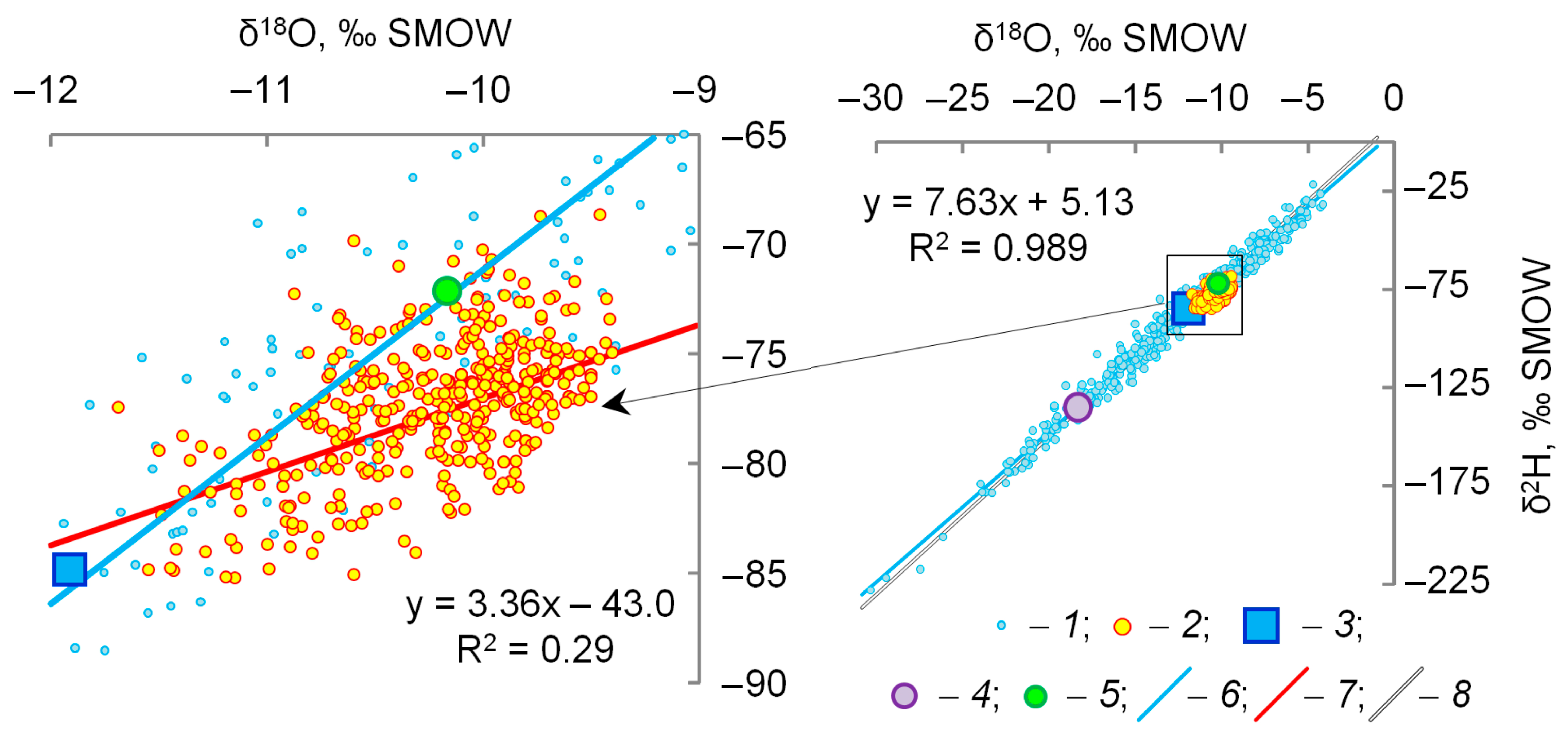
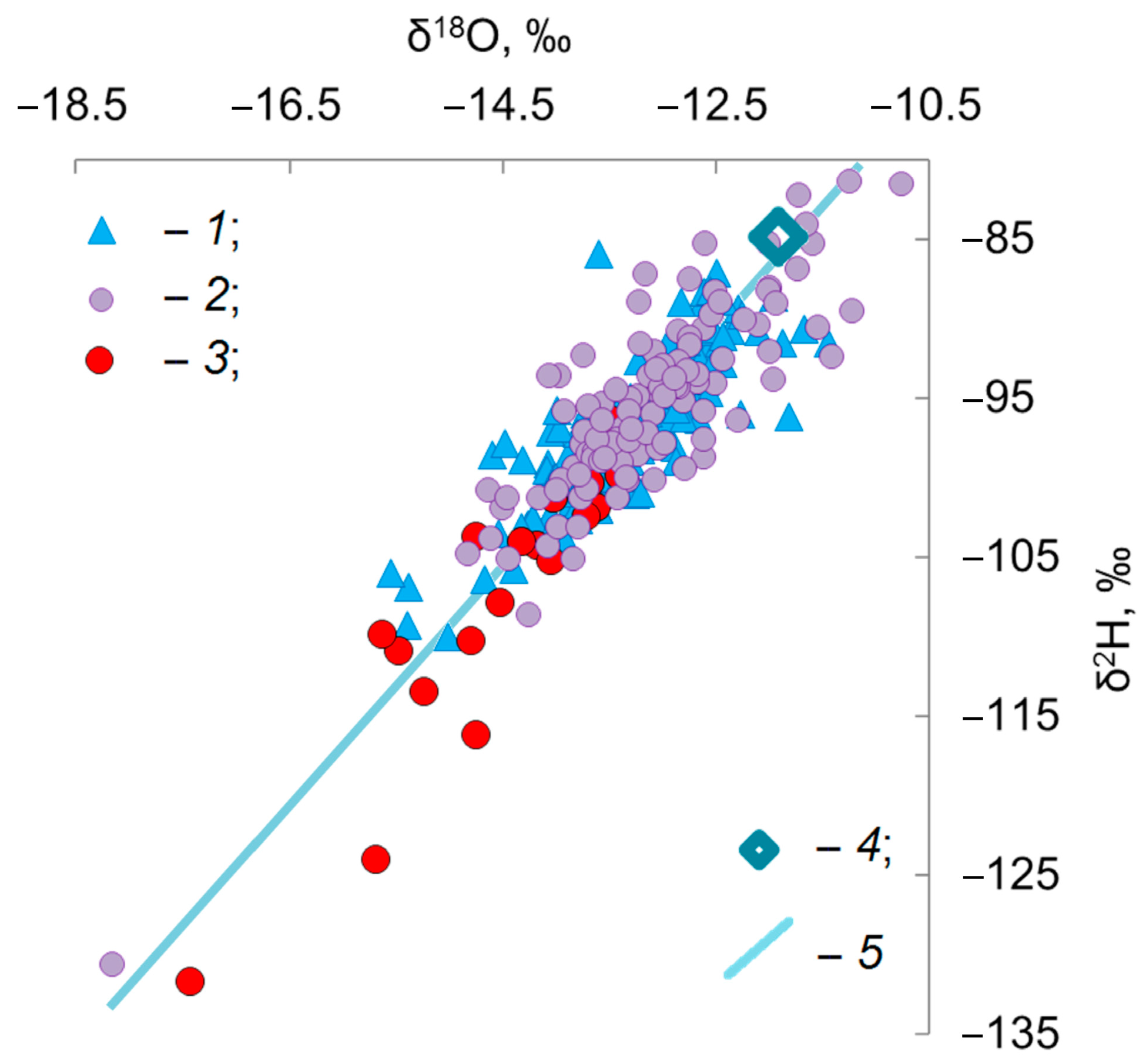
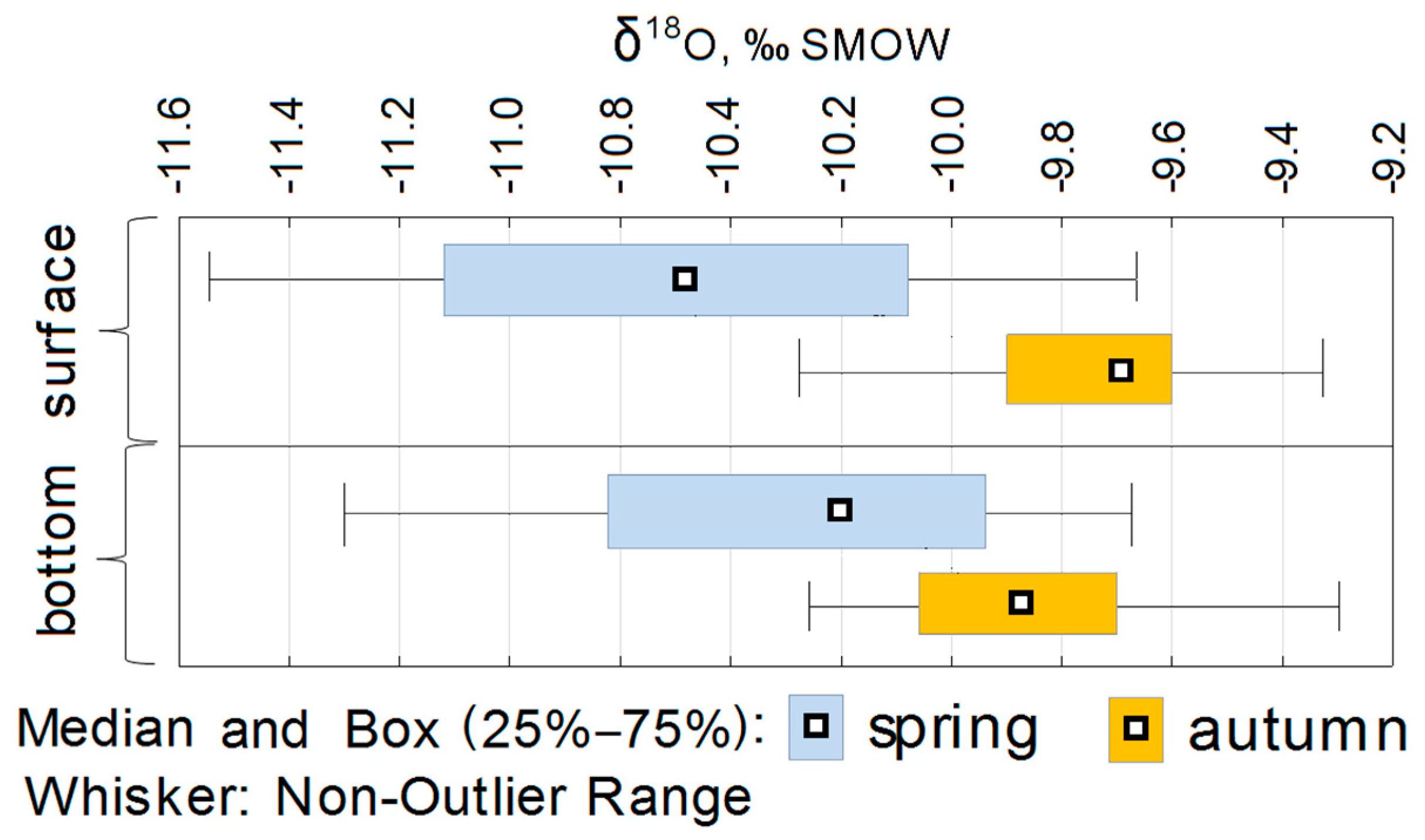
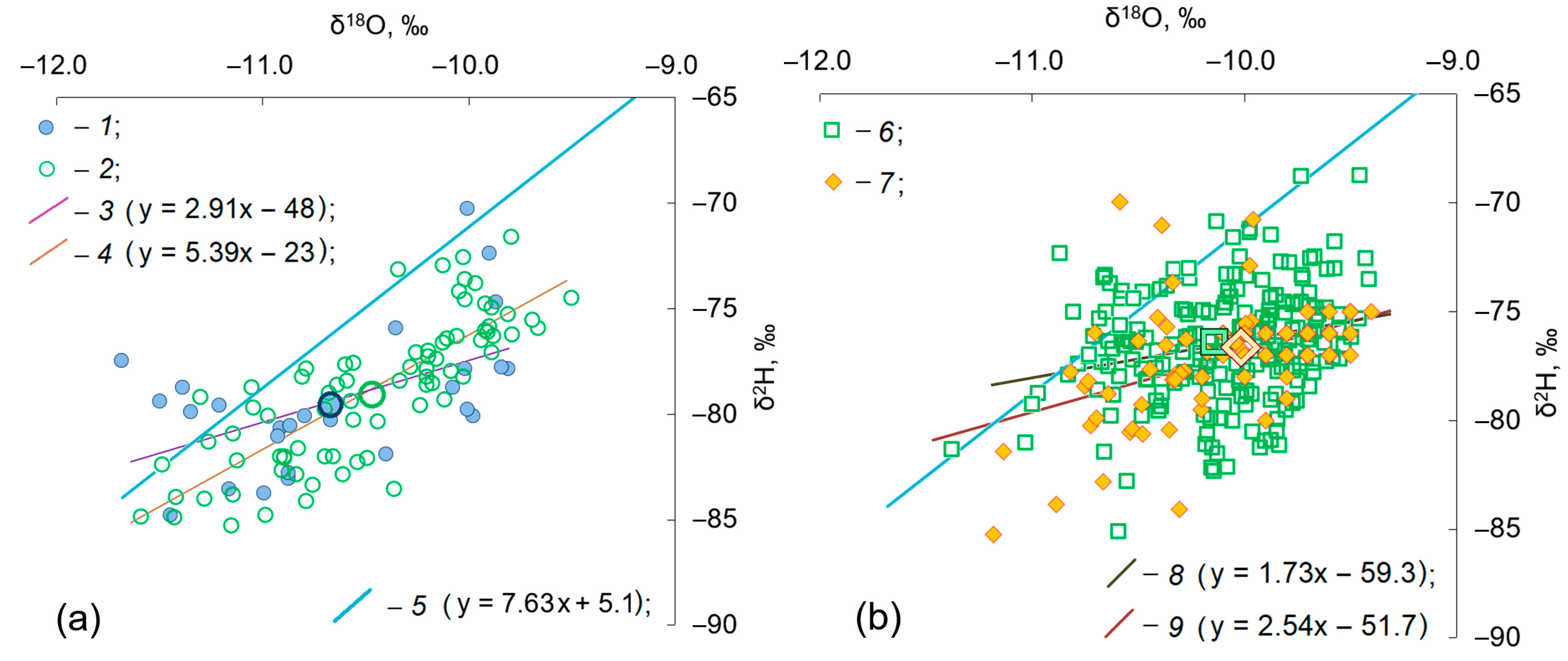
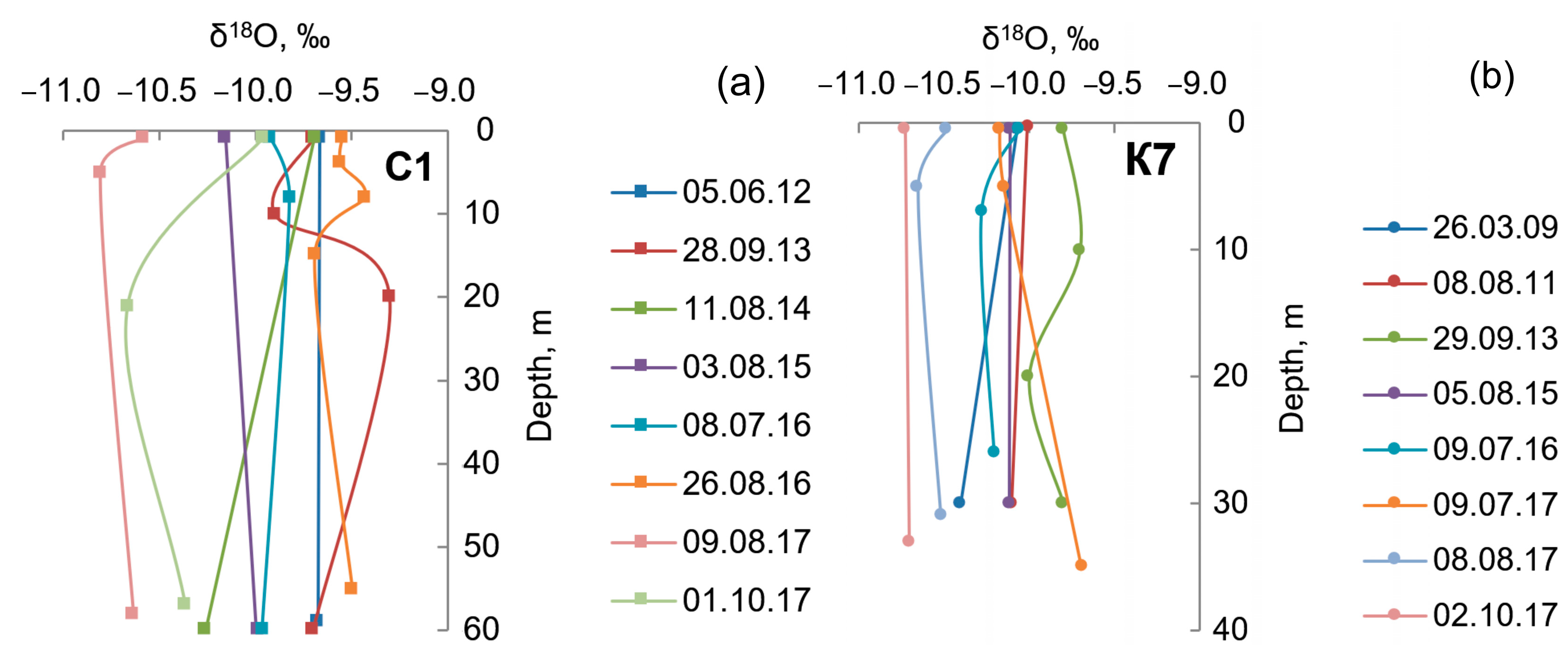
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Filatov, N.N.; Kalinkina, N.M.; Kulikova, T.P.; Litvinenko, A.V.; Lozovik, P.A. The Current State and Changes of Ecosystems of Large Lakes- Reservoirs of the North-West European Territory of Russia under Climate Change and Human Impact; Karelian Research Centre of the RAS: Petrozavodsk, Russia, 2015. [Google Scholar]
- Bogachev, M.A.; Borodulina, G.S.; Igonin, A.; Karpechko, V.A.; Katra, K.; Kotkasaari, T.; Litvinenko, A.V.; Litvinova, I.A.; Lozovik, P.A.; Morozov, A.K.; et al. Water Resources of the Republic of Karelia and Their Use for Drinking Water Supply. Experience in Karelian-Finnish Cooperation; Petrozavodsk-Kuopio; Karelian Research Center of RAS: Petrozavodsk, Russia, 2006. [Google Scholar]
- Lozovik, P.A. The Largest Lakes-Reservoirs of the North-West of the European Territory of Russia: The Current State and Changes in Ecosystems under Climatic and Man-Induced Impact; KarRC RAS: Petrozavodsk, Russia, 2015; pp. 88–95. [Google Scholar]
- Efremova, T.A.; Sabylina, A.V.; Lozovik, P.A.; Slaveykova, V.I.; Zobkova, M.V.; Pasche, N. Seasonal and spatial variation in hydrochemical parameters of Lake Onego (Russia): Insights from 2016 field monitoring. Inland Waters 2019, 9, 227–238. [Google Scholar] [CrossRef]
- Vodogretsky, V.E. Karelia and Northwest Russia; Surface Water Resources of the USSR: Hydrometeoizdat, Leningrad, 1972. [Google Scholar]
- Filatov, N.N. Lake Onega. In Atlas; Karelian Research Centre of the RAS: Petrozavodsk, Russia, 2010. [Google Scholar]
- Podsechin, V.; Kaipainen, H.; Filatov, N.; Bilaletdin, Ä.; Frisk, T.; Paananen, A.; Terzhevik, A.; Vuoristo, H. Development of Water Protection of Lake Onega; Final Report; Pirkanmaa Regional Environment Centre: Tampere, Finland, 2009.
- Moiseenko, T.; Sharov, A. Large Russian Lakes Ladoga, Onega, and Imandra under Strong Pollution and in the Period of Revitalization: A Review. Geosciences 2019, 9, 492. [Google Scholar] [CrossRef]
- Filatov, N.; Baklagin, V.; Efremova, T.; Nazarova, L.; Palshin, N. Climate change impacts on the watersheds of Lakes Onego and Ladoga from remote sensing and in situ data. Inland Waters 2019, 9, 130–141. [Google Scholar] [CrossRef]
- Livingstone, D.M.; Adrian, R.; Arvola, L.; Blenckner, T.; Dokulil, M.T.; Hari, R.E.; George, G.; Jankowski, T.; Järvinen, M.; Jennings, E.; et al. Regional and supra-regional coherence in limnological variables. In The Impact of Climate Change on European Lakes; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Sabylina, A.V.; Lozovik, P.A.; Zobkov, M.B. Water chemistry in Lake Onega and its tributaries. Water Resour. 2010, 37, 842–853. [Google Scholar] [CrossRef]
- Sabylina, A.V.; Ryzhakov, A.V. Hydrochemical Characteristic of the Littoral Zone of Lake Onega. Water Resour. 2018, 45, 213–221. [Google Scholar] [CrossRef]
- Filatov, N.N.; Vyruchalkina, T.Y. Many-year level variations in the Great Lakes of Eurasia and North America. Water Resour. 2017, 44, 685–696. [Google Scholar] [CrossRef]
- Mook, W.G. Environmental isotopes in the hydrological cycle. In Principles and Applications; UNESCO: Paris, France, 2001. [Google Scholar]
- Ferronsky, V.; Polyakov, V. Isotopes of the Earth’s Hydrosphere; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar] [CrossRef]
- Ferronsky, V.I.; Dubinchuk, V.T.; Polyakov, V.A.; Seletsky, Y.B.; Kuptsov, V.M.; Yakubovsky, A.V. Natural Isotopes of the Hydrosphere; Nedra: Moscow, Russia, 1975. [Google Scholar]
- Gibson, J.; Birks, S.; Jeffries, D.; Yi, Y. Regional trends in evaporation loss and water yield based on stable isotope mass balance of lakes: The Ontario Precambrian Shield surveys. J. Hydrol. 2017, 544, 500–510. [Google Scholar] [CrossRef]
- Kendall, C.; McDonnell, J.J. Isotope Tracers in Catchment Hydrology; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Gibson, J.; Prepas, E.; McEachern, P. Quantitative comparison of lake throughflow, residency, and catchment runoff using stable isotopes: Modelling and results from a regional survey of Boreal lakes. J. Hydrol. 2002, 262, 128–144. [Google Scholar] [CrossRef]
- Horita, J.; Rozanski, K.; Cohen, S. Isotope effects in the evaporation of water: A status report of the Craig–Gordon model. Isot. Environ. Health Stud. 2008, 44, 23–49. [Google Scholar] [CrossRef]
- Chen, K.; Meng, Y.; Liu, G.; Xia, C.; Zhou, J.; Li, H. Identifying hydrological conditions of the Pihe River catchment in the Chengdu Plain based on spatio-temporal distribution of 2H and 18O. J. Radioanal. Nucl. Chem. 2020, 324, 1125–1140. [Google Scholar] [CrossRef]
- McGuire, K.J.; McDonnell, J.J. A review and evaluation of catchment transit time modeling. J. Hydrol. 2006, 330, 543–563. [Google Scholar] [CrossRef]
- Niinikoski, P.I.A.; Hendriksson, N.M.; Karhu, J.A. Using stable isotopes to resolve transit times and travel routes of river water: A case study from southern Finland. Isot. Environ. Health Stud. 2016, 52, 380–392. [Google Scholar] [CrossRef]
- Rodgers, P.; Soulsby, C.; Waldron, S.; Tetzlaff, D. Using stable isotope tracers to assess hydrological flow paths, residence times and landscape influences in a nested mesoscale catchment. Hydrol. Earth Syst. Sci. 2005, 9, 139–155. [Google Scholar] [CrossRef]
- Timsic, S.; William, P. Spatial variability in stable isotope values of surface waters of Eastern Canada and New England. J. Hydrol. 2014, 511, 594–604. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, G.; Meng, Y.; Xia, C.; Chen, K.; Chen, Y. Using stable isotopes as tracer to investigate hydrological condition and estimate water residence time in a plain region, Chengdu, China. Sci. Rep. 2021, 11, 2812. [Google Scholar] [CrossRef]
- Kulik, N.; Efremenko, N.; Strakhovenko, V.; Belkina, N.; Borodulina, G.; Gatalskaya, E.; Malov, V.; Tokarev, I. Geochemical Features of River Runoff and Their Effect on the State of the Aquatic Environment of Lake Onego. Water 2023, 15, 964. [Google Scholar] [CrossRef]
- Lozovik, P.A.; Zobkov, M.B.; Borodulina, G.S.; Tokarev, I.V. Assessing External Water Exchange of Lake Bays by Water Chemistry Characteristics. Water Resour. 2019, 46, 94–102. [Google Scholar] [CrossRef]
- Rukhovets, L.; Filatov, N. (Eds.) Ladoga and Onego—Great European lakes. In Observations and Modeling; Springer: Berlin/Heidelberg, Germany, 2010; 302p. [Google Scholar]
- Borodulina, G.S. Role of groundwater flow to lakes of the Onega watershed in formation of the chemical composition of lake water. Proc. Karelian Res. Cent. RAS Limnol. Ser. 2011, 4, 108–116. [Google Scholar]
- Borodulina, G.S.; Tokarev, I.V. Geochemical and isotopic characteristics of groundwater and surface water in the Paleoproterozoic Onega structure. In Integrated Problems in Hydrogeology; St. Petersburg State University: St. Petersburg, FL, USA, 2013. [Google Scholar]
- Borodulina, G.S.; Tokarev, I.V.; Levichev, M.A. Isotopic Composition (δ18O, δ2H) of Karelian Snow Cover. Water Resour. 2022, 49 (Suppl. 1), S90–S98. [Google Scholar] [CrossRef]
- Filatov, N.N.; Kukharev, V.I. (Eds.) Lakes of Karelia; A reference book; KarRC RAS: Petrozavodsk, Russia, 2013; 461p. [Google Scholar]
- Lozovik, P.A.; Kulikova, T.P.; Martynova, N.N. Condition of Water Bodies in the Republic of Karelia: The Results of 1998–2006 Monitoring; Karelian Research Center of the RAS: Petrozavodsk, Russia, 2007. [Google Scholar]
- Efremova, T.; Palshin, N.; Zdorovennov, R. Long-term characteristics of ice phenology in Karelian lakes. Est. J. Earth Sci. 2013, 62, 33. [Google Scholar] [CrossRef]
- Sabylina, A.V.; Efremova, T.A. The chemical composition of ice and water under ice of Lake Onega (the case of Petrozavodsk Bay). Ice Snow 2018, 58, 417–428. [Google Scholar] [CrossRef]
- Leppäranta, M. Freezing of Lakes and the Evolution of Their Ice Cover; Springer: Berlin/Heidelberg, Germany, 2015; pp. 84–265. [Google Scholar]
- Salo, Y.A.; Nazarova, L.E.; Balagansky, A.F. Computations of evaporation from watersheds of North-Western Russia. Proc. Karelian Res. Cent. RAS Limnol. 2016, 9, 95–101. [Google Scholar] [CrossRef]
- Martínez, D.E.; Londoño, O.M.Q.; Solomon, D.K.; Dapeña, C.; Massone, H.E.; Benavente, M.A.; Panarello, H.O. Hydrogeochemistry, Isotopic Composition and Water Age in the Hydrologic System of a Large Catchment within a Plain Humid Environment (Argentine Pampas): Quequén Grande River, Argentina. River Res. Appl. 2017, 33, 438–449. [Google Scholar] [CrossRef]
- Ogrinc, N.; Kocman, D.; Miljević, N.; Vreča, P.; Vrzel, J.; Povinec, P. Distribution of H and O stable isotopes in the surface waters of the Sava River, the major tributary of the Danube River. J. Hydrol. 2018, 565, 365–373. [Google Scholar] [CrossRef]
- Malov, A.; Tokarev, I. Using stable isotopes to characterize the conditions of groundwater formation on the eastern slope of the Baltic Shield (NW Russia). J. Hydrol. 2019, 578, 124130. [Google Scholar] [CrossRef]
- Voronyuk, G.Y.; Borodulina, G.S.; Krainyukova, I.A.; Tokarev, I.V. Water exchange on the Baltic Shield margin and in adjacent artesian basins: Isotope and chemical data (scientific and applied aspects). Karelian Isthmus. Proc. Karelian Res. Cent. RAS Limnol. Ser. 2016, 9, 46–56. [Google Scholar] [CrossRef]
- Tokarev, I.; Rumyantsev, V.; Rybakin, V.; Yakovlev, E. Inflow of surface and groundwater to Lake Ladoga based on stable isotope (2H, 18O) composition. J. Great Lakes Res. 2022, 48, 890–902. [Google Scholar] [CrossRef]
- Borodulina, G.; Tokarev, I.; Avramenko, I. Investigation of small river watershed hydrology in Karelia (North- West Russia) by high-resolution record of δ2H and δ18O in precipitation and river discharge, including experimental estimates of evaporation. In Book of Extended Synopses. International Symposium on Isotope Hydrology: Revisiting Foundations and Exploring Frontiers; IAEA: Vienna, Austria, 2015; Volume 3, pp. 174–176. [Google Scholar]
- Uhlenbrook, S.; Frey, M.; Leibundgut, C.; Maloszewski, P. Hydrograph separations in a mesoscale mountainous basin at event and seasonal timescales. Water Resour. Res. 2002, 38, 1096. [Google Scholar] [CrossRef]
- Vasil’chuk, Y.K.; Rets, E.; Chizhova, J.N.; Tokarev, I.; Frolova, N.L.; Budantseva, N.A.; Kireeva, M.; Loshakova, N.A. Hydrograph separation of the Dzhankuat River, North Caucasus, with the use of isotope methods. Water Resour. 2016, 43, 847–861. [Google Scholar] [CrossRef]
- Sarkkola, S.; Nieminen, M.; Koivusalo, H.; Laurén, A.; Kortelainen, P.; Mattsson, T.; Palviainen, M.; Piirainen, S.; Starr, M.; Finér, L. Iron concentrations are increasing in surface waters from forested headwater catchments in eastern Finland. Sci. Total. Environ. 2013, 463–464, 683–689. [Google Scholar] [CrossRef]




| Winter (114) * | Spring (75) | Summer (126) | Autumn (98) | For 2009–2018 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| δ18O | δ2H | δ18O | δ2H | δ18O | δ2H | δ18O | δ2H | δ18O | δ2H | |
| Weighted average | −18.3 | −135 | −10.6 | −76 | −10.1 | −72 | −12.9 | −91 | −11.9 | −85 |
| Average | −17.2 | −126 | −11.1 | −80 | −9.6 | −68 | −13.4 | −96 | −12.9 | −93 |
| Minimum | −30.9 | −239 | −22.5 | −174 | −16.4 | −123 | −27.4 | −217 | −30.9 | −239 |
| Maximum | −9.1 | −67 | −4.7 | −22 | −4.1 | −26 | −6.2 | −40 | −4.7 | −22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borodulina, G.; Tokarev, I.; Yakovlev, E. Isotope Composition of Natural Water in Lake Onega Basin. Water 2023, 15, 1855. https://doi.org/10.3390/w15101855
Borodulina G, Tokarev I, Yakovlev E. Isotope Composition of Natural Water in Lake Onega Basin. Water. 2023; 15(10):1855. https://doi.org/10.3390/w15101855
Chicago/Turabian StyleBorodulina, Galina, Igor Tokarev, and Evgeny Yakovlev. 2023. "Isotope Composition of Natural Water in Lake Onega Basin" Water 15, no. 10: 1855. https://doi.org/10.3390/w15101855
APA StyleBorodulina, G., Tokarev, I., & Yakovlev, E. (2023). Isotope Composition of Natural Water in Lake Onega Basin. Water, 15(10), 1855. https://doi.org/10.3390/w15101855






