Enhanced Electrocatalytic Oxidation of Phenol by SnO2-Sb2O3/GAC Particle Electrodes in a Three-Dimensional Electrochemical Oxidation System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SnO2-Sb2O3/GAC Particle Electrodes
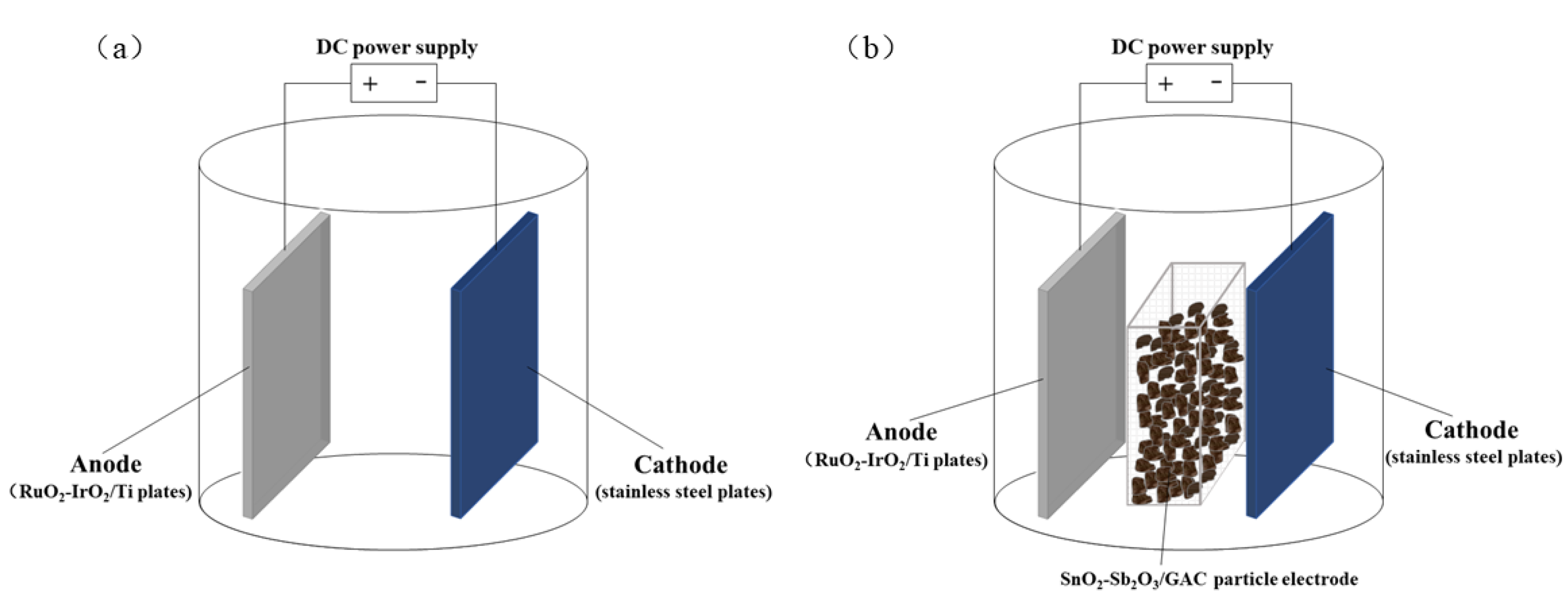
2.3. Reactor Configuration
2.4. Reactor Operation
2.5. Analytical Methods
3. Results
3.1. Characterization of Particle Electrodes
3.1.1. Morphological Analysis Using SEM
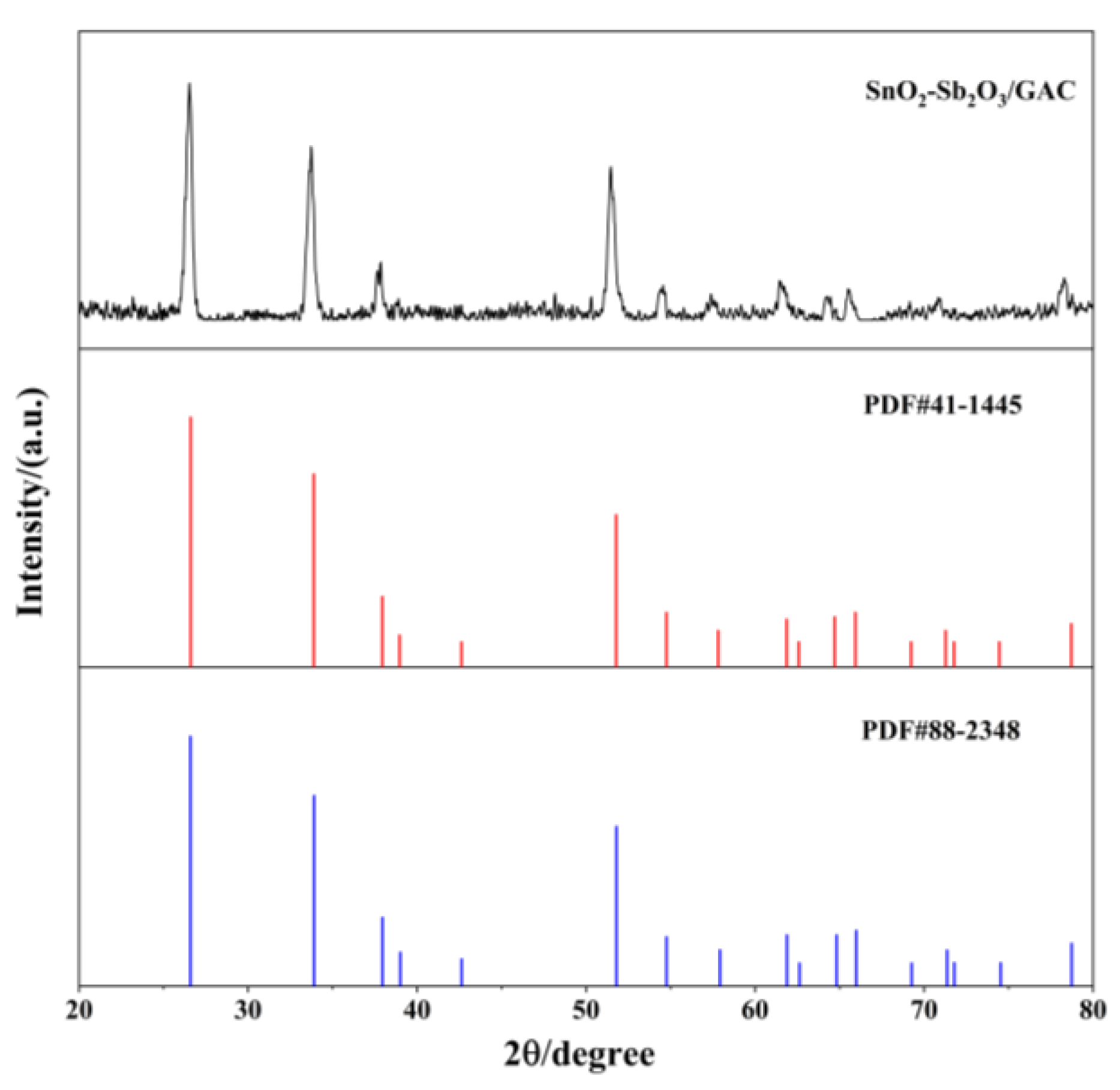
3.1.2. Structural Analysis Using XRD
3.1.3. Surface Area Analysis Using BET
3.1.4. Thermogravimetric Analysis
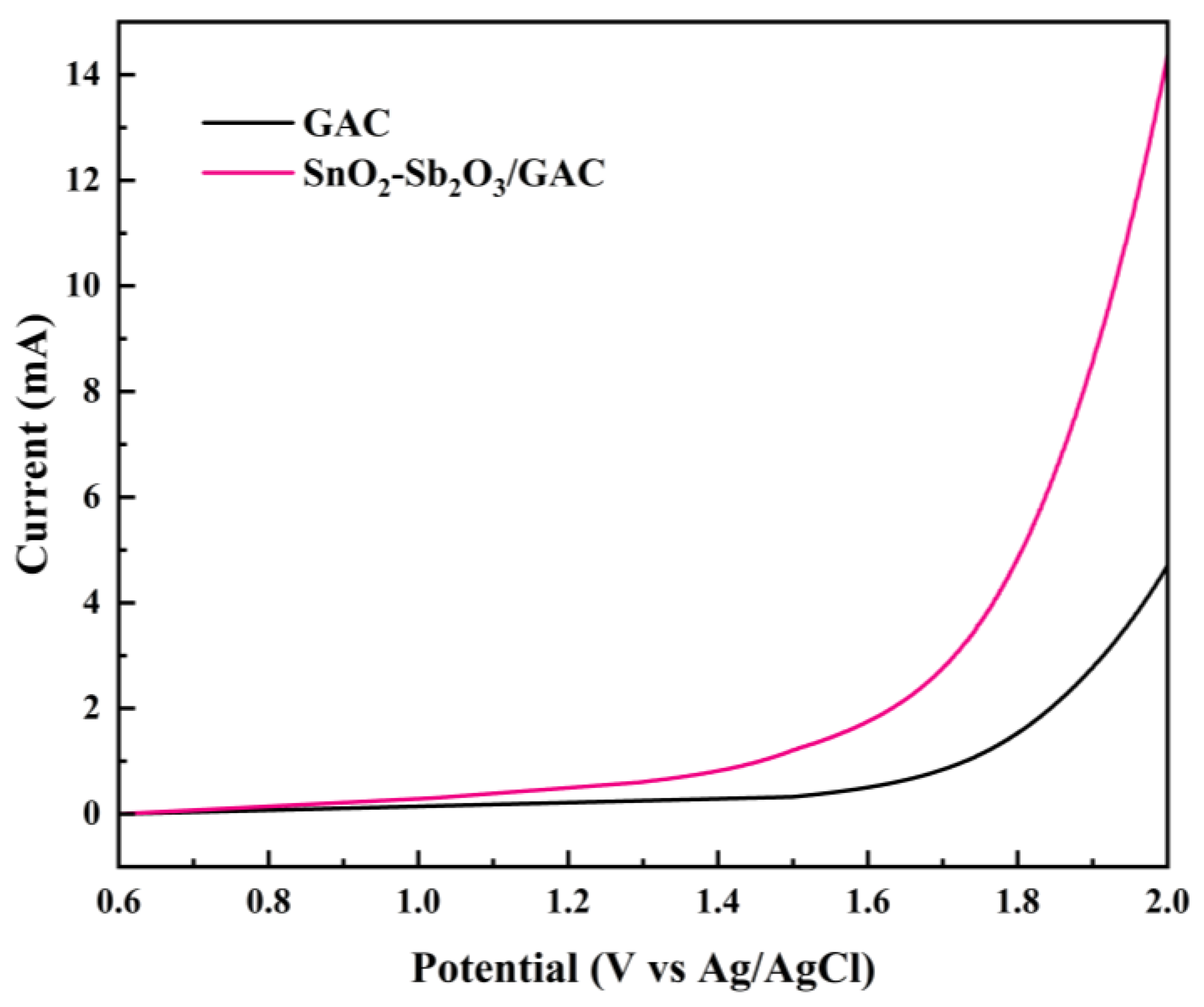
3.1.5. LSV Testing and Analysis
3.2. Factors Influencing the Removal of Phenol by 3D Electrochemical Oxidation
3.2.1. Effect of the Initial pH
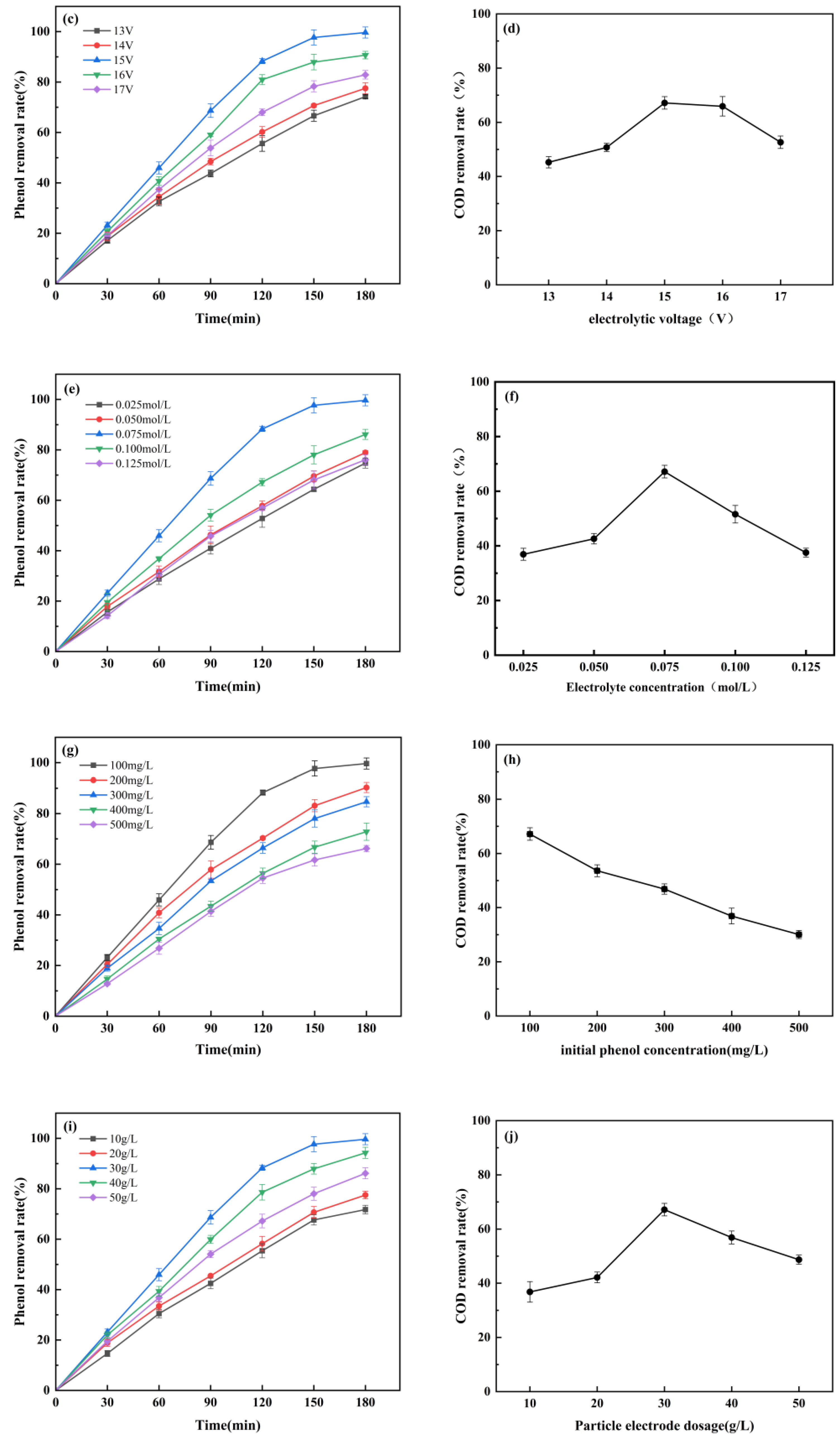
3.2.2. Effect of Electrolytic Voltage
3.2.3. Effect of Electrolyte Concentration
3.2.4. Effect of Electrolyte Concentration
3.2.5. Effect of the Particle Electrode Dosage
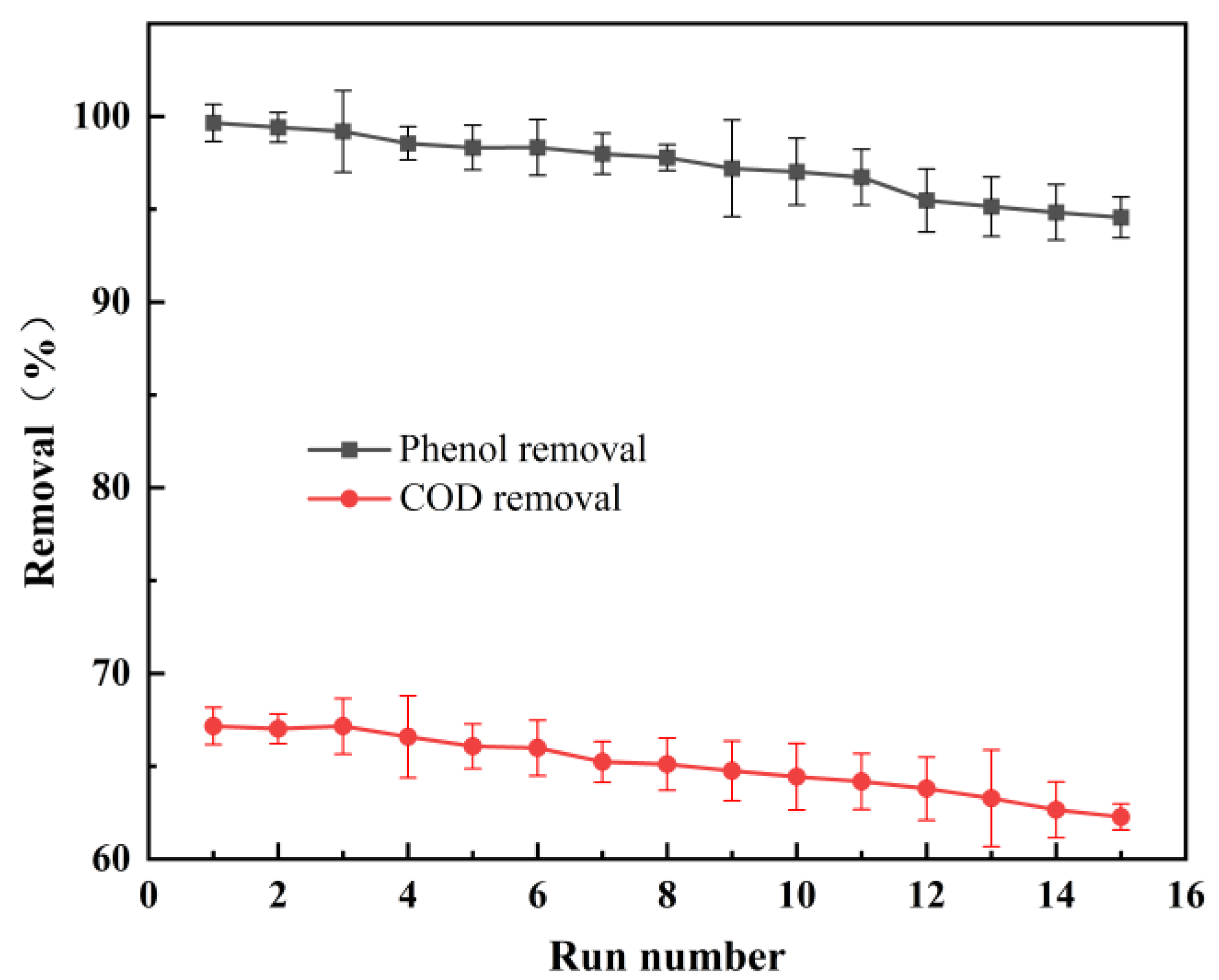
3.3. Stability Analysis of SnO2-Sb2O3/GAC Particle Electrodes
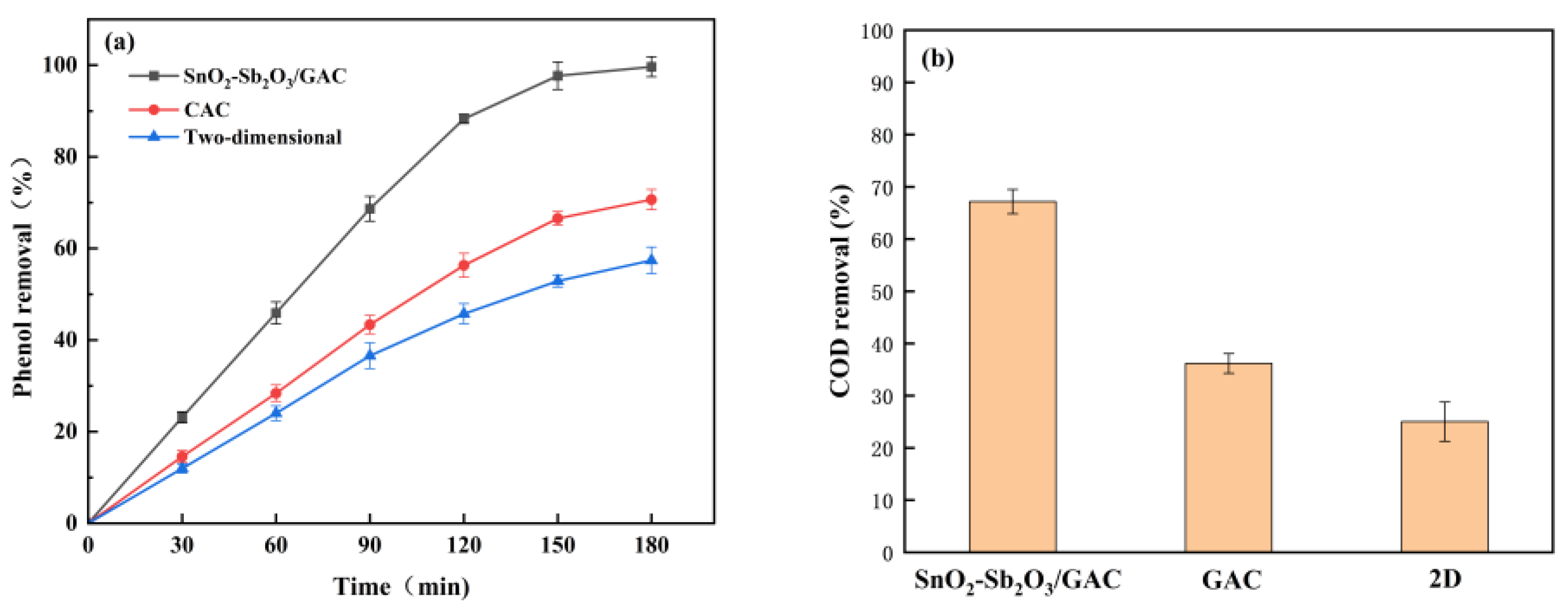
3.4. Comparison of Treatment Effects between the 2D and 3D Electrode Systems
3.5. Mechanism of Phenol Degradation by the 3D Electrode System
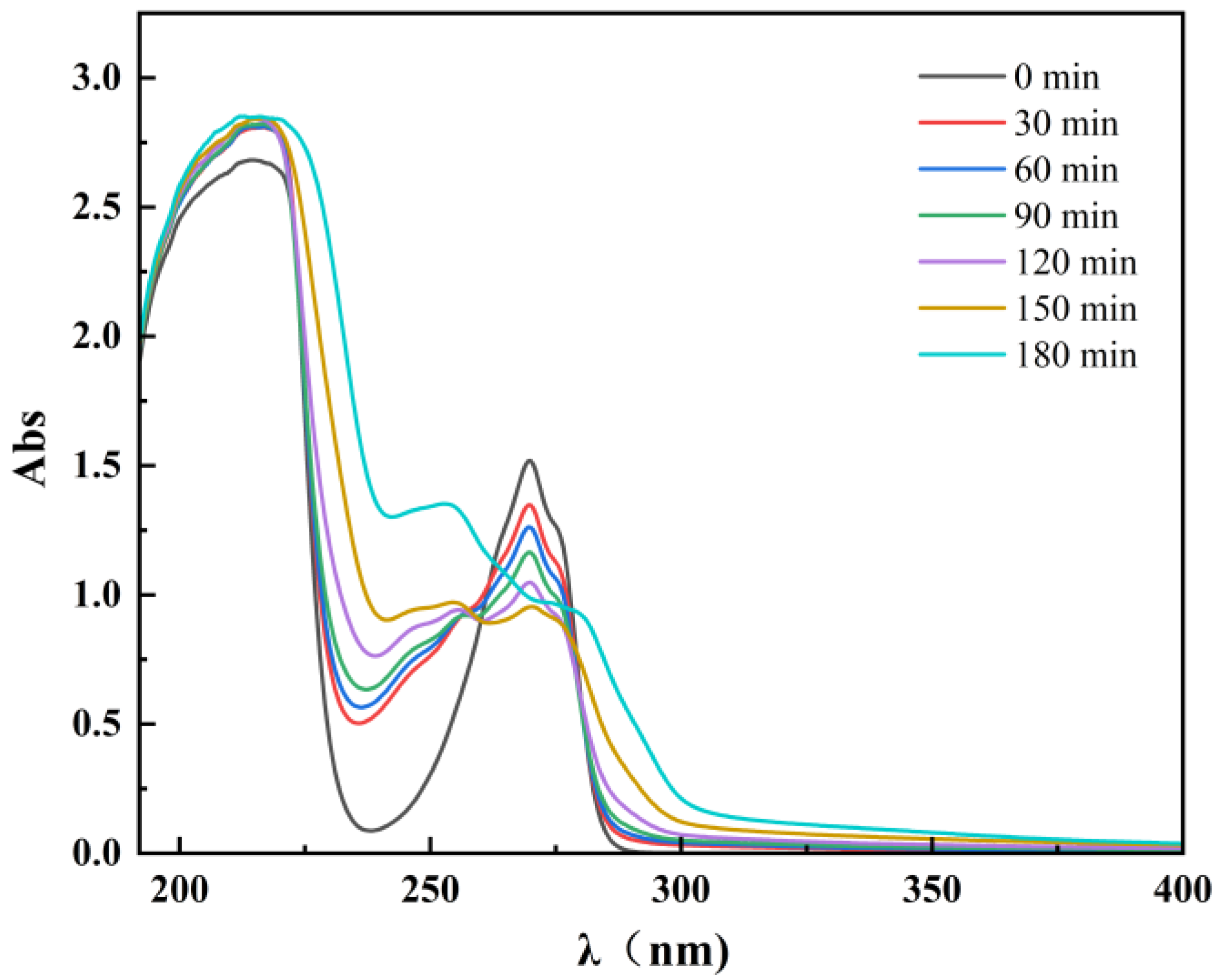
3.5.1. Ultraviolet–Visible Spectrum Analysis
3.5.2. GC-MS Analysis of the Phenol Degradation Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brillas, E.; Garcia-Segura, S. Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: A review on the relevance of phenol as model molecule. Sep. Purif. Technol. 2020, 237, 116337. [Google Scholar] [CrossRef]
- Li, H.; Meng, F.; Duan, W.; Lin, Y.; Zheng, Y. Biodegradation of phenol in saline or hypersaline environments by bacteria: A review. Ecotoxicol. Environ. Saf. 2019, 184, 109658. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.; Zhao, H.; Deng, W.; Feng, X.; Li, Y. Photocatalytic degradation of phenol in water under simulated sunlight by an ultrathin MgO coated Ag/TiO2 nanocomposite. Chemosphere 2019, 216, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, Z.; Jiang, W.; Yuan, B.; Huang, T.; Wang, L.; Tang, J. Degradation of phenol using a peroxidase mimetic catalyst through conjugating deuterohemin-peptide onto metal-organic framework with enhanced catalytic activity. Catal. Commun. 2020, 134, 105859. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Heterogeneous photocatalytic degradation of phenols in wastewater: A review on current status and developments. Desalination 2010, 261, 3–18. [Google Scholar] [CrossRef]
- Xiong, Y.; He, C.; Karlsson, H.T.; Zhu, X. Performance of three-phase three-dimensional electrode reactor for the reduction of COD in simulated wastewater-containing phenol. Chemosphere 2003, 50, 131–136. [Google Scholar] [CrossRef]
- Duan, W.; Meng, F.; Cui, H.; Lin, Y.; Wang, G.; Wu, J. Ecotoxicity of phenol and cresols to aquatic organisms: A review. Ecotoxicol. Environ. Saf. 2018, 157, 441–456. [Google Scholar] [CrossRef]
- Mortada, W.I.; Mohamed, R.A.; Monem, A.A.A.; Awad, M.M.; Hassan, A.F. Effective and Low-Cost Adsorption Procedure for Removing Chemical Oxygen Demand from Wastewater Using Chemically Activated Carbon Derived from Rice Husk. Separations 2023, 10, 43. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef]
- Jung, K.-W.; Hwang, M.-J.; Park, D.-S.; Ahn, K.-H. Performance evaluation and optimization of a fluidized three-dimensional electrode reactor combining pre-exposed granular activated carbon as a moving particle electrode for greywater treatment. Sep. Purif. Technol. 2015, 156, 414–423. [Google Scholar] [CrossRef]
- He, Y.; Lin, H.; Guo, Z.; Zhang, W.; Li, H.; Huang, W. Recent developments and advances in boron-doped diamond electrodes for electrochemical oxidation of organic pollutants. Sep. Purif. Technol. 2019, 212, 802–821. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Panizza, M. Electrochemical oxidation of organic pollutants for wastewater treatment. Curr. Opin. Electrochem. 2018, 11, 62–71. [Google Scholar] [CrossRef]
- Quang, H.H.P.; Nguyen, T.P.; Nguyen, D.D.D.; Bao, L.T.N.; Nguyen, V.-H. Advanced electro-Fenton degradation of a mixture of pharmaceutical and steel industrial wastewater by pallet-activated-carbon using three-dimensional electrode reactor. Chemosphere 2022, 297, 134074. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Deng, Z.; Liu, H.; Li, T.; Yang, Y.; Zhong, S. Bimetallic modified halloysite particle electrode enhanced electrocatalytic oxidation for the degradation of sulfanilamide. J. Environ. Manag. 2022, 312, 114975. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, Y. Electro-catalytic oxidation of phenacetin with a three-dimensional reactor: Degradation pathway and removal mechanism. Chemosphere 2016, 152, 17–22. [Google Scholar] [CrossRef]
- Li, X.-Y.; Xu, J.; Cheng, J.-P.; Feng, L.; Shi, Y.-F.; Ji, J. TiO2-SiO2/GAC particles for enhanced electrocatalytic removal of acid orange 7 (AO7) dyeing wastewater in a three-dimensional electrochemical reactor. Sep. Purif. Technol. 2017, 187, 303–310. [Google Scholar] [CrossRef]
- Pedersen, N.L.; Fini, M.N.; Molnar, P.K.; Muff, J. Synergy of combined adsorption and electrochemical degradation of aqueous organics by granular activated carbon particulate electrodes. Sep. Purif. Technol. 2019, 208, 51–58. [Google Scholar] [CrossRef]
- Sun, W.; Sun, Y.; Shah, K.J.; Zheng, H.; Ma, B. Electrochemical degradation of oxytetracycline by Ti-Sn-Sb/γ-Al2O3 three-dimensional electrodes. J. Environ. Manag. 2019, 241, 22–31. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Y.; Li, Y.; Hu, Z.; Zhou, L.; Zhou, M. Three-dimensional electrochemical process for wastewater treatment: A general review. Chem. Eng. J. 2013, 228, 455–467. [Google Scholar] [CrossRef]
- Zhan, J.; Li, Z.; Yu, G.; Pan, X.; Wang, J.; Zhu, W.; Han, X.; Wang, Y. Enhanced treatment of pharmaceutical wastewater by combining three-dimensional electrochemical process with ozonation to in situ regenerate granular activated carbon particle electrodes. Sep. Purif. Technol. 2019, 208, 12–18. [Google Scholar] [CrossRef]
- Ji, J.; Liu, Y.; Yang, X.-Y.; Xu, J.; Li, X.-Y. Multiple response optimization for high efficiency energy saving treatment of rhodamine B wastewater in a three-dimensional electrochemical reactor. J. Environ. Manag. 2018, 218, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Mahardika, D.; Park, H.-S.; Choo, K.-H. Ferrihydrite-impregnated granular activated carbon (FH@GAC) for efficient phosphorus removal from wastewater secondary effluent. Chemosphere 2018, 207, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.L.; Zayas, T.; Contreras, O.E.; Salgado, L. Effect of Sn precursor on the synthesis of SnO2 and Sb-doped SnO2 particles via polymeric precursor method. Front. Mater. Sci. 2013, 7, 387–395. [Google Scholar] [CrossRef]
- Fockedey, E.; Van Lierde, A. Coupling of anodic and cathodic reactions for phenol electro-oxidation using three-dimensional electrodes. Water Res. 2002, 36, 4169–4175. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, P.; Zheng, H.; Zhao, C.; Xiao, X.; Xu, Y.; Sun, W.; Wu, H.; Ren, M. Electrochemical treatment of chloramphenicol using Ti-Sn/γ-Al2O3 particle electrodes with a three-dimensional reactor. Chem. Eng. J. 2017, 308, 1233–1242. [Google Scholar] [CrossRef]
- Oliveira, R.; Bento, F.; Geraldo, D. Aromatic hydroxylation reactions by electrogenerated HO radicals: A kinetic study. J. Electroanal. Chem. 2012, 682, 7–13. [Google Scholar] [CrossRef]
- An, T.-C.; Zhu, X.-H.; Xiong, Y. Feasibility study of photoelectrochemical degradation of methylene blue with three-dimensional electrode-photocatalytic reactor. Chemosphere 2002, 46, 897–903. [Google Scholar] [CrossRef]
- Wang, Z.; Song, B.; Li, J.; Teng, X. Degradation of norfloxacin wastewater using kaolin/steel slag particle electrodes: Performance, mechanism and pathway. Chemosphere 2021, 270, 128652. [Google Scholar] [CrossRef]
- Jia, Z.; Zhao, X.; Yu, C.; Wan, Q.; Liu, Y. Design and properties of Sn–Mn–Ce supported activated carbon composite as particle electrode for three-dimensionally electrochemical degradation of phenol. Environ. Technol. Innov. 2021, 23, 101554. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Li, J.; Zhao, C.; He, X.; Yang, G. Fabrication of slag particle three-dimensional electrode system for methylene blue degradation: Characterization, performance and mechanism study. Chemosphere 2018, 213, 377–383. [Google Scholar] [CrossRef]
- Zheng, Y.; Qiu, S.; Deng, F.; Zhu, Y.; Li, G.; Ma, F. Three-dimensional electro-Fenton system with iron foam as particle electrode for folic acid wastewater pretreatment. Sep. Purif. Technol. 2019, 224, 463–474. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Li, P.; Zhang, Y.; Yan, Q.; Zhao, Y. Electrochemical treatment of industrial wastewater using a novel layer-upon-layer bipolar electrode system (nLBPEs). Chem. Eng. J. 2013, 215–216, 157–161. [Google Scholar] [CrossRef]
- Lv, G.; Wu, D.; Fu, R. Performance of carbon aerogels particle electrodes for the aqueous phase electro-catalytic oxidation of simulated phenol wastewaters. J. Hazard. Mater. 2009, 165, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Nady, H.; El-Rabiei, M.; El-Hafez, G.A. Electrochemical oxidation behavior of some hazardous phenolic compounds in acidic solution. Egypt. J. Pet. 2017, 26, 669–678. [Google Scholar] [CrossRef]
- Li, X.-Y.; Cui, Y.-H.; Feng, Y.-J.; Xie, Z.-M.; Gu, J.-D. Reaction pathways and mechanisms of the electrochemical degradation of phenol on different electrodes. Water Res. 2005, 39, 1972–1981. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Shim, Y.-B.; Lee, B.-S.; Choi, S.-Y.; Won, M.-S. Electrochemical degradation of phenol and 2-chloro phenol using Pt/Ti and boron-doped diamond electrodes. Bull. Korean Chem. Soc. 2012, 33, 2274–2278. [Google Scholar] [CrossRef]




| Sample | Pore Size (nm) | Pore Volume (cm3/g) | Specific Surface Area (m2/g) |
|---|---|---|---|
| blank GAC | 10.2457 | 0.1263 | 978.3 |
| SnO2-Sb2O3/GAC | 8.6933 | 0.0779 | 769.5 |
| SN | Retention Time | Intermediate Products | Chemical Structure | Formula |
|---|---|---|---|---|
| 1 | 3.825 | Toluene | C7H8 | 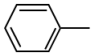 |
| 2 | 4.091 | Oxalic acid | C2H2O4 |  |
| 3 | 5.283 | Hydroquinone | C6H6O2 | 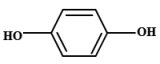 |
| 4 | 5.407 | o-diphenol | C6H6O2 |  |
| 5 | 5.755 | Styrene | C8H8 | 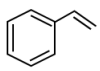 |
| 6 | 5.968 | Benzoquinone | C6H4O2 | 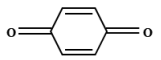 |
| 7 | 7.225 | Phenol | C6H6O | 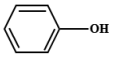 |
| 8 | 7.379 | 2,2,3,5-tetramethyl | C11H24 | 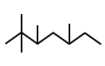 |
| 9 | 8.040 | 3-hydroxybenzyl alcohol | C7H8O2 | 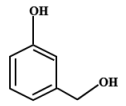 |
| 10 | 8.448 | Maleic acid | C4H4O4 |  |
| 11 | 9.109 | Undecane | C11H24 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, Y.; Zhao, F.; Chen, Y.; Lin, X.; Yin, H.; Tang, X. Enhanced Electrocatalytic Oxidation of Phenol by SnO2-Sb2O3/GAC Particle Electrodes in a Three-Dimensional Electrochemical Oxidation System. Water 2023, 15, 1844. https://doi.org/10.3390/w15101844
Pu Y, Zhao F, Chen Y, Lin X, Yin H, Tang X. Enhanced Electrocatalytic Oxidation of Phenol by SnO2-Sb2O3/GAC Particle Electrodes in a Three-Dimensional Electrochemical Oxidation System. Water. 2023; 15(10):1844. https://doi.org/10.3390/w15101844
Chicago/Turabian StylePu, Yu, Fan Zhao, Yini Chen, Xiangyu Lin, Haoran Yin, and Xinhua Tang. 2023. "Enhanced Electrocatalytic Oxidation of Phenol by SnO2-Sb2O3/GAC Particle Electrodes in a Three-Dimensional Electrochemical Oxidation System" Water 15, no. 10: 1844. https://doi.org/10.3390/w15101844
APA StylePu, Y., Zhao, F., Chen, Y., Lin, X., Yin, H., & Tang, X. (2023). Enhanced Electrocatalytic Oxidation of Phenol by SnO2-Sb2O3/GAC Particle Electrodes in a Three-Dimensional Electrochemical Oxidation System. Water, 15(10), 1844. https://doi.org/10.3390/w15101844






