Potential of Epipremnum aureum and Bacopa monnieri (L.) Wettst for Saline Phytoremediation in Artificial Wetlands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Vegetable Matter
2.3. Greenhouse Experiment
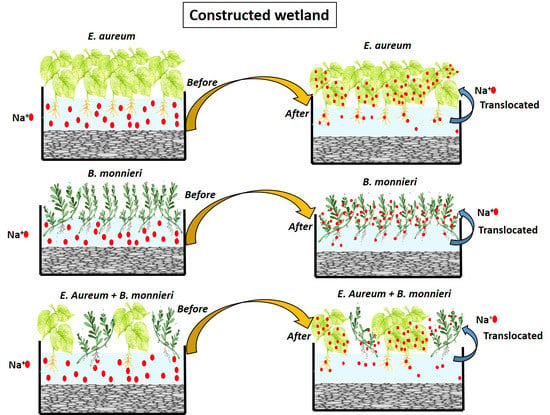
2.4. Treatments
2.5. Chemical Characteristics of the Irrigation Water Used for the Experiment
2.6. Fertilization
2.7. Chemical Analysis of the Water
2.8. Phytoremediation Capacity (PHC)
2.9. Statistical Analysis
3. Results
3.1. Cations
3.2. Phytoremediation Capacity (PHC)
3.3. Chemical Characteristics of the Irrigation Water
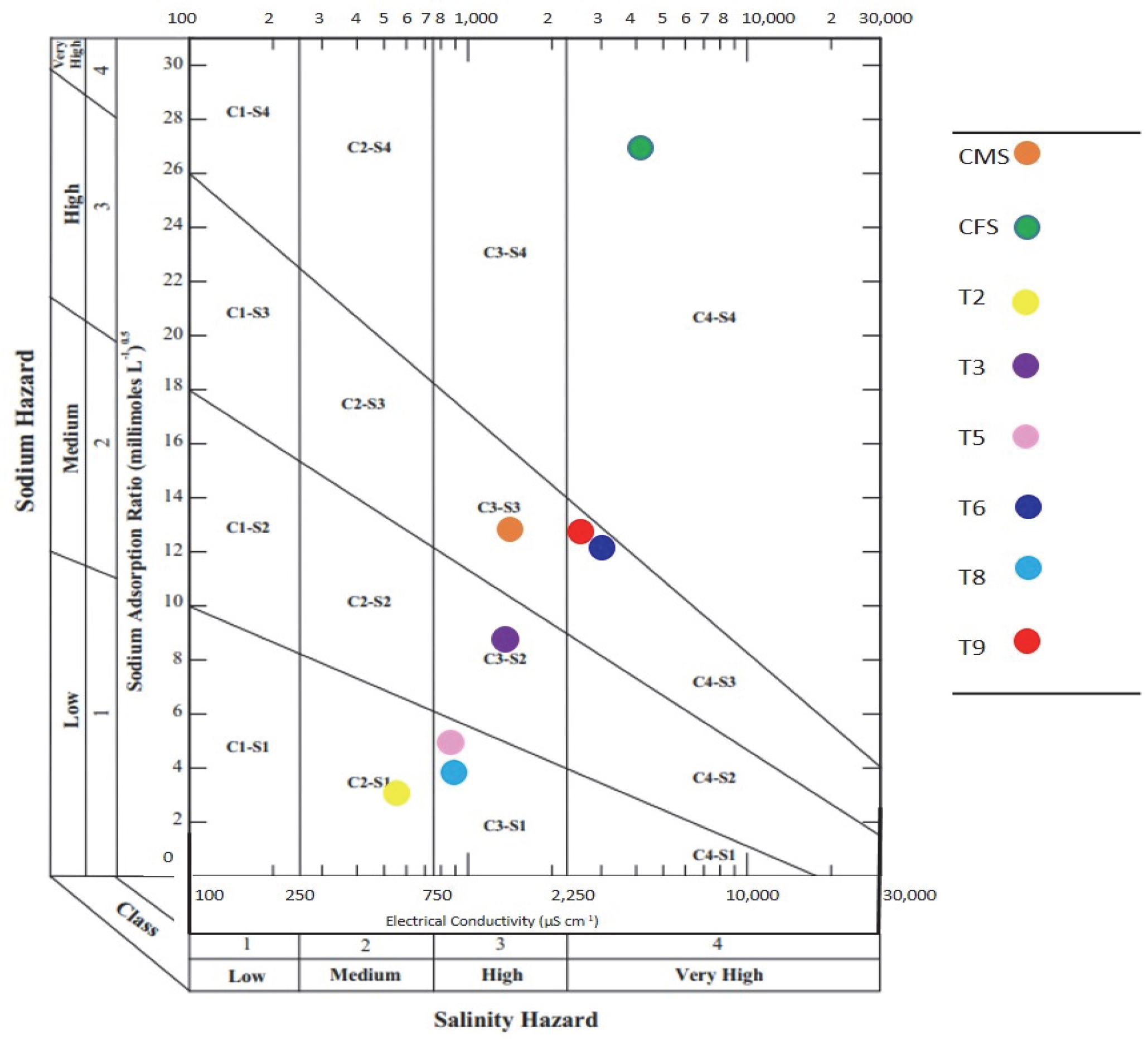
3.4. Degree of Restriction of Irrigation Water for Agricultural Use
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Al-Yahyai, R.A.; Al-Kharusi, L.M.; Khan, M.M.; Al-Adawi, A.O.; Al-Subhi, A.M.; AL-Kalbani, B.S.; Al-Sadi, A.M. Biotic and Abiotic Stresses of Major Fruit Crops in Oman: A Review. JAMS 2022, 27, 16–37. [Google Scholar]
- Song, J.; Shi, W.; Liu, R.; Xu, Y.; Sui, N.; Zhou, J.; Feng, G. The role of the seed coat in adaptation of dimorphic seeds of the euhalophyte Suaeda salsa to salinity. Plant Species Biol. 2017, 32, 107–114. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhuang, X.; Ahmad, S.; Sung, S.; Ni, S.Q. Biotreatment of high-salinity wastewater: Current methods and future directions. World J. Microbiol. Biotechnol. 2020, 36, 37. [Google Scholar] [CrossRef]
- Wali, S.U.; Gada, M.A.; Umar, K.J.; Abba, A.; Umar, A. Understanding the causes, effects, and remediation of salinity in irrigated fields: A review. J. Environ. Assess Policy Manag. 2021, 1, 9–42. [Google Scholar]
- Ondrasek, G.; Rathod, S.; Manohara, K.K.; Gireesh, C.; Anantha, M.S.; Sakhare, A.S.; Horvatinec, J. Salt Stress in Plants and Mitigation Approaches. Plants 2022, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhu, H.; Banuelos, G.; Yan, B.; Zhou, Q.; Yu, X.; Cheng, X. Constructed wetlands for saline wastewater treatment: A review. Ecol. Eng. 2017, 98, 275–285. [Google Scholar] [CrossRef]
- Freedman, A.; Gross, A.; Shelef, O.; Rachmilevitch, S.; Arnon, S. Salt uptake and evapotranspiration under arid conditions in horizontal subsurface flow constructed wetland planted with halophytes. Ecol. Eng. 2014, 70, 282–286. [Google Scholar] [CrossRef]
- Karungamye, P.N. Potential of Canna indica in Constructed Wetlands for Wastewater Treatment: A Review. Conservation 2022, 2, 499–513. [Google Scholar] [CrossRef]
- Turcios, A.E.; Miglio, R.; Vela, R.; Sánchez, G.; Bergier, T.; Wlodyka-Bergier, A.; Papenbrock, J. From natural habitats to successful application-Role of halophytes in the treatment of saline wastewater in constructed wetlands with a focus on Latin America. Environ. Exp. Bot. 2021, 190, 104583. [Google Scholar] [CrossRef]
- Chavan, R.; Mutnuri, S. Domestic wastewater treatment by constructed wetland and microalgal treatment system for the production of value-added products. Environ. Technol. 2021, 42, 3304–3317. [Google Scholar] [CrossRef]
- Polińska, W.; Kotowska, U.; Kiejza, D.; Karpińska, J. Insights into the use of phytoremediation processes for the removal of organic micropollutants from water and wastewater: A review. Water 2021, 13, 2065. [Google Scholar] [CrossRef]
- Hassan, I.; Chowdhury, S.R.; Prihartato, P.K.; Razzak, S.A. Wastewater treatment using constructed wetland: Current trends and future potential. Processes 2021, 9, 1917. [Google Scholar] [CrossRef]
- Farzi, A.; Borghei, S.M.; Vossoughi, M. The use of halophytic plants for salt phytoremediation in constructed wetlands. Int. J. Phytoremed. 2017, 19, 643–650. [Google Scholar] [CrossRef]
- Fountoulakis, M.S.; Sabathianakis, G.; Kritsotakis, I.; Kabourakis, E.M.; Manios, T. Halophytes as vertical-flow constructed wetland vegetation for domestic wastewater treatment. Sci. Total Environ. 2017, 583, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Jesus, J.; Cassoni, A.; Danko, A.; Fiuza, A.; Borges, M. Role of three different plants on simultaneous salt and nutrient reduction from saline synthetic wastewater in lab-scale constructed wetlands. Sci. Total Environ. 2017, 579, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Guesdon, G.; de Santiago-Martín, A.; Galvez-Cloutier, R. Phytodesalinization potential of Typha angustifolia, Juncus maritimus, and Eleocharis palustris for removal of de-icing salts from runoff water. Environ. Sci. Pollut. Res. 2016, 23, 19634–19644. [Google Scholar] [CrossRef]
- Buhmann, A.K.; Waller, U.; Wecker, B.; Papenbrock, J. Optimization of culturing conditions and selection of species for the use of halophytes as biofilter for nutrient-rich saline water. Agric. Water Manag. 2015, 149, 102–114. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Bhowmik, P.C.; Hossain, M.A.; Rahman, M.M.; Prasad, M.N.V.; Ozturk, M.; Fujita, M. Potential use of halophytes to remediate saline soils. Biomed Res. Int. 2014, 2014, 589341. [Google Scholar] [CrossRef]
- Flores-Olvera, H.; Czaja, A.; Estrada-Rodríguez, J.L.; Méndez, U.R. Floristic diversity of halophytic plants of Mexico. In Sabkha Ecosystems, 1st ed.; Khan, M.A., Boër, B., Ȫzturk, M., Clüsener-Godt, M., Gul, B., Breckle, S.W., Eds.; Springer: New York, NY, USA, 2016; Volume 5, pp. 299–327. [Google Scholar]
- Lastiri-Hernández, M.A.; Álvarez-Bernal, D.; Ochoa-Estrada, S.; Contreras-Ramos, S.M. Potential of Bacopa monnieri (L.) Wettst and Sesuvium verrucosum Raf. as an agronomic management alternative to recover the productivity of saline soils. Int. J. Phytoremed. 2020, 22, 343–352. [Google Scholar] [CrossRef]
- Maheshwaran, M.V.; Hyness, N.R.J.; Senthamaraikannan, P.; Saravanakumar, S.S.; Sanjay, M.R. Characterization of natural cellulosic fiber from Epipremnum aureum stem. J. Nat. Fibers 2018, 15, 789–798. [Google Scholar] [CrossRef]
- Yadav, R.K.; Sahoo, S.; Yadav, A.K.; Patil, S.A. Epipremnum aureum is a promising plant candidate for developing nature-based technologies for nutrients removal from wastewaters. J. Environ. Chem. Eng. 2021, 9, 106134. [Google Scholar] [CrossRef]
- Baltazar-Bernal, O.; Gaytán-Acuña, E.A.; Rodríguez-Elizalde, M.A.; Becerra-García, J.; García-Balderrama, V.B.; López-Hernández, N.A.; Moreno-Morelos, G. Production of pothos (Epipremnum aureum) in pots. Agroproductividad 2018, 11, 19–25. [Google Scholar]
- Sarma, P.J.; Mohanty, K. Epipremnum aureum and Dracaena braunii as indoor plants for enhanced bio-electricity generation in a plant microbial fuel cell with electrochemically modified carbon fiber brush anode. J. Biosci. Bioeng. 2018, 126, 404–410. [Google Scholar] [CrossRef]
- De Tar, W.R. Using a subsurface drip irrigation system to measure crop water use. Irrig. Sci. 2004, 23, 11. [Google Scholar]
- Allen, S.E. (Ed.) Analysis of vegetation and other organic materials. In Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: London, UK, 1989; pp. 46–61. [Google Scholar]
- USSL Staff. Diagnosis and Improvement of Saline and Alkali Soils; USDA Handbook No 60; USSL: Washington, DC, USA, 1954; 160p. [Google Scholar]
- Shahid, S.A.; Mahmoudi, H. National strategy to improve plant and animal production in the United Arab Emirates. Soil and water resources Annexes, 2014. [Google Scholar]
- APHA (American Public Health Association); AWWA-WEF (American Water Works). Standard Methods, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Eaton, F.M. Significance of carbonates in irrigation waters. Soil Sci. 1950, 69, 123–133. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture. Water Quality for Agriculture; Irrigation and Drainage Paper 29; FAO: Rome, Italy, 1994. [Google Scholar]
- Raju, N.J.; Shukla, U.K.; Ram, P. Hydrogeochemistry for the assessment of groundwater quality in Varanasi: A fast-urbanizing center in Uttar Pradesh, India. Environ. Monit. Assess. 2011, 73, 279–300. [Google Scholar] [CrossRef]
- Castellón-Gómez, J.J.; Bernal-Muñoz, R.; Hernández-Rodríguez, M.D.L. Calidad del agua para riego en la agricultura protegida en Tlaxcala. Ingeniería 2015, 19, 39–50. [Google Scholar]
- Rabhi, M.; Ferchichi, S.; Jouini, J.; Hamrouni, M.H.; Koyro, H.W.; Ranieri, A.; Smaoui, A. Phytodesalination of a salt-affected soil with the halophyte Sesuvium portulacastrum L. to arrange in advance the requirements for the successful growth of a glycophytic crop. Bioresour. Technol. 2010, 101, 6822–6828. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS 9.1. 3 Help and Documentation; SAS Institute Inc.: Cary, NC, USA, 2004; p. 5136. [Google Scholar]
- Sarwar, A.G.; Tinne, F.J.; Islam, N.; Islam, M.M.; Haque, M.S. Effects of Salt Stress on Growth and Accumulation of Na+, K+ and Ca2+ Ions in Different Accessions of Sesbania. Bangladesh J. Bot. 2022, 51, 157–167. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, K.; Cakmak, I.; Wang, S.; Zhang, F.; Guo, S. Synergistic and antagonistic interactions between potassium and magnesium in higher plants. Crop J. 2021, 9, 249–256. [Google Scholar] [CrossRef]
- Akter, M.; Oue, H. Effect of saline irrigation on accumulation of Na+, K+, Ca2+, and Mg2+ ions in rice plants. Agriculture 2018, 8, 164. [Google Scholar] [CrossRef] [Green Version]
- Ludwiczak, A.; Osiak, M.; Cárdenas-Pérez, S.; Lubińska-Mielińska, S.; Piernik, A. Osmotic stress or ionic composition: Which affects the early growth of crop species more? Agronomy 2021, 11, 435. [Google Scholar] [CrossRef]
- Breś, W.; Kleiber, T.; Markiewicz, B.; Mieloszyk, E.; Mieloch, M. The Effect of NaCl Stress on the Response of Lettuce (Lactuca sativa L.). Agronomy 2022, 12, 244. [Google Scholar] [CrossRef]
- Alaoui, I.; Serbouti, S.; Ahmed, H.; Mansouri, I.; El Kamari, F.; Taroq, A.; Farah, A. The mechanisms of absorption and nutrients transport in plants: A review. Trop. J. Nat. Prod. 2022, 6, 8–14. [Google Scholar]
- Debnath, M. Responses of Bacopa monnieri to salinity and drought stress in vitro. J. Med. Plant Res. 2008, 2, 347–351. [Google Scholar]
- Aslam, R.; Bostansup, N.; Mariasup, M.; Safdar, W. A critical review on halophytes: Salt tolerant plants. J. Med. Plant Res. 2011, 5, 7108–7118. [Google Scholar]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Ahire, M.L.; Walunj, P.R.; Kishor, P.K.; Nikam, T.D. Effect of sodium chloride-induced stress on growth, proline, glycine betaine accumulation, antioxidative defence and bacoside A content in in vitro regenerated shoots of Bacopa monnieri (L.) Pennell. Acta Physiol. Plant. 2013, 35, 1943–1953. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz-Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Jahan, B.; Iqbal, N.; Fatma, M.; Sehar, Z.; Masood, A.; Sofo, A.; Khan, N.A. Ethylene supplementation combined with split application of nitrogen and sulfur protects salt-inhibited photosynthesis through optimization of proline metabolism and antioxidant system in mustard (Brassica juncea L.). Plants 2021, 10, 1303. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, S.A.; El-Bialy, N.M. Glycine betaine enhancing plant growth and antioxidant activity of fenugreek (Trigonella foenum-graecum L.) under salt stress. J. Biol. Res. 2022, 1, 1–21. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Banerjee, A. Endogenous glycine betaine accumulation mediates abiotic stress tolerance in plants. Trop. Plant Res. 2016, 3, 105–111. [Google Scholar]
- Panda, A.; Rangani, J.; Parida, A.K. Unraveling salt responsive metabolites and metabolic pathways using non-targeted metabolomics approach and elucidation of salt tolerance mechanisms in the xero-halophyte Haloxylon salicornicum. Plant Physiol. Biochem. 2021, 158, 284–296. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Koźmińska, A.; Hanus-Fajerska, E.; Dziurka, K.; Dziurka, M. Insight into phytohormonal modulation of defense mechanisms to salt excess in a halophyte and a glycophyte from Asteraceae family. Plant Soil 2021, 463, 55–76. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef]
- Zhou, S.; Zhao, R.; Li, Q.; Du, J.; Chen, C.; Lu, Q.; An, S. Influent salinity affects substrate selection in surface flow constructed wetlands. Environ. Sci. Pollut. Res. 2021, 28, 62235–62245. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Baek, K.H. Physiological and biochemical perspectives of non-salt tolerant plants during bacterial interaction against soil salinity. Plant Physiol. Biochem. 2017, 116, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Ahkami, A.H.; White, R.A., III; Handakumbura, P.P.; Jansson, C. Rhizosphere engineering: Enhancing sustainable plant ecosystem productivity. Rhizosphere 2017, 3, 233–243. [Google Scholar] [CrossRef]
- de la Fuente, C.C.; Simonin, M.; King, E.; Moulin, L.; Bennett, M.J.; Castrillo, G.; Laplaze, L. An extended root phenotype: The rhizosphere, its formation and impacts on plant fitness. Plant J. 2020, 103, 951–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merino-Martín, L.; Stokes, A.; Gweon, H.S.; Moragues-Saitua, L.; Staunton, S.; Plassard, C.; Griffiths, R.I. Interacting effects of land use type, microbes and plant traits on soil aggregate stability. Soil Biol. Biochem. 2021, 154, 108072. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z. The importance of Cl− exclusion and vacuolar Cl− sequestration: Revisiting the role of Cl− transport in plant salt tolerance. Front. Plant Sci. 2019, 10, 1418. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tester, M.; Gilliham, M. Chloride on the move. Trends Plant Sci. 2017, 22, 236–248. [Google Scholar] [CrossRef] [PubMed]
- López-García, A.D.; Ortega-Escobar, H.M.; Ramírez-Ayala, C.; Sánchez-Bernal, E.I.; Can-Chulim, Á.; Gómez-Meléndez, D.J.; Vázquez-Alvarado, R.E. Caracterización fisicoquímica del agua residual urbano-industrial y su importancia en la agricultura. Tecnol. Cienc. Agua 2016, 7, 139–157. [Google Scholar]
- Ilangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Vega, M.I.; Can Chulim, Á.; Romero Bañuelos, C.A.; Cruz Crespo, E.; Madueño Molina, A. Water quality for agricultural use of Mololoa river, Mexico. Terra Latinoam. 2019, 37, 185–195. [Google Scholar]
- Sahab, S.; Suhani, I.; Srivastava, V.; Chauhan, P.S.; Singh, R.P.; Prasad, V. Potential risk assessment of soil salinity to agroecosystem sustainability: Current status and management strategies. Sci. Total Environ. 2021, 764, 144164. [Google Scholar] [CrossRef]
| mg g−1 DW | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | Na+ | Ca2+ | Mg2+ | K+ | Cl− | |||||
| I | F | I | F | I | F | I | F | I | F | |
| T1 | 18.52 a ± 0.38 | 29.15 g ± 0.61 | 8.32 c ± 0.18 | 82.49 a ± 1.69 | 3.93 a ± 0.1 | 51.84 a ± 1.08 | 6.04 c ± 0.12 | 98.19 a ± 2.06 | 32.72 a ± 0.68 | 51.50 g ± 1.08 |
| T2 | 18.52 a ± 0.38 | 102.14 d ± 2.14 | 8.32 c ± 0.18 | 66.67 c ± 1.35 | 3.93 a ± 0.1 | 38.25 c ± 0.80 | 6.04 c ± 0.12 | 83.45 c ± 1.75 | 32.72 a ± 0.68 | 180.48 d ± 3.79 |
| T3 | 18.52 a ± 0.38 | 195.68 a ± 4.10 | 8.32 c ± 0.18 | 47.54 e ± 0.91 | 3.93 a ± 0.1 | 23.61 f ± 0.45 | 6.04 c ± 0.12 | 64.92 d ± 1.36 | 32.72 a ± 0.68 | 345.76 a ± 7.26 |
| T4 | 3.78 c ± 0.12 | 12.26 i ± 0.25 | 16.51 a ± 0.34 | 62.17 d ± 1.30 | 3.15 c ± 0.06 | 34.78 d ± 0.73 | 11.07 a ± 0.23 | 79.44 c ± 1.79 | 6.67 c ± 0.14 | 21.66 i ± 0.45 |
| T5 | 3.78 c ± 0.12 | 53.39 f ± 1.12 | 16.51 a ± 0.34 | 23.81 g ± 0.92 | 3.15 c ± 0.06 | 17.27 h ± 0.36 | 11.07 a ± 0.23 | 37.35 g ± 1.41 | 6.67 c ± 0.14 | 94.34 f ± 1.98 |
| T6 | 3.78 c ± 0.12 | 117.32 c ± 2.46 | 16.51 a ± 0.34 | 19.26 h ± 0.61 | 3.15 c ± 0.06 | 12.19 i ± 0.25 | 11.07 a ± 0.23 | 25.58 h ± 0.95 | 6.67 c ± 0.14 | 207.30 c ± 4.35 |
| T7 | 11.15 b ± 0.23 | 20.61 h ± 0.43 | 12.41 b ± 0.27 | 74.32 b ± 1.56 | 3.54 b ± 0.08 | 45.69 b ± 0.95 | 8.55 b ± 0.17 | 87.83 b ± 1.82 | 19.70 b ± 0.41 | 36.41 h ± 0.76 |
| T8 | 11.15 b ± 0.23 | 76.27 e ± 1.60 | 12.41 b ± 0.27 | 46.77 e ± 1.19 | 3.54 b ± 0.08 | 29.76 e ± 0.62 | 8.55 b ± 0.17 | 60.66 e ± 1.48 | 19.70 b ± 0.41 | 134.77 e ± 2.83 |
| T9 | 11.15 b ± 0.23 | 143.68 b ± 3.01 | 12.41 b ± 0.27 | 28.93 f ± 0.81 | 3.54 b ± 0.08 | 20.05 g ± 0.43 | 8.55 b ± 0.17 | 43.48 f ± 1.12 | 19.70 b ± 0.41 | 253.88 b ± 5.33 |
| Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Treatments | Final FW per Plant (g) | Final DW per Plant (g) | Initial FW per Plant (g) | Initial DW per Plant (g) | PHC (g Plant−1) | PHC CW (g) | PHC (g m−2) |
| T1 | 284.13 a ± 5.68 | 26.08 d ± 0.49 | 83.42 a ± 1.73 | 7.66 c ± 0.14 | 0.195 f ± 0.006 | 1.170 f ± 0.041 | 5.655 f ± 0.171 |
| T2 | 257.09 c ± 5.14 | 23.60 e ± 0.43 | 83.61 a ± 1.68 | 7.66 c ± 0.12 | 1.332 c ± 0.027 | 7.992 c ± 0.167 | 38.628 c ± 0.812 |
| T3 | 221.83 e ± 4.43 | 20.36 f ± 0.38 | 83.55 a ± 1.62 | 7.66 c ± 0.15 | 2.249 a ± 0.047 | 13.494 a ± 0.283 | 65.221 a ± 1.369 |
| T4 | 270.94 b± 5.41 | 31.48 a ± 0.57 | 83.35 a ± 1.72 | 9.69 a± 0.17 | 0.184 f ± 0.006 | 1.104 f ± 0.034 | 5.336 f ± 0.155 |
| T5 | 228.68 e ± 4.57 | 26.57 c ± 0.50 | 83.47 a ± 1.59 | 9.69 a ± 0.20 | 0.837 e ± 0.016 | 5.022 e ± 0.105 | 24.273 e ± 0.509 |
| T6 | 182.56 g ± 3.65 | 21.21 f ± 0.42 | 83.43 a± 1.64 | 9.69 a± 0.19 | 1.307 c ± 0.025 | 7.842 c ± 0.164 | 37.903 c ± 0.795 |
| T7 | 277.19 b ± 5.54 | 28.82 b ± 0.54 | 83.46 a ± 1.73 | 8.67 b ± 0.16 | 0.190 f ± 0.005 | 1.140 f ± 0.029 | 5.510 f ± 0.147 |
| T8 | 241.93 d ± 4.83 | 25.16 d ± 0.47 | 83.39 a ± 1.65 | 8.67 b ± 0.15 | 1.073 d ± 0.022 | 6.438 d ± 0.135 | 31.117 d ± 0.653 |
| T9 | 203.37 f ± 4.06 | 21.15 f ± 0.40 | 83.34 a ± 1.56 | 8.67 b ± 0.15 | 1.655 b ± 0.034 | 9.930 b ± 0.208 | 47.995 b ± 1.007 |
| Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|
| CMS | T2 | T5 | T8 | CFS | T3 | T6 | T9 | |
| Parameters | I | F | F | F | I | F | F | F |
| pH | 7.96 c ± 0.02 | 7.75 f ± 0.02 | 7.84 e ± 0.02 | 7.81 e ± 0.03 | 8.14 a ± 0.02 | 7.91 d ± 0.03 | 8.06 b ± 0.02 | 7.98 c ± 0.02 |
| EC (μS cm−1) | 2000 d ± 50 | 670 h ± 50 | 1170 f ± 50 | 920 g ± 50 | 4000 a ± 50 | 1730 e ± 50 | 2690 b ± 50 | 2340 c ± 50 |
| Na+ (mmol L−1) | 19.18 d ±0.12 | 6.82 h ± 0.12 | 10.35 f ± 0.18 | 9.34 g ±0.17 | 38.26 a ±0.12 | 16.66 e ±0.29 | 25.63 b ± 0.46 | 23.34 c ± 0.42 |
| Ca2+ (mmol L−1) | 0.42 e ± 0.03 | 1.58 d ± 0.03 | 1.72 b ± 0.03 | 1.67 c ± 0.03 | 0.42 e ± 0.03 | 1.67 c ± 0.32 | 1.83 ª ± 0.03 | 1.76 b ± 0.03 |
| Mg2+ (mmol L−1) | 3.57 f ± 0.08 | 4.49 e ± 0.08 | 4.79 d ± 0.08 | 4.61 c ± 0.08 | 3.57 f ± 0.08 | 5.61 b ± 0.10 | 5.97 ª ± 0.11 | 5.83 a ± 0.10 |
| K+ (mmol L−1) | 0.34 g ± 0.09 | 5.08 f ± 0.09 | 6.16 d ± 0.11 | 5.58 e ± 0.10 | 0.34 g ± 0.09 | 6.97 c ± 0.12 | 8.53 ª ± 0.15 | 7.94 b ± 0.14 |
| SO42− (mmol L−1) | 2.47 a ± 0.04 | 0.91 f ± 0.01 | 1.32 e ± 0.02 | 1.18 e ± 0.02 | 2.47 a ± 0.04 | 1.55 d ± 0.02 | 2.15 b ± 0.03 | 1.87 c ± 0.03 |
| CO32− (mmol L−1) | 1.16 a ± 0.02 | 0.55 g ± 0.01 | 0.81 e ± 0.02 | 0.67 f ± 0.02 | 1.16 a ± 0.02 | 0.93 d ± 0.02 | 1.10 b ± 0.02 | 1.04 c ± 0.02 |
| HCO3− (mmol L−1) | 3.58 a ± 0.07 | 1.57 g± 0.03 | 1.92 e ± 0.03 | 1.75 f ± 0.03 | 3.58 a ± 0.07 | 2.45 d ± 0.04 | 3.29 b ± 0.06 | 2.91 c ± 0.05 |
| Cl− (mmol L−1) | 19.15 c ± 0.10 | 6.04 g ± 0.10 | 9.18 e ± 0.17 | 8.28 f ± 0.15 | 33.89 c ±0.10 | 14.76 d ±0.26 | 22.70 a ± 0.41 | 20.67 b ± 0.37 |
| RSC (mmol L−1) | 0.75 a ± 4.51 | ND | ND | ND | 0.75 a ± 4.51 | ND | ND | ND |
| SAR (mmol L−1)0.5 | 13.54 b ± 0.07 | 3.91 h ± 0.07 | 5.73 f ± 0.12 | 5.27 g ± 0.09 | 27.35 a ±0.07 | 8.73 e ± 0.16 | 12.98 c ± 0.24 | 12.02 d ± 0.21 |
| ESP | 20.17 b ± 0.10 | 5.83 h ± 0.10 | 8.54 f ± 0.15 | 7.85 g ± 0.14 | 40.35 a ±0.10 | 13.01 e ±0.23 | 19.34 c ± 0.35 | 17.91 d ± 0.33 |
| ES (mmol L−1) | 19.52 f ± 0.23 | 15.85 h ± 0.19 | 19.29 e ± 0.27 | 18.78 g ±0.2 | 38.60 a ±0.42 | 27.53 d ±0.28 | 37.57 b ± 0.36 | 34.92 c ± 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lastiri-Hernández, M.A.; Álvarez-Bernal, D.; Cruz-Cárdenas, G.; Silva-García, J.T.; Conde-Barajas, E.; Oregel-Zamudio, E. Potential of Epipremnum aureum and Bacopa monnieri (L.) Wettst for Saline Phytoremediation in Artificial Wetlands. Water 2023, 15, 194. https://doi.org/10.3390/w15010194
Lastiri-Hernández MA, Álvarez-Bernal D, Cruz-Cárdenas G, Silva-García JT, Conde-Barajas E, Oregel-Zamudio E. Potential of Epipremnum aureum and Bacopa monnieri (L.) Wettst for Saline Phytoremediation in Artificial Wetlands. Water. 2023; 15(1):194. https://doi.org/10.3390/w15010194
Chicago/Turabian StyleLastiri-Hernández, Marcos Alfonso, Dioselina Álvarez-Bernal, Gustavo Cruz-Cárdenas, J. Teodoro Silva-García, Eloy Conde-Barajas, and Ernesto Oregel-Zamudio. 2023. "Potential of Epipremnum aureum and Bacopa monnieri (L.) Wettst for Saline Phytoremediation in Artificial Wetlands" Water 15, no. 1: 194. https://doi.org/10.3390/w15010194