Catalytic Ozonation Combined with Conventional Treatment Technologies for the Recycling of Automobile Service Station Wastewater
Abstract
:1. Introduction
2. Material and Methods
2.1. Sampling
2.2. Analytical Methods
2.3. Chemicals and Reagents
2.4. Experimental Setup
2.5. Treatment Procedure
2.6. Ozonation Treatment Optimization
2.7. Catalysts Preparation and Characterization
2.8. Treatment Combinations
3. Results and Discussion
3.1. Initial Characterization of ASSWW
3.2. RH and GAC Characterization
3.3. Ozonation Treatment
3.4. Comparison of Treatment Combinations
3.4.1. Effect of Turbidity Removal
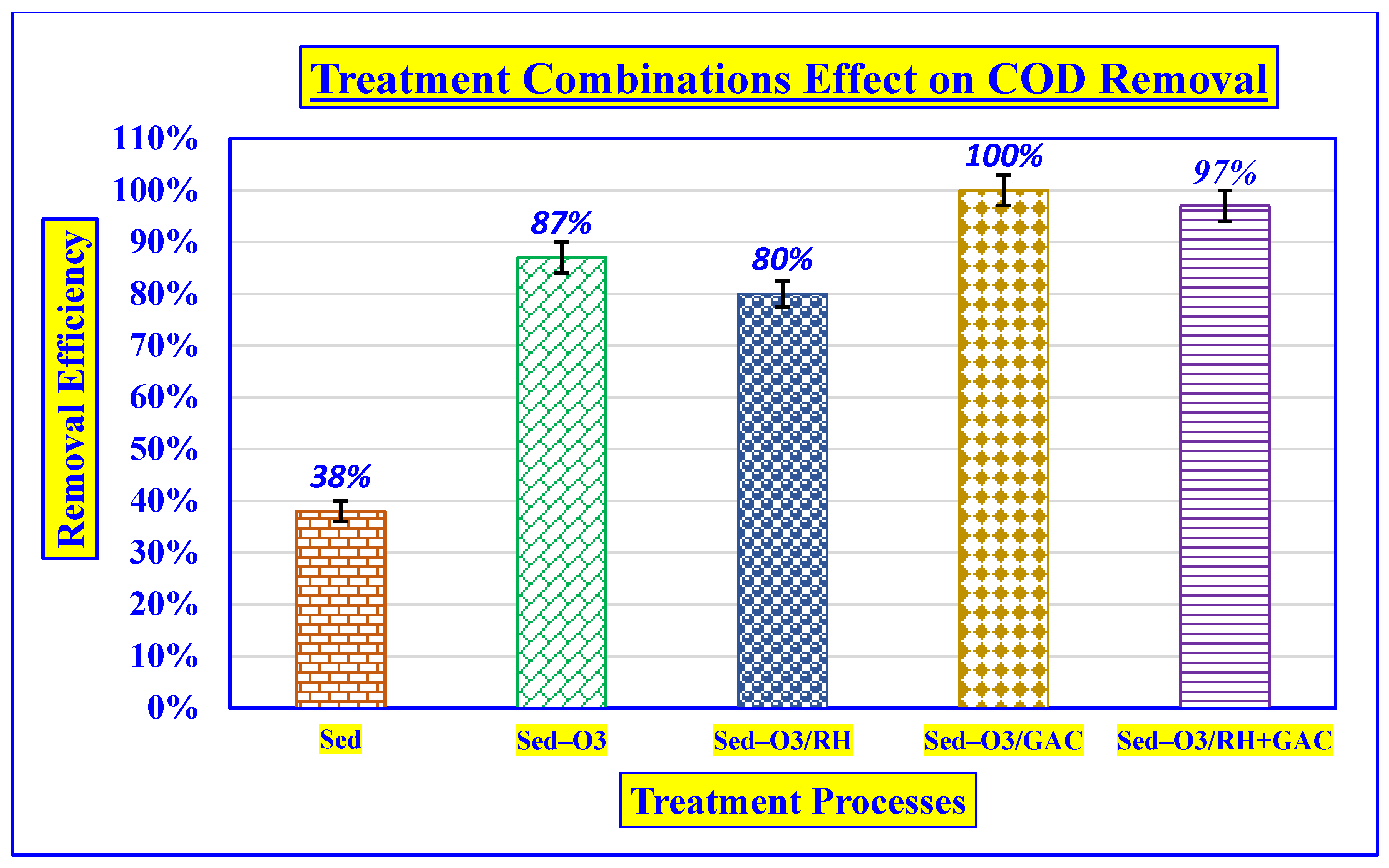
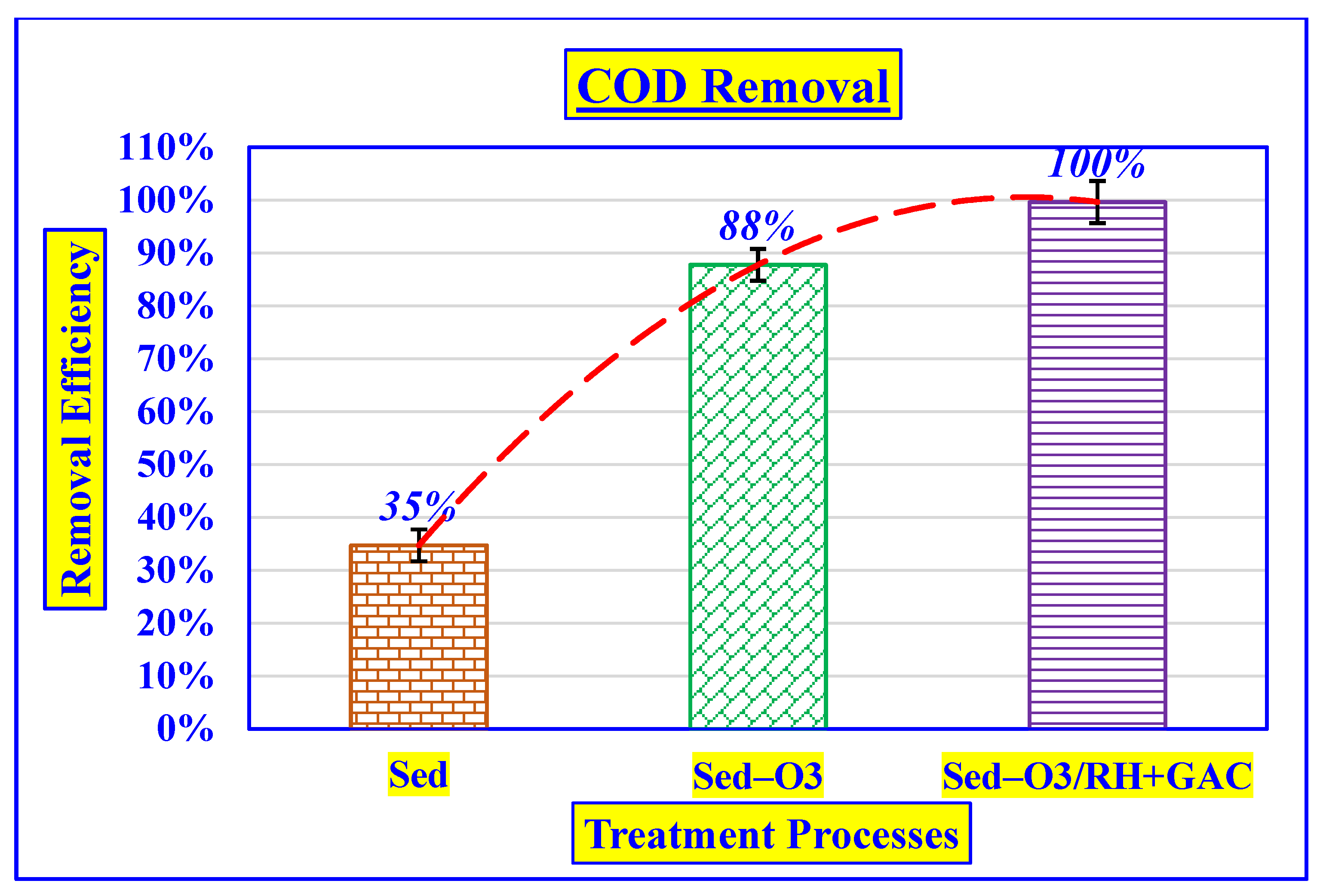
3.4.2. Effect of COD Removal
3.4.3. Effect of BOD5 Removal
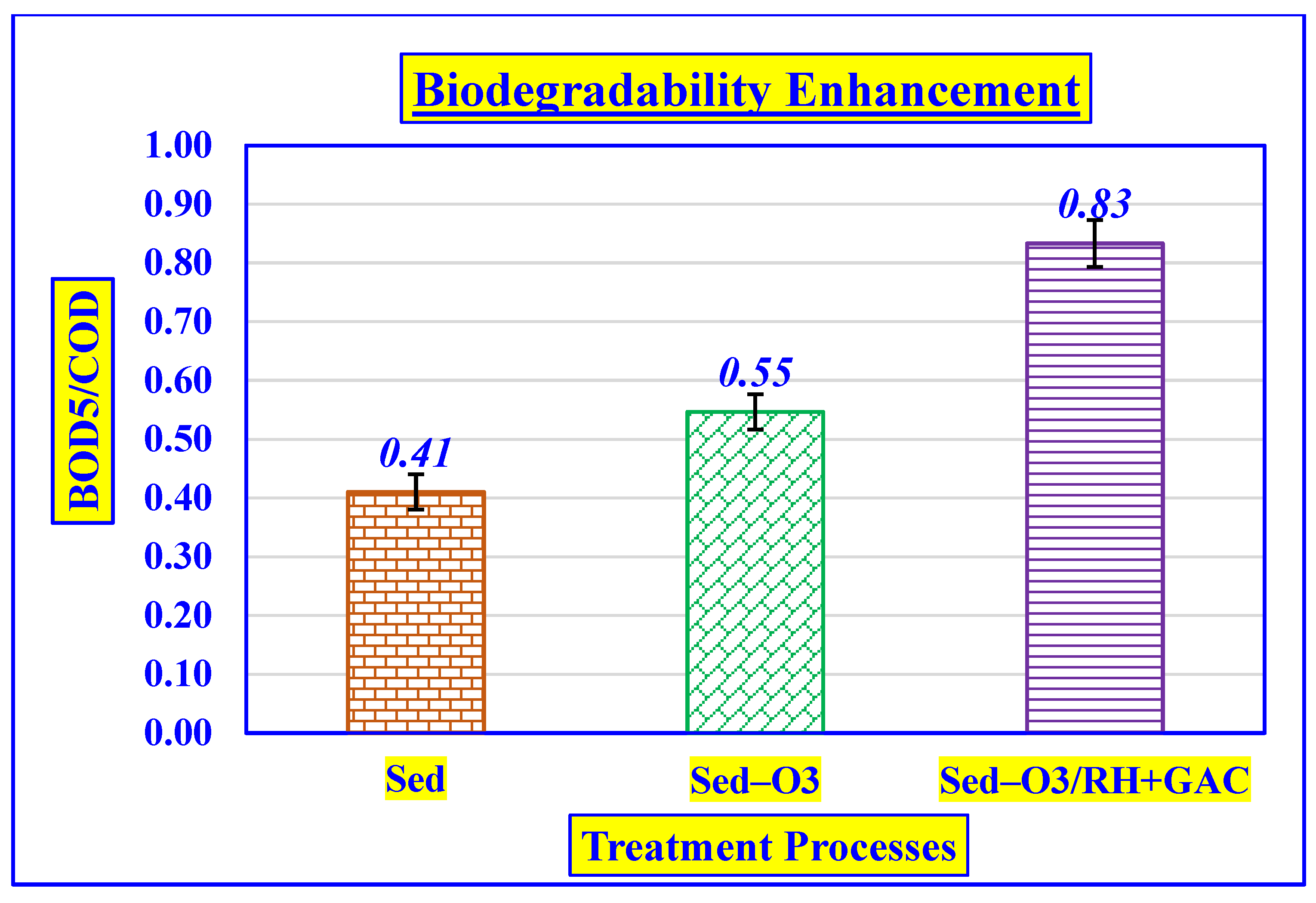
3.4.4. Effect of Biodegradability Enhancement
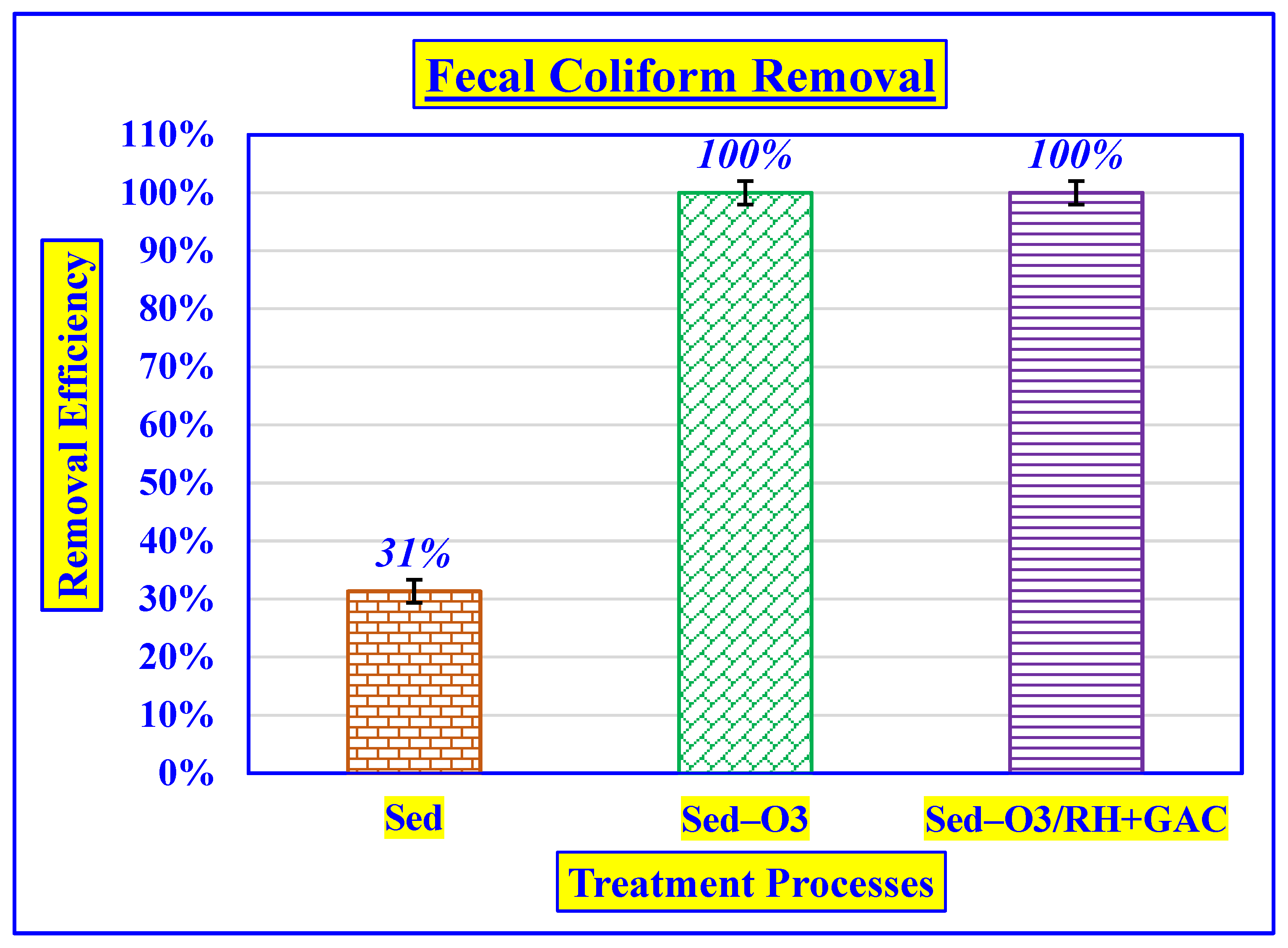
3.4.5. Effect of Fecal Coliform Removal
3.4.6. Effect of Oil and Grease Removal
3.4.7. Effect of Potentially Toxic Metals Removal
3.5. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahil, T.; Albiac, J.; Fischer, G.; Strokal, M.; Tramberend, S.; Greve, P.; Tang, T.; Burek, P.; Burtscher, R.; Wada, Y. A nexus modeling framework for assessing water scarcity solutions. Curr. Opin. Environ. Sustain. 2019, 40, 72–80. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Liu, Z.; Wu, J.; Pan, X.; Fang, Z.; Li, J.; Bryan, B.A.J.N.C. Future global urban water scarcity and potential solutions. Nat. Commun. 2021, 12, 4667. [Google Scholar] [CrossRef]
- Biswas, A.K.; Tortajada, C. Assessing global water megatrends. In Assessing Global Water Megatrends; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–26. [Google Scholar]
- van Vliet, M.T.; Jones, E.R.; Flörke, M.; Franssen, W.H.; Hanasaki, N.; Wada, Y.; Yearsley, J.R.J.E.R.L. Global water scarcity including surface water quality and expansions of clean water technologies. Environ. Res. Lett. 2021, 16, 024020. [Google Scholar] [CrossRef]
- Masood, Z.; Ikhlaq, A.; Akram, A.; Qazi, U.Y.; Rizvi, O.S.; Javaid, R.; Alazmi, A.; Madkour, M.; Qi, F. Application of Nanocatalysts in Advanced Oxidation Processes for Wastewater Purification: Challenges and Future Prospects. Catalysts 2022, 12, 741. [Google Scholar] [CrossRef]
- Hossaini, H.; Rahimi, S.; Usefi, S. Evaluation of carwashes in Kermanshah metro-city with regard to water resources, consumption, and existence of treatment facility. Int. J. Health Life Sci. 2017, 3, 1–5. [Google Scholar]
- Zaneti, R.; Etchepare, R.; Rubio, J.J.R. Conservation. Car wash wastewater reclamation. Full-scale application and upcoming features. Resour. Conserv. Recycl. 2011, 55, 953–959. [Google Scholar] [CrossRef]
- Bhatti, Z.A.; Mahmood, Q.; Raja, I.A.; Malik, A.H.; Khan, M.S.; Wu, D. Chemical oxidation of carwash industry wastewater as an effort to decrease water pollution. Phys. Chem. Earth Parts A/B/C 2011, 36, 465–469. [Google Scholar] [CrossRef]
- Hashim, N.H.; Zayadi, N. Pollutants characterization of car wash wastewater. In Proceedings of the MATEC Web of Conferences, Amsterdam, The Netherlands, 23–25 March 2016; p. 05008. [Google Scholar]
- Bhatti, S.; Siddiqui, Z.; Memon, S.; Kandhir, I.; Memon, M. Analysis and treatment wash off water from vehicular service station in Hyderabad. Sindh Univ. Res. J.-SURJ 2017, 49, 473–478. [Google Scholar] [CrossRef]
- Ahmad, J.; Khan, N.A.; Shafiq, M.A.; Khan, N. A low-cost wastewater treatment unit for reducing the usage of fresh water at car wash stations in Pakistan. Pak. J. Sci. Ind. Res. Ser. A Phys. Sci. 2019, 62, 57–66. [Google Scholar]
- Monney, I.; Donkor, E.A.; Buamah, R. Clean vehicles, polluted waters: Empirical estimates of water consumption and pollution loads of the carwash industry. Heliyon 2020, 6, e03952. [Google Scholar] [CrossRef]
- Mazumder, D.; Mukherjee, S. Treatment of automobile service station wastewater by coagulation and activated sludge process. Int. J. Environ. Sci. Dev. 2011, 2, 64. [Google Scholar] [CrossRef]
- Boluarte, I.A.R.; Andersen, M.; Pramanik, B.K.; Chang, C.-Y.; Bagshaw, S.; Farago, L.; Jegatheesan, V.; Shu, L.J.I.B. Reuse of car wash wastewater by chemical coagulation and membrane bioreactor treatment processes. Int. Biodeterior. Biodegrad. 2016, 113, 44–48. [Google Scholar] [CrossRef]
- Gönder, Z.B.; Balcıoğlu, G.; Vergili, I.; Kaya, Y. An integrated electrocoagulation–nanofiltration process for carwash wastewater reuse. Chemosphere 2020, 253, 126713. [Google Scholar] [CrossRef] [PubMed]
- Zaneti, R.; Etchepare, R.; Rubio, J. More environmentally friendly vehicle washes: Water reclamation. J. Clean. Prod. 2012, 37, 115–124. [Google Scholar] [CrossRef]
- Etchepare, R.; Zaneti, R.; Azevedo, A.; Rubio, J. Application of flocculation–flotation followed by ozonation in vehicle wash wastewater treatment/disinfection and water reclamation. Desalin. Water Treat. 2015, 56, 1728–1736. [Google Scholar] [CrossRef]
- Subtil, E.L.; Rodrigues, R.; Hespanhol, I.; Mierzwa, J.C. Water reuse potential at heavy-duty vehicles washing facilities–the mass balance approach for conservative contaminants. J. Clean. Prod. 2017, 166, 1226–1234. [Google Scholar] [CrossRef]
- Gönder, Z.B.; Balcıoğlu, G.; Vergili, I.; Kaya, Y. Electrochemical treatment of carwash wastewater using Fe and Al electrode: Techno-economic analysis and sludge characterization. J. Environ. Manag. 2017, 200, 380–390. [Google Scholar] [CrossRef]
- Gönder, Z.; Balcıoğlu, G.; Kaya, Y.; Vergili, I. Treatment of carwash wastewater by electrocoagulation using Ti electrode: Optimization of the operating parameters. Int. J. Environ. Sci. Technol. 2019, 16, 8041–8052. [Google Scholar] [CrossRef]
- Boussu, K.; Kindts, C.; Vandecasteele, C.; Van der Bruggen, B. Applicability of nanofiltration in the carwash industry. Sep. Purif. Technol. 2007, 54, 139–146. [Google Scholar] [CrossRef]
- Moazzem, S.; Wills, J.; Fan, L.; Roddick, F.; Jegatheesan, V. Performance of ceramic ultrafiltration and reverse osmosis membranes in treating car wash wastewater for reuse. Environ. Sci. Poll. Res. 2018, 25, 8654–8668. [Google Scholar] [CrossRef]
- Bakacs, M.E.; Yergeau, S.E.; Obropta, C.C. Assessment of car wash runoff treatment using bioretention mesocosms. J. Environ. Eng. 2013, 139, 1132–1136. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Dos Santos, E.V.; de Araújo Costa, E.C.T.; Martínez-Huitle, C.A. Electrochemical advanced oxidation processes (EAOPs) as alternative treatment techniques for carwash wastewater reclamation. Chemosphere 2018, 211, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Enoh, B.S.; Christopher, W.J.A. Adsorption of metal ions from carwash wastewater by phosphoric acid modified clay: Kinetics and thermodynamic studies. Adsorption 2015, 7, 1–8. [Google Scholar]
- Tony, M.A.; Bedri, Z. Experimental design of photo-Fenton reactions for the treatment of car wash wastewater effluents by response surface methodological analysis. Adv. Environ. Chem. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Singh, P.; Berawala, N.; Patil, Y. Automobile service station waste assessment and promising biological treatment alternatives: A review. Environ. Monit. Assess. 2022, 194, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Li, R.; Li, C.; Wang, W. Piezo-promoted regeneration of Fe2+ boosts peroxydisulfate activation by Bi2Fe4O9 nanosheets. Appl. Catal. B Environ. 2022, 310, 121330. [Google Scholar] [CrossRef]
- Chen, L.; He, X.-X.; Gong, Z.-H.; Li, J.-L.; Liao, Y.; Li, X.-T.; Ma, J.J.R.M. Significantly improved photocatalysis-self-Fenton degradation performance over g-C3N4 via promoting Fe (III)/Fe (II) cycle. Rare Metals 2022, 41, 1–10. [Google Scholar] [CrossRef]
- Zhou, Y.; Ouyang, W.; Wang, Y.; Wang, H.; Wu, Z. Core-shell structured NH2-UiO-66@ TiO2 photocatalyst for the degradation of toluene under visible light irradiation. Acta Phys.-Chim. Sin. 2021, 37, 8. [Google Scholar]
- Javed, F.; Tariq, A.; Ikhlaq, A.; Rizvi, O.S.; Ikhlaq, U.; Masood, Z.; Qazi, U.Y.; Qi, F. Application of Laboratory-Grade Recycled Borosilicate Glass Coated with Iron and Cobalt for the Removal of Methylene Blue by Catalytic Ozonation Process. Arab. J. Sci. Eng. 2022, 1–16. Available online: https://link.springer.com/article/10.1007/s13369-022-07437-6 (accessed on 1 November 2022). [CrossRef]
- Qazi, U.Y.; Ikhlaq, A.; Akram, A.; Rizvi, O.S.; Javed, F.; Ul-Hasan, I.; Alazmi, A.; Ibn Shamsah, S.M.; Javaid, R. Novel vertical flow wetland filtration combined with Co-zeotype material based catalytic ozonation process for the treatment of municipal wastewater. Water 2022, 14, 3361. [Google Scholar] [CrossRef]
- Rizvi, O.S.; Ikhlaq, A.; Ashar, U.U.; Qazi, U.Y.; Akram, A.; Kalim, I.; Alazmi, A.; Shamsah, S.M.I.; Al-Sodani, K.A.A.; Javaid, R. Application of poly aluminum chloride and alum as catalyst in catalytic ozonation process after coagulation for the treatment of textile wastewater. J. Environ. Manag. 2022, 323, 115977. [Google Scholar] [CrossRef] [PubMed]
- Ikhlaq, A.; Qazi, U.Y.; Akram, A.; Rizvi, O.S.; Sultan, A.; Javaid, R.; Al-Sodani, K.A.A.; Ibn Shamsah, S.M. Potable Water Treatment in a Batch Reactor Benefited by Combined Filtration and Catalytic Ozonation. Water 2022, 14, 2357. [Google Scholar] [CrossRef]
- Kuan, W.-H.; Hu, C.-Y.; Ke, L.-W.; Wu, J.-M. A Review of On-Site Carwash Wastewater Treatment. Sustainability 2022, 14, 5764. [Google Scholar] [CrossRef]
- Qazi, U.Y.; Javaid, R.; Ikhlaq, A.; Al-Sodani, K.A.A.; Rizvi, O.S.; Alazmi, A.; Asiri, A.M.; Ibn Shamsah, S.M. Synergistically Improved Catalytic Ozonation Process Using Iron-Loaded Activated Carbons for the Removal of Arsenic in Drinking Water. Water 2022, 14, 2406. [Google Scholar] [CrossRef]
- APHA, A.P.H.A.; Washington, D.C. WEF, Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Water Works Association, Water Environment Federation: Denver, CO, USA, 2017. [Google Scholar]
- Ikhlaqa, A.; Aslama, T.; Zafara, A.M.; Javedb, F.; Munirc, H.M.S. Combined ozonation and adsorption system for the removal of heavy metals from municipal wastewater: Effect of COD removal. Desalin. Water Treat. 2019, 159, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Ikhlaq, A.; Raashid, M.; Akram, A.; Kazmi, M.; Farman, S.J.D. Removal of methylene blue dye from aqueous solutions by adsorption in combination with ozonation on iron loaded sodium zeolite: Role of adsorption. Desalin. Water Treat. 2021, 237, 302–306. [Google Scholar] [CrossRef]
- Clayton, W.S.; Petri, B.G.; Huling, S.G. Fundamentals of ISCO using ozone. In In Situ Chemical Oxidation for Groundwater Remediation; Springer: Berlin/Heidelberg, Germany, 2011; pp. 193–232. [Google Scholar]
- Ikhlaq, A.; Munir, H.M.S.; Khan, A.; Javed, F.; Joya, K.S. Comparative study of catalytic ozonation and Fenton-like processes using iron-loaded rice husk ash as catalyst for the removal of methylene blue in wastewater. Ozone Sci. Eng. 2019, 41, 250–260. [Google Scholar] [CrossRef]
- WWF National Surface Water Classification Criteria & Irrigation Surface Water Guidelines for Pakistan, WWF-Pakistan, Ferozepur road, Lahore-54600, Pakistan. 2007. Available online: https://mocc.gov.pk/SiteImage/Misc/files/National%20Surface%20Water%20Classification%20Criteria.pdf (accessed on 1 November 2022).
- Metcalf, E. Wastewater Engineering: Treatment and Reuse, 4th ed.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Sarmadi, M.; Foroughi, M.; Najafi Saleh, H.; Sanaei, D.; Zarei, A.A.; Ghahrchi, M.; Bazrafshan, E. Efficient technologies for carwash wastewater treatment: A systematic review. Environ. Sci. Pollut. Res. 2020, 27, 34823–34839. [Google Scholar] [CrossRef]








| Reference | pH | Turbidity (NTU) | Phosphate (mg/L) | Alkalinity (mg/L) | COD (mg/L) | BOD5 (mg/L) | Coliform (CFU/100 mL) | TSS (mg/L) | Oil and Grease (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| [7] | 7.4–7.7 | 89–103 | - | - | 191–241 | 68–133 | 0.47–1.8 × 106 | 68–89 | 6–11 |
| [8] | 7.89–8.75 | 73–772 | 141–1019 | 110–5856 | 1.3–83.7 | ||||
| [9] | 7.96–8.33 | - | 8.35–10.23 | 108–300 | 398–490 | - | - | 268–333 | 79–89 |
| [10] | - | 82.4–93 | - | - | - | - | - | - | 11–49 |
| [11] | 6.90 | 253 | - | - | - | - | - | 1000 | 27 |
| [12] | 7.6–8.6 | - | - | - | 990–1413 | 297–565 | 2.3–4.7 × 103 | 1260–3417 | - |
| Parameters | Units | Mean Concentration | IWQGs Class D [42] |
|---|---|---|---|
| pH | - | 6.9 ± 0.2 | 6.5–8.4 |
| TSS | mg/L | 217 ± 6 | - |
| TDS | mg/L | 1037 ± 35 | 1000 |
| Turbidity | NTU | 669 ± 16 | - |
| BOD5 | mg/L | 225 ± 9 | 8 |
| COD | mg/L | 490 ± 11.5 | - |
| Total Nitrogen | mg/L | 28 ± 3.5 | - |
| Chlorides | mg/L | 150 ± 5.5 | 100 |
| Total Hardness | mg/L | 374 ± 17 | - |
| Fecal Coliform | MPN/100 mL | 5170 ± 95 | 1000 |
| Oil and Grease | mg/L | 200 ± 7.5 | - |
| Cadmium | mg/L | 0.056 ± 0.001 | 0.01 |
| Lead | mg/L | 0.012 ± 0.001 | 0.10 |
| Arsenic | mg/L | 0.100 ± 0.001 | 0.10 |
| Catalysts | Surface Area (m2/g) | Pore Size (Å) | pHpzc |
|---|---|---|---|
| RH | 90.4 | 18.2 | 2.9 ± 0.1 |
| GAC | 1080 | 9.1 | 8.7 ± 0.2 |
| Parameters | Units | Initial Values | Sed | Sed-O3 | Sed-O3/RH + GAC |
|---|---|---|---|---|---|
| pH | - | 6.9 ± 0.2 | 6.9 ± 0.2 | 7.3 ± 0.2 | 7.3 ± 0.2 |
| Turbidity | NTU | 669 ± 16 | 375 ± 11 | 155 ± 4 | 7.5 ± 0.5 |
| BOD5 | mg/L | 225 ± 9 | 131.3 ± 3.7 | 32.8 ± 1.5 | 1.5 ± 0.1 |
| COD | mg/L | 490 ± 11.5 | 320 ± 7 | 60 ± 2 | 1.8 ± 0.1 |
| BOD5/COD | ratio | 0.46 ± 0.01 | 0.44 ± 0.01 | 0.82 ± 0.01 | 0.83 ± 0.01 |
| Fecal Coliform | MPN/100 mL | 5170 ± 95 | 3550 ± 69 | 0.015 ± 0.001 | 0.007 ± 0.001 |
| Cadmium | mg/L | 0.056 ± 0.001 | 0.055 ± 0.001 | 0.053 ± 0.001 | BDL |
| Lead | mg/L | 0.012 ± 0.001 | 0.011 ± 0.001 | 0.010 ± 0.001 | BDL |
| Arsenic | mg/L | 0.100 ± 0.001 | 0.087 ± 0.001 | 0.07 ± 0.001 | BDL |
| Oil and Grease | mg/L | 200 ± 7.5 | 189 ± 6 | 52 ± 3 | 0.9 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikhlaq, A.; Fiaz, U.; Rizvi, O.S.; Akram, A.; Qazi, U.Y.; Masood, Z.; Irfan, M.; Al-Sodani, K.A.A.; Kanwal, M.; Shamsah, S.M.I.; et al. Catalytic Ozonation Combined with Conventional Treatment Technologies for the Recycling of Automobile Service Station Wastewater. Water 2023, 15, 171. https://doi.org/10.3390/w15010171
Ikhlaq A, Fiaz U, Rizvi OS, Akram A, Qazi UY, Masood Z, Irfan M, Al-Sodani KAA, Kanwal M, Shamsah SMI, et al. Catalytic Ozonation Combined with Conventional Treatment Technologies for the Recycling of Automobile Service Station Wastewater. Water. 2023; 15(1):171. https://doi.org/10.3390/w15010171
Chicago/Turabian StyleIkhlaq, Amir, Umar Fiaz, Osama Shaheen Rizvi, Asia Akram, Umair Yaqub Qazi, Zafar Masood, Mobeen Irfan, Khaled A. Alawi Al-Sodani, Mamoona Kanwal, Sami M. Ibn Shamsah, and et al. 2023. "Catalytic Ozonation Combined with Conventional Treatment Technologies for the Recycling of Automobile Service Station Wastewater" Water 15, no. 1: 171. https://doi.org/10.3390/w15010171







