Abstract
High-resolution multibeam sonar allows estimating movements of pelagic fish schools at short range. Taking advantage of this methodology, we calculated a Straightness Index (SI) to quantify the proportion of schools migrating actively from those residents in lagoon channels. This information enhances our knowledge of both fish school displacements and migration processes, which are essential to improve our understanding of ecosystem functioning. Most fish schools (65%) exhibited a SI value demonstrating oriented swimming behavior through the channels displayed by schools reaching the sea during fall migration. This trend appears as an intrinsic property of school movements, allowing monitoring of the school migration process in a channel to provide information for manager vs. fishing regulation measures or lagoon planning. The result strengthens the ‘multi-transit’ hypothesis, as 35% of schools show sinuous trajectories representative of schools staying in the channel or displaying high exploratory behaviors. Lastly, the fish school Exploration Swimming Speed (ESS) was tested as a fishery-independent sampling method to evaluate the proportion of different fish species monitored using hydroacoustics. This approach demonstrates the interest in using swimming behavioral characteristics of fish schools for ecological and management purposes.
1. Introduction
Fish movements are involved in a wide range of behavioral processes such as migration, space use, food searching, and reproduction [1,2]. Multibeam sonars in fisheries science [3] are usually applied to pelagic fish schools to scrutinize their tridimensional morphology [4,5,6]. Multibeam sonar observations in shallow waters allow the measurement of fish school swimming speeds and movements at different temporal scales [7,8,9], as well as calculation of the straightness index (SI) [1], which corresponds to the ratio between net and gross distances patrolled by a school. This index aims to characterize the path sinuosity of a school for given biological, ecological and social conditions. Moreover, the SI value could be an indicator of the efficiency for a fish school to orient its movement to reach a goal (e.g., [1,10,11]). Indeed, in a patchy food environment, foraging behaviors are often characterized by an increased turning rate and, therefore, by sinuous tracks, whereas migration behaviors should be more efficient when straight line movements are used [12,13,14]. In this work, we scrutinized the swimming patterns under the assumption that exploratory and migration behaviors show different trajectories, i.e., zigzag vs. straight line, respectively. Then, the path sinuosity of schools inferred from SI values is discussed with reference to the school migration behavior in lagoon channels and its consequences on school study methodologies using acoustic remote systems.
Otherwise, the fish swimming speed is often a key factor in ecological studies (e.g., [15,16,17]) and several approaches to measure this metric were experimentally developed in laboratories, water tanks and swim tunnels [18]. In situ, the swimming speed is usually estimated from electronic and ultrasonic tags on individual fish [19]. Studies of path orientation on individual fish were carried out using this technology [20]. For studying fish school velocity, one approach is to use multibeam acoustic systems [8,21,22] but very few studies are available in the literature, particularly at short range. Due to hydrodynamic constraints, the sizes of fish are homogeneous in a school [23]. Under this assumption, fish sizes by species obtained from both fishing data and the Exploratory Swimming Speed (ESS) [9] of each school estimated by the multibeam sonar tracking method were used to explore the relationship between school velocity and species. Thus, we proposed a new method to estimate the relative abundance of different groups of schooling species in a lagoon.
2. Materials and Methods
Two 24-h acoustic surveys were carried out consecutively in early autumn inside two Mediterranean lagoon channels in 1999 (Figure 1). The study area was located in the south of France (43°44′ N; 03°79′ E and 43°52′ N; 03°90′ E) in two coastal shallow water lagoons (Ingril, 549 ha and Prévost, 380 ha) connected to the sea by a channel [8]. These two lagoons are part of a series of shallow ponds with similar fish species communities [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. The common species of these lagoons are Anguilla anguilla, Atherina boyeri, Dicentrarchus labrax, Sparus aurata, and various mullets (Liza ramada, Liza aurata, Mugil cephalus, Chelon labrosus) [8,25,26]. Only schooling species were considered in this work. During the acoustic surveys, cast net samplings were performed in the channel for fish identification [8]. During this sampling period, the dominant gregarious fish groups were represented by juveniles of mugilidae (fork length ‘FL1’~7.5 cm), of S. aurata and D. labrax (mean FL2~13 cm), and the last group was composed of sub-adults of S. aurata, D. labrax and of several mugilidae species (mean FL3~20 cm) [8], identified by local experts. Considering the ethical rules on animal welfare, no dissection or experiment was carried out on fish. These species encountered in lagoons are known to migrate to the sea in autumn for physiological (e.g., decrease in water lagoon temperature) and spawning reasons, whereas the spring migration to lagoons is considered to be linked to trophic motivations, lagoons being considered as a nursery for juveniles of several species [27].
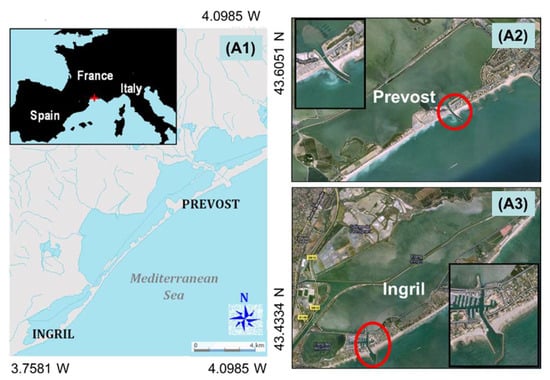
Figure 1.
(A1) Map of the study area: the two French Mediterranean shallow water lagoons are located in the same watershed; they are part of a series of shallow ponds, and are both linked to the sea by a single channel. (A2,A3) On the right, an aerial view of the two lagoons (source: Google earth), with the channels (circle and zoom) where the sonar system was set up.
A Reson Seabat 6012 multibeam sonar, operating at a frequency of 455 kHz with a pulse duration of 0.06 ms, was deployed at a fixed position on the channel bank. The sonar was used in horizontal beaming [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] across both lagoon channels, width less than 25 m (20 m for the Ingril channel and 24 m for the Prévost channel) and 1.5 m depth (Appendix A). The efficient horizontal angle of echo reception is 90° (horizontal plan: 60 contiguous 1.5° beams; vertical angle of 15°), the sonar resolution was around 45 cm [29]. The sonar detection, i.e., echo traces, allows observing the kinematics of fish schools [8] over both 24-h surveys. Fish schools showing splitting/merging events were removed from the analysis to avoid double counting. The total time of continuous school detection (residence time) is variable due to the loss of school echo traces. Indeed, the swimming trajectory inside the sonar beams generates the variation in the observation time between fish schools.
The lowest elementary linear distances (l in meter) were estimated between two consecutive positions of the geometric center of fish school echo traces on a time interval of 2 s between consecutive position records. The sum ‘L’ of elementary ‘l’ distances for a given school corresponds to the gross displacement or path length of this school. Finally, the beeline distance ‘D’ (in meters) was measured [9] from the starting point (i.e., first detection) to the last position of the school in the sonar beams (net displacement). Fish school path lengths L and beeline distances D were estimated for each school with a minimum of three echo traces [9] reported from sonar detections.
To compare the gross to net path lengths, we calculated the straightness index (SI) [1], which corresponds to the ratio between the net and the gross distances patrolled by a fish school and computed as the ratio between D and L (SI = D/L). This index ranging between 0 and 1 is a reliable measure of the sinuosity of the path, i.e., a SI close to 1 illustrates directed movements linked to the efficiency of an orientation mechanism to reach a goal [1,30]. Moreover, the Exploratory Swimming Speed (ESS in m s−1) of each school was estimated in [9] by dividing the beeline distance D between the starting point to the last point of the school in the beam with the time interval between these two extreme records of the school in the sonar beams. Previous processing on the same data set have shown that the distance values between fish school echo traces were recorded for time intervals ranging from 1 to 8 s (average of 4 s) [9]. ESS mean values were 1.19 m s−1 (standard deviation: σ = 0.77) and 1.34 m s−1 (σ = 0.79) for the Ingril and Prévost channels, respectively; no difference was found between the Ingril and Prévost channels [9]. These Exploratory Swimming Speed (ESS) data were compared to the fish lengths by species obtained from fishing data [8] in order to explore the relationship between school velocity and species.
The difference in beeline distances (D), path length (L), Straightness Index (SI) and Exploratory Swimming Speed (ESS) of schools between both lagoons were tested using analysis of variance (ANOVA) tests. All the assumptions for the models were previously checked. If the assumptions were not met, a non-parametric Kruskal–Wallis test was used. Linear regression models were used to analyze the relationships between the straightness index and the total time of the school observation or the path length L in the sonar beams. All statistical tests were performed with R software (R Core Team, 2021; version 3.6.2, https://www.R-project.org/ (accessed on 16 July 2021)).
3. Results
3.1. Fish School Data Collection
A total of 164 fish schools was analyzed over both 24-h surveys [9], 41 originated from the Ingril channel and 123 from the Prévost one. The number of sampled school echo traces reached 621 (n = 174 in the Ingril channel and n = 447 in the Prévost channel). The residence time in the acoustic beam varies from 2 to 34 s (average of 10 s for both channels) [9]. No statistical difference (ANOVA, F(1,162) = 0.8469, p-value = 0.359) was found for the residence time between both channels.
The beeline value (D) of schools ranged between 0.34 and 22.1 m (average = 8.59 m; SD = 5.04) for the Ingril channel and between 1.57 to 37.1 m (average = 10.7 m; SD = 5.62) for the Prévost channel [9]. A statistical difference was found for the beeline distances (D) between channels (ANOVA, F(1, 162) = 4.37, p-value = 0.03815). However, no significant difference was found for the path length between channels (ANOVA, F (1, 162) = 2.811, p-value = 0.09553) (Figure 2b).
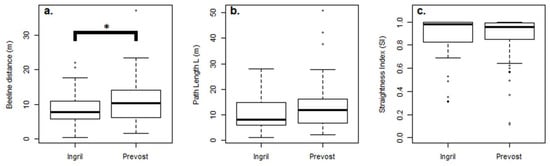
Figure 2.
(a) The beeline value (D) of fish schools for the Ingril and Prévost channels. A significant difference (*) was found between D values for channels. (b) However, no statistical difference was found for the path length (L) between the Ingril and Prévost channels. (c) Straightness index (SI) for the Ingril and Prévost channels, where no significant difference was found.
3.1.1. Straightness Index
For the Ingril and Prévost channels, SI values for schools ranged from 0.31 to 1 (average = 0.87, SD = 0.20) and from 0.12 to 1 (average = 0.89, SD = 0.16), respectively. No statistical difference for the SI was found between the Ingril and Prévost channels (Kruskal–Wallis, 0.50632, df = 1, p-value = 0.4767) (Figure 2c). Similar cumulated frequency distributions of SI values were found for both lagoons (Appendix C; Figure A4).
A linear significant relationship was found between the SI and the total time of the school observation in the sonar beams for the Prevost channel (p-value = 0.0127) but with a low adjusted R-squared (R2 = 0.04). No significant linear relationship was found for the Ingril channel (p-value = 0.162). Finally, a linear significant relationship was found between the SI and the path length (L) for the Prevost channel (R2 = 0.25, p-value = 3.36 × 10−9). No significant linear relationship was found for the Ingril channel (p-value = 0.273) (Figure 3).
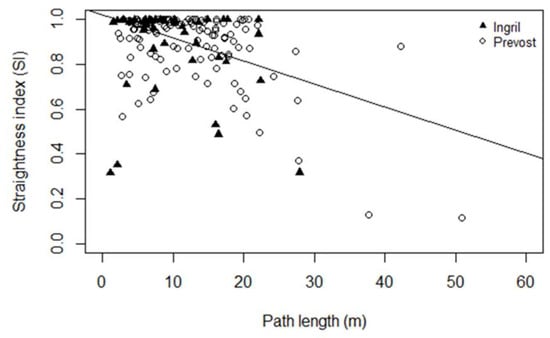
Figure 3.
Relationship between the net to gross displacement ratio ‘straightness index’ and the fish school path length (Full triangles: the Ingril lagoon. Empty circles: the Prévost lagoon). The black line represents the single significant linear relationship found for the Prévost lagoon.
3.1.2. Exploratory Swimming Speed (ESS) of Fish Schools
The scatterplot of ESS values versus the residence time showed the effect of a limiting factor (Figure 4). This limiting factor suggests maximal speed values of schools for an oriented path according to the residence time in the beams and regarding the fish community in concern. The theoretical limits of the observations given by the sampling protocol was a power trend curve (Figure 4).
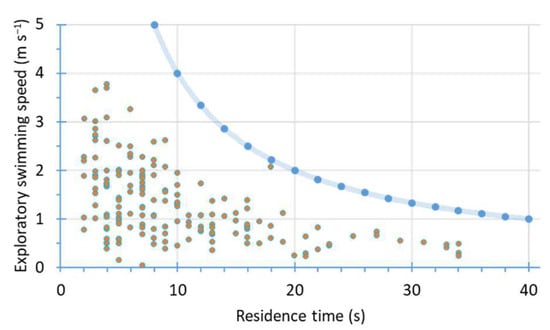
Figure 4.
Relationship between the fish school residence time (in seconds) and their exploratory swimming speed (ESS in m s−1). The scatterplot of ESS values versus the residence time showed the effect of a limiting factor, highlighted by the bold curve, which represents the theoretical limits (bold point) of the observations given by the sampling protocol, here a power trend curve of the theoretical function (see, Appendix D).
According to the average sizes of the three groups of migratory gregarious fish sampled by fishing (FL1~7.5 cm, FL2~13 cm and FL3~20 cm), ESS values were divided into three groups (Figure 5) with an upper limit of ESS by group corresponding to a body length speed of about 19 Bl s−1. This limit value of 19 Bl s−1 was selected because, for a school with both a short residence time in the beams and an oriented path (i.e., major part of the observations in this study), ESS might be considered close to instantaneous speed values. Considering this limit value, individual fish swimming speeds for the three groups correspond to ESS values of 1.4 m s−1, 2.5 m s−1 and 3.8 m s−1, respectively.
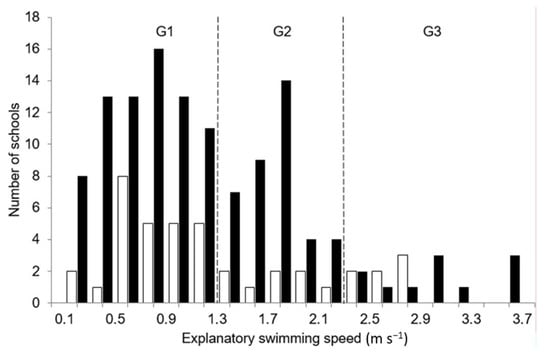
Figure 5.
Histogram of average Exploratory Swimming Speed of fish schools observed in the Prevost (black) and Ingril (white) lagoons. Three groups (G1; G2 and G3) might be distinguished at a similar swimming speed interval (~1.25 m s−1), which were assumed to be related to main migratory gregarious species: mugilidae juveniles (G1: Fork length ‘FL’ < 7.5 cm), juveniles of Sparus aurata and Dicentrarchus labrax (G2: FL~13 cm), and G3 composed of sub-adults of several species (mullets, S. aurata and D. labrax) with an average FL size around 20 cm.
4. Discussion
We used a high-resolution multibeam sonar in horizontal beaming to better understand the movements of pelagic fish schools during the migration period in shallow waters. Our approach demonstrates the interest in using swimming behavioral characteristics of fish schools for ecological and management purposes.
4.1. Straigtness Index and Fish Migration
If we assume that the oriented swimming behavior corresponds to a certain form of an active migration (continuous swimming activity), our results estimated that ~65% of fish schools (Appendix B, Figure A3) exhibited an active migration movement through the lagoon channels. Up to a threshold value of 0.9 for SI, the school was considered displaying an oriented swimming behavior. A significant linear relationship was observed between the path length (L) and the total time in the sonar beam for a given school. Rather than being dependent of one of the two previous variables considered, this movement pattern appeared as an intrinsic characteristic of the observed schools since such a general trend of school movements in both channels was observed regardless of the path length (Figure 3). Furthermore, as the time of the school observation in the beam depends essentially on the distance between the school and the beam-emitting source (the sonar transducer), this rules out the hypothesis of a likely relationship between SI values and the residence time of schools in the beam. The factor observed in Figure 4 represents the maximum distance covered by the sonar beam, knowing that it covers a maximum distance of 20 and 24 m perpendicular to the channels (the width of the lagoon channel at Ingrid and Prevost, respectively) and therefore (with a sonar opening angle of 90°) a maximum distance (parallel to the channel) between 40 and 48 m. Hence, the general pattern observed for the orientation of school displacements in lagoon channels would be independent of the sampling protocol.
This estimation is reliable under the hypothesis that schools exhibited this movement pattern throughout their transfer within the lagoon channels during the fall migration of mugilidae, sparidae and centrarchidae. Fish schools not having a well-defined migration behavior regarding their SI value are thus susceptible to be counted several times by the acoustic system (what has been called the multi-transit hypothesis [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]) and thus overestimate the school passage in the channel. They reached ~35% of analyzed fish schools, which could represent resident schools in lagoon channels having an exploratory behavior rather than a migratory one.
No statistical difference was found for the SI values between the two channels. Hence, we cannot conclude about differences in swimming tracks of the fish species. Nevertheless, the beeline distance was significantly higher for the Prevost channel than for the Ingril channel. The path length was also higher for Prevost than for the Ingril channel although no statistical difference was found between the two channels, which is consistent with the fact that the SI were close to 1. Even if similar fish species communities were present in both the Prévost and Ingril channels [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24], this could be related to a change in abundance of fish species. Indeed, during the sampling period, the dominant gregarious fish species were represented by S. aurata and D. labrax and several mugilidae species. According to [8], we observed that among species that could be detected by the multibeam sonar, D. Labrax was in higher abundance in the catches during the fall survey (according to catches per unit of effort values, Figure A4). This result is consistent with the fact that the global school shapes were significantly different between the lagoons as shown in [8]. Furthermore, based on these data, 92.4% of the fish could display a migration behavior in fall for both channels, which is consistent with the SI values. However, further investigations would be required to establish a link between the fish species discriminations and the sonar detections.
We found according to the beeline distance that fish schools from both lagoons did not exhibit the same swimming behavior (Figure 2) in the lagoon channels. Such difference can be linked to the difference observed in fish species composition in the two lagoons (Appendix C, Figure A4). An alternative explanation for the difference in swimming behaviors, as seen for the beeline distance of fish schools from both lagoons could be the presence of resident fish in the lagoon channels. Indeed, we could assume that migratory fish schools (lagoon towards sea at the studied season) get a straighter displacement than the resident ones, which should lead to more exploration of the channels for e.g., trophic reasons. The absence of difference in the SI could be explained by the fact that the fall migration started in both channels. Nevertheless, additional information on the migration of each species is necessary to state such a hypothesis.
4.2. Do Exploratory Swimming Speed Values Can Help to Discriminate Fish Species?
Relative abundances of each size group in the lagoon channels were inferred from ESS distributions. On this basis, the proportion of each group (G1, G2 and G3) will be 60, 30 and 10% in the Prévost lagoon channel and 68, 20 and 12% in the Ingril one, respectively. Obviously, further surveys must be carried out to assess the relevance of this remote sensing approach, which is an interesting independent-fishery sampling method of gregarious fish communities in shallow-waters, i.e., an alternative method to usual lethal fish sampling like gillnets or fyke nets. Monitoring fish communities in sensitive zones like Marine Protected Areas or essential habitats using such methodology is obviously relevant. In combination with the e-DNA method [31], underwater video observations [32,33,34], such as complementary non-invasive approaches, might be used in the future for fish monitoring to gather diversity, quantification and behavioral knowledge.
To gather our data, the operating system carried out was time consuming (Appendix A, Figure A1). An automation of working sequences using dedicated software is suitable. As echo traces were easily identifiable, a discrimination algorithm of useful echoes might be technically easy to develop [35,36,37].
5. Conclusions
Combining fish school path sinuosity and exploratory swimming speed will bring interesting perspectives in ecological studies of pelagic fish schools. First, this information enhances our knowledge of both fish school displacements and migration processes, which are essential to improve our understanding of ecosystem functioning and therefore its modelling at the mesoscale [38,39]. The method is non-invasive and allows monitoring of the school migration process in a channel to provide information for manager vs. fishing regulation measures or lagoon planning. Second, the size structure of the fish community can be inferred from this approach forward as a fishery-independent sampling method. Thus, it will allow helping decision making for management measures at the seashore. Third, this kind of information remains rare in the literature but can be applied for other purposes, e.g., to estimate the fish school coefficient of diffusion in situ at a fine scale. Both straightness index (SI) and Exploratory Swimming Speed (ESS) should be proposed as indicators for various purposes: (i) to discriminate fish school species, (ii) characterize their behavioral motivation (feeding, spawning, and migration), (iii) identify the structure of the fish community into size groups, and (iv) even bring elementary information on fish activity rates for fish bioenergetic models.
Author Contributions
Conceptualization, P.B. (Patrice Brehmer); methodology, P.B. (Patrice Brehmer) and M.S.; software, P.B. (Patrice Brehmer); validation, P.B. (Pascal Bach) and J.G.; formal analysis, P.B. (Patrice Brehmer), V.D. and P.I.C.P.; investigation, P.B. (Patrice Brehmer); resources, P.B. (Patrice Brehmer); data curation, P.I.C.P.; writing—original draft preparation, P.B. (Patrice Brehmer) and M.S.; writing—review and editing, P.B. (Pascal Bach), P.B. (Patrice Brehmer), V.D., N.D. and J.G.; supervision, P.B. (Patrice Brehmer) and P.B. (Pascal Bach); project administration, P.B. (Patrice Brehmer); funding acquisition, P.B. (Patrice Brehmer) and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by planning agreement between the national and regional governments CPER XI (Occitanie, France), and ended within the AWA project funded by French National Research Institute for Sustainable Development “IRD” and the German Federal Ministry of Education and Research BMBF grant number 01DG12073E and the European Maritime and Fisheries Fund “Acapela”.
Data Availability Statement
Not applicable.
Acknowledgments
We dedicate this work to Thang Do Chi † (Univ Montpellier). Thank to Simon Benhamou (CNRS) for early discussion, to David Mouillot (Univ Montpellier) for friendly work within this project, François Gerlotto (retired Orstom-IRD) for his early work on the use of multibeam sonar in shallow water to monitor fish school and Pascal Cotel (retired Orstom-IRD) as well as all volunteers for support during the field measurements and the French National Research Institute for Sustainable Development ‘IRD’ staff of IRD DR Ouest and DR Occitanie.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Fish school dynamic observations using high-resolution multibeam sonar (according to [8,9].
The RESON Seabat 6012 multi-beam sonar used for data acquisition emits on 60 contiguous beams of 1.5° each. The efficient horizontal angle of echo receptions is 90° with a vertical angle of 15°. The sonar frequency was 455 kHz with pulse duration of 0.06 ms; all the data were continuously stored on video recording supports. The sonar characteristics and the environmental parameters determine the threshold of the sonar resolution, in our case 45 cm [29]. A preliminary study of acoustic data, intended to quantify the migratory fish school flows collected from the school echo traces [40,41] count from acoustic imagery, was performed using the same acoustic equipment in horizontal beaming [8].
The video recordings were replayed at the laboratory to select the sonar sequences including fish school echo traces (Figure A1), which correspond to specific detections of homogeneous continuous responses clearly discriminated on the screen. For both sampling areas, we were able to observe both mobile and stationary echo traces [9]. Dynamic echo traces and characteristics of fish school detections were discriminated from fixed bottom echo traces (Figure A2). In this way, each selected series of sonar images corresponding to a detection of a school identified by an individual code was stored in a fish school library. Each separate fish school datum was extracted from the sonar images using the ‘Infobancs’ software [37]. For each fish school, we collected the number of consecutive echo traces ‘N’, the total time of observation of echo traces within the beam (in seconds) and the Euclidian position (x; y) of the center of the fish school, defined as the gravity center of the surface defining the detected biological structure. From this information and scale factor of observations on the screen [8] we estimated all distances traveled by the fish schools. The selected time interval was set at one second for the shortest observation, without restriction in the total time of observation above three seconds.
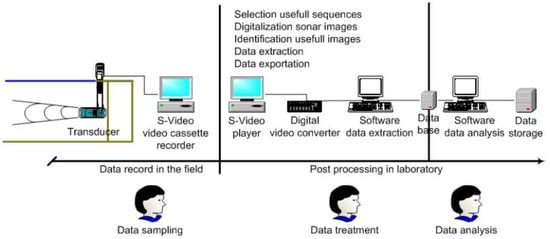
Figure A1.
Scheme representing the sonar data collection, their treatment, which include several steps (selection of sonar sequence, digitalization, identification of echo traces on sonar images, data extraction and then exportation for final analysis on ad hoc software), and their analysis to obtain the swimming speed measurements.

Figure A2.
Diagram of the experimental set up, showing the sonar beams covering the channel, bank to bank. On the right, an example of a sonar picture where the dynamic echoes of fish school (inside the circle, in t1, t2 and t3) were discriminated from the fixed echoes of the bottom (Reprinted with permission from [9]).
Appendix B
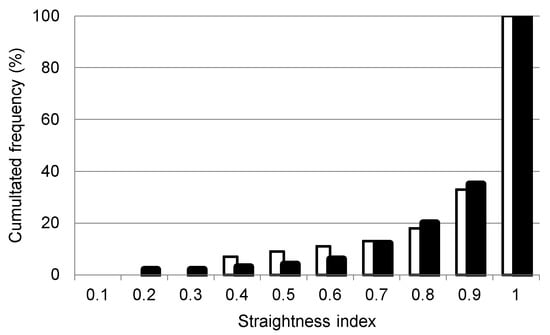
Figure A3.
Cumulative frequency of the fish school net to gross displacement ratio ‘straightness index’; the schools having a SI above 0.9 (~65% of the schools) were considered to exhibit an oriented swimming behavior that corresponds to a certain form of active migration. In white, the Ingril lagoon schools, and in black the Prevost ones.
Appendix C
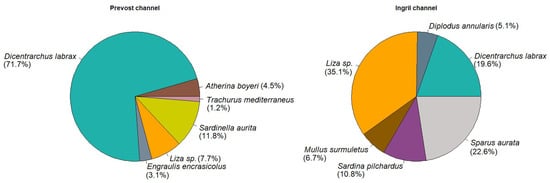
Figure A4.
Composition of 15 fish species commercially caught in the two Prévost and Ingril lagoons. Data extracted from [8].
Appendix D

Table A1.
Raw experimental data used for this study and extracted from [8,9], the Straightness index (SI) was the Beeline value divided (D) by the Path Length (L), calculated as SI = D/L. Below the theoretical function of limiting factor to the sampling protocol.
Table A1.
Raw experimental data used for this study and extracted from [8,9], the Straightness index (SI) was the Beeline value divided (D) by the Path Length (L), calculated as SI = D/L. Below the theoretical function of limiting factor to the sampling protocol.
| Lagoons | Residence Time (s) | ESS (m s−1) | Beeline Value (D) (m) | Path Length (L) (m) | Straigthness Index (SI) |
|---|---|---|---|---|---|
| Ingril | 2 | 0.777 | 1.55 | 1.57 | 0.988 |
| Ingril | 2 | 1.023 | 2.05 | 2.06 | 0.994 |
| Ingril | 2 | 2.275 | 4.55 | 4.57 | 0.996 |
| Ingril | 3 | 2.615 | 7.84 | 8.79 | 0.893 |
| Ingril | 3 | 1.968 | 5.90 | 6.05 | 0.976 |
| Ingril | 3 | 1.266 | 3.80 | 3.85 | 0.987 |
| Ingril | 3 | 1.897 | 5.69 | 5.75 | 0.989 |
| Ingril | 3 | 2.702 | 8.11 | 8.14 | 0.995 |
| Ingril | 3 | 2.718 | 8.15 | 8.16 | 1.000 |
| Ingril | 4 | 1.657 | 6.63 | 6.63 | 1.000 |
| Ingril | 4 | 1.720 | 6.88 | 7.16 | 0.961 |
| Ingril | 5 | 0.149 | 0.74 | 2.12 | 0.351 |
| Ingril | 5 | 1.022 | 5.11 | 7.43 | 0.688 |
| Ingril | 5 | 1.106 | 5.53 | 5.81 | 0.952 |
| Ingril | 5 | 0.592 | 2.96 | 2.96 | 1.000 |
| Ingril | 5 | 2.009 | 10.04 | 10.25 | 0.980 |
| Ingril | 5 | 0.486 | 2.43 | 3.43 | 0.707 |
| Ingril | 6 | 2.484 | 14.91 | 14.91 | 1.000 |
| Ingril | 7 | 0.048 | 0.34 | 1.08 | 0.313 |
| Ingril | 7 | 0.878 | 6.14 | 6.14 | 1.000 |
| Ingril | 7 | 1.064 | 7.45 | 17.20 | 1.000 |
| Ingril | 8 | 0.785 | 6.28 | 7.25 | 0.867 |
| Ingril | 8 | 2.580 | 20.64 | 22.09 | 0.934 |
| Ingril | 8 | 2.215 | 17.72 | 17.72 | 1.000 |
| Ingril | 9 | 0.836 | 7.53 | 7.58 | 0.993 |
| Ingril | 9 | 0.832 | 7.49 | 7.49 | 1.000 |
| Ingril | 9 | 1.578 | 14.20 | 17.51 | 0.811 |
| Ingril | 11 | 1.076 | 11.83 | 13.24 | 0.894 |
| Ingril | 11 | 0.928 | 10.21 | 10.21 | 1.000 |
| Ingril | 12 | 0.736 | 8.84 | 27.92 | 0.316 |
| Ingril | 13 | 0.525 | 6.82 | 6.88 | 0.992 |
| Ingril | 16 | 0.854 | 13.66 | 16.49 | 0.828 |
| Ingril | 16 | 1.383 | 22.12 | 22.12 | 1.000 |
| Ingril | 17 | 0.498 | 8.47 | 16.05 | 0.528 |
| Ingril | 18 | 0.605 | 10.90 | 11.26 | 0.968 |
| Ingril | 19 | 0.524 | 9.96 | 10.15 | 0.981 |
| Ingril | 21 | 0.639 | 13.42 | 13.62 | 0.985 |
| Ingril | 22 | 0.363 | 7.99 | 16.41 | 0.487 |
| Ingril | 23 | 0.450 | 10.35 | 12.70 | 0.815 |
| Ingril | 23 | 0.473 | 10.88 | 11.57 | 0.941 |
| Ingril | 31 | 0.523 | 16.22 | 22.38 | 0.725 |
| Prevost | 2 | 3.068 | 6.14 | 6.44 | 0.953 |
| Prevost | 2 | 2.187 | 4.37 | 4.58 | 0.955 |
| Prevost | 2 | 1.886 | 3.77 | 3.82 | 0.988 |
| Prevost | 3 | 2.259 | 6.78 | 8.23 | 0.824 |
| Prevost | 3 | 1.515 | 4.54 | 4.96 | 0.915 |
| Prevost | 3 | 1.851 | 5.55 | 5.73 | 0.969 |
| Prevost | 3 | 2.854 | 8.56 | 8.56 | 1.000 |
| Prevost | 3 | 1.001 | 3.00 | 3.00 | 1.000 |
| Prevost | 3 | 1.619 | 4.86 | 4.86 | 1.000 |
| Prevost | 3 | 3.012 | 9.04 | 9.04 | 1.000 |
| Prevost | 3 | 3.637 | 10.91 | 11.13 | 0.980 |
| Prevost | 4 | 0.391 | 1.56 | 2.76 | 0.568 |
| Prevost | 4 | 0.789 | 3.15 | 5.03 | 0.627 |
| Prevost | 4 | 1.093 | 4.37 | 6.78 | 0.645 |
| Prevost | 4 | 0.493 | 1.97 | 2.63 | 0.748 |
| Prevost | 4 | 0.819 | 3.28 | 3.95 | 0.829 |
| Prevost | 4 | 3.774 | 15.10 | 17.86 | 0.845 |
| Prevost | 4 | 3.692 | 14.77 | 15.90 | 0.928 |
| Prevost | 4 | 0.515 | 2.06 | 2.20 | 0.938 |
| Prevost | 4 | 1.801 | 7.20 | 7.67 | 0.939 |
| Prevost | 4 | 1.046 | 4.19 | 4.37 | 0.958 |
| Prevost | 4 | 1.700 | 6.80 | 7.05 | 0.964 |
| Prevost | 4 | 1.998 | 7.99 | 8.06 | 0.991 |
| Prevost | 4 | 1.043 | 4.17 | 4.20 | 0.993 |
| Prevost | 4 | 3.083 | 12.33 | 12.41 | 0.993 |
| Prevost | 4 | 1.721 | 6.88 | 6.91 | 0.996 |
| Prevost | 4 | 2.596 | 10.38 | 10.42 | 0.997 |
| Prevost | 4 | 0.580 | 2.32 | 2.53 | 0.916 |
| Prevost | 5 | 0.564 | 2.82 | 3.75 | 0.753 |
| Prevost | 5 | 1.426 | 7.13 | 7.51 | 0.949 |
| Prevost | 5 | 0.958 | 4.79 | 4.90 | 0.978 |
| Prevost | 5 | 0.906 | 4.53 | 4.63 | 0.980 |
| Prevost | 5 | 1.968 | 9.84 | 9.99 | 0.986 |
| Prevost | 5 | 1.904 | 9.52 | 9.64 | 0.987 |
| Prevost | 5 | 1.235 | 6.18 | 6.22 | 0.993 |
| Prevost | 5 | 2.506 | 12.53 | 12.19 | 1.000 |
| Prevost | 5 | 1.387 | 6.93 | 9.04 | 0.767 |
| Prevost | 6 | 2.269 | 13.61 | 14.20 | 0.959 |
| Prevost | 6 | 1.109 | 6.65 | 6.75 | 0.986 |
| Prevost | 6 | 1.942 | 11.65 | 11.82 | 0.986 |
| Prevost | 6 | 2.337 | 14.02 | 14.05 | 0.998 |
| Prevost | 6 | 1.676 | 10.06 | 10.06 | 1.000 |
| Prevost | 6 | 1.557 | 9.34 | 9.34 | 1.000 |
| Prevost | 6 | 3.256 | 19.54 | 19.54 | 1.000 |
| Prevost | 6 | 0.826 | 4.96 | 4.96 | 1.000 |
| Prevost | 7 | 1.663 | 11.64 | 20.41 | 0.570 |
| Prevost | 7 | 1.602 | 11.21 | 18.57 | 0.604 |
| Prevost | 7 | 1.870 | 13.09 | 20.28 | 0.646 |
| Prevost | 7 | 1.898 | 13.28 | 19.62 | 0.677 |
| Prevost | 7 | 1.914 | 13.40 | 18.76 | 0.714 |
| Prevost | 7 | 1.512 | 10.58 | 14.82 | 0.714 |
| Prevost | 7 | 0.826 | 5.78 | 6.81 | 0.850 |
| Prevost | 7 | 0.685 | 4.80 | 5.27 | 0.911 |
| Prevost | 7 | 1.244 | 8.71 | 9.07 | 0.959 |
| Prevost | 7 | 1.834 | 12.84 | 15.49 | 0.829 |
| Prevost | 7 | 1.739 | 12.17 | 13.44 | 0.905 |
| Prevost | 7 | 2.265 | 15.86 | 17.28 | 0.918 |
| Prevost | 7 | 1.220 | 8.54 | 8.79 | 0.971 |
| Prevost | 7 | 1.987 | 13.91 | 13.97 | 0.996 |
| Prevost | 8 | 2.096 | 16.77 | 19.20 | 0.873 |
| Prevost | 8 | 0.610 | 4.88 | 4.92 | 0.992 |
| Prevost | 8 | 0.478 | 3.82 | 3.84 | 0.996 |
| Prevost | 8 | 1.064 | 8.51 | 8.54 | 0.997 |
| Prevost | 8 | 1.052 | 8.42 | 8.43 | 0.998 |
| Prevost | 8 | 1.385 | 11.08 | 22.30 | 0.497 |
| Prevost | 8 | 1.895 | 15.16 | 15.95 | 0.950 |
| Prevost | 9 | 0.518 | 4.66 | 5.33 | 0.874 |
| Prevost | 9 | 0.382 | 3.44 | 3.77 | 0.914 |
| Prevost | 9 | 0.693 | 6.24 | 6.46 | 0.966 |
| Prevost | 9 | 1.370 | 12.33 | 12.56 | 0.982 |
| Prevost | 9 | 2.610 | 23.49 | 27.38 | 0.858 |
| Prevost | 9 | 1.560 | 14.04 | 14.80 | 0.949 |
| Prevost | 9 | 2.061 | 18.55 | 19.00 | 0.976 |
| Prevost | 10 | 1.693 | 16.93 | 17.20 | 0.985 |
| Prevost | 10 | 0.450 | 4.50 | 4.52 | 0.994 |
| Prevost | 10 | 1.300 | 13.00 | 13.00 | 1.000 |
| Prevost | 10 | 1.489 | 14.89 | 17.79 | 0.837 |
| Prevost | 10 | 1.942 | 19.42 | 20.28 | 0.957 |
| Prevost | 10 | 1.253 | 12.53 | 12.91 | 0.970 |
| Prevost | 10 | 0.941 | 9.41 | 9.42 | 0.999 |
| Prevost | 10 | 1.330 | 13.30 | 13.30 | 1.000 |
| Prevost | 11 | 0.572 | 6.29 | 7.53 | 0.836 |
| Prevost | 11 | 0.857 | 9.43 | 9.66 | 0.976 |
| Prevost | 12 | 1.078 | 12.93 | 14.70 | 0.879 |
| Prevost | 12 | 0.829 | 9.95 | 10.05 | 0.990 |
| Prevost | 12 | 0.773 | 9.27 | 10.63 | 0.872 |
| Prevost | 12 | 1.725 | 20.70 | 20.70 | 1.000 |
| Prevost | 12 | 1.067 | 12.81 | 12.81 | 1.000 |
| Prevost | 13 | 0.682 | 8.87 | 10.09 | 0.879 |
| Prevost | 13 | 1.093 | 14.20 | 14.37 | 0.988 |
| Prevost | 13 | 0.704 | 9.15 | 9.21 | 0.994 |
| Prevost | 13 | 0.349 | 4.54 | 6.12 | 0.742 |
| Prevost | 13 | 0.883 | 11.48 | 13.94 | 0.823 |
| Prevost | 13 | 0.816 | 10.61 | 12.20 | 0.870 |
| Prevost | 13 | 1.362 | 17.71 | 27.67 | 0.640 |
| Prevost | 14 | 1.054 | 14.75 | 14.74 | 1.000 |
| Prevost | 14 | 1.409 | 19.73 | 19.78 | 0.997 |
| Prevost | 15 | 0.700 | 10.50 | 10.80 | 0.972 |
| Prevost | 15 | 0.967 | 14.51 | 15.60 | 0.930 |
| Prevost | 15 | 1.112 | 16.68 | 18.49 | 0.902 |
| Prevost | 16 | 0.612 | 9.78 | 13.11 | 0.747 |
| Prevost | 16 | 0.491 | 7.85 | 7.86 | 1.000 |
| Prevost | 16 | 0.784 | 12.55 | 16.09 | 0.780 |
| Prevost | 16 | 0.815 | 13.05 | 13.70 | 0.953 |
| Prevost | 16 | 1.208 | 19.33 | 13.34 | 1.000 |
| Prevost | 16 | 0.881 | 14.10 | 16.81 | 0.839 |
| Prevost | 17 | 0.951 | 16.17 | 16.19 | 1.000 |
| Prevost | 18 | 0.853 | 15.35 | 16.02 | 0.958 |
| Prevost | 18 | 2.063 | 37.13 | 42.24 | 0.879 |
| Prevost | 19 | 1.124 | 21.36 | 21.97 | 0.972 |
| Prevost | 20 | 0.245 | 4.91 | 7.26 | 0.676 |
| Prevost | 21 | 0.283 | 5.94 | 50.83 | 0.117 |
| Prevost | 21 | 0.229 | 4.81 | 37.74 | 0.127 |
| Prevost | 22 | 0.823 | 18.11 | 24.29 | 0.746 |
| Prevost | 25 | 0.644 | 16.10 | 16.11 | 1.000 |
| Prevost | 27 | 0.746 | 20.13 | 20.10 | 1.000 |
| Prevost | 27 | 0.672 | 18.14 | 20.85 | 0.870 |
| Prevost | 29 | 0.549 | 15.92 | 16.09 | 0.990 |
| Prevost | 33 | 0.419 | 13.83 | 15.86 | 0.872 |
| Prevost | 33 | 0.482 | 15.89 | 17.28 | 0.920 |
| Prevost | 34 | 0.301 | 10.24 | 27.84 | 0.368 |
| Prevost | 34 | 0.241 | 8.19 | 10.47 | 0.782 |
| Prevost | 34 | 0.497 | 16.90 | 18.11 | 0.933 |

Table A2.
Assuming that fish can only be tracked over a maximum distance of 40 m (e.g., fish passing along the bank opposite to the sonar bank, i.e., where it was immersed) and setting an obvious limit constraint at ESS < 5m s−1 (above which swimming speed was not biologically realistic in our case study) and a Residence time ≥ 1 s, the theoretical function is as follows (maximum distance (m) of the sampling protocol divided by the residence time (s)).
Table A2.
Assuming that fish can only be tracked over a maximum distance of 40 m (e.g., fish passing along the bank opposite to the sonar bank, i.e., where it was immersed) and setting an obvious limit constraint at ESS < 5m s−1 (above which swimming speed was not biologically realistic in our case study) and a Residence time ≥ 1 s, the theoretical function is as follows (maximum distance (m) of the sampling protocol divided by the residence time (s)).
| Residence time (s) | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | 24 | 26 | 28 | 30 | 32 | 34 | 36 | 38 | 40 |
| ESS (m s−1) | 20.0 | 10.0 | 6.7 | 5.0 | 4.0 | 3.3 | 2.9 | 2.5 | 2.2 | 2.0 | 1.8 | 1.7 | 1.5 | 1.4 | 1.3 | 1.3 | 1.2 | 1.1 | 1.1 | 1.0 |
References
- Benhamou, S. How to reliably estimate the tortuosity of an animal’s path: Straightness, sinuosity, or fractal dimension? J. Theor. Biol. 2004, 229, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, S. Detecting an orientation component in animal paths when the preferred direction is individual-dependent. Ecology 2006, 87, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, E.J.; MacLennan, D.N. Fisheries Acoustics: Theory and Practice; Blackwell Publishing: London, UK, 2005. [Google Scholar]
- Brehmer, P.; Gerlotto, F.; Rouault, A. In Situ Inter-Standardization of Acoustics Data: An Integrated Database for Fish School Behaviour Studies. Acta Acust. United Acust. 2002, 88, 730–734. [Google Scholar]
- Guillard, J.; Fernandes, P.; Laloë, T.; Brehmer, P. Three-dimensional internal spatial structure of young-of-the-year pelagic freshwater fish provides evidence for the identification of fish school species. Limnol. Oceanogr. Methods 2011, 9, 322–328. [Google Scholar] [CrossRef]
- David, V.; Mouget, A.; Perrot, Y.; Le Goff, L.; Thiriet, P.; Diogoul, N.; Feunteun, E.; Acou, A.; Brehmer, P. Insights from a multibeam echosounder to survey pelagic fish shoals and their spatio-temporal distribution in ultra-shallow waters. Estuar. Coast. Shelf Sci. 2022, 264, 107705. [Google Scholar] [CrossRef]
- Brehmer, P. Fisheries Acoustics: Theory and Practice, 2nd edn. Fish Fish. 2006, 7, 227–228. [Google Scholar] [CrossRef]
- Brehmer, P.; Mouillot, D.; Chi, T.D. Amphidromus fish school diel flow in two Mediterranean lagoons by combining sonar and fishing data. J. Exp. Mar. Biol. Ecol. 2006, 334, 139–150. [Google Scholar] [CrossRef]
- Brehmer, P.; Guillard, J.; Pinzon, P.I.C.; Bach, P. Exploratory and Instantaneous Swimming Speeds of Amphidromous Fish School in Shallow-Water Coastal Lagoon Channels. Estuaries Coasts 2011, 34, 739–744. [Google Scholar] [CrossRef][Green Version]
- Stasko, A.B.; Horrall, R.M.; Hasler, A. Coastal Movements of Adult Fraser River Sockeye Salmon (Oncorhynchus nerka) Observed by Ultrasonic Tracking. Trans. Am. Fish. Soc. 1976, 105, 64–71. [Google Scholar] [CrossRef]
- Hawkes, L.A.; Broderick, A.C.; Coyne, M.S.; Godfrey, M.H.; Lopez-Jurado, L.-P.; Lopez-Suarez, P.; Merino, S.E.; Varo-Cruz, N.; Godley, B.J. Phenotypically Linked Dichotomy in Sea Turtle Foraging Requires Multiple Conservation Approaches. Curr. Biol. 2006, 16, 990–995. [Google Scholar] [CrossRef]
- Weimerskirch, H.; Bonadonna, F.; Bailleul, F.; Mabille, G.; Dell’Omo, G.; Lipp, H.P. GPS tracking of foraging albatrosses. Science 2002, 295, 1259. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, V.; D’Anna, G.; Pipitone, C.; Badalamenti, F. Movements and residence time of spiny lobsters, Palinurus elephas released in a marine protected area: An investigation by ultrasonic telemetry. J. Mar. Biol. Assoc. U. K. 2006, 86, 1101–1106. [Google Scholar] [CrossRef]
- Klimley, A.P.; Beavers, S.C.; Curtis, T.C.; Jorgensen, S.J. Movements and Swimming Behavior of Three Species of Sharks in La Jolla Canyon, California. Environ. Biol. Fishes 2002, 63, 117–135. [Google Scholar] [CrossRef]
- Ware, D.M. Bioenergetics of Pelagic Fish: Theoretical Change in Swimming Speed and Ration with Body Size. J. l’Office Rech. Pêcheries Can. 1978, 35, 220–228. [Google Scholar] [CrossRef]
- Brochier, T.; Auger, P.-A.; Pecquerie, L.; Machu, E.; Capet, X.; Thiaw, M.; Mbaye, B.C.; Braham, C.-B.; Ettahiri, O.; Charouki, N.; et al. Complex small pelagic fish population patterns arising from individual behavioral responses to their environment. Prog. Oceanogr. 2018, 164, 12–27. [Google Scholar] [CrossRef]
- Videler, J.J. Fish Swimming; Chapman & Hall: London, UK, 1993. [Google Scholar]
- Marcinek, D.J.; Blackwell, S.B.; Dewar, H.; Freund, E.V.; Farwell, C.; Dau, D.; Seitz, A.C.; Block, B.A. Depth and muscle temperature of Pacific bluefin tuna examined with acoustic and pop-up satellite archival tags. Mar. Biol. 2001, 138, 869–885. [Google Scholar] [CrossRef]
- Zydlewski, G.B.; Haro, A.; Whalen, K.G.; McCormick, S.D. Performance of stationary and portable passive transponder detection systems for monitoring of fish movements. J. Fish Biol. 2001, 58, 1471–1475. [Google Scholar] [CrossRef]
- Dunlop, E.S.; Milne, S.W.; Ridgway, M.S.; Condiotty, J.; Higginbottom, I. In Situ Swimming Behavior of Lake Trout Observed Using Integrated Multibeam Acoustics and Biotelemetry. Trans. Am. Fish. Soc. 2010, 139, 420–432. [Google Scholar] [CrossRef]
- Trygonis, V.; Georgakarakos, S.; Dagorn, L.; Brehmer, P. Spatiotemporal distribution of fish schools around drifting fish aggregating devices. Fish. Res. 2016, 177, 39–49. [Google Scholar] [CrossRef]
- Fréon, P.; Misund, O.A. Dynamics of Pelagic Fish Distribution and Behaviour: Effects on Fisheries and Stock Assessment; Blackwell Science: London, UK, 1999. [Google Scholar]
- Mouillot, D.; Laune, J.; Tomasini, J.A.; Aliaume, C.; Brehmer, P.; Dutrieux, E.; Chi, T.D. Assessment of coastal lagoon quality with taxonomic diversity indices of fish, zoobenthos and macrophyte communities. Hydrobiologia 2005, 550, 121–130. [Google Scholar] [CrossRef]
- Bourquard, C. Structure et Mécanisme de Mise en Place, de Maintien et d’Evolution des Peuplements Ichthyologiques Lagunaires du Golfe du Lion. Ph.D. Thesis, Université Montpellier, Montpellier, France, 1985. [Google Scholar]
- Bach, P.; Legendre, P.; Amanieu, M.; Lasserre, G. Strategy of eel (Anguilla anguilla, L.) exploitation in the Thau lagoon. Estuar. Coast. Shelf Sci. 1992, 35, 55–73. [Google Scholar] [CrossRef]
- Quignard, J.P.; Mazoyer, C.; Vianet, R.; Wai, R.M.; Benharrat, K. Un exemple d’exploitation lagunaire en Languedoc: L’étang de l’Or (Mauguio)—Pêche et production halieutique. Sci. Pêche 1983, 336, 3–23. [Google Scholar]
- Brehmer, P.; Chi, T.D.; Laugier, T.; Galgani, F.; Laloë, F.; Darnaude, A.M.; Fiandrino, A.; Mouillot, D. Field investigations and multi-indicators for shallow water lagoon management: Perspective for societal benefit. Aquat. Conserv. 2011, 21, 728–742. [Google Scholar] [CrossRef]
- Brehmer, P.; Vercelli, C.; Gerlotto, F.; Sanguinède, F.; Pichot, Y.; Buestel, D.; Guénnegan, Y. Multibeam sonar three-dimensional monitoring of mussel culture grounds in open sea for management purpose. Aquaculture 2006, 252, 234–241. [Google Scholar] [CrossRef]
- Epstein, N. On tortuosity and the tortuosity factor in flow and diffusion through porous media. Chem. Eng. Sci. 1989, 44, 777–779. [Google Scholar] [CrossRef]
- Cronkite, G.; Mulligan, T.; Holmes, J.; Enzenhofer, H. Categorising salmon migration behaviour using characteristics of split-beam acoustic data. Aquat. Living Resour. 2007, 20, 205–212. [Google Scholar] [CrossRef]
- Thalinger, B.; Wolf, E.; Traugott, M.; Wanzenböck, J. Monitoring spawning migrations of potamodromous fish species via eDNA. Sci. Rep. 2019, 9, 15388. [Google Scholar] [CrossRef]
- Brehmer, P.; Soncho, G.; Trygornis, V.; Itano, D.; Dalen, J.; Fuchs, A.; Faraj, A.; Taquet, M. Toward an autonomous observatory of pelagic environment: Experiences from fish communities monitoring around drifting FADs. Thalass. Int. J. Mar. Sci. 2019, 35, 177–189. [Google Scholar] [CrossRef]
- Minart, C.; David, V.; Mouget, A.; Brehmer, P.; Acou, A.; Le Goff, L.; Feunteun, E.; Thiriet, P. An innovative sampling protocol for fish species identification methods in shallow waters: Towed diver, towed video and stereoscopic camera system. In Proceedings of the Oceans 2021, San Diego, CA, USA, 20–23 September 2021; pp. 1–10. [Google Scholar] [CrossRef]
- Gladstone, W.; Lindfield, S.; Coleman, M.; Kelaher, B. Optimisation of baited remote underwater video sampling designs for estuarine fish assemblages. J. Exp. Mar. Biol. Ecol. 2012, 429, 28–35. [Google Scholar] [CrossRef]
- Weill, A.; Scalabrin, C.; Diner, N. MOVIES-B: An acoustic detection description software. Application to shoal species’ classification. Aquat. Living Resour. 1993, 6, 255–267. [Google Scholar] [CrossRef]
- Perrot, Y.; Brehmer, P.; Habasque, J.; Roudaut, G.; Behagle, N.; Sarre, A.; Lebourges Dhaussy, A. Matecho: An Open-Source Tool for Processing Fisheries Acoustics Data. Acoust. Aust. 2018, 46, 241–248. [Google Scholar] [CrossRef]
- Brehmer, P.; Lafont, T.; Georgakarakos, S.; Josse, E.; Gerlotto, F.; Collet, C. Omnidirectional multibeam sonar monitoring: Applications in fisheries science. Fish Fish. 2006, 7, 165–179. [Google Scholar] [CrossRef]
- Cury, P.M.; Shin, Y.-J.; Planque, B.; Durant, J.M.; Fromentin, J.M.; Kramer-Schadt, S.; Stenseth, N.C.; Travers, M.; Grimm, V. Ecosystem oceanography for global change in fisheries. Trends Ecol. Evol. 2008, 23, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Fulton, E.A.; Link, J.S.; Kaplan, I.C.; Savina-Rolland, M.; Johnson, P.; Ainsworth, C.; Horne, P.; Gorton, R.; Gamble, R.J.; Smith, A.D.M.; et al. Lessons in modelling and management of marine ecosystems: The Atlantis experience. Fish Fish. 2011, 12, 171–188. [Google Scholar] [CrossRef]
- Moreno, G.; Josse, E.; Brehmer, P.; Nøttestad, L. Echotrace classification and spatial distribution of pelagic fish aggregations around drifting fish aggregating devices (DFAD). Aquat. Living Resour. 2007, 20, 343–356. [Google Scholar] [CrossRef]
- Olsen, K. A note on estimating school size from echotraces. FAO Fish. Rep. 1969, 78, 37–48. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).