Hydrogeochemical Characteristics of Bottled Waters Sourced from Bedrock Aquifers in South Korea: Evaluation of Water Type and Natural Background Levels
Abstract
:1. Introduction
2. Study Area: Outline of the Geology and Hydrogeology of South Korea
3. Materials and Methods
3.1. Hydrochemical Analysis
3.2. Statistical Analysis
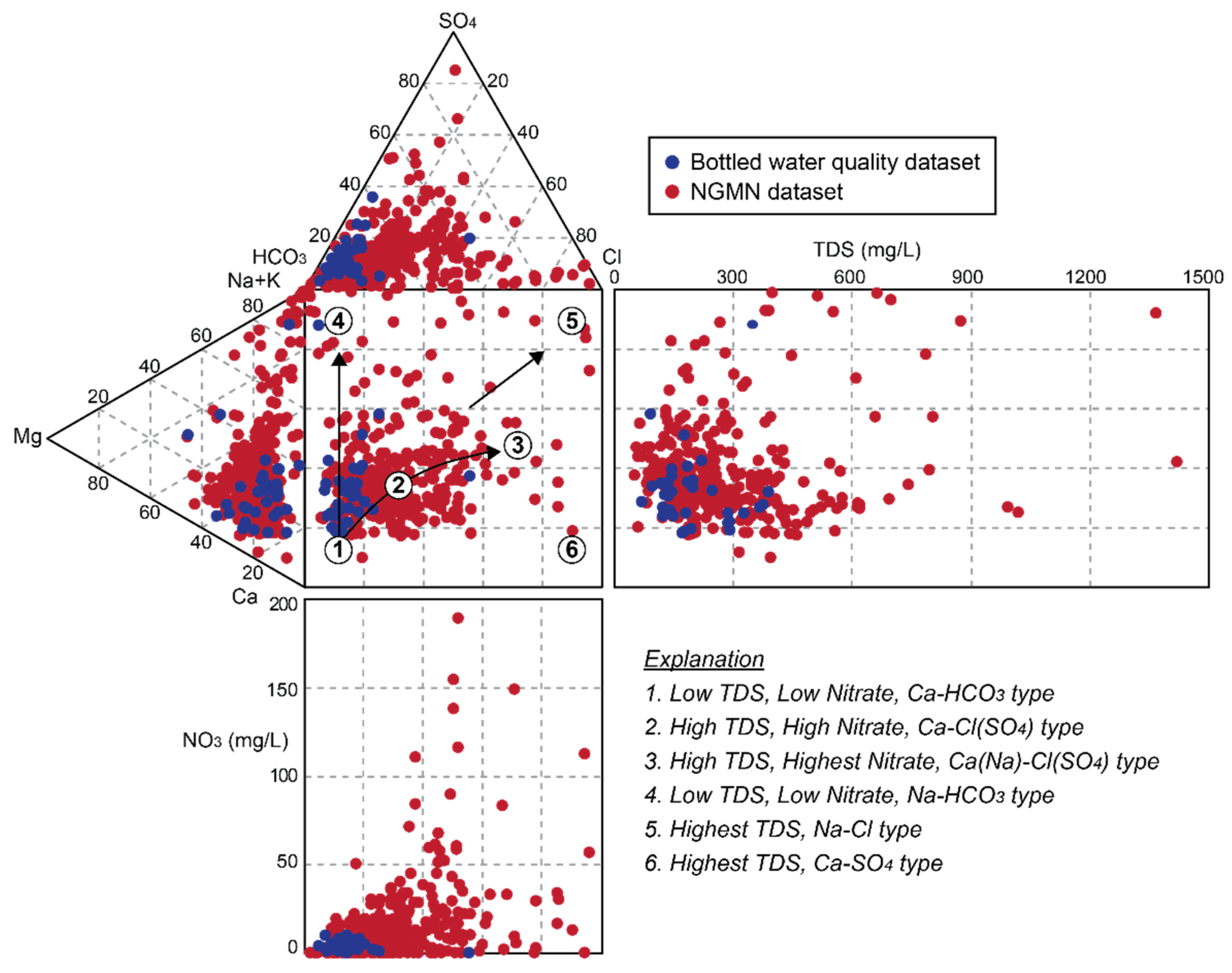
4. Results and Discussion
4.1. Hydrochemical Properties
4.1.1. Major Constituents
4.1.2. Trace Elements
4.2. Major Hydrogeochemical Processes for Ca-HCO3-Type Bottled Water
4.2.1. Major Hydrochemical Processes
4.2.2. Causes for Short-Circulating Pathways
4.2.3. Trace Elements in Major Hydrochemical Processes
4.3. Natural Background Levels in Shallow to Moderately Deep Bedrock Groundwater in South Korea
5. Conclusions and Suggestions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banks, D.; Reimann, C.; Røyset, O.; Skarphagen, H.; Sæther, O.M. Natural concentrations of major and trace elements in some Norwegian bedrock groundwaters. Appl. Geochem. 1995, 10, 1–16. [Google Scholar] [CrossRef]
- Müller, D.; Blum, A.; Hart, A.; Hookey, J.; Kunkel, R.; Scheidleder, A.; Tomlin, C.; Wendland, F. Final Proposal for a Methodology to Set up Groundwater Threshold Values in Europe. Report to the EU Project “BRIDGE”. 2006. Available online: http://www.keriel.org/BIB/Publ_UNESCO/SOG_BRIDGE/Deliverables/WP3/D18.pdf (accessed on 26 March 2022).
- Sellerino, M.; Forte, G.; Ducci, D. Identification of the natural background levels in the Phlaegrean fields groundwater body (Southern Italy). J. Geochem. Explor. 2019, 200, 181–192. [Google Scholar] [CrossRef]
- Jiang, H.H.; Cai, L.M.; Wen, H.H.; Luo, J. Characterizing pollution and source identification of heavy metals in soils using geochemical baseline and PMF approach. Sci. Rep. 2020, 10, 1–11. [Google Scholar]
- Bondu, R.; Humez, P.; Mayer, B.; Chaste, E.; Naumenko-Dèzes, M.O.; Cloutier, V.; Rosa, E.; Kloppmann, W. Estimating natural background concentrations for dissolved constituents in groundwater: A methodological review and case studies for geogenic fluoride. J. Geochemical Explor. 2022, 233, 106906. [Google Scholar] [CrossRef]
- Voutchkova, D.D.; Ernstsen, V.; Schullehner, J.; Hinsby, K.; Thorling, L.; Hansen, B. Roadmap for determining natural background levels of trace metals in groundwater. Water 2021, 13, 1267. [Google Scholar] [CrossRef]
- Molinari, A.; Guadagnini, L.; Marcaccio, M.; Guadagnini, A. Natural background levels and threshold values of chemical species in three large-scale groundwater bodies in Northern Italy. Sci. Total Environ. 2012, 425, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.V.; Andrade, C. Natural background groundwater composition in the Azores archipelago (Portugal): A hydrogeochemical study and threshold value determination. Sci. Total Environ. 2015, 520, 127–135. [Google Scholar] [CrossRef]
- Reimann, C.; Garrett, R.G. Geochemical background—concept and reality. Sci. Total Environ. 2005, 350, 12–27. [Google Scholar] [CrossRef]
- Marcussen, H.; Holm, P.E.; Hansen, H.C.B. Composition, flavor, chemical foodsafety, and consumer preferences of bottled water. Compr. Rev. Food Sci. Food Saf. 2013, 12, 333–352. [Google Scholar] [CrossRef]
- Reimann, C.; Birke, M. Geochemistry of European Bottled Water; Borntraeger Science Publishers: Stuttgart, Germany, 2010; ISBN 978-3-443-01067-6. [Google Scholar]
- Hinsby, K.; Condesso de Melo, M.T.; Dahl, M. European case studies supporting the derivation of natural background levels and groundwater threshold values for the protection of dependent ecosystems and human health. Sci. Total Environ. 2008, 401, 1–20. [Google Scholar] [CrossRef]
- Wendland, F.; Berthold, G.; Blum, A.; Elsass, P.; Fritsche, J.G.; Kunkel, R.; Wolter, R. Derivation of natural background levels and threshold values for groundwater bodies in the Upper Rhine Valley (France, Switzerland and Germany). Desalination 2008, 226, 160–168. [Google Scholar] [CrossRef]
- Griffioen, J.; Passier, H.F.; Klein, J. Comparison of selection methods to deduce natural background levels for groundwater units. Environ. Sci. Technol. 2008, 42, 4863–4869. [Google Scholar] [CrossRef] [PubMed]
- Coetsiers, M.; Blaser, P.; Martens, K.; Walraevens, K. Natural background levels and threshold values for groundwater in fluvial Pleistocene and Tertiary marine aquifers in Flanders, Belgium. Environ. Geol. 2009, 57, 1155–1168. [Google Scholar] [CrossRef]
- European Commission. Common Implementation Strategy for the Water Framework Directive (2000/60/EC)—Guidance Document No. 18 Guidance on Groundwater Status and Trend Assessment; European Communities: Luxembourg, 2009. [Google Scholar]
- Rotiroti, M.; Fumagalli, L. Derivation of preliminary natural background levels for naturally Mn, Fe, As and NH4+ rich groundwater: The case study of Cremona area (Northern Italy). Rend. Online Soc. Geol. Ital. 2013, 24, 284–286. [Google Scholar]
- Preziosi, E.; Parrone, D.; Del Bon, A.; Ghergo, S. Natural background level assessment in groundwaters: Probability plot versus pre-selection method. J. Geochemical Explor. 2014, 143, 43–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Sun, J.; Wang, J. Natural background levels of chemical components in groundwater of Hutuo River catchment area, North China Plain. Environ. Forensics 2017, 18, 62–73. [Google Scholar] [CrossRef]
- Apollaro, C.; Di Curzio, D.; Fuoco, I.; Buccianti, A.; Dinelli, E.; Vespasiano, G.; Castrignanò, A.; Rusi, S.; Barca, D.; Figoli, A.; et al. A multivariate non-parametric approach for estimating probability of exceeding the local natural background level of arsenic in the aquifers of Calabria region (Southern Italy). Sci. Total Environ. 2022, 806, 150345. [Google Scholar] [CrossRef]
- Hawkes, H.E.; Webb, J.S. Geochemistry in Mineral Exploration; Harper and Row: New York, NY, USA, 1962; ISBN 978-0060427108. [Google Scholar]
- Reimann, C.; Filzmoser, P.; Garrett, R.G. Background and threshold: Critical comparison of methods of determination. Sci. Total Environ. 2005, 346, 1–16. [Google Scholar] [CrossRef]
- Wendland, F.; Hannappel, S.; Kunkel, R.; Schenk, R.; Voigt, H.J.; Wolter, R. A procedure to define natural groundwater conditions of groundwater bodies in Germany. Water Sci. Technol. 2005, 51, 249–257. [Google Scholar] [CrossRef]
- Panno, S.; Kelly, W.; Martinsek, A.; Hackley, K. Estimating background and threshold nitrate concentrations using probability graphs. Ground Water 2006, 44, 697–709. [Google Scholar] [CrossRef]
- Walter, T.; Beer, A.; Brose, D.; Budziak, D.; Clos, P.; Dreher, T.; Fritsche, H.G.; Hübschmann, M.; Marczinek, S.; Peters, A.; et al. Determining natural background values with probability plots. In Groundwater Quality Sustainability; Piotr, M., Stanislaw, W., Grzegorz, M., Eds.; CRC Press: London, UK, 2012; pp. 331–342. [Google Scholar]
- Atega, P.L.E.; Vinches, M.; Casiot, C.; Pistre, S. Development and implementation of a multi-criteria aggregation operator to estimate the contributions of the natural geochemical background and anthropogenic inputs in groundwater in former mining regions: An application to arsenic and antimony in the Gardon river watershed (southern France). Sci. Total Environ. 2022, 814, 151936. [Google Scholar]
- NGII. The National Atlas of Korea; Volume 2. 2017. Available online: http://nationalatlas.ngii.go.kr/pages/page_668.php (accessed on 24 January 2022).
- Turek, A.; Kim, C.B. U-Pb zircon ages for Precambrian rocks in southwestern Ryeongnam and southwestern Gyeonggi massifs, Korea. Geochem. J. 1996, 30, 231–249. [Google Scholar] [CrossRef]
- Cho, B.W.; Kim, M.S.; Kim, T.S.; Yun, U.; Lee, B.D.; Hwang, J.H.; Choo, C.O. Characteristics of occurrence and distribution of natural radioactive materials, uranium and radon in groundwater of the Danyang area. J. Eng. Geol. 2013, 23, 399–407. (In Korean) [Google Scholar]
- Cho, B.W.; Sung, H.I.; Choo, C.O.; Lee, B.D.; Kim, T.K. Hydrochemical characteristics of natural mineral water in the Daebo and Bulguksa granites. J. Eng. Geol. 1998, 8, 247–259. (In Korean) [Google Scholar]
- Tsusue, A.; Mizuta, T.; Watanabe, M.; Min, K.G. Jurassic and Cretaceous granitic rocks in South Korea. Min. Geol. 1981, 31, 261–280. [Google Scholar]
- Uchida, E.; Choi, S.G.; Baba, D.; Wakisaka, Y. Petrogenesis and solidification depth of the Jurassic Daebo and Cretaceous Bulguksa granitic rocks in South Korea. Resour. Geol. 2012, 62, 281–295. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, J.; Park, C.; Moutte, J. Geochemistry of peridotite xenoliths in alkali basalts from Jeju Island, Korea. Isl. Arc 2002, 11, 221–235. [Google Scholar] [CrossRef]
- Tatsumi, Y.; Shukuno, H.; Yoshikawa, M.; Chang, Q.; Sato, K.; Lee, M. The petrology and geochemistry of volcanic rocks on Jeju Island: Plume magmatism along the Asian continental margin. J. Petrol. 2004, 46, 523–553. [Google Scholar] [CrossRef]
- Jeon, Y.M.; Sohn, Y.K. Sedimentary characteristics and stratigraphic implications of the Kusandong Tuff, Cretaceous Gyeongsang Basin, Korea. Geosci. J. 2003, 7, 53–64. [Google Scholar] [CrossRef]
- Hwang, B.H.; Son, M.; Yang, K.; Yoon, J.; Ernst, W.G. Tectonic evolution of the Gyeongsang Basin, southeastern Korea from 140 Ma to the present, based on a strike-slip and block rotation tectonic model. Int. Geol. Rev. 2008, 50, 343–363. [Google Scholar] [CrossRef]
- Chough, S.K. Geology and Sedimentology of the Korean Peninsula; Elsevier: London, UK, 2013; ISBN 9780124055124. [Google Scholar]
- MOCT. Master Plan for Groundwater Management of Korea: 2007~2011 (in Korean); Ministry of Construction & Transportation: Sejong, Korea, 2007.
- Jeon, S.K.; Koo, M.H.; Kim, Y.; Kang, I.O. Statistical analysis of aquifer characteristics using pumping test data of national groundwater monitoring well of Korea. J. Korean Soc. Environ. Eng. 2005, 10, 32–44. (In Korean) [Google Scholar]
- Chae, G.T.; Yun, S.T.; Mayer, B.; Kim, K.H.; Kim, S.Y.; Kwon, J.S.; Kim, K.; Koh, Y.K. Fluorine geochemistry in bedrock groundwater of South Korea. Sci. Total Environ. 2007, 385, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, W.M.; Smedley, P.L. Fluoride in natural waters. In Essentials of Medical Geology; Selinus, O., Alloway, B., Centeno, J.A., Finkelman, R.B., Fuge, R., Lindh, U., Smedley, P.L., Eds.; Springer: Amsterdam, The Netherlands, 2013; pp. 311–336. [Google Scholar]
- Lee, J.U.; Chon, H.T.; John, Y.W. Geochemical characteristics of deep granitic groundwater in Korea. J. Korean Soc. Groundw. Environ. (Korea) 1997, 4, 199–211. (In Korean) [Google Scholar]
- Cho, B.W.; Lee, B.D.; Lee, I.H.; Choo, C.O. Speculation on the water quality for the natural mineral water. J. Eng. Geol. 2002, 12, 395–404. (In Korean) [Google Scholar]
- Son, D.; Park, S.; Jeong, G.C. A Study on the water withdrawal permit rate and monitoring well management of bottled water manufacturers. J. Eng. Geol. 2019, 29, 329–337. (In Korean) [Google Scholar]
- KIGAM. Study on an Improvement Plan for Proper Management of Drinking Water Production Wells (in Korean); Ministry of Environment: Sejong, Korea, 2019.
- Koh, D.C.; Ko, K.S. Recent trends of domestic and international management and research of natural mineral water used for bottled water. J. Korean Soc. Environ. Eng. 2018, 23, 9–27. (In Korean) [Google Scholar]
- Ra, W.C. Analysis of the Chinese bottled water market and export strategy for Korean firms. Korea Trade Rev. 2014, 39, 77–108. (In Korean) [Google Scholar]
- Bong, Y.S.; Ryu, J.S.; Lee, K.S. Characterizing the origins of bottled water on the South Korean market using chemical and isotopic compositions. Anal. Chim. Acta 2009, 631, 189–195. [Google Scholar] [CrossRef]
- Kim, G.E.; Ryu, J.S.; Shin, W.J.; Bong, Y.S.; Lee, K.S.; Choi, M.S. Chemical and isotopic compositions of bottled waters sold in Korea: Chemical enrichment and isotopic fractionation by desalination. Rapid Commun. Mass Spectrom. 2012, 26, 25–31. [Google Scholar] [CrossRef]
- Kim, G.E.; Shin, W.J.; Ryu, J.S.; Choi, M.S.; Lee, K.S. Identification of the origin and water type of various Korean bottled waters using strontium isotopes. J. Geochem. Explor. 2013, 132, 1–5. [Google Scholar] [CrossRef]
- Lee, B.D.; Oh, Y.H.; Cho, B.W.; Yun, U.; Choo, C.O. Hydrochemical properties of ground water used for Korea bottled waters in relation to geology. Water 2019, 11, 1043. [Google Scholar] [CrossRef]
- Shin, W.J.; Ryu, J.S.; Shin, H.S.; Jung, Y.Y.; Ko, K.S.; Lee, K.S. Major and trace element geochemistry of Korean bottled waters. Water 2020, 12, 2585. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yi, M.J.; Yoo, Y.K.; Ahn, K.H.; Kim, G.B.; Won, J.H. A review of the national groundwater monitoring network in Korea. Hydrol. Process. 2007, 21, 907–919. [Google Scholar] [CrossRef]
- Westerhoff, P.; Prapaipong, P.; Shock, E.; Hillaireau, A. Antimony leaching from polyethylene terephthalate (PET) plastic used for bottled drinking water. Water Res. 2008, 42, 551–556. [Google Scholar] [CrossRef]
- Helena, B.; Pardo, R.; Vega, M.; Barrado, E.; Fernandez, J.M.; Fernandez, L. Temporal evolution of groundwater composition in an alluvial aquifer (Pisuerga River, Spain) by principal component analysis. Water Res. 2000, 34, 807–816. [Google Scholar] [CrossRef]
- Güler, C.; Thyne, G.; McCray, J.; Turner, K. Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeol. J. 2002, 10, 455–474. [Google Scholar] [CrossRef]
- Thyne, G.; Güler, C.; Poeter, E. Sequential analysis of hydrochemical data for watershed characterization. Ground Water 2004, 42, 711–723. [Google Scholar] [CrossRef]
- Kim, K.H.; Yun, S.T.; Choi, B.Y.; Chae, G.T.; Joo, Y.; Kim, K.; Kim, H.S. Hydrochemical and multivariate statistical interpretations of spatial controls of nitrate concentrations in a shallow alluvial aquifer around oxbow lakes (Osong area, central Korea). J. Contam. Hydrol. 2009, 107, 114–127. [Google Scholar] [CrossRef]
- Holland, S.M. Principal components analysis (PCA); Department of Geology, University of Georgia: Athens, Georgia, 2008. [Google Scholar]
- Bolks, A.; DeWire, A.; Harcum, J.B. Baseline Assessment of Left-Censored Environmental Data Using R.; Tech Notes 10; 2014. Available online: https://www.epa.gov/polluted-runoff-nonpointsource-pollution/nonpoint-source-monitoring-technical-notes (accessed on 26 March 2022).
- Palarea-Albaladejo, J.; Martín-Fernández, J.A. ZCompositions—R package for multivariate imputation of left-censored data under a compositional approach. Chemom. Intell. Lab. Syst. 2015, 143, 85–96. [Google Scholar] [CrossRef]
- Husson, A.F.; Josse, J.; Le, S.; Mazet, J.; Husson, M.F. Package ‘FactoMineR’. 2020. Available online: https://cran.r-project.org/web/packages/FactoMineR/FactoMineR.pdf (accessed on 26 March 2022).
- Kim, K.H.; Yun, S.T.; Kim, H.K.; Kim, J.W. Determination of natural backgrounds and thresholds of nitrate in South Korean groundwater using model-based statistical approaches. J. Geochem. Explor. 2015, 148, 196–205. [Google Scholar] [CrossRef]
- Maghrebi, M.; Noori, R.; Partani, S.; Araghi, A.; Barati, R.; Farnoush, H.; Haghighi, A.T. Iran’s groundwater hydrochemistry. Earth Space Sci. 2021, 8, e2021EA001793. [Google Scholar] [CrossRef]
- Join, J.L.; Coudray, J.; Longworth, K. Using principal components analysis and Na/Cl ratios to trace groundwater circulation in a volcanic island: The example of Reunion. J. Hydrol. 1997, 190, 1–18. [Google Scholar] [CrossRef]
- Koh, D.C.; Ko, K.S.; Kim, Y.; Lee, S.G.; Chang, H. Effect of agricultural land use on the chemistry of groundwater from basaltic aquifers, Jeju Island, South Korea. Hydrogeol. J. 2007, 15, 727–743. [Google Scholar] [CrossRef]
- Jung, H.W.; Yun, S.T.; Kim, K.H.; Oh, S.S.; Kang, K.G. Role of an impermeable layer in controlling groundwater chemistry in a basaltic aquifer beneath an agricultural field, Jeju Island, South Korea. Appl. Geochem. 2014, 45, 82–93. [Google Scholar] [CrossRef]
- Chae, G.T.; Yun, S.T.; Kim, K.; Mayer, B. Hydrogeochemistry of sodium-bicarbonate type bedrock groundwater in the Pocheon spa area, South Korea: Water–rock interaction and hydrologic mixing. J. Hydrol. 2006, 321, 326–343. [Google Scholar] [CrossRef]
- Gascoyne, M.; Kamineni, D.C. The hydrogeochemistry of fractured plutonic rocks in the Canadian Shield. Appl. Hydrogeol. 1994, 2, 43–49. [Google Scholar] [CrossRef]
- Kim, K.H.; Yun, S.T.; Yu, S.; Choi, B.Y.; Kim, M.J.; Lee, K.J. Geochemical pattern recognitions of deep thermal groundwater in South Korea using self-organizing map: Identified pathways of geochemical reaction and mixing. J. Hydrol. 2020, 589, 125202. [Google Scholar] [CrossRef]
- Toran, L.E.; Saunders, J.A. Modeling alternative paths of chemical evolution of Na-HCO3-type groundwater near Oak Ridge, Tennessee, USA. Hydrogeol. J. 1999, 7, 355–364. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 3rd ed.; Volume 1—Recommendations Incorporating the First and Second Addenda; World Health Organization: Geneva, Swiss, 2008; ISBN 978-92-4-154761-1. [Google Scholar]
- ME. Drinking Water Quality Standards of South Korea (in Korean); Ministry of Environment: Sejong, Korea, 2019.
- Lee, P.K.; Chae, B.G.; Yu, S.; Choi, J.; Oh, C.; Ji, S.W. Assessment of the alteration and acid-generating potential of deep boreholes for geological disposal. Environ. Earth Sci. 2016, 75, 572. [Google Scholar] [CrossRef]
- Welch, A.H.; Stollenwerk, K.G. Arsenic in Ground Water—Geochemistry and Occurrence; Kluwer Academic Publishers Group: Dordrecht, The Netherlands, 2003; ISBN 978-1402073175. [Google Scholar]
- Ahmed, K.M.; Bhattacharya, P.; Hasan, M.A.; Akhter, S.H.; Alam, S.M.M.; Bhuyian, M.A.H.; Imam, M.B.; Khan, A.A.; Sracek, O. Arsenic enrichment in groundwater of the alluvial aquifers in Bangladesh: An overview. Appl. Geochem. 2004, 19, 181–200. [Google Scholar] [CrossRef]
- Fuoco, I.; De Rosa, R.; Barca, D.; Figoli, A.; Gabriele, B.; Apollaro, C. Arsenic polluted waters: Application of geochemical modelling as a tool to understand the release and fate of the pollutant in crystalline aquifers. J. Environ. Manage. 2022, 301, 113796. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Oh, J.; Choung, S.; Cho, B.W.; Lee, K.S.; Yun, U.; Woo, N.C.; Kim, H.K. Distribution and potential health risk of groundwater uranium in Korea. Chemosphere 2016, 163, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.A.; Osmond, J.K.; Rogers, J.J. The geochemistry of thorium and uranium. Phys. Chem. Earth 1959, 3, 298–348. [Google Scholar] [CrossRef]
- Hwang, J. Occurrence of U-minerals and Source of U in groundwater in daebo granite, Daejeon area. J. Eng. Geol. 2013, 23, 399–407. (In Korean) [Google Scholar] [CrossRef]
- Drever, J.I. The Geochemistry of Natural Waters: Surface and Groundwater Environments; Prentice Hall: Upper Saddle River, NJ, USA, 1997; ISBN 978-0132727907. [Google Scholar]
- Nordstrom, D.K.; Andrews, J.N.; Carlsson, L.; Fontes, J.C.; Frits, P.; Moser, H.; Olsson, T. Hydrogeological and Hydrogeochemical Investigations in Boreholes—Final Report of the Phase I Geochemical Investigations of the Stripa Groundwaters; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 1985. [Google Scholar]
- Nordstroem, D.K.; Lindblom, S.; Donahoe, R.J.; Barton, C.C. Fluid inclusions in the Stripa granite and their possible influence on the groundwater chemistry. Geochim. Cosmochim. Acta 1989, 53, 1741–1755. [Google Scholar] [CrossRef]
- Fontes, J.C.; Fritz, P.; Louvat, D.; Michelot, J.L. Aqueous sulphates from the Stripa groundwater system. Geochim. Cosmochim. Acta 1989, 53, 1783–1789. [Google Scholar] [CrossRef]
- Al-Khashman, O.A. Chemical characteristics of rainwater collected at a western site of Jordan. Atmos. Res. 2009, 91, 53–61. [Google Scholar] [CrossRef]
- Yu, S.; Chae, G.; Oh, J.; Kim, S.H.; Kim, D.I.; Yun, S.T. Hydrochemical and isotopic difference of spring water depending on flow type in a stratigraphically complex karst area of South Korea. Front. Earth Sci. 2021, 736, 712865. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Ramanathan, A.L. Geochemical assessment of groundwater quality in vicinity of Bhalswa landfill, Delhi, India, using graphical and multivariate statistical methods. Environ. Geol. 2007, 53, 1509–1528. [Google Scholar] [CrossRef]
- Kumar, M.; Ramanathan, A.; Keshari, A.K. Understanding the extent of interactions between groundwater and surface water through major ion chemistry and multivariate. Hydrol. Process. 2009, 23, 297–310. [Google Scholar] [CrossRef]
- Luo, W.; Gao, X.; Zhang, X. Geochemical processes controlling the groundwater chemistry and fluoride contamination in the yuncheng basin, China—An area with complex hydrogeochemical conditions. PLoS ONE 2018, 13, e0199082. [Google Scholar] [CrossRef] [PubMed]
- Frengstad, B.; Banks, D. The natural inorganic chemical quality of crystalline bedrock groundwaters of Norway. In Natural Groundwater Quality; Edmunds, W.M., Shand, P., Eds.; Blackwell Publishing Ltd.: Malden, MA, USA, 2008; pp. 421–440. [Google Scholar]
- Fuoco, I.; Marini, L.; De Rosa, R.; Figoli, A.; Gabriele, B.; Apollaro, C. Use of reaction path modelling to investigate the evolution of water chemistry in shallow to deep crystalline aquifers with a special focus on fluoride. Sci. Total Environ. 2022, 830, 154566. [Google Scholar] [CrossRef] [PubMed]
- Frengstad, B.S.; Lax, K.; Tarvainen, T.; Jæger, Ø.; Wigum, B.J. The chemistry of bottled mineral and spring waters from Norway, Sweden, Finland and Iceland. J. Geochem. Explor. 2010, 107, 350–361. [Google Scholar] [CrossRef]
- Chae, G.T.; Yun, S.T.; Kwon, M.J.; Kim, S.Y.; Mayer, B. Batch dissolution of granite and biotite in water: Implication for fluorine geochemistry in groundwater. J. Hydrol. 2006, 40, 95–102. [Google Scholar] [CrossRef]
- Sung, I.H.; Choo, C.O.; Cho, B.W.; Lee, B.D.; Kim, T.K.; Lee, I.H. Hydrochemical properties of the groundwater used for the natural mineral waters in Precambrian metamorphic terrains, Korea. J. Korean Soc. Environ. Eng. 1998, 5, 203–209. (In Korean) [Google Scholar]
- Wakatsuki, T.; Tanaka, Y.; Matsukura, Y. Soil slips on weathering-limited slopes underlain by coarse-grained granite or fine-grained gneiss near Seoul, Republic of Korea. Catena 2005, 60, 181–203. [Google Scholar] [CrossRef]
- Kim, K.; Jeong, G.Y. Factors influencing natural occurrence of fluoride-rich groundwaters: A case study in the southeastern part of the Korean Peninsula. Chemosphere 2005, 58, 1399–1408. [Google Scholar] [CrossRef]
- Yun, S.T.; Chae, G.T.; Koh, Y.K.; Kim, S.R.; Choi, B.Y.; Lee, B.H.; Kim, S.Y. Hydrogeochemical and environmental isotope study of groundwaters in the Pungki area. J. Kor. Soc. Environ. Eng. 1998, 5, 177–191. (In Korean) [Google Scholar]
- Lee, J.Y.; Lee, K.K. Use of hydrologic time series data for identification of recharge mechanism in a fractured bedrock aquifer system. J. Hydrol. 2000, 229, 190–201. [Google Scholar] [CrossRef]
- Joo, Y.; Brumback, B.; Lee, K.; Yun, S.T.; Kim, K.H.; Joo, C. Clustering of temporal profiles using a Bayesian logistic mixture model: Analyzing groundwater level data to understand the characteristics of urban groundwater recharge. J. Agric. Biol. Environ. Stat. 2009, 14, 356–373. [Google Scholar] [CrossRef]
- Chae, G.T.; Yun, S.T.; Kim, D.S.; Kim, K.H.; Joo, Y. Time-series analysis of three years of groundwater level data (Seoul, South Korea) to characterize urban groundwater recharge. Q. J. Eng. Geol. Hydrogeol. 2010, 4, 117–127. [Google Scholar] [CrossRef]
- Koo, J.; Kim, G.; Hwang, Y. Groundwater age analysis using tritium in gneiss of the Samkwang mine in Korea. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 1995, 30, 2093–2109. [Google Scholar] [CrossRef]
- Choi, B.Y.; Yun, S.T.; Yu, S.; Lee, P.K.; Park, S.S.; Chae, G.T.; Mayer, B. Hydrochemistry of urban groundwater in Seoul, South Korea: Effects of land-use and pollutant recharge. Environ. Geol. 2005, 48, 979–990. [Google Scholar] [CrossRef]
- Lee, J.M.; Ko, K.S.; Woo, N.C. Characterization of groundwater level and water quality by classification of aquifer types in South Korea. Econ. Environ. Geol. (Korea) 2020, 53, 619–629. (In Korean) [Google Scholar]
- Edmunds, W.M.; Smedley, P.L. Residence time indicators in groundwater: The East Midlands Triassic sandstone aquifer. Appl. Geochem. 2000, 15, 737–752. [Google Scholar] [CrossRef]
- Ford, R.G.; Wilkin, R.T.; Puls, R.W. Monitored Natural Attenuation of Inorganic Contaminants in Ground Water—Volume 2: Assessment for non-Radionuclides Including Arsenic, Cadmium, Chromium, Copper, Lead, Nickel, Nitrate, Perchlorate and Selenium; USA Environmental Protection Agency: Washington, DC, USA, 2007.
- Shand, P.; Edmunds, W.M. The baseline inorganic chemistry of European groundwaters. In Natural groundwater quality; Edmunds, W.M., Shand, P., Eds.; Blackwell Publishing Ltd.: Malden, MA, USA, 2008; pp. 22–58. [Google Scholar]
- White, A.F.; Bullen, T.D.; Vivit, D.V.; Schulz, M.S.; Clow, D.W. The role of disseminated calcite in the chemical weathering of granitoid rocks. Geochim. Cosmochim. Acta 1999, 63, 1939–1953. [Google Scholar] [CrossRef]
- White, A.; Schulz, M.; Lowenstern, J.; Vivit, D.; Bullen, T. The ubiquitous nature of accessory calcite in granitoid rocks: Implications for weathering, solute evolution, and petrogenesis. Geochim. Cosmochim. Acta 2005, 69, 1455–1471. [Google Scholar] [CrossRef]
- Goldich, S.S. A study in rock-weathering. J. Geol. 1938, 46, 17–58. [Google Scholar] [CrossRef]
- Choi, B.Y.; Yun, S.T.; Kim, K.H.; Kim, J.W.; Kim, H.M.; Koh, Y.K. Hydrogeochemical interpretation of South Korean groundwater monitoring data using Self-Organizing Maps. J. Geochem. Explor. 2014, 137, 73–84. [Google Scholar] [CrossRef]
- Zaidi, F.; Nazzal, Y.; Jafri, M.; Naeem, M.; Ahmed, I. Reverse ion exchange as a major process controlling the groundwater chemistry in an arid environment: A case study from northwestern Saudi Arabia. Environ. Monit. Assess. 2015, 187, 607. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.H.; Thao, N.T.; Batsaikhan, B.; Yun, S.T. Hydrochemical assessment of freshening saline groundwater using multiple end-members mixing modeling: A study of Red River delta aquifer, Vietnam. J. Hydrol. 2017, 549, 703–714. [Google Scholar] [CrossRef]
- Hornung, R.W.; Reed, L.D.; Thao, N.T. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. 1990, 5, 46–51. [Google Scholar] [CrossRef]
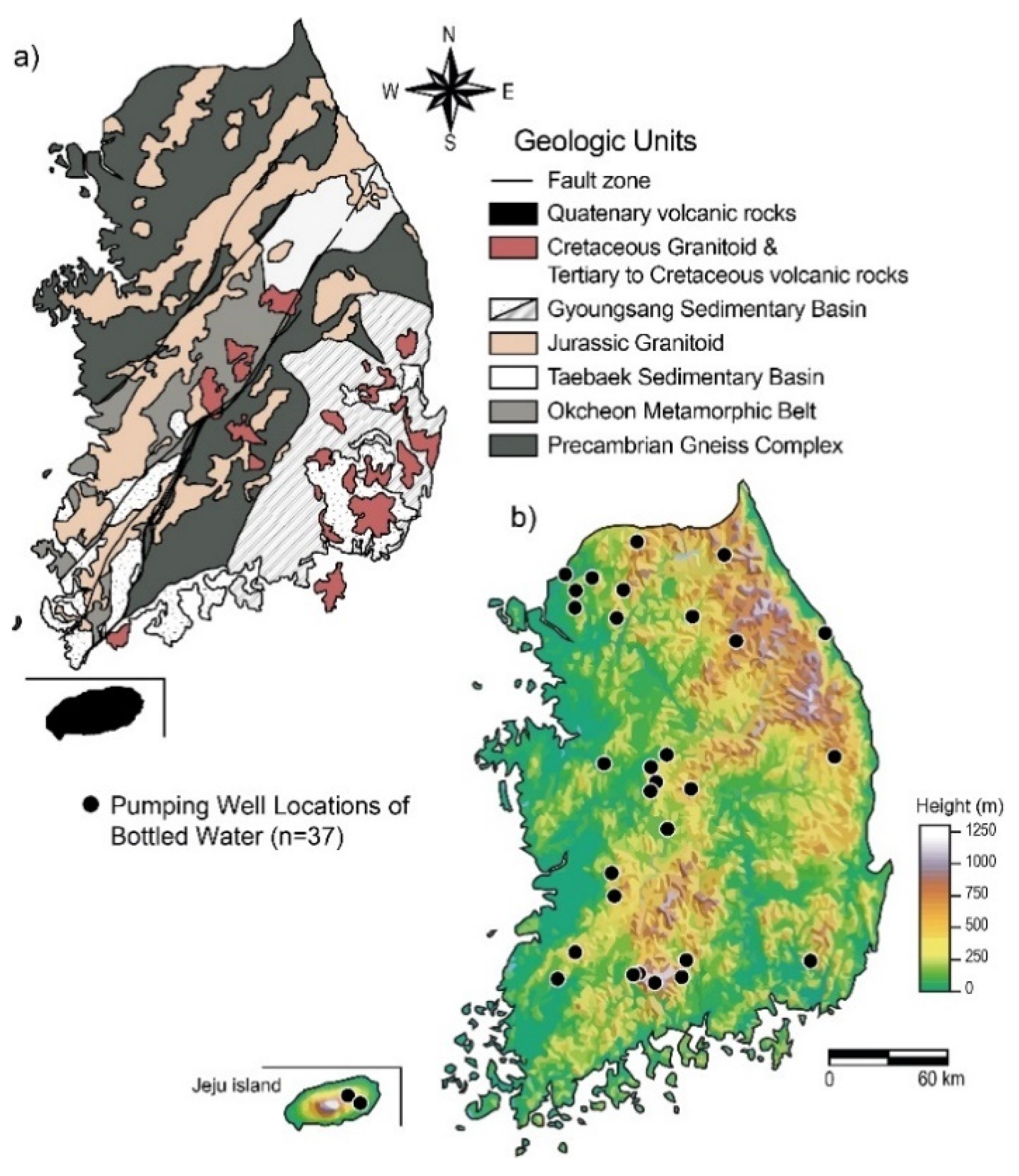
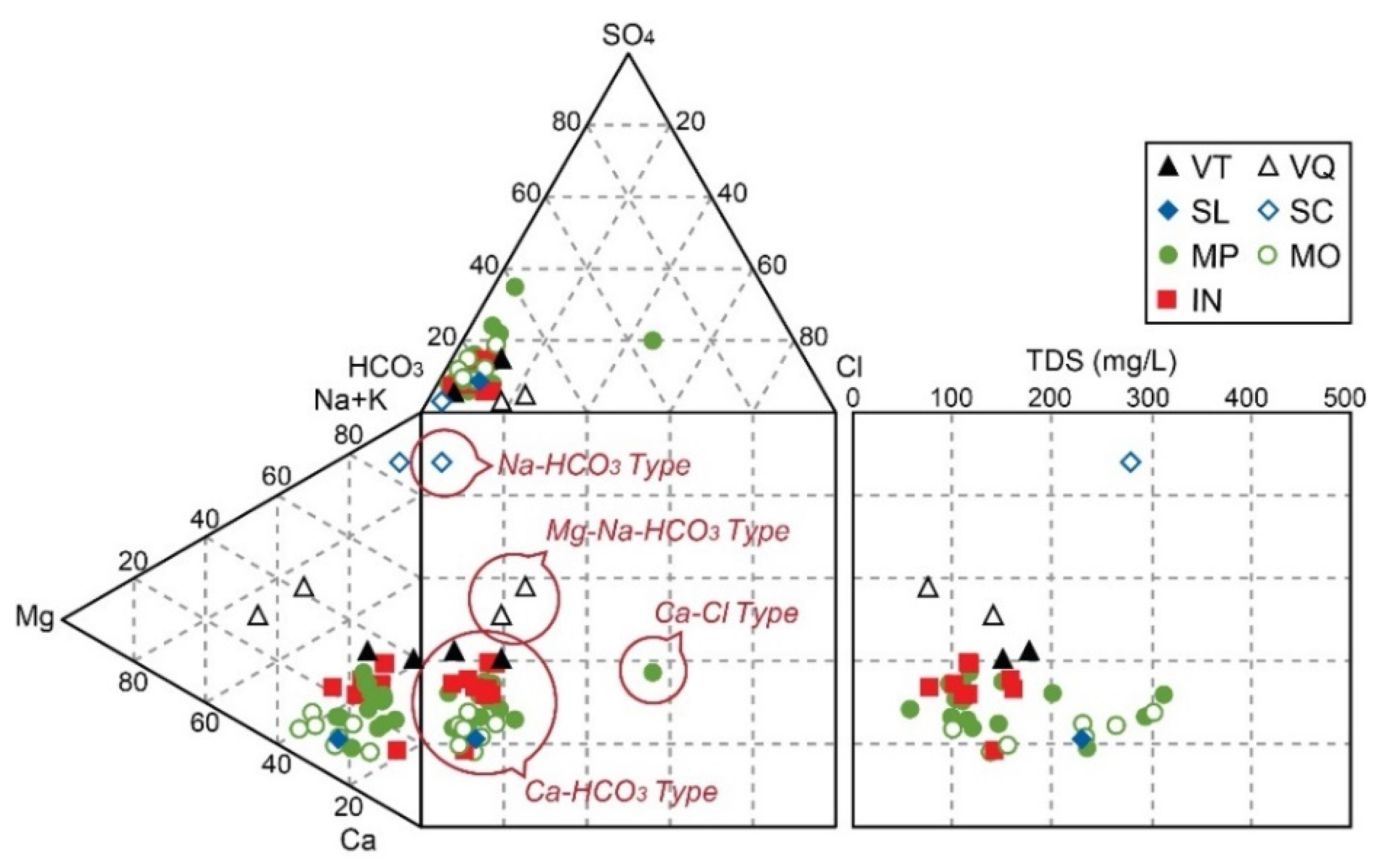

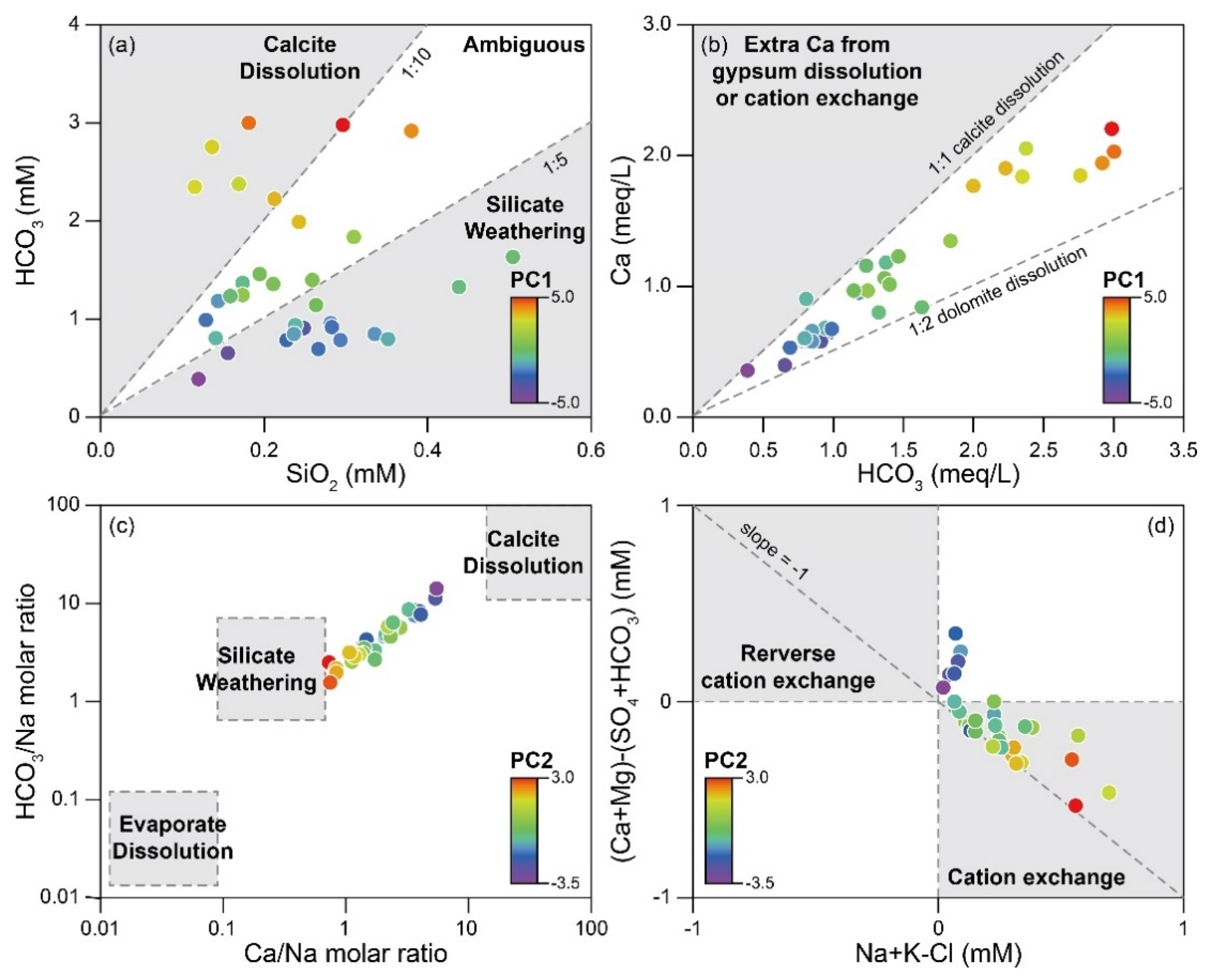

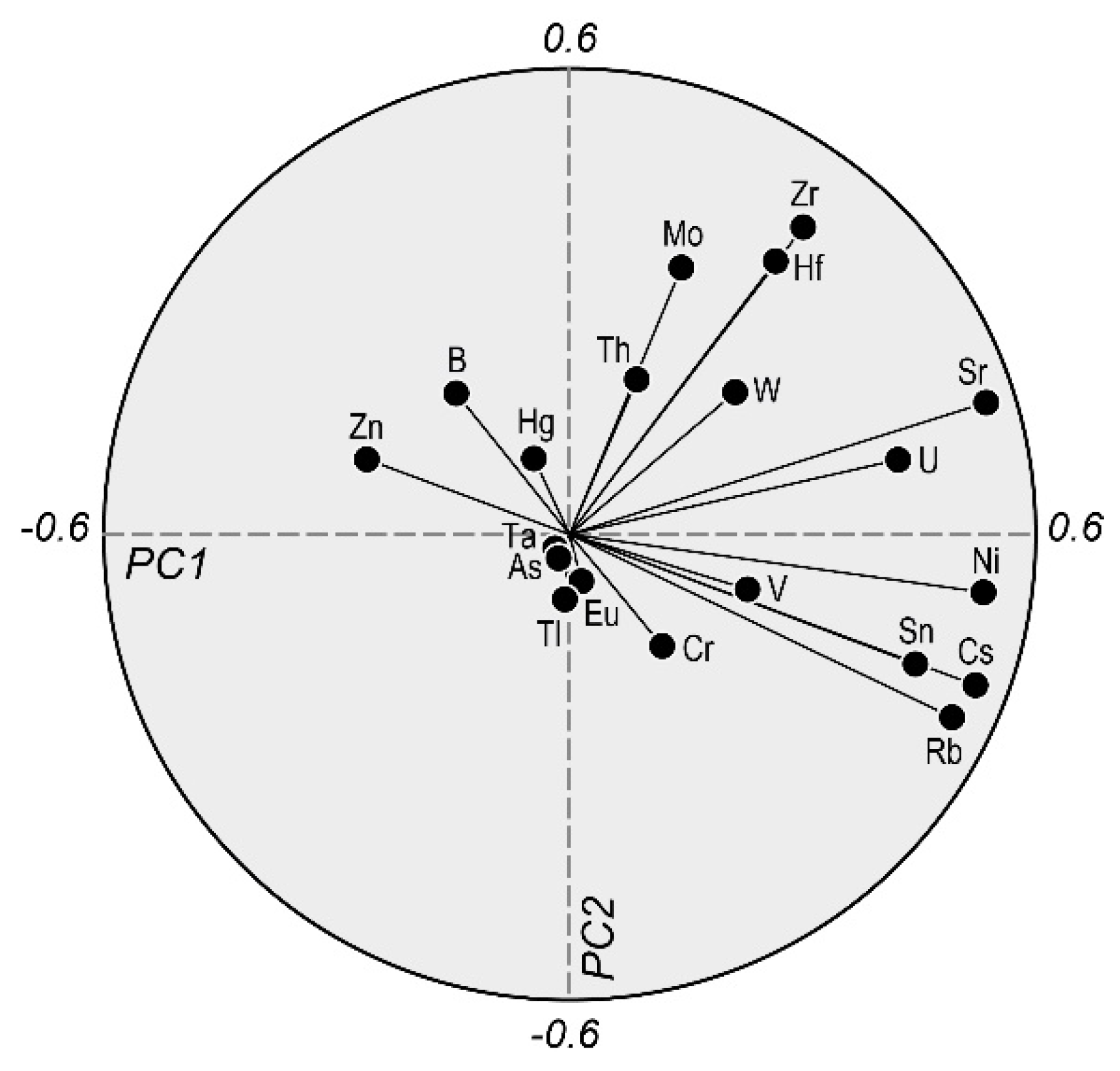
| Hydrogeological Units | Geological Units | Lithology | Abbreviations | Bottled Water Samples for This Study |
|---|---|---|---|---|
| Volcanic rocks | Quaternary volcanic rocks | Basalt, trachybasalt, tuff | VQ | N1, N2 |
| Tertiary to Cretaceous volcanic rocks | Rhyolite, andesite, basaltic andesite, tuff | VT | N16, N27 | |
| Intrusive rocks | Cretaceous (Bulguksa) granitoids | Mainly granite with minor diorite, gabbro, and hypabyssal igneous rocks | IN | N4, N5, N6, N7, N8, N20, N21, N29, N36 |
| Jurassic (Daebo) granitoids | ||||
| Sedimentary rocks | Cretaceous clastic sedimentary rocks in Gyeongsang sedimentary basin | Mainly fluvial and lacustrine sedimentary rocks (sandstone, shale, others) with volcanic intercalations | SC | N22 |
| Cambro–Ordovician carbonate rocks of Great Limestone groups in Taebaeksan basin | Marine carbonate rocks with interbedded clastic sedimentary rocks | SL | N3 | |
| Metamorphic rocks | Early Paleozoic metasedimentary rocks of Okcheon metamorphic belt | Phyllite, slate, schist, quartzite, etc. | MO | N28, N31, N32, N33, N34, N35, N37 |
| Precambrian basement rocks of metamorphic complex | Mainly gneiss and schist with minor metabasite | MP | N9, N10, N11, N12, N13, N14, N15, N17, N18, N19, N23, N24, N25, N26, N30 |
| Variables | Mean | Std.dev. | Minimum | Median | Q90 a | Maximum | EU Q90 b | WHO c | South Korea d | p-Value e |
|---|---|---|---|---|---|---|---|---|---|---|
| EC (μS/cm) | 177.3 | 83.4 | 65.0 | 152.5 | 298.6 | 356.3 | 2546.0 | 0.04 | ||
| TDS (mg/L) | 158.4 | 68.1 | 57.3 | 142.0 | 258.6 | 312.2 | - | 0.17 | ||
| pH | 7.5 | 0.4 | 6.7 | 7.5 | 8.1 | 8.2 | 7.8 | 6.5–9.5 | 5.8–8.5 (4.5–9.5) f | 0.04 |
| Ca (mg/L) | 24.0 | 12.4 | 8.0 | 21.2 | 43.0 | 49.1 | 282.0 | 0.02 | ||
| Mg (mg/L) | 4.1 | 3.4 | 0.4 | 2.9 | 8.7 | 14.5 | 71.0 | 0.00 | ||
| Na (mg/L) | 7.9 | 4.4 | 1.6 | 6.5 | 13.1 | 22.3 | 290.0 | 0.82 | ||
| K (mg/L) | 0.9 | 0.5 | 0.2 | 0.7 | 1.3 | 2.8 | 15.0 | 0.00 | ||
| SiO2 (mg/L) | 14.3 | 5.6 | 6.9 | 14.2 | 20.9 | 30.3 | 34.2 | 0.05 | ||
| Cl (mg/L) | 4.6 | 2.8 | 1.4 | 3.8 | 8.5 | 12.3 | 180.0 | 250 | 250 | 0.69 |
| SO4 (mg/L) | 10.5 | 6.3 | 3.5 | 8.5 | 18.7 | 27.5 | 447.0 | - | 200 | 0.08 |
| NO3 (mg/L) | 4.2 | 2.8 | 0.1 | 3.0 | 7.9 | 10.0 | 8.8 | 50 | 44.3 | 0.38 |
| HCO3 (mg/L) | 87.3 | 45.4 | 23.7 | 75.3 | 163.9 | 183.3 | - | 0.05 | ||
| F (mg/L) | 0.4 | 0.3 | 0.03 | 0.2 | 0.9 | 1.1 | 0.9 | 1.5 | 1.5 (2.0) f | 0.25 |
| Br (mg/L) | 0.02 | 0.01 | 0.001 | 0.01 | 0.0 | 0.06 | 313.0 | 0.15 |
| Element (μg/L) | N a | Mean b | Mean c | Std.Dev | Minimum | Median | Q90 e | Maximum | EU Q90 f | WHOg | South Korea h | Max. from PET i | p-Value l |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | 0 | 4.41 | - | 3.08 | 0.67 | 3.56 | 9.18 | 11.69 | 13 | 200 | 200 | 36 | |
| As | 0 | 3.16 | - | 2.81 | 0.09 | 2.26 | 5.55 | 10.94 | 2.3 | 10 | 10 | - | 0.31 |
| B | 0 | 6.48 | - | 8.80 | 0.69 | 3.88 | 11.86 | 46.06 | 602 | 500 | 1000 | - | 0.55 |
| Ba | 0 | 17.24 | - | 41.45 | 0.70 | 3.76 | 36.11 | 228.26 | 181 | 700 | - | 36 | |
| Hf | 0 | 0.30 | - | 0.06 | 0.22 | 0.28 | 0.36 | 0.47 | 0.004 | - | - | 0.0014 | 0.75 |
| Hg | 0 | 0.38 | - | 0.08 | 0.29 | 0.35 | 0.51 | 0.59 | - | 6 | 1 | - | 0.75 |
| Rb | 0 | 1.55 | - | 1.62 | 0.12 | 1.14 | 2.57 | 8.95 | 38 | - | - | 0.01 | 0.02 |
| Sb | 0 | 0.46 | - | 0.41 | 0.04 | 0.34 | 0.77 | 2.09 | 0.53 | 20 | - | -k | |
| Sn | 0 | 0.10 | - | 0.02 | 0.06 | 0.10 | 0.13 | 0.16 | 0.031 | - | - | - | 0.46 |
| Sr | 0 | 215.40 | - | 195.72 | 49.21 | 115.72 | 387.96 | 843.05 | 4360 | - | - | 37 | 0.78 |
| Ta | 0 | 0.13 | - | 0.02 | 0.10 | 0.13 | 0.15 | 0.16 | <0.005 | - | - | 0.005 | 0.88 |
| Th | 0 | 0.56 | - | 0.18 | 0.42 | 0.50 | 0.78 | 1.29 | 0.004 | - | - | - | 0.38 |
| Tl | 0 | 0.11 | - | 0.05 | 0.06 | 0.08 | 0.17 | 0.17 | 0.039 | - | - | 0.01 | 1.00 |
| Zr | 0 | 0.29 | - | 0.05 | 0.21 | 0.27 | 0.35 | 0.45 | 0.29 | - | - | 0.073 | 0.57 |
| Co | 14 | 0.09 | 0.05 | 0.05 d | <0.01 | 0.04 | 0.11 | 0.17 | 0.12 | - | - | 0.16 | |
| Cr | 13 | 0.83 | 0.53 | 0.61 d | <0.14 | 0.30 | 1.09 | 3.03 | 0.97 | 50 | 50 | - | 0.96 |
| Cs | 13 | 0.20 | 0.12 | 0.16 d | <0.03 | 0.05 | 0.38 | 0.57 | 6.6 | - | - | 0.015 | 0.01 |
| Cu | 11 | 0.19 | 0.14 | 0.26 d | <0.03 | 0.06 | 0.25 | 1.47 | 1.6 | 2000 | 1000 | 0.68 | |
| Eu | 14 | 0.03 | 0.02 | 0.01 d | <0.01 | 0.01 | 0.04 | 0.04 | 0.004 | - | - | 0.009 | 0.17 |
| Fe | 14 | 2.20 | 1.47 | 1.75 d | <0.89 | 1.10 | 2.00 | 8.37 | 12 | - | 300 | 599 | |
| Mo | 1 | 3.68 | 3.57 | 6.04 d | <0.11 | 1.15 | 9.71 | 25.86 | 2 | 70 | 70 | 0.006 | 0.45 |
| Ni | 3 | 0.40 | 0.37 | 0.25 d | <0.02 | 0.31 | 0.69 | 1.02 | 2.4 | - | - | - | 0.29 |
| Ti | 9 | 0.15 | 0.12 | 0.10 d | <0.05 | 0.08 | 0.28 | 0.37 | 0.3 | - | - | 0.07 | |
| U | 2 | 1.94 | 1.82 | 3.82 d | <0.06 | 0.42 | 4.63 | 20.25 | 2.4 | 15 | 30 | 0.06 | 0.74 |
| V | 5 | 0.97 | 0.83 | 0.87 d | <0.02 | 0.63 | 2.27 | 3.55 | 0.88 | - | - | 0.06 | 0.67 |
| W | 8 | 0.44 | 0.35 | 0.56 d | <0.11 | 0.20 | 0.61 | 2.80 | 0.099 | - | - | - | 0.36 |
| Zn | 2 | 4.43 | 4.16 | 8.67 d | <0.03 | 1.54 | 5.46 | 38.97 | 11 | 3000 | 3000 | - | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-J.; Yu, S.; Kim, K.-H.; Kang, K.-G.; Moon, S.-H.; Kim, M.-S.; Yun, S.-T. Hydrogeochemical Characteristics of Bottled Waters Sourced from Bedrock Aquifers in South Korea: Evaluation of Water Type and Natural Background Levels. Water 2022, 14, 1457. https://doi.org/10.3390/w14091457
Lee K-J, Yu S, Kim K-H, Kang K-G, Moon S-H, Kim M-S, Yun S-T. Hydrogeochemical Characteristics of Bottled Waters Sourced from Bedrock Aquifers in South Korea: Evaluation of Water Type and Natural Background Levels. Water. 2022; 14(9):1457. https://doi.org/10.3390/w14091457
Chicago/Turabian StyleLee, Kyung-Jin, Soonyoung Yu, Kyoung-Ho Kim, Kyoung-Gu Kang, Su-Hyung Moon, Moon-Su Kim, and Seong-Taek Yun. 2022. "Hydrogeochemical Characteristics of Bottled Waters Sourced from Bedrock Aquifers in South Korea: Evaluation of Water Type and Natural Background Levels" Water 14, no. 9: 1457. https://doi.org/10.3390/w14091457
APA StyleLee, K.-J., Yu, S., Kim, K.-H., Kang, K.-G., Moon, S.-H., Kim, M.-S., & Yun, S.-T. (2022). Hydrogeochemical Characteristics of Bottled Waters Sourced from Bedrock Aquifers in South Korea: Evaluation of Water Type and Natural Background Levels. Water, 14(9), 1457. https://doi.org/10.3390/w14091457






