Biodiversity of Non-Marine Ostracoda (Crustacea) of Botswana: An Annotated Checklist with Notes on Distribution
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Checklist of Non-Marine Living and Fossil (Late Pleistocene to Holocene) Ostracoda of Botswana
- Szwarc et al. [22]: Thamalakane river near the city of Maun, site no SA-97/coord. 19°55′52″ S, 23°30′38″ E, 940 m a.s.l./FEOW: 569 Okavango/Habitat: river side channel/Coll. date: 13 September 2012/2 ♀♀, 9 juv;
- Present paper: Botanic Garden in the city of Gabarone, site no SA-112/coord. 24°39′56″ S, 25°56′40″ E, 987 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: artificial rock-pool/Coll. date: 19 September 2012/2 ♀♀.
- Barnard [19]: 1 mile north of Tsotsoroga Pan, localities nos 1204a and 1214/approx. coord. 18°43′00″ S, 24°21′00″ E, 940 m a.s.l./FEOW: 569 Okavango/Habitat: not indicated/Coll. dates: 19–20 June 1930/number of specimens unspecified;
- Barnard [19]: N’kate (= Nekati) Pan, locality no 1505/approx. coord. 20°05′00″ S, 26°01′00″ E, 920 m a.s.l./FEOW: 570 Kalahari/Habitat: shallow pan in limestone formation/Coll. date: 7 August 1930;
- McKenzie [16]: Makarikari expedition, no further details given/FEOW: 570 Kalahari/Habitat: not indicated/Coll. date: 2.10.1958/number of specimens unspecified, det. D.H. Eccles;
- Barnard [19]: N’kate (=Nekati) Pan, locality no 1510a/approx. coord. 20°05′00″ S, 26°01′00″ E, 920 m a.s.l./FEOW: 570 Kalahari, Habitat: a pan/Coll. date: 8 August 1930/two empty valves;
- Present paper: Kang Nkisi Guest House in the village of Kang, site no SA-95/coord. 23°04′50″ S, 22°45′55″ E, 1142 m a.s.l./FEOW: 570 Kalahari/Habitat: artificial pool/Coll. date: 12 September 2012/7 ♀♀, 5 juv;
- Present paper: Botanic Garden in the city of Gabarone, site no SA-112/coord. 24°39′56″ S, 25°56′40″ E, 987 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: artificial rock-pool/Coll. date: 19 September 2012/11 ♀♀, 28 juv;
- Present paper: Kolobeng river near the village of Manyana, site no SA-113/coord. 24°46′08″ S, 25°35′22″ E, 1133 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: river/Coll. date: 20 September 2012/3 ♀♀, 2 juv;
- Present paper: Ramatlabama river near the village of Ramatlabama, site no SA-117/coord. 25°38′29″ S, 25°34′27″ E, 1276 m a.s.l./FEOW: 571 Southern Kalahari/Habitat: river/Coll. date: 20 September 2012/2 ♀♀.
- McCulloch et al. [21] as Plesiocypridopsis aldabrae: North Basin of Makgadikgadi Pan/approx. coord. 20°24′00″ S, 26°12′00″ E, 890 m a.s.l./FEOW: 570 Kalahari/Habitat: ephemeral saline lake (water conductivity 320-24400 µS/cm, pH: 8.6–10.1)/Coll. dates: beginning of the 1999–2000 flood/low abundance;
- Szwarc et al. [22]: floodplains south of Okavango Delta near the city of Maun, site no SA-103/coord: 19°52′12″ S, 23°20′23″ E, 940 m a.s.l./FEOW: 569 Okavango/Habitat: grassy shore of temporary pond/Coll. date: 15 September 2012/3 ♀♀, 2 ♂♂;
- Present paper: Tati river near the town of Francistown, site no SA-108/coord. 21°10′48″ S, 27°30′44″ E, 982 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: pools in riverbed/Coll. date: 18 September 2012/3 juv;
- Present paper: Botanic Garden in the city of Gabarone, site no SA-112/coord. 24°39′56″ S, 25°56′40″ E, 987 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: artificial rock-pool/Coll. date: 19 September 2012/61 ♀♀ and ♂♂, 283 juv.
- Jocqué et al. [20] as Sarscypridopsis cf. gregaria: Kgale Siding near Gabarone/coord. 24°40′30″ S, 25°50′20″ E, 1040 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: nine temporary granite rock pools/Coll. dates: entire inundation cycle of the rainy season of 2002–2003/number of specimens unspecified;
- Jocqué et al. [20,37] as Sarscypridopsis cf. gregaria: near Thamaga/coord. 24°41′50″ S, 25°31′00″ E in [20] and 24°40′30″ S, 25°31′00″ E in [37], 1105 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: nine temporary granite rock pools/Coll. dates: entire inundation cycle of the rainy season of 2002–2003/number of specimens unspecified.
- Szwarc et al. [22]: Lake Ngami, site no SA-96/coord: 20°28′57″S, 22°42′08″E, 930 m a.s.l./FEOW: 569 Okavango/Habitat: endorheic lake/Coll. date: 12 September 2012/1 juv;
- Szwarc et al. [22]: Thamalakane river near the city of Maun, site no SA-97/coord. 19°55′52″ S, 23°30′38″ E, 940 m a.s.l./FEOW: 569 Okavango/Habitat: river side channel/Coll. date: 13 September 2012/11 ♀♀, 1 juv;
- Szwarc et al. [22]: floodplains south of Okavango Delta near the city of Maun, site no SA-98/coord: 19°52′15″ S, 23°21′06″ E, 940 m a.s.l/FEOW: 569 Okavango/Habitat: temporary channel/Coll. date: 14 September 2012/6 ♀♀;
- Szwarc et al. [22]: floodplains south of Okavango Delta near the city of Maun, site no SA-99/coord: 19°52′15″ S, 23°20′45″ E, 940 m a.s.l/FEOW: 569 Okavango/Habitat: temporary channel/Coll. date: 14 September 2012/16 ♀♀, 1 juv;
- Szwarc et al. [22]: floodplains south of Okavango Delta near the city of Maun, site no SA-100/coord: 19°52′04″ S, 23°20′38″ E, 940 m a.s.l/FEOW: 569 Okavango/Habitat: flooded swamp and grassland/Coll. date: 14 September 2012/11 ♀♀;
- Szwarc et al. [22]: floodplains south of Okavango Delta near the city of Maun, site no SA-101/coord: 19°51′39″ S, 23°19′41″ E, 940 m a.s.l/FEOW: 569 Okavango/Habitat: isolated pool in flooded grassland/Coll. date: 15 September 2012/6 ♀♀;
- Szwarc et al. [22]: floodplains south of Okavango Delta near the city of Maun, site no SA-102/coord: 19°52′06″ S, 23°20′41″ E, 940 m a.s.l/FEOW: 569 Okavango/Habitat: floodplain channel/Coll. date: 15 September 2012/1 ♀;
- Szwarc et al. [22]: North-West District, floodplains south of Okavango Delta near the city of Maun, site no SA-103/coord: 19°52′12″ S, 23°20′23″ E, 940 m a.s.l./FEOW: 569 Okavango/Habitat: grassy shore of temporary pond/Coll. date: 15 September 2012/28 ♀♀.
- Barnard [19] as Cyprilla producta Sars: Kaotwe Pan, locality no 374/approx. coord. 22°33′00″ S, 23°15′00″ E, 1011 m a.s.l./FEOW: 570 Kalahari/Habitat: a pan/Coll. date: 10 April 1930/very numerous;
- Olszewski et al. [34]: Makgadikgadi depression/coord: 20°08′02″ S, 25°33′41″ E, 934 m a.s.l./FEOW: 570 Kalahari/Habitat: raised from a sample of dry sediment from temporary shallow salt lake/Coll. date: 19 September 2012/number of specimens unspecified;
- Szwarc et al. [22]: Thamalakane river near the city of Maun, site no SA-97/coord. 19°55′52″ S, 23°30′38″ E, 940 m a.s.l./FEOW: 569 Okavango/Habitat: river side channel/Coll. date: 13 September 2012/697 ♀♀ and ♂♂, 135 juv;
- Szwarc et al. [22]: floodplains south of Okavango Delta near the city of Maun, site no SA-99/coord: 19°52′15″ S, 23°20′45″ E, 940 m a.s.l/FEOW: 569 Okavango/Habitat: temporary channel/Coll. date: 14 September 2012/1 ♀;
- Present paper: Mahalapye river near the town of Mahalapye, site no SA-110/coord. 23°06′09″ S, 26°50′21″ E, 1006 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: small river dam/Coll. date: 18 September 2012/8 ♀♀, 11 juv;
- Present paper: Botanic Garden in the city of Gabarone, site no SA-112/coord. 24°39′56″ S, 25°56′40″ E, 987 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: artificial rock-pool/Coll. date: 19 September 2012/595 ♀♀ and ♂♂, 679 juv;
- Present paper: Bathoen near the town of Kanye, site no SA-114/coord. 24°56′54″ S, 25°20′39″ E, 1255 m a.s.l./FEOW: 575 Southern Temperate Highveld/Habitat: river flood pool/Coll. date: 20 September 2012/1 ♀.
- 1.
- Daday [18] as Cyprinotus inversus: Ku-Gudië between Phitshane and Kooa, South Kalahari, site no 5/approx. coord. 25°40′00″ S, 25°06′00″ E, 1100–1200 m a.s.l./FEOW: 571 Southern Kalahari/Habitat: unspecified/Coll. date: January 1905/numerous females and males;
- 2.
- 3.
- Szwarc et al. [22]: Lake Ngami, site no SA-96/coord: 20°28′57″ S, 22°42′08″ E, 930 m a.s.l./FEOW: 569 Okavango/Habitat: endorheic lake/Coll. date: 12 September 2012/2 ♀♀.
- 1.
- Savatenalinton & Martens [31] as Heterocypris giesbrechti: Ku-Gudië between Phitshane and Kooa, South Kalahari, site no 5/approx. coord. 25°40′00″ S, 25°06′00″ E, 1100–1200 m a.s.l./FEOW: 571 Southern Kalahari/Habitat: unspecified/Coll. date: January 1905/numerous females and males;
- 2.
- Szwarc et al. [22]: Lake Ngami, site no SA-96/coord: 20°28′57″ S, 22°42′08″ E, 930 m a.s.l./FEOW: 569 Okavango/Habitat: endorheic lake/Coll. date: 12 September 2012/1 ♀, 3 ♂♂, 7 juv;
- 3.
- Szwarc et al. [22]: floodplains south of Okavango Delta near the city of Maun, site no SA-98/coord: 19°52′15″ S, 23°21′06″ E, 940 m a.s.l/FEOW: 569 Okavango/Habitat: temporary channel/Coll. date: 14 September 2012/2 ♀♀, 9 juv.
- Szwarc et al. [22]: Thamalakane river near the city of Maun, site no SA-97/coord. 19°55′52″ S, 23°30′38″ E, 940 m a.s.l./FEOW: 569 Okavango/Habitat: river side channel/Coll. date: 13 September 2012/132 ♀♀ and ♂♂, 90 juv;
- Szwarc et al. [22]: floodplains south of Okavango Delta near the city of Maun, site no SA-99/coord: 19°52′15″ S, 23°20′45″ E, 940 m a.s.l/FEOW: 569 Okavango/Habitat: temporary channel/Coll. date: 14 September 2012/153 ♀♀, 106 juv;
- Szwarc et al. [22]: floodplains south of Okavango Delta near the city of Maun, site no SA-100/coord: 19°52′04″ S, 23°20′38″ E, 940 m a.s.l/FEOW: 569 Okavango/Habitat: flooded swamp and grassland/Coll. date: 14 September 2012/14 ♀♀, 6 juv;
- Szwarc et al. [22]: floodplains south of Okavango Delta near the city of Maun, site no SA-101/coord: 19°51′39″ S, 23°19′41″ E, 940 m a.s.l/FEOW: 569 Okavango/Habitat: isolated pool in flooded grassland/Coll. date: 15 September 2012/25 ♀♀, 17 ♂♂, 1 juv;
- Szwarc et al. [22]: floodplains south of Okavango Delta near the city of Maun, site no SA-102/coord: 19°52′06″ S, 23°20′41″ E, 940 m a.s.l/FEOW: 569 Okavango/Habitat: floodplain channel/Coll. date: 15 September 2012/1 ♀, 2 juv;
- Szwarc et al. [22]: North-West District, floodplains south of Okavango Delta near the city of Maun, site no SA-103/coord: 19°52′12″ S, 23°20′23″ E, 940 m a.s.l./FEOW: 569 Okavango/Habitat: grassy shore of temporal pond/Coll. date: 15 September 2012/16 ♀♀, 2 juv.
- Barnard [19] as Herpetocypris ovularis Sars: 2 miles north of Tsotsoroga, localities nos 1204b/approx. coord. 18°40′00″ S, 24°19′00″ E, 1058 m a.s.l./FEOW: 569 Okavango/Habitat: not specified/Coll. dates: 19 June 1930/mostly empty valves;
- Barnard [19] as Herpetocypris ovularis Sars: Kaotwe Pan, locality no 374a/approx. coord. 22°33′00″ S, 23°15′00″ E, 1011 m a.s.l./FEOW: 570 Kalahari/Habitat: a pan/Coll. date: 10 April 1930/a few specimens.
- Jocqué et al. [20]: Kgale Siding near Gabarone/coord. 24°40′30″ S, 25°50′20″ E, 1040 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: nine temporary granite rock pools/Coll. dates: entire inundation cycle of the rainy season of 2002–2003/number of specimens unspecified;
- Jocqué et al. [20] and Jocqué et al. [37] as Heterocypris sp. nov.: near Thamaga/coord. 24°41′50″ S, 25°31′00″ E, 1105 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: nine temporary granite rock pools/Coll. dates: entire inundation cycle of the rainy season of 2002–2003/number of specimens unspecified.
- Jocqué et al. [20] as Amphibolocypris sp. n. sp.: Kgale Siding near Gabarone/coord. 24°40′30″ S, 25°50′20″ E, 1040 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: nine temporary granite rock pools/Coll. dates: entire inundation cycle of the rainy season of 2002–2003/number of specimens unspecified;
- Jocqué et al. [20] as Amphibolocypris sp. n. sp.: near Thamaga/coord. 24°41′50″ S, 25°31′00″ E, 1105 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: nine temporary granite rock pools/Coll. dates: entire inundation cycle of the rainy season of 2002–2003/number of specimens unspecified;
- Szwarc et al. [22]: Thamalakane river near the city of Maun, site no SA-97/coord. 19°55′52″ S, 23°30′38″ E, 940 m a.s.l./FEOW: 569 Okavango/Habitat: river side channel/Coll. date: 13 September 2012/2 ♀♀, 1 juv;
- Present paper: Bathoen reservoir near the town of Kanye, site no SA-115/coord. 24°57′06″ S, 25°20′33″ E, 1267 m a.s.l./FEOW: 575 Southern Temperate Highveld/Habitat: reservoir/Coll. date: 20 September 2012/50 ♀♀, 353 juv.
- Szwarc et al. [22]: Thamalakane river near the city of Maun, site no SA-97/coord. 19°55′52″ S, 23°30′38″ E, 940 m a.s.l./FEOW: 569 Okavango/Habitat: river side channel/Coll. date: 13 September 2012/2 ♀♀, 3 juv;
- Szwarc et al. [22]: floodplains south of Okavango Delta near the city of Maun, site no SA-100/coord: 19°52′04″ S, 23°20′38″ E, 940 m a.s.l/FEOW: 569 Okavango/Habitat: flooded swamp and grassland/Coll. date: 14 September 2012/1 ♀.
- Martens [32] and Seaman et al. [33] as Sclerocypris excerta Sars (typographical error of exserta): Makgadikgadi Pan/approx. coord. 20°42′00″ S, 24°57′00″ E, 905 m a.s.l./FEOW: 570 Kalahari/Habitat: huge, temporary salt pan endorheic system/Coll. date: 4 May 1957, collected by Rhodesian Schools Exploration Society/number of specimens unspecified;
- McCulloch et al. [21]: North Basin of Makgadikgadi Pan/approx. coord. 20°24′00″ S, 26°12′00″ E, 905 m a.s.l./FEOW: 570 Kalahari/Habitat: ephemeral saline lake system (water conductivity 320-24400 µS/cm, pH: 8.6–10.1)/Coll. dates: December 1999–June 2001/number of specimens unspecified;
- McCulloch et al. [21]: Middle Basin of Makgadikgadi Pan/approx. coord. 20°39′00″ S, 26°04′00″ E, 905 m a.s.l./FEOW: 570 Kalahari/Habitat: ephemeral saline lake system (water conductivity 730-91600 µS/cm, pH: 8.6–10.1)/Coll. dates: December 1999–June 2001/number of specimens unspecified.
- Present paper: Shashe river near the village of Shashe, site no SA-109/coord. 21°23′20″ S, 27°27′20″ E, 956 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: pools in riverbed/Coll. date: 18 September 2012/97 ♀♀;
- Present paper: Mahalapye river near the town of Mahalapye, site no SA-110/coord. 23°06′09″ S, 26°50′21″ E, 1006 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: small river dam/Coll. date: 18 September 2012/1 ♀;
- Present paper: Bathoen near the town of Kanye, site no SA-114/coord. 24°56′54″ S, 25°20′39″ E, 1255 m a.s.l./FEOW: 575 Southern Temperate Highveld/Habitat: river flood pool/Coll. date: 20 September 2012/10 ♀♀.
- Szwarc et al. [22]: Thamalakane river near the city of Maun, site no SA-97/coord. 19°55′52″ S, 23°30′38″ E, 940 m a.s.l./FEOW: 569 Okavango/Habitat: river side channel/Coll. date: 13 September 2012/1 ♀;
- Present paper: Kolobeng river near the village of Manyana, site no SA-113/coord. 24°46′08″ S, 25°35′22″ E, 1133 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: river/Coll. date: 20 September 2012/15 ♀♀, 46 juv;
- Present paper: Bathoen near the town of Kanye, site no SA-114/coord. 24°56′54″ S, 25°20′39″ E, 1255 m a.s.l./FEOW: 575 Southern Temperate Highveld/Habitat: river flood pool/Coll. date: 20 September 2012/5 ♀♀, 3 juv.
- Present paper: Tati river near the town of Francistown, site no SA-108/coord. 21°10′48″ S, 27°30′44″ E, 982 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: pools in riverbed/Coll. date: 18 September 2012/2 ♀♀;
- Present paper: Mahalapye river near the town of Mahalapye, site no SA-110/coord. 23°06′09″ S, 26°50′21″ E, 1006 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: small river dam/Coll. date: 18 September 2012/8 ♀♀, 2 juv;
- Present paper: Bathoen near the town of Kanye, site no SA-114/coord. 24°56′54″ S, 25°20′39″ E, 1255 m a.s.l./FEOW: 575 Southern Temperate Highveld/Habitat: river flood pool/Coll. date: 20 September 2012/1 ♀, 1 juv.
- Riedel et al. [35]: an outcrop on Kubu Island at the south-western edge of Sua Pan in Makgadikgadi Basin/coord. 20°53′29″ S, 25°51′14″ E, 901 m a.s.l./FEOW: 570 Kalahari/Habitat: palaeo-mega-lake system (sediments representing relics of the 8.5 ka B.P. last “mega-lake event”)/Coll. dates: September 2007, April 2008, July 2010/fossil valves;
- Riedel et al. [36]: sediment core 1.5 km east of Kubu Island at the south-western edge of Sua Pan in Makgadikgadi Basin/coord. 20°53′30″ S, 25°50′30″ E, 900–903 m a.s.l./FEOW: 570 Kalahari/Habitat: palaeo-mega-lake system (core to 3.0 m depth)/Coll. dates: September 2007, April 2008, July 2010/fossil valves in a sedimentary sequence dated from ca. 37 ka to 2 ka cal. BP.
- Present paper: Bathoen reservoir near the town of Kanye, site no SA-115/coord. 24°57′06″ S, 25°20′33″ E, 1267 m a.s.l./FEOW: 575 Southern Temperate Highveld/Habitat: reservoir/Coll. date: 20 September 2012/subfossil empty valves;
- Present paper: Moshenang Dam near the village of Kanye, site no SA-116/coord. 24°54′50″ S, 25°16′15″ E, 1282 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: reservoir/Coll. date: 20 September 2012/subfossil empty valves.
- Szwarc et al. [22]: Thamalakane river near the city of Maun, site no SA-97/coord. 19°55′52″ S, 23°30′38″ E, 940 m a.s.l./FEOW: 569 Okavango/Habitat: river side channel/Coll. date: 13 September 2012/8 ♀♀, 2 juv;
- Szwarc et al. [22]: North-West District, floodplains south of Okavango Delta near the city of Maun, site no SA-103/coord: 19°52′12″ S, 23°20′23″ E, 940 m a.s.l./FEOW: 569 Okavango/Habitat: grassy shore of temporary pond/Coll. date: 15 September 2012/1 ♀;
- Present paper: Shashe river near the village of Shashe, site no SA-109/coord. 21°23′20″ S, 27°27′20″ E, 956 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: pools in riverbed/Coll. date: 18 September 2012/10 ♀♀, 1 juv;
- Present paper: Mahalapye river near the town of Mahalapye, site no SA-110/coord. 23°06′09″ S, 26°50′21″ E, 1006 m a.s.l./FEOW: 576 Zambezian Lowveld/Habitat: small river dam/Coll. date: 18 September 2012/3 ♀♀;
- Present paper: Bathoen reservoir near the town of Kanye, site no SA-115/coord. 24°57′06″ S, 25°20′33″ E, 1267 m a.s.l./FEOW: 575 Southern Temperate Highveld/Habitat: reservoir/Coll. date: 20 September 2012/28 ♀♀.
- Riedel et al. [35] as Limnocythere thomasi–group: an outcrop on Kubu Island at the south-western edge of Sua Pan in Makgadikgadi Basin/coord. 20°53′309″ S, 25°49′00″ E, 908 m a.s.l./FEOW: 570 Kalahari/Habitat: palaeo-mega-lake system (sediments representing relics of the 8.5 ka B.P. last “mega-lake event”)/Coll. dates: September 2007, April 2008, July 2010/fossil valves;
- Riedel et al. [36]: sediment core 1.5 km east of Kubu Island at the south-western edge of Sua Pan in Makgadikgadi Basin/coord. 20°53′30″ S, 25°50′30″ E, 900–903 m a.s.l./FEOW: 570 Kalahari/Habitat: palaeo-mega-lake system (core to 3.0 m depth)/Coll. dates: September 2007, April 2008, July 2010/fossil valves in a sedimentary sequence dated from ca. 37 ka to 2 ka cal. BP.
- McCulloch et al. [21]: North Basin of Makgadikgadi Pan/approx. coord. 20°25′00″ S, 26°11′00″ E, 890 m a.s.l./FEOW: 570 Kalahari/Habitat: temporary salt pan/Coll. dates: December 1999–June 2001/number of specimens unspecified;
- McCulloch et al. [21]: Middle Basin of Makgadikgadi Pan/approx. coord. 20°41′00″ S, 26°06′00″ E, 904 m a.s.l./FEOW: 570 Kalahari/Habitat: temporary salt pan/Coll. dates: December 1999–June 2001/number of specimens unspecified;
- McCulloch et al. [21]: South Basin of Makgadikgadi Pan/approx. coord. 21°00′00″ S, 26°12′00″ E, 904 m a.s.l./FEOW: 570 Kalahari/Habitat: temporary salt pan/Coll. dates: December 1999–June 2001/number of specimens unspecified.
3.2. Biodiversity of Non-Marine Ostracods of Botswana
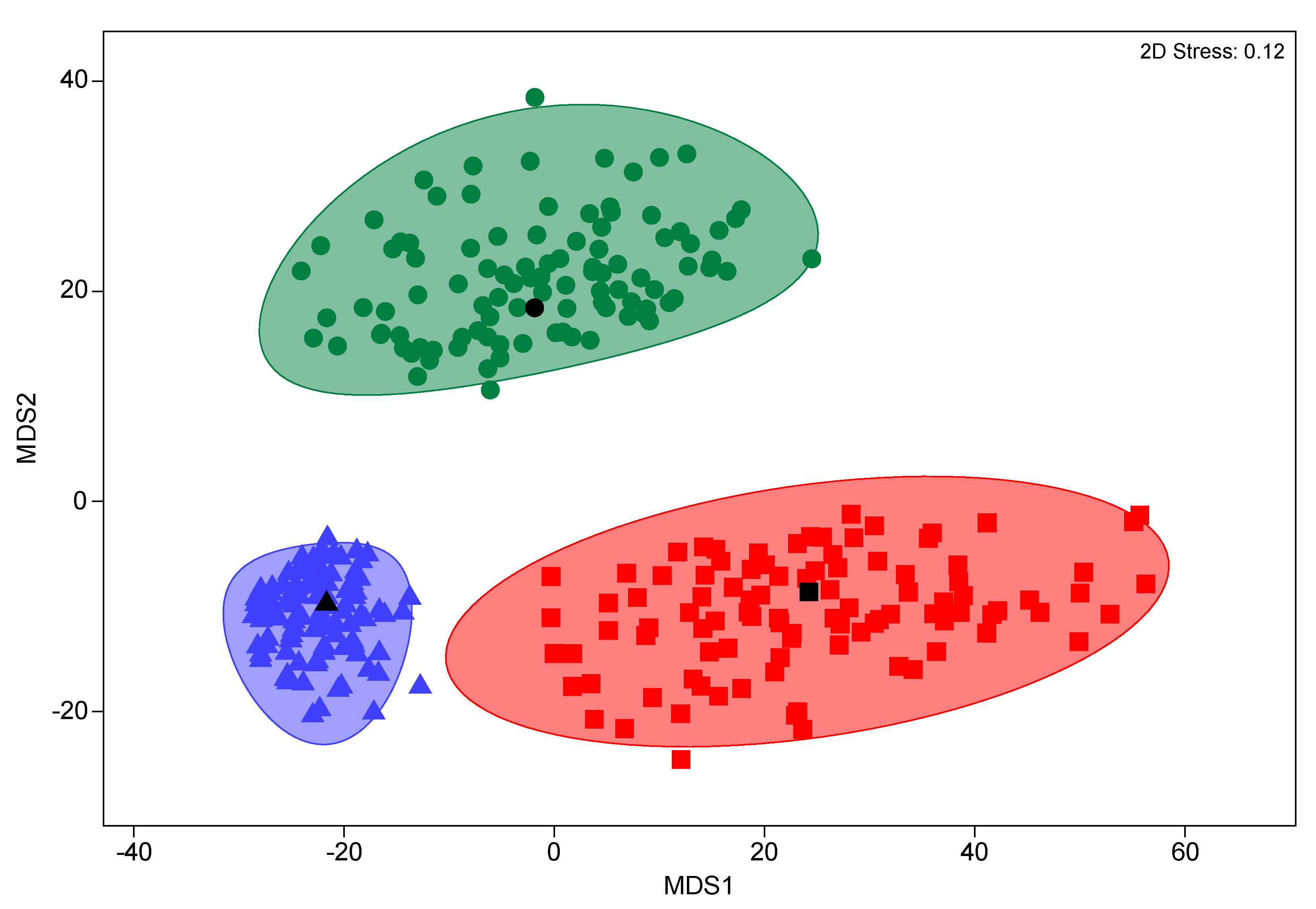
4. Discussion
Note
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Almond, R.E.A.; Grooten, M.; Petersen, T. WWF Living Planet Report 2020—Bending the Curve of Biodiversity Loss; WWF: Gland, Switzerland, 2020; p. 159. [Google Scholar]
- Tickner, D.; Opperman, J.J.; Abell, R.; Acreman, M.; Arthington, A.H.; Bunn, S.E.; Cooke, S.J.; Dalton, J.; Darwall, W.; Edwards, G.; et al. Bending the curve of global freshwater biodiversity loss: An emergency recovery plan. Bioscience 2020, 70, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.S.; Jenkins, D.G. Temporary aquatic habitats: Constraints and opportunities. Aquat. Ecol. 2000, 34, 3–8. [Google Scholar] [CrossRef]
- Williams, D.D. Temporary water crustaceans: Biodiversity and habitat loss. In Modern Approaches to the Study of Crustacea; Escobar-Briones, E., Alvarez, F., Eds.; Springer: Boston, MA, USA, 2002; pp. 223–233. [Google Scholar] [CrossRef]
- Williams, D.D. The Biology of Temporary Waters; University Press: Oxford, UK, 2006; p. 337. [Google Scholar] [CrossRef]
- Calhoun, A.J.K.; Mushet, D.M.; Bell, K.P.; Boix, D.; Fitzsimons, J.A.; Isselin-Nondedeu, F. Temporary wetlands: Challenges and solutions to conserving a ‘disappearing’ ecosystem. Biol. Conserv. 2017, 211, 3–11. [Google Scholar] [CrossRef]
- Harrison, I.; Abell, R.; Darwall, W.; Thieme, M.L.; Tickner, D.; Timboe, I. The freshwater biodiversity crisis. Science 2018, 362, 1369. [Google Scholar] [CrossRef]
- Thieme, M.L.; Abell, R.; Stiassny, M.L.J.; Skelton, P.H.; Lehner, B.; Teugels, G.G.; Dinerstein, E.; Toham, A.K.; Burgess, N.D.; Olson, D. Freshwater Ecoregions of Africa and Madagascar: A Conservation Assessment; Island Press: Washington, DC, USA, 2005; p. 431. [Google Scholar]
- Bird, M.S.; Mlambo, M.C.; Wasserman, R.J.; Dalu, T.; Holland, A.J.; Day, J.A.; Villet, M.H.; Bilton, D.T.; Barber-James, H.M.; Brendonck, L. Deeper knowledge of shallow waters: Reviewing the invertebrate fauna of southern African temporary wetlands. Hydrobiologia 2019, 827, 89–121. [Google Scholar] [CrossRef]
- Thomas, D.S.G.; Shaw, P.A. The Kalahari Environment; Cambridge University Press: Cambridge, UK, 1991; p. 284. [Google Scholar]
- Hughes, R.H.; Hughes, J.S. A Directory of African Wetlands; International Union for Conservation of Nature and Natural Resources (IUCN), Gland-Cambridge, United Nations Environment Programme (UNEP): Nairobi, Kenya; World Conservation Monitoring Centre (WCMC): Cambridge, UK, 1992; p. 820. [Google Scholar]
- UNEP. Africa Water Atlas; Division of Early Warning and Assessment (DEWA); United Nations Environment Programme (UNEP): Nairobi, Kenya, 2010; p. 314. [Google Scholar]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryger, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Contreras Balderas, S.; Bussing, W.; et al. Freshwater Ecoregions of the World: A new map of biogeographic units for freshwater biodiversity conservation. BioScience 2008, 58, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.J.; Horne, D.J.; Martens, K.; Schön, I. Class Ostracoda. In Ecology and General Biology. Thorp and Covich’s Freshwater Invertebrates; Thorp, J.H., Rogers, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume I, pp. 757–780. [Google Scholar] [CrossRef]
- McKenzie, K.G. Species list of South African freshwater Ostracoda with an appendix listing museum collections and some further determinations. Ann. S. Afr. Mus. 1971, 57, 147–213. [Google Scholar]
- Martens, K. Volume 3: Crustacea II. In Guides to the freshwater invertebrates of Southern Africa; Day, J.A., de Moor, I.J., Stewart, B.A., Louw, A.E., Eds.; Water Research Commission Report: Pretoria, South Africa, 2001; TT 148/01; pp. 9–77. [Google Scholar]
- Daday, E. Cladoceren und Ostracoden aus Süd- und Südwestafrika. Denkschr. Med.-Naturwiss. Ges. Jena 1913, 17, 89–102. [Google Scholar]
- Barnard, K.H. Scientific results of the Vernay-Lang Kalahari Expedition, March to September, 1930. Ann. Transvaal. Mus. 1935, 16, 481–492. [Google Scholar]
- Jocqué, M.; Martens, K.; Riddoch, B.; Brendonck, L. Faunistics of ephemeral rock pools in southeastern Botswana. Arch. Hydrobiol. 2006, 165, 415–431. [Google Scholar] [CrossRef]
- McCulloch, G.P.; Irvine, K.; Eckardt, F.D.; Bryant, R. Hydrochemical fluctuations and crustacean community composition in an ephemeral saline lake (Sua Pan, Makgadikgadi Botswana). Hydrobiologia 2008, 596, 31–46. [Google Scholar] [CrossRef]
- Szwarc, A.; Martens, K.; Namiotko, N. On two new non-marine species of the subfamily Cypridopsinae Kaufmann, 1900 (Crustacea, Ostracoda) from southern Africa. ZooKeys 2021, 1076, 83–107. [Google Scholar] [CrossRef] [PubMed]
- Meisch, C.; Smith, R.J.; Martens, K. A subjective global checklist of the extant non-marine Ostracoda (Crustacea). Eur. J. Taxon. 2019, 492, 1–135. [Google Scholar] [CrossRef] [Green Version]
- Martens, K. Taxonomic revision of African Cypridini. Part I: The genera Cypris O.F. Müller, Pseudocypris Daday and Globocypris Klie (Crustacea, Ostracoda). Bull. Inst. R. Sci. Nat. Belg. 1990, 60, 127–172. [Google Scholar]
- Namiotko, T.; Danielopol, D.L.; Baltanás, A. Soft body morphology, dissection and slide-preparation of Ostracoda: A primer. Joannea Geol. Paläontol. 2011, 11, 327–343. [Google Scholar]
- Sars, G.O. The fresh-water Entomostraca of Cape Province (Union of South Africa). Part 2. Ostracoda. Ann. S. Afr. Mus. 1924, 20, 105–193. [Google Scholar]
- Sars, G.O. The contributions to a knowledge of the fauna of South-West Africa (Union of South Africa). Part 3. Ann. S. Afr. Mus. 1924, 20, 194–211. [Google Scholar]
- Martens, K. Revision of African Limnocythere s.s. Brady, 1867 (Crustacea, Ostracoda), with special reference to the Rift Valley Lakes: Morphology, taxonomy, evolution and (palaeo) ecology. Arch. Hydrobiol. 1990, 4, 453–524. [Google Scholar]
- Meisch, C. Freshwater Ostracoda of Western and Central Europe; Spektrum Akademischer Verlag: Heidelberg, Germany, 2000; p. 522. [Google Scholar]
- Rumes, B. Regional Diversity, Ecology and Palaeoecology of Aquatic Invertebrate Communities in East African Lakes. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2010; p. 257. [Google Scholar]
- Savatenalinton, S.; Martens, K. Redescription of Hemicypris mizunoi Okubo, 1990 (Crustacea, Ostracoda) from Thailand, with a reassessment of the validity of the genera Hemicypris and Heterocypris. Bull. Inst. R. Sci. Nat. Belg. 2008, 78, 17–27. [Google Scholar]
- Martens, K. Seven new species and two new subspecies of Sclerocypris Sars, 1924 from Africa, with new records of some other Megalocypridinids (Crustacea, Ostracoda). Hydrobiologia 1988, 162, 243–273. [Google Scholar] [CrossRef]
- Seaman, M.T.; Ashton, P.J.; Williams, W.D. Inland salt waters of southern Africa. Hydrobiologia 1991, 210, 75–91. [Google Scholar] [CrossRef]
- Olszewski, P.; Bruhn-Olszewska, B.; Namiotko, L.; Sell, J.; Namiotko, T. Co-cultured non-marine ostracods from a temporary wetland harbor host-specific microbiota of different metabolic profiles. Hydrobiologia 2020, 847, 2503–2519. [Google Scholar] [CrossRef]
- Riedel, F.; Erhardt, S.; Chauke, C.; Kossler, A.; Shemang, E.; Tarasov, P. Evidence for a permanent lake in Sua Pan (Kalahari, Botswana) during the early centuries of the last millennium indicated by distribution of Baobab trees (Adansonia digitata) on “Kubu Island”. Quat. Int. 2012, 253, 67–73. [Google Scholar] [CrossRef]
- Riedel, F.; Henderson, A.C.G.; Heußner, K.U.; Kaufmann, G.; Kossler, A.; Leipe, C.; Shemang, E.; Taft, L. Dynamics of a Kalahari long-lived mega-lake system: Hydromorphological and limnological changes in the Makgadikgadi Basin (Botswana) during the terminal 50 ka. Hydrobiologia 2014, 739, 25–53. [Google Scholar] [CrossRef]
- Jocqué, M.; Brendonck, L.; Riddoch, B.J.; Martens, K. On Amphibolocypris arida sp.nov. (Crustacea, Ostracoda), from rock pools in Botswana (southern Africa). Zootaxa 2010, 2408, 47–58. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial, 1st ed.; PRIMER-E: Plymouth, UK, 2015; p. 296. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods, 1st ed.; PRIMER-E: Plymouth, UK, 2008; p. 214. [Google Scholar]
- Schultze, L. Aus Namaland und Kalahari; Gustav Fischer Verlag: Jena, Germany, 1907. [Google Scholar]
- Smith, R.J. The morphology of the upper lip of Cypridoidea ostracods: Taxonomic and phylogenetic significance. Hydrobiologia 2000, 418, 169–184. [Google Scholar] [CrossRef]
- Kempf, E. Juxilyocypris gen. nov. and replacement names for homonym species or genera of Ostracoda (Arthropoda: Crustacea). Munis Entomol. Zool. J. 2011, 6, 955–969. [Google Scholar]
- Hajek-Tadesse, V. Juxilyocypris kempfi, a new species of the genus Juxilocyprus (Ostracoda, Ilyocyprididae) from the Late Pleistocene-Holocene of Eastern Adriatic coast (Croatia). Hist. Biol. 2018, 32, 880–885. [Google Scholar] [CrossRef]
- Martens, K.; Davies, B.R.; Baxter, A.J.; Meadows, M.E. A contribution to the taxonomy and ecology of the Ostracoda (Crustacea) from Verlorenvlei (Western Cape, South Africa). S. Afr. J. Zool. 1996, 31, 23–36. [Google Scholar] [CrossRef]
- Curtis, B. Freshwater macro-invertebrates of Namibia. Madoqua 1991, 17, 163–187. [Google Scholar]
- Curtis, B.; Roberts, K.S.; Griffin, M.; Bethune, S.; Hay, C.J.; Kolberg, H. Species richness and conservation of Namibian freshwater macro-invertebrates, fish and amphibians. Biodivers. Conserv. 1998, 7, 447–466. [Google Scholar] [CrossRef]
- Martens, K. Annotated checklist of non-marine ostracods Crustacea, Ostracoda from African inland waters. Doc. Zool. Mus. Roy. Afr. Centr. 1984, 1–51. [Google Scholar]
- Siziba, N.; Chimbari, M.J.; Masundire, H.; Mosepele, K. Spatial and temporal variations of microinvertebrates across temporary floodplains of the lower Okavango Delta, Botswana. Phys. Chem. Earth. 2011, 36, 939–948. [Google Scholar] [CrossRef]
- Davidson, T.A.; Mackay, A.W.; Wolski, P.; Mazebedi, R.; Murray-Hudson, M.; Todd, M. Seasonal and spatial hydrological variability drives aquatic biodiversity in a flood-pulsed, sub-tropical wetland. Freshw. Biol. 2012, 57, 1253–1265. [Google Scholar] [CrossRef] [Green Version]
- Dallas, H.; Mosepele, B. Spatial variability of aquatic macroinvertebrate assemblages in the Okavango Delta, Botswana: Considerations for developing a rapid bioassessment tool. Afr. J. Aquat. Sci. 2020, 45, 350–363. [Google Scholar] [CrossRef]
- Alonso, L.E. ; Nordin, L-A. A Rapid Biological Assessment af the Aquatic Ecosystems of the Okavango Delta, Botswana: High Water Survey. RAP Bulletin of Biological Assessment 27; Conservation International Center for Applied Biodiversity Science, Department of Conservation Biology: Washington, DC, USA, 2003; p. 248. [Google Scholar]
- Kipping, J. The dragonflies and damselflies of Botswana—an annotated checklist with notes on distribution, phenology, habitats and Red List status of the species (Insecta: Odonata). Mauritiana 2010, 21, 126–204. [Google Scholar]
- Martens, K.; de Moor, F. The fate of the Rhino Ridge pool at Thomas Baines Nature Reserve: A cautionary tale for nature conservationists. S. Afr. J. Sci. 1995, 91, 385–387. [Google Scholar]
- West, D.T.; van As, L.L. Zooplankton composition of temporary pools within the lower Nata River channel, Botswana, during dry season. S. Afr. J. Sci. 2021, 46, 241–245. [Google Scholar] [CrossRef]
- Williams, W.D. Salnity as a determinant of the structure of biological communities in salt lakes. Hydrobiologia 1998, 381, 191–201. [Google Scholar] [CrossRef]
- Scheffer, M.; Van Geest, G.J.; Zimmer, K.; Jeppesen, E.; Søndergaard, M.; Butler, M.G.; Hanson, M.A.; Declerck, S.; De Meester, L. Small habitat size and isolation can promote species richness: Second-order effects on biodiversity in shallow lakes and ponds. Oikos 2006, 112, 227–231. [Google Scholar] [CrossRef]
- Timms, B.V. Drivers restricting biodiveristy in Australian saline lakes: A review. Mar. Freshw. Res. 2021, 72, 462–468. [Google Scholar] [CrossRef]
- Meyer-Milne, E.; Brendonck, L.; Pinceel, T. Egg banks in dryland wetlands provide information on the diversity and vulnerability of branchiopod communities along a longitudinal aridity gradient. Wetl. Ecol. Manag. 2022. [Google Scholar] [CrossRef]
- Vanschoenwinkel, B.; Waterkeyn, A.; Jocqué, M.; Boven, L.; Seaman, M.; Brendonck, L. Species sorting in space and time—the impact of disturbance regime on community assembly in a temporary rock pool metacommunity. J. North. Am. Benthol. Soc. 2010, 29, 1267–1278. [Google Scholar] [CrossRef]
- Echeverria-Galingo, P.; Anslan, S.; Frenzel, P.; Künzel, S.; Vences, M.; Pérez, L.; Börner, N.; Kang, W.; Schwarz, A.; Wang, J.; et al. High-throughput identification of non-marine Ostracoda from the Tibetan Plateau: Evaluating the success of various primers on sedimentary DNA samples. Environ. DNA. 2021, 3, 982–996. [Google Scholar] [CrossRef]
- Darwall, W.R.T.; Smith, K.G.; Tweddle, D.; Skelton, P. The Status and Distribution of Freshwater Biodiversity in Southern Africa; IUCN: Gland, Switzerland; SAIAB: Grahamstown, South Africa, 2009; p. viii+120. [Google Scholar]
- Vandekerkhove, J.; Martens, K.; Rossetti, G.; Mesquita-Joanes, F.; Namiotko, T. Extreme tolerance to environmental stress of sexual and parthenogenetic eggs of Eucypris virens (Crustacea, Ostracoda). Freshw. Biol. 2013, 58, 237–247. [Google Scholar] [CrossRef]
- Martins, M.J.F.; Vandekerkhove, J.; Namiotko, T. Environmental stability and the distribution of the sexes: Insights from life history experiments with the geographic parthenogen Eucypris virens (Crustacea, Ostracoda). Oikos 2008, 117, 829–836. [Google Scholar] [CrossRef]
- Brendonck, L.; Pinceel, T.; Ortells, R. Dormancy and dispersal as mediators of zooplankton population and community dynamics along a hydrological disturbance gradient in inland temporary pools. Hydrobiologia 2017, 796, 201–222. [Google Scholar] [CrossRef]
- Franchi, F.; Cavalazzi, B.; Evans, M.; Fillippidou, S.; Mackay, R.; Malaspina, P.; Mosekiemang, G.; Price, A.; Rossi, V. Late Pleistocene–Holocene palaeoenvironmental evolution of the Makgadikgadi Basin, Central Kalahari, Botswana: New evidence from shallow sediments and ostracod fauna. Front. Ecol. Evol. 2022, 10, 818417. [Google Scholar] [CrossRef]
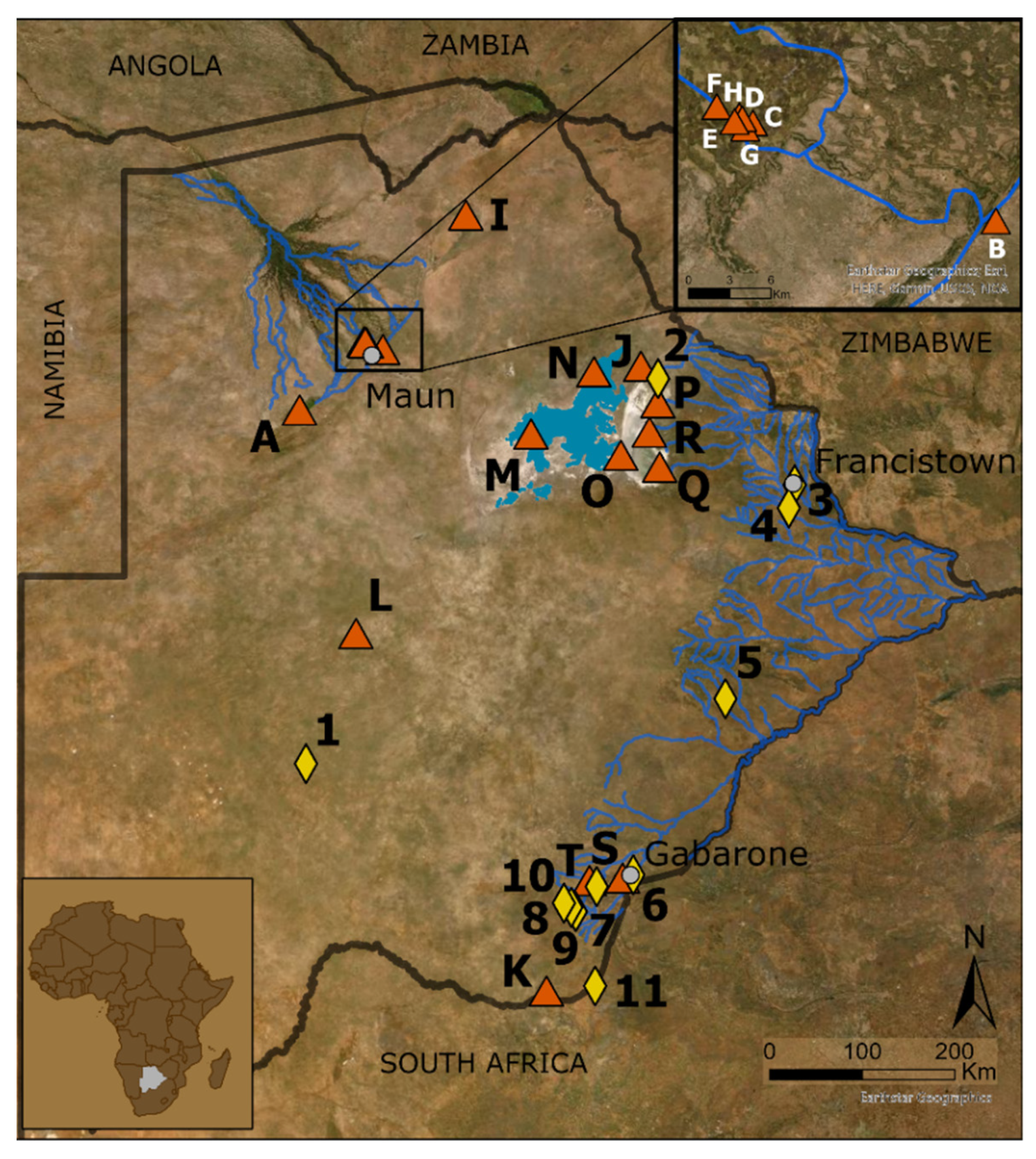

| No. | Species | References | Fossil/ Living | No. | Species | References | Fossil/Living |
|---|---|---|---|---|---|---|---|
| 1. | Amphibolocypris arida | [20,37] | L | 28. | *Oncocypris muelleri | X | SF |
| 2. | Apatelecypris schultzei | [19] ([16]) | L | 29. | Physocypria cf. capensis | [22], X | L |
| 3. | *Candonopsis nama | X | L | 30. | Plesiocypridopsis newtoni | [21,22], X | L |
| 4. | Candonopsis navicula | [22] | L | 31. | Potamocypris deflexa | [22] | L |
| 5. | Chrissia fascigera | [19] ([16]) | L | 32. | Potamocypris mastigophora | [19,22,34], X ([16]) | L |
| 6. | *Chrissia cf. pectinata | X | L | 33. | Potamocypris variegata | [36] | F |
| 7. | Chrissia cf. perarmata | [22] | L | 34. | Potamocypris new sp. ? | [21] | L |
| 8. | *Cypricercus cf. cuneatus | X | L | 35. | Potamocypris sp. | [20,37] | L |
| 9. | Cypricercus inermis | [20] | L | 36. | *Pseudocandona sp. | X | L |
| 10. | *Cypridopsis vidua | X | L | 37. | Pseudocypris circularis | [16,19,24] ([16]) | L |
| 11. | Hemicypris inversa | [18,22,41] ([16]) | L | 38. | Pseudocypris gibbera | [19,24] ([16]) | L |
| 12. | Hemicypris reticulata | [20] | L | 39. | Sarscypridopsis cf. elizabethae | [22] | L |
| 13. | Heterocypris giesbrechti | [22,31] | L | 40. | Sarscypridopsis glabrata | [36] | F |
| 14. | Heterocypris incongruens | [37] | L | 41. | Sarscypridopsis cf. gregaria | [20,37] | L |
| 15. | Heterocypris cf. incongruens | X | L | 42. | Sarscypridopsis harundineti | [22] | L |
| 16. | Heterocypris oblonga | [22] | L | 43. | *Sarscypridopsis cf. reniformis | X | L |
| 17. | Heterocypris ovularis | [19] ([16]) | L | 44. | Sarscypridopsis sp. | [20] | L |
| 18. | Heterocypris sp. | [20,37] | L | 45. | Sclerocypris clavularis | [20] | L |
| 19. | *Ilyocypris cf. gibba | X | L | 46. | Sclerocypris exserta makarikarensis | [21,32,33] | L |
| 20. | Ilyocypris sp. | [35,36] | F | 47. | Sclerocypris methueni | [34] | L |
| 21. | *Ilyocypris sp. n. | X | SF | 48. | Sclerocypris sp. | [22] | L |
| 22. | Isocypris cf. priomena | [22], X | L | 49. | Stenocypris malayica | [22] | L |
| 23. | Limnocythere inopinata | [36] | F | 50. | Strandesia cf. prava | [22], X | L |
| 24. | Limnocythere cf. stationis | [22], X | L | 51. | Strandesia n. sp. (gr. sudanica) | [20] | L |
| 25. | Limnocythere thomasi | [35,36] | F | 52. | Strandesia sp. | [36] | F |
| 26. | Limnocythere tudoranceai | [21,44] | L | 53. | Zonocypris costata | [22] | L |
| 27. | Limnocythere sp. | X | SF | 54. | Zonocypris tuberosa | [22] | L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwarc, A.; Namiotko, T. Biodiversity of Non-Marine Ostracoda (Crustacea) of Botswana: An Annotated Checklist with Notes on Distribution. Water 2022, 14, 1441. https://doi.org/10.3390/w14091441
Szwarc A, Namiotko T. Biodiversity of Non-Marine Ostracoda (Crustacea) of Botswana: An Annotated Checklist with Notes on Distribution. Water. 2022; 14(9):1441. https://doi.org/10.3390/w14091441
Chicago/Turabian StyleSzwarc, Agata, and Tadeusz Namiotko. 2022. "Biodiversity of Non-Marine Ostracoda (Crustacea) of Botswana: An Annotated Checklist with Notes on Distribution" Water 14, no. 9: 1441. https://doi.org/10.3390/w14091441
APA StyleSzwarc, A., & Namiotko, T. (2022). Biodiversity of Non-Marine Ostracoda (Crustacea) of Botswana: An Annotated Checklist with Notes on Distribution. Water, 14(9), 1441. https://doi.org/10.3390/w14091441







