Interception and Redistribution of Precipitation by Parkinsonia aculeata L.: Implications for Palo Verde National Park Wetlands, Costa Rica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Data Analysis
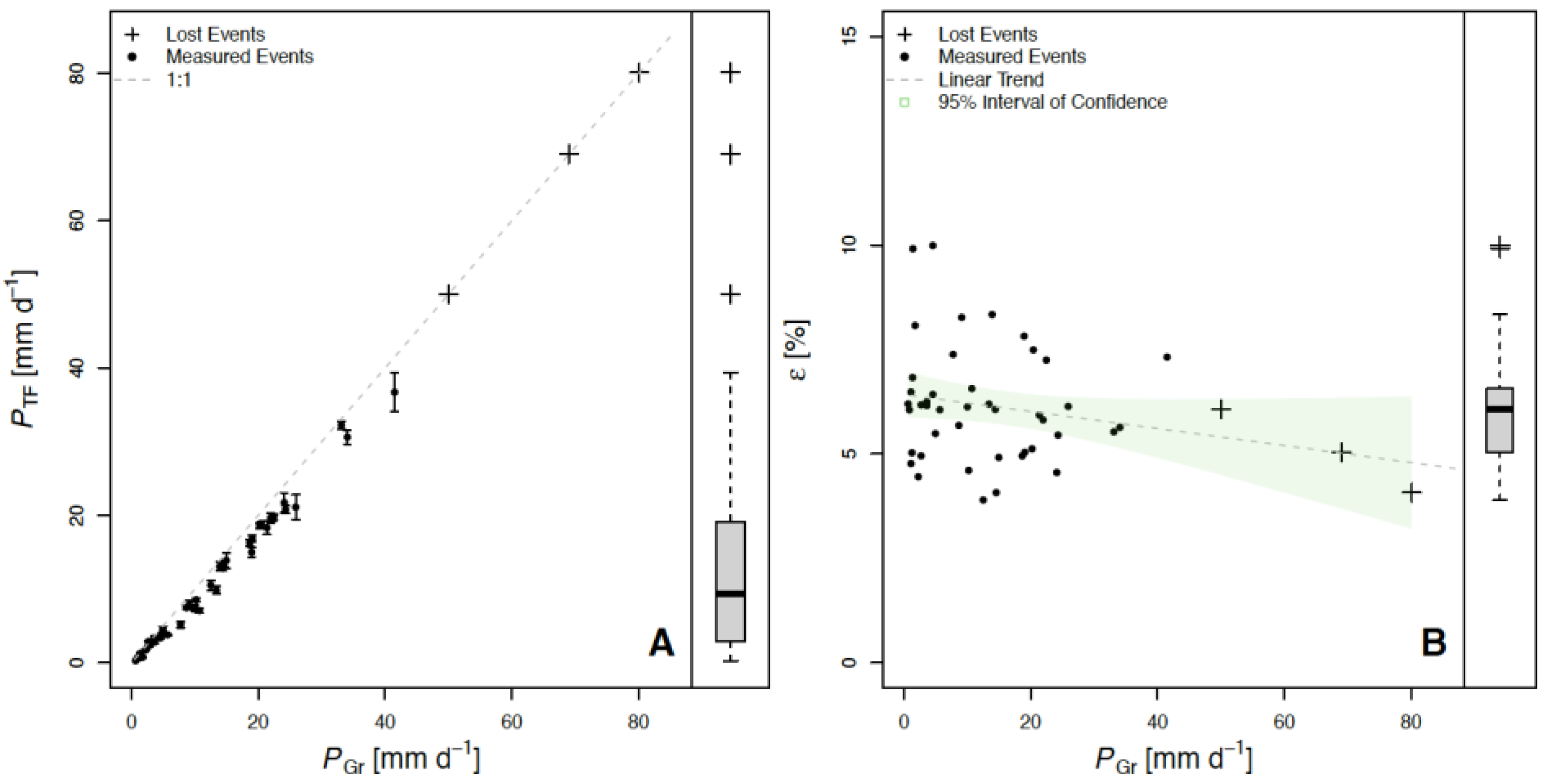
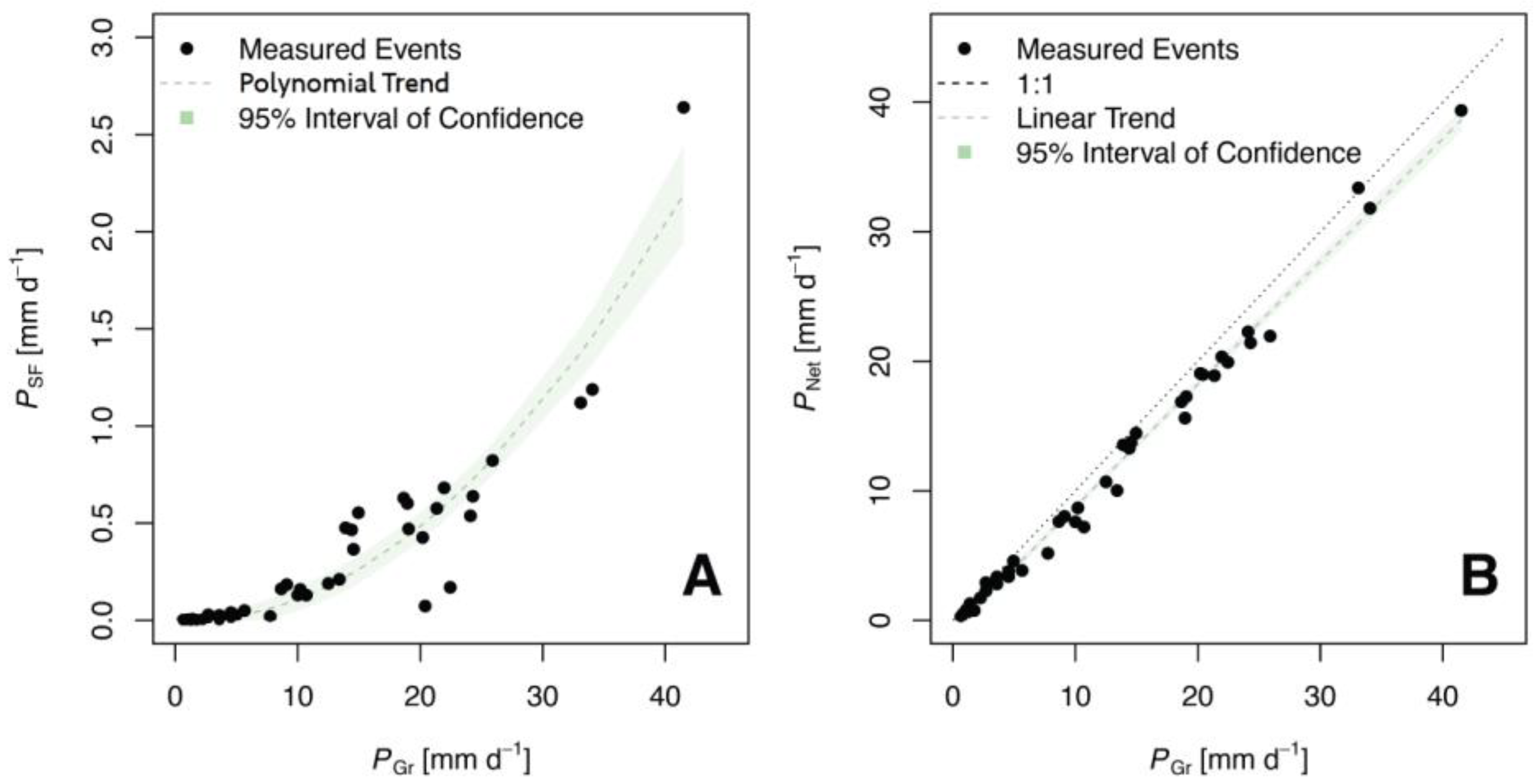
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quirós, R.G.; Solano, M.M.; Gamboa, E.J. Ramsar Sites Information Service: Ficha Técnica para Ampliación del Sitio Ramsar Palo Verde, Costa Rica. 2001. Available online: https://rsis.ramsar.org/RISapp/files/RISrep/CR540RIS.pdf (accessed on 1 April 2021).
- Mateo-Vega, J. Características generales de la Cuenca del Río Tempisque. In La cuenca del Río Tempisque: Perspectivas para el Manejo Integrado; Jiménez, J., González, E., Eds.; Organización para Estudios Tropicales: Durham, NC, USA, 2001; pp. 32–72. [Google Scholar]
- Osland, M.J.; González, E.; Richardson, C.J. Coastal Freshwater Wetland Plant Community Response to Seasonal Drought and Flooding in Northwestern Costa Rica. Wetlands 2011, 31, 641–652. [Google Scholar] [CrossRef]
- RAMSAR. Sitio Ramsar Parque Nacional Palo Verde Costa Rica: El Procedimiento de Orientación para la Gestión es un Mecanismo de Apoyo Técnico a Las Partes Contratantes de la Convención Sobre Los Humedales; Ramsar: Cham, Switzerland, 1998; p. 34. [Google Scholar]
- Calvo Alvarado, J.C.; Arias, O. Restauración hidrológica del humedal Palo Verde. Ambientico 2004, 129, 7–8. [Google Scholar]
- Castillo, M.; Guzman, J. Cambios en la cobertura vegetal en el humedal Palo Verde segun SIG. Ambientico 2004, 129, 46. [Google Scholar]
- Horn, S.; Kennedy, L. Pollen evidence of the prehistoric presence of cattail (Typha: Typhaceae) in Palo Verde National Park, Costa Rica. Brenesia 2006, 66, 85–87. [Google Scholar]
- Vaughan, C.; McCoy, M.; Fallas, J.; Barboza, G.; Wong, G.; Rau, J.; Chaves, H.; Carbonell, M. Plan de Manejo de Desarrollo Parque Nacional Palo Verde y Reserva Biológica Lomas Barbudal; Technical Report; Universidad Nacional: Heredia, Costa Rica, 1994. [Google Scholar]
- McCoy, M. The Seasonal, Freshwater Marsh at Palo Verde National Park. In Wetlands, Biodiversity and the Ramsar Convention; Hails, A., Ed.; RAMSAR Convention Bureau, Ministry of Environment and Forest: Amnedabad, India, 1996; Volume 133, p. 137. [Google Scholar]
- Trama, F.A. Manejo Activo y Restauración del Humedal Palo Verde: Cambios en Las Coberturas de Vegetación y Respuesta de Las Aves Acuáticas; Universidad Nacional de Costa Rica: Heredia, Costa Rica, 2005. [Google Scholar]
- Gallaher, C.; Stiles, C. Using Soils to Understand Ecosystem Change in Wetlands in Palo Verde National Park, Costa Rica. Limnetica 2003, 35, 131. [Google Scholar]
- Sasa, M.; Armengol Díaz, J.; Bonilla, F.; Mesquita Joanes, F.; Piculo, R.; Rojo García-Morato, C.; Rueda, R.; Monrós González, J.S. Seasonal wetlands in the Pacific coast of Costa Rica and Nicaragua: Environmental characterisation and conservation state. Limnetica 2015, 34, 116. [Google Scholar]
- Solano, J. Estudio de la distribución y la abundancia de Parkinsonia aculeata y Typha dominguensis en el humedal Palo Verde, Parque Nacional Palo Verde, Costa Rica. In Informe de Proyecto de Graduación Para Optar por el Grado de Bachiller en Ingeniería Forestal; Escuela de Ingeniería Forestal, Instituto Tecnológico de Costa Rica: Cartago, Costa Rica, 2004; p. 97. [Google Scholar]
- Stiles, F.G.; Smith, S.M. New Information on Costa Rican Waterbirds. Condor 1977, 79, 91–97. Available online: https://academic.oup.com/condor/articlepdf/79/1/91/28179608/condor0091.pdf (accessed on 15 November 2021). [CrossRef]
- Guzmán Alvarez, J. Effect of Land Cover Changes on the Water Balance of the Palo Verde Wetland, Costa Rica. Master’s Thesis, International Institute for Geo-Information Science and Earth Observation, Enschede, The Netherlands, 2007. [Google Scholar]
- Calvo Alvarado, J.C.; Arias, O. Estudio de Evapotraspiración de la Tifa (Typha domingensis) en el Parque Nacional Palo Verde, Guanacaste–Costa Rica. In Programa de Manejo Integrado de Recursos Naturales. Tecnológico de Costa Rica; Escuela de Ingeniería Forestal: Cartago, Costa Rica, 2006; pp. 1–15. [Google Scholar]
- Jiménez-Rodríguez, C.D.; Esquivel-Vargas, C.; Coenders-Gerrits, M.; Sasa-Marín, M. Quantification of the Evaporation Rates from Six Types ofWetland Cover in Palo Verde National Park, Costa Rica. Water 2019, 11, 674. [Google Scholar] [CrossRef] [Green Version]
- CABI. Parkinsonia aculeata (Mexican palo-verde). In Invasive Species Compendium; CAB International: Wallingford, UK, 2021. [Google Scholar]
- Francis, J.K. Parkinsonia aculeata L. In Wildland Shrubs of the United States and Its Territories: Thamnic Descriptions; Gen. Tech. Rep. IITF-GTR-26; Francis, J.K., Ed.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2004; pp. 529–530. [Google Scholar] [CrossRef]
- van Klinken, R.D.; Campbell, S.D.; Heard, T.A.; McKenzie, J.; March, N. The Biology of Australian Weeds: 54. ‘Parkinsonia aculeata’ L. Plant Prot. Q. 2009, 24, 100–117. [Google Scholar] [CrossRef]
- Adhikari, A.; White, J.D. Plant water use characteristics of five dominant shrub species of the Lower Rio Grande Valley, Texas, USA: Implications for shrubland restoration and conservation. Conserv. Physiol. 2014, 2, cou005. [Google Scholar] [CrossRef]
- van Klinken, R.D.; Heard, T.A. Parkinsonia aculeata L.—parkinsonia. In Biological Control of Weeds in Australia; Cullen, J., Julien, M., McFadyen, R., Eds.; CSIRO Publishing: Clayton, Australia, 2012; pp. 437–447. [Google Scholar] [CrossRef]
- Aselmann, I.; Crutzen, P.J. Global distribution of natural freshwater wetlands and rice paddies, their net primary productivity, seasonality and possible methane emissions. J. Atmos. Chem. 1989, 8, 307–358. [Google Scholar] [CrossRef]
- Davidson, T.A.; Mackay, A.W.; Wolski, P.; Mazebbedi, R.; Murray-Hudson, M.; Todd, M. Seasonal and spatial hydrological variability drives aquatic biodiversity in a flood-pulsed, sub-tropical wetland. Freshw. Biol. 2012, 57, 1253–1265. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1365-2427.2012.02795.x (accessed on 15 November 2021). [CrossRef] [Green Version]
- Goodwin, E.J. Convention on Wetlands of International Importance, Especially asWaterfowl Habitat 1971 (Ramsar). In Elgar Encyclopedia of Environmental Law; Edward Elgar Publishing Limited: Cheltenham, UK, 2017; pp. 101–108. [Google Scholar]
- Calvo-Obando, A.; Ibarra-Vargas, A. Caracterización Topográfica y Estimación de la Biomasa, Carbono y Dióxido de Carbono Equivalente, Mediante el Uso de Sensores Remotos, en la Laguna Palo Verde, Parque Nacional Palo Verde, Guanacaste, Costa Rica; Universidad de Costa Rica: San Jose, Costa Rica, 2021. [Google Scholar]
- Hartshorn, G. Plants. In Costa Rican Natural History; Janzen, D., Ed.; The University of Chicago Press: Chicago, IL, USA, 1983; pp. 118–157. [Google Scholar]
- Staff, S.S. Illustrated Guide to Soil Taxonomy, 12th ed.; U.S. Department of Agriculture, Natural Resources Conservation Service, National Soil Survey Center: Lincoln, NE, USA; USDA—Natural Resources Conservation Service: Washington, DC, USA, 2014; p. 372.
- Holdridge, L.R. Life Zone Ecology; Tropical Science Center: San Jose, Costa Rica, 1967; p. 206. [Google Scholar]
- Skau, C.M. Interception, Throughfall, and Stemflow in Utah and Alligator Juniper Cover Types of Northern Arizona. For. Sci. 1964, 10, 283–287. Available online: https://academic.oup.com/forestscience/article-pdf/10/3/283/23083742/forestscience0283.pdf (accessed on 15 November 2021). [CrossRef]
- Tobón Marin, C.; Bouten, W.; Sevink, J. Gross rainfall and its partitioning into throughfall, stemflow and evaporation of intercepted water in four forest ecosystems in western Amazonia. J. Hydrol. 2000, 237, 40–57. [Google Scholar] [CrossRef]
- Loescher, H.W.; Powers, J.S.; Oberbauer, S.F. Spatial Variation of Throughfall Volume in an Old-Growth Tropical Wet Forest, Costa Rica. J. Trop. Ecol. 2002, 18, 397–407. [Google Scholar] [CrossRef]
- Jiménez-Rodríguez, C.; Calvo-Alvarado, J. An evaluation of rainfall interception in secondary tropical dry forests. In Tropical Dry Forests in the Americas; Sanchez-Azofeifa, A., Powers, J.S., Fernandes, G.W., Quesada, M., Eds.; CRC Press: Boca Raton, FL, USA, 2013; Chapter 14; pp. 249–266. [Google Scholar]
- Klamerus-Iwan, A.; Link, T.E.; Keim, R.F.; Van Stan II, J.T. Storage and Routing of Precipitation Through Canopies. In Precipitation Partitioning by Vegetation: A Global Synthesis; Van Stan, J.T., II, Gutmann, E., Friesen, J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 17–34. [Google Scholar] [CrossRef]
- Ahmed, A.; Tomar, J.; Mehta, H.; Alam, N.; Chaturvedi, O. Influence of canopy architecture on stemflow in agroforestry trees in Western Himalayas. Curr. Sci. 2015, 109, 759–764. [Google Scholar]
- Herwitz, S.R. Interception storage capacities of tropical rainforest canopy trees. J. Hydrol. 1985, 77, 237–252. [Google Scholar] [CrossRef]
- Hölscher, D.; Köhler, L.; van Dijk, A.I.; Bruijnzeel, L. The importance of epiphytes to total rainfall interception by a tropical montane rain forest in Costa Rica. J. Hydrol. 2004, 292, 308–322. [Google Scholar] [CrossRef]
- Llorens, P.; Gallart, F. A simplified method for forest water storage capacity measurement. J. Hydrol. 2000, 240, 131–144. [Google Scholar] [CrossRef]
- Fathizadeh, O.; Attarod, P.; Pypker, T.G.; Darvishsefat, A.A.; Zahedi Amiri, G.A. Seasonal Variability of Rainfall Interception and Canopy Storage Capacity Measured under Individual Oak (Quercus brantii) Trees in Western Iran. J. Agric. Sci. Technol. 2013, 15, 175–188. Available online: http://jast.modares.ac.ir/article-23-4073-en.pdf (accessed on 15 November 2021).
- Klamerus-Iwan, A.; Błońska, E. Canopy storage capacity and wettability of leaves and needles: The effect of water temperature changes. J. Hydrol. 2018, 559, 534–540. [Google Scholar] [CrossRef]
- Návar, J.; Bryan, R. Interception loss and rainfall redistribution by three semi–arid growing shrubs in northeastern Mexico. J. Hydrol. 1990, 115, 51–63. [Google Scholar] [CrossRef]
- Tamez-Ponce, C.; Cantú-Silva, I.; González-Rodríguez, H.; Yáñez-Díaz, M.I.; Uvalle-Sauceda, J.I. Pérdidas por intercepción en cuatro especies de matorral en el noreste de México. Rev. Mex. Cienc. For. 2018, 9, 126–147. [Google Scholar] [CrossRef]
- Venkatraman, K.; Ashwath, N. Canopy Rainfall Intercepted by Nineteen Tree Species Grown on a Phytocapped Landfill. Int. J. Waste Resour. 2016, 6, 1000202. [Google Scholar] [CrossRef]
- Spencer, S.A.; van Meerveld, H.J. Double funnelling in a mature coastal British Columbia forest: Spatial patterns of stemflow after infiltration. Hydrol. Processes 2016, 30, 4185–4201. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1002/hyp.10936 (accessed on 15 November 2021). [CrossRef]

| Subplot | Trees per Plot | Tree Density (tree ha−1) | Basal Area (m2 ha−1) | Tree Diameter (cm) | Tree Height (m) |
|---|---|---|---|---|---|
| 1 | 9 | 900 | 9.09 | 11.34 | 4.6 |
| 2 | 32 | 3200 | 4.66 | 4.31 | 3.95 |
| 3 | 40 | 4000 | 6.44 | 4.53 | 3.84 |
| Whole Plot | 27 | 2700 | 9.59 | 6.73 | 4.13 |
| 2003 Forest Survey [13] | 16 | 1600 | 8.42 | 8.12 | 3.88 |
| Subplot | PTF (mm) | PSF (mm) | PNet (mm) | PI (mm) | PTF (%) | PSF (%) | PNet (%) | PI (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 471.8 | 3.5 | 475.2 | 40.9 | 91.40 | 0.68 | 92.07 | 7.93 |
| 2 | 450.2 | 16.7 | 466.9 | 49.3 | 87.21 | 3.24 | 90.45 | 9.55 |
| 3 | 452.0 | 20.6 | 472.6 | 43.6 | 87.57 | 3.99 | 91.56 | 8.44 |
| Whole Plot | 458.0 | 13.6 | 471.6 | 44.6 | 88.73 | 2.63 | 91.36 | 8.64 |
| Water Fluxes | n | Regression Parameters | Regression Coefficients | |||||
|---|---|---|---|---|---|---|---|---|
| R2adj | F | p | SE | |||||
| PTF | 43 | 0.99 | 5336 | 0.000 | 0.83 | −0.5346 * | 0.9318 ** | |
| PSF | 43 | 0.89 | 240 | 0.000 | 0.16 | 0.0014 ** | −0.0023 ** | |
| PNet | 43 | 0.97 | 9675 | 0.000 | 0.97 | −0.7074 ** | 0.9475 ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Alvarado, J.C.; Jiménez-Rodríguez, C.D.; Solano, J.C.; Arias-Rodríguez, O. Interception and Redistribution of Precipitation by Parkinsonia aculeata L.: Implications for Palo Verde National Park Wetlands, Costa Rica. Water 2022, 14, 311. https://doi.org/10.3390/w14030311
Calvo-Alvarado JC, Jiménez-Rodríguez CD, Solano JC, Arias-Rodríguez O. Interception and Redistribution of Precipitation by Parkinsonia aculeata L.: Implications for Palo Verde National Park Wetlands, Costa Rica. Water. 2022; 14(3):311. https://doi.org/10.3390/w14030311
Chicago/Turabian StyleCalvo-Alvarado, Julio César, César Dionisio Jiménez-Rodríguez, Juan Carlos Solano, and Oscar Arias-Rodríguez. 2022. "Interception and Redistribution of Precipitation by Parkinsonia aculeata L.: Implications for Palo Verde National Park Wetlands, Costa Rica" Water 14, no. 3: 311. https://doi.org/10.3390/w14030311
APA StyleCalvo-Alvarado, J. C., Jiménez-Rodríguez, C. D., Solano, J. C., & Arias-Rodríguez, O. (2022). Interception and Redistribution of Precipitation by Parkinsonia aculeata L.: Implications for Palo Verde National Park Wetlands, Costa Rica. Water, 14(3), 311. https://doi.org/10.3390/w14030311






