Hydrogeochemical Characteristics of Karst Areas: A Case Study of Dongzhuang Reservoir Area in Jinghe River
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Methods
- (1)
- ICP-AES
- (2)
- Silver nitrate titration
- (3)
- EDTA titration
- (4)
- Acid–base titration
- (5)
- Glass electrode method
- (6)
- Gravimetric method
3. Results and Discussion
3.1. Characteristics of Hydrogeochemical Components in Different Water Bodies
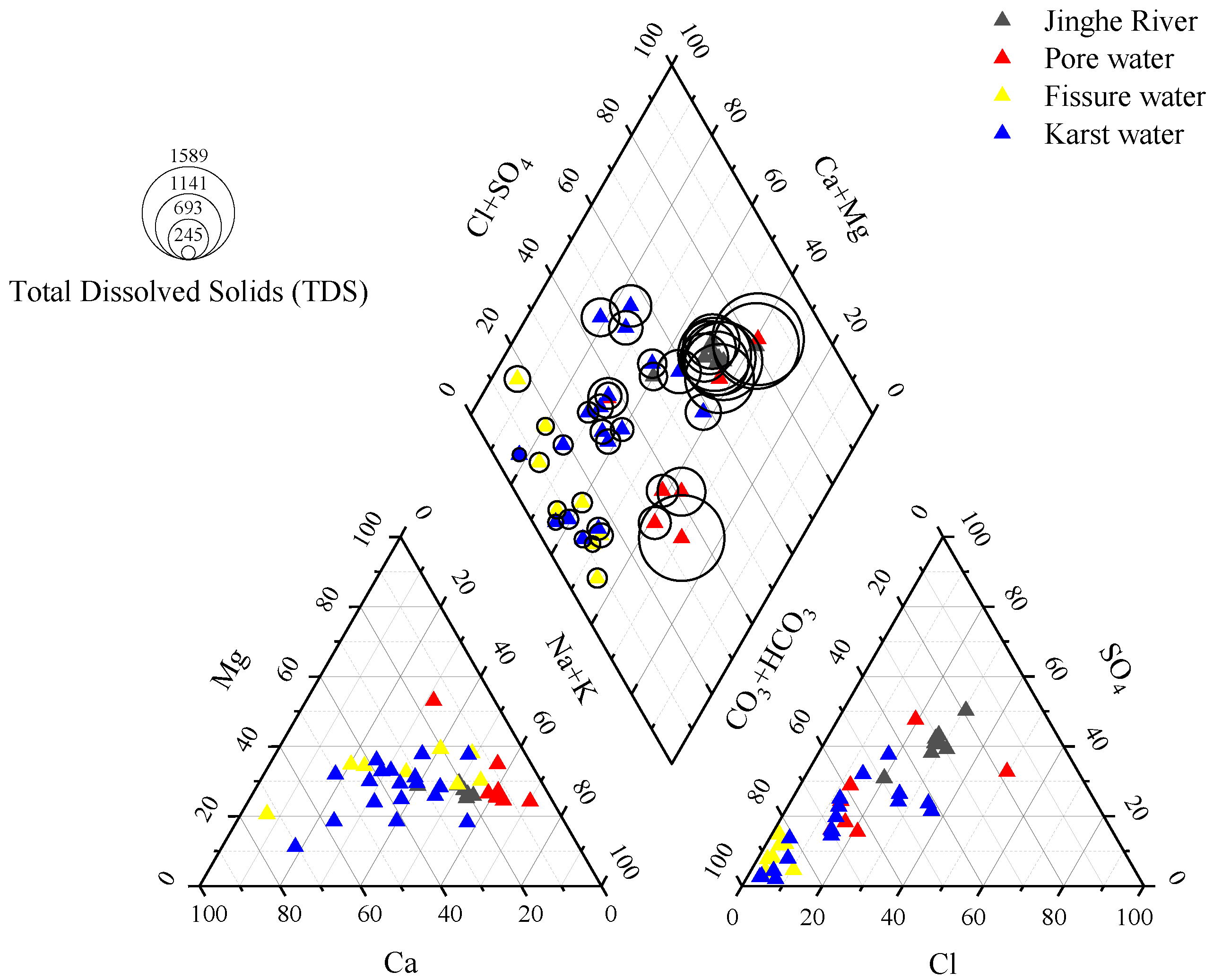
3.2. Characteristics of Hydrogeochemical Types in Different Water Bodies
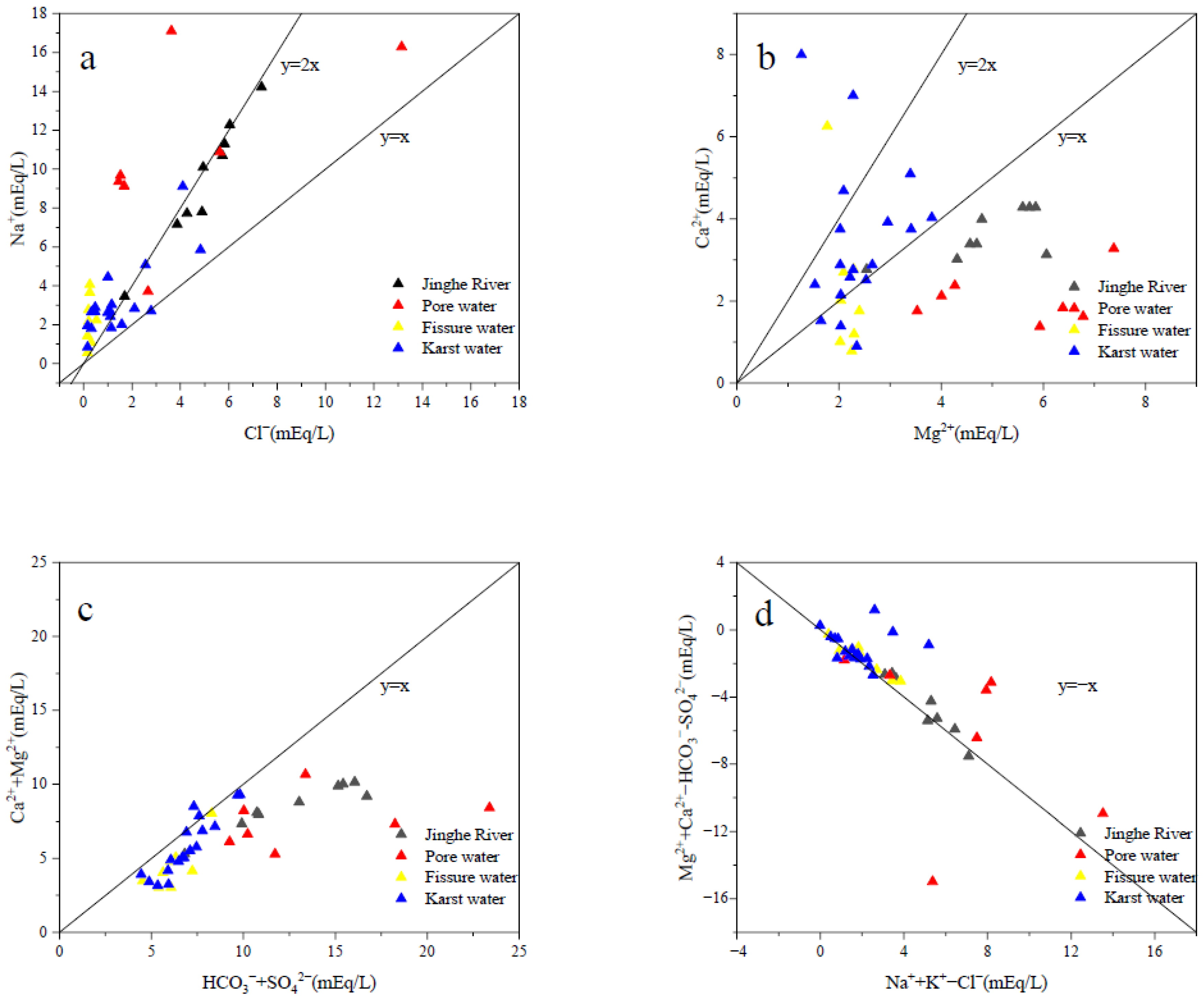
3.3. Hydrogeochemical Origin of Different Water Bodies
3.4. Hydraulic Connection of Different Water Bodies
4. Conclusions
- (1)
- In general, Jinghe River and pore water are weakly alkaline, with high TDS value (brackish water). Na+ and K+ are the main cations in Jinghe River, while Cl− and SO42− are the main anions. HCO3− and SO42− are the main anions of pore water, fissure water and karst water, and the content of cations is different, indicating that the formation mechanism of the hydrochemistry is complex.
- (2)
- There are many hydrogeochemical types of water bodies in the study area. Jinghe River and some pore water are mainly subjected to evaporation-concentration. Fissure water and karst water are mainly subjected to rock-weathering. The main source of Ca2+ and Mg2+ in karst water and fissure water should be the weathering dissolution of carbonate minerals. The main reason for the unstable distribution of cations is that the water body in the study area has undergone different degrees of cation exchange.
- (3)
- According to the hydrogeochemical origin and cluster analysis, the chemical composition of the Jinghe River is obviously different from the hydrogeochemical composition of the fissure water and karst water in the area. It can be inferred that the Jinghe River has hydraulic connection with pore water, but there is almost no hydraulic connection between Jinghe River and the regional karst water. This shows that it is difficult for the Jinghe River to recharge karst groundwater through leakage. However, the specific hydraulic recharge and discharge relationship between water bodies needs to be further analyzed by means of runoff characteristics and isotope chemistry.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ren, A.W.; Ke, B.R.; Cheng, J.S.; Sun, P.; Duan, Q.W.; Shao, Y. Barrier effect of covered sheet pile wharfs with diaphragm walls. J. Hydraul. Eng. 2014, 45 (Suppl. 2), 119–124. [Google Scholar] [CrossRef]
- Teng, Y.G.; Zuo, R.; Wang, J.S.; Lin, X.Y. Progress in geochemistry of regional groundwater evolution. Adv. Water Sci. 2010, 21, 127–136. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, X.W.; Wan, L.; Han, G.L.; Guo, H.M. Hydrogeochemical characterization of groundwater flow systems in the discharge area of a river basin. J. Hydrol. 2015, 527, 433–441. [Google Scholar] [CrossRef]
- Guo, X.; Wang, H.W.; Shi, J.S.; Wang, W. Hydrochemical characteristics and evolution pattern of groundwater system in Baiyangdian wetland, North China Plain. Acta Geol. Sin. 2022, 96, 656–672. [Google Scholar] [CrossRef]
- Chen, B.; Su, W.C. Analysis on the Karst Seepage of Maguan Underground Reservoir. Earth Environ. 2005, 33, 237–241. [Google Scholar] [CrossRef]
- Tang, J. Research on the Karst Developing Characteristics and Seepage in Dam Area of Charikou Hydropower Station. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2014. [Google Scholar]
- Tong, J. Research on Hydrogeological Conditions of Leakage in Qianwei Hydropower Station Dam Site. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2011. [Google Scholar]
- Dai, Z.H.; Zhu, Y.H.; Lu, P.; Li, Z.S.; Zhao, X.R. Development characteristics and leakage of atypical concealed karst: Taking Daxueshan reservoir as an example. J. Guilin Univ. Technol. 2021, 41, 518–524. [Google Scholar] [CrossRef]
- Mao, C.P.; Yang, Z.Y.; Li, G.K.; Zhang, L.; Rao, W.B. Major ion chemistry of Pumped Storage Power Station Reservoir: Controlling mechanisms and tracing implications for Reservoir Water Leakage. J. Hohai Univ. Nat. Sci. 2022. Available online: https://kns.cnki.net/kcms/detail/32.1117.TV.20220527.1850.004.html (accessed on 4 December 2022).
- Wan, W.F.; Wang, Q.W.; Zou, J.F.; Miao, W. Discussion on Several Key Problems about Karst Leakage of Dongzhuang Reservoir. Yellow River 2015, 37, 99–103. [Google Scholar] [CrossRef]
- Lei, C.R.; Song, X.L.; Wang, Q.W. Research on Impact of Underground Water Environment of Dongzhuang Reservoir of Jinghe River. Yellow River 2018, 40, 97–99. [Google Scholar] [CrossRef]
- Wang, Y.W. The Karst Leakage Study on Jinghe Dongzhuang Reservoir. Ph.D. Thesis, China University of Geosciences (Beijing), Beijing, China, 2014. [Google Scholar]
- Du, P.Z.; Lei, C.R.; Gao, P. Research on Controlling Factors and Development Rules of Karst in Carbonate Rocks of Dongzhuang Reservoir. J. N. China Univ. Water Resour. Electr. Power Nat. Sci. Ed. 2018, 39, 88–92. [Google Scholar] [CrossRef]
- Zhao, R.; Zuo, S.Y.; Li, H.; Zhang, Y. Characteristics of Sedimentary Facies of Lower Paleozoic Ordovician Reservoir in Weibei Dongzhuang Area. J. Guizhou Univ. Nat. Sci. 2015, 32, 43–48. [Google Scholar] [CrossRef]
- Li, Q.B.; Wan, W.F.; Liu, J.L.; Zhou, Y.M. Study on Karst Leakage of Dongzhuang Reservoir in Jinghe River; China Water & Power Press: Beijing, China, 2021; pp. 45–47. [Google Scholar]
- Reddy, A.G.; Kumar, K.N. Identification of the Hydrogeo-Chemical Processes in Groundwater Using Major Ion Chemistry: A Case Study of Penna-Chitravathi River Basins in Southern India. Environ. Monit. Assess. 2010, 170, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, O.; Larbi, D.; Rihab, H. Geochemical characterization of groundwater from shallow aquifer surrounding Fetzara Lake N.E. Algeria. Arab. J. Geosci. 2012, 5, 1–13. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, J.L.; Jin, M.G. Hydrochemical Characteristics and Formation Causes of Karst Water in Jinan Spring Catchment. Earth Sci. 2016, 42, 821–831. [Google Scholar] [CrossRef]
- Han, Y.P.; Zhang, D.Q.; Liu, Z.P. Study on the Hydrochemical Characteristics of Groundwater in the People’s Victory Canal Irrigation Area. Yellow River 2018, 40, 85–90. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Liang, X.; Jin, M.G.; Wan, L.; Yu, Q.C. General Hydrogeology, 3rd ed.; Geology Press: Beijing, China, 2010; pp. 54–69. [Google Scholar]
- Du, Q.H.; Qu, J.H.; Song, Q.X. An Analysis of Chemical Characteristics and Causes of Groundwater near the Yellow River in Kaifeng City. China Rural Water Hydropower 2020, 62, 172–176. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Y.Z. An Analysis of the Ions Ratio Coefficients of Groundwater in Shenzhou Area. China Rural Water Hydropower 2014, 56, 18–24. [Google Scholar] [CrossRef]
- Han, Y.; Bian, J.M.; Gu, T.X.; Tian, X.P.; Fang, Z. Hydrochemical Parameter Characteristics and Evolution Laws of Groundwater in Songyuan City. J. Jilin Agric. Univ. 2016, 38, 175–181. [Google Scholar] [CrossRef]



| Test Items | Test Method | Apparatus (Model) | Test Specification |
|---|---|---|---|
| Na+ | ICP-AES | ICP-AES (optima2100DV) | GB/T5750.6-2006(CN) |
| K+ | ICP-AES | ICP-AES (optima2100DV) | GB/T5750.6-2006(CN) |
| Ca2+ | ICP-AES | ICP-AES (optima2100DV) | GB/T5750.6-2006(CN) |
| Mg2+ | ICP-AES | ICP-AES (optima2100DV) | GB/T5750.6-2006(CN) |
| Cl− | Silver nitrate titration | Burette | DZ/T0064.50-1993(CN) |
| SO42− | EDTA titration | Burette | DZ/T0064.64-1993(CN) |
| HCO3− | Acid–base titration | Burette | DZ/T0064.49-1993(CN) |
| pH | Glass electrode method | Ionometer (PXSJ-226) | DZ/T0064.5-1993(CN) |
| TDS | Gravimetric method | Electronic balance (AE160) | DZ/T0064.9-1993(CN) |
| Water Body | Statistics | Na+ (mg/L) | K+ (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | Cl− (mg/L) | SO42− (mg/L) | HCO3− (mg/L) | Ph (−) | TDS (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| Jinghe River | Minimum | 19.39 | 3.01 | 55.31 | 30.50 | 60.26 | 126.32 | 255.06 | 8.10 | 498.6 |
| Maximum | 326.80 | 9.45 | 85.57 | 72.78 | 260.91 | 581.06 | 396.63 | 8.30 | 1483.3 | |
| Mean | 216.43 | 6.33 | 72.23 | 58.89 | 176.03 | 357.01 | 323.74 | 8.14 | 1080.0 | |
| CV 1 | 0.01 | 0.29 | 0.17 | 0.22 | 0.32 | 0.37 | 0.18 | 0.29 | 0.28 | |
| Pore water | Minimum | 85.22 | 0.91 | 27.45 | 42.40 | 51.44 | 83.41 | 286.79 | 7.80 | 570.00 |
| Maximum | 393.30 | 7.28 | 65.53 | 88.57 | 466.52 | 663.44 | 869.54 | 8.30 | 1589.72 | |
| Mean | 250.11 | 3.01 | 40.98 | 65.64 | 150.59 | 253.14 | 517.06 | 7.97 | 996.24 | |
| CV 1 | 0.42 | 0.76 | 0.31 | 0.28 | 0.99 | 0.84 | 0.35 | 0.02 | 0.43 | |
| Fissure water | Minimum | 12.64 | 0.21 | 15.55 | 21.26 | 5.67 | 11.32 | 262.14 | 7.30 | 294.00 |
| Maximum | 93.22 | 6.21 | 125.05 | 28.80 | 18.84 | 60.04 | 428.97 | 8.40 | 469.31 | |
| Mean | 51.51 | 3.20 | 46.14 | 25.74 | 9.26 | 27.50 | 342.47 | 7.91 | 363.81 | |
| CV 1 | 0.54 | 0.74 | 0.76 | 0.09 | 0.46 | 0.63 | 0.15 | 0.04 | 0.17 | |
| Karst water | Minimum | 19.21 | 0.59 | 17.85 | 15.19 | 5.67 | 6.24 | 263.00 | 7.30 | 249.34 |
| Maximum | 209.50 | 7.05 | 159.92 | 45.81 | 171.58 | 215.65 | 369.78 | 8.30 | 764.09 | |
| Mean | 73.27 | 2.96 | 69.03 | 28.36 | 52.06 | 80.27 | 320.36 | 7.70 | 470.38 | |
| CV 1 | 0.60 | 0.66 | 0.54 | 0.28 | 0.91 | 0.77 | 0.08 | 0.03 | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhan, J.; Wan, W.; Wang, J. Hydrogeochemical Characteristics of Karst Areas: A Case Study of Dongzhuang Reservoir Area in Jinghe River. Water 2022, 14, 4111. https://doi.org/10.3390/w14244111
Zhang H, Zhan J, Wan W, Wang J. Hydrogeochemical Characteristics of Karst Areas: A Case Study of Dongzhuang Reservoir Area in Jinghe River. Water. 2022; 14(24):4111. https://doi.org/10.3390/w14244111
Chicago/Turabian StyleZhang, Haifeng, Jiang Zhan, Weifeng Wan, and Junzhi Wang. 2022. "Hydrogeochemical Characteristics of Karst Areas: A Case Study of Dongzhuang Reservoir Area in Jinghe River" Water 14, no. 24: 4111. https://doi.org/10.3390/w14244111
APA StyleZhang, H., Zhan, J., Wan, W., & Wang, J. (2022). Hydrogeochemical Characteristics of Karst Areas: A Case Study of Dongzhuang Reservoir Area in Jinghe River. Water, 14(24), 4111. https://doi.org/10.3390/w14244111






