Genesis Mechanisms of Geothermal Resources in Mangkang Geothermal Field, Tibet, China: Evidence from Hydrochemical Characteristics of Geothermal Water
Abstract
:1. Introduction
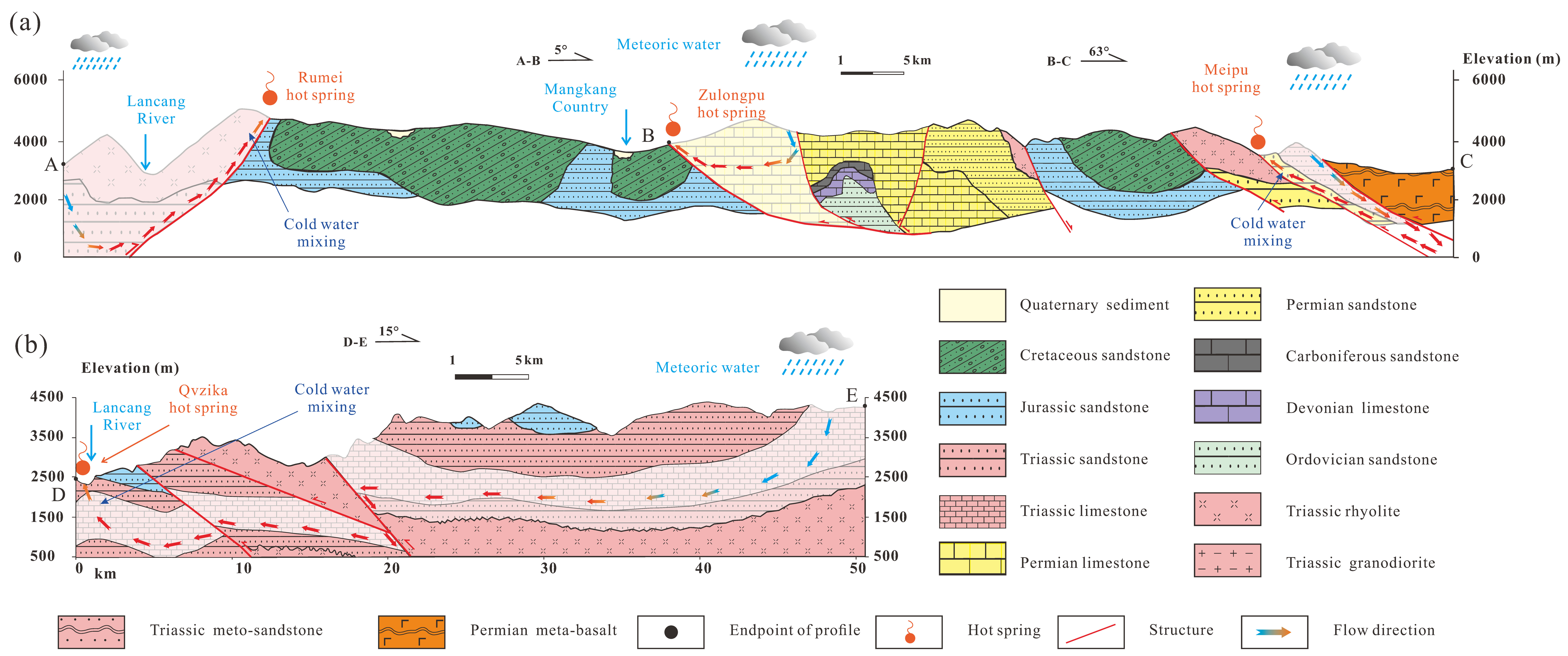
2. Geological Setting
3. Sampling and Methods
4. Results and Discussion
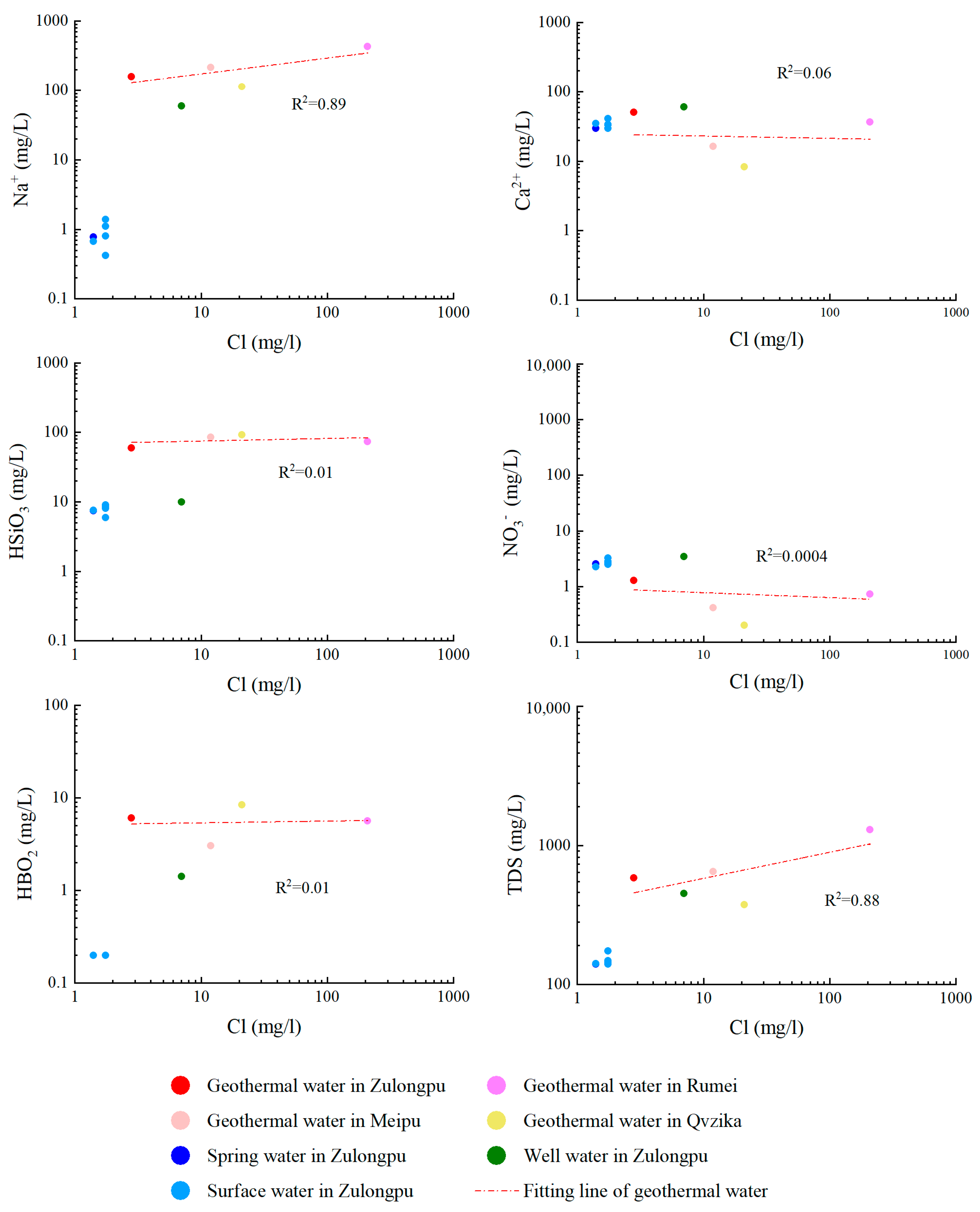
4.1. Water Geochemistry
4.2. Geothermal Reservoir Temperature
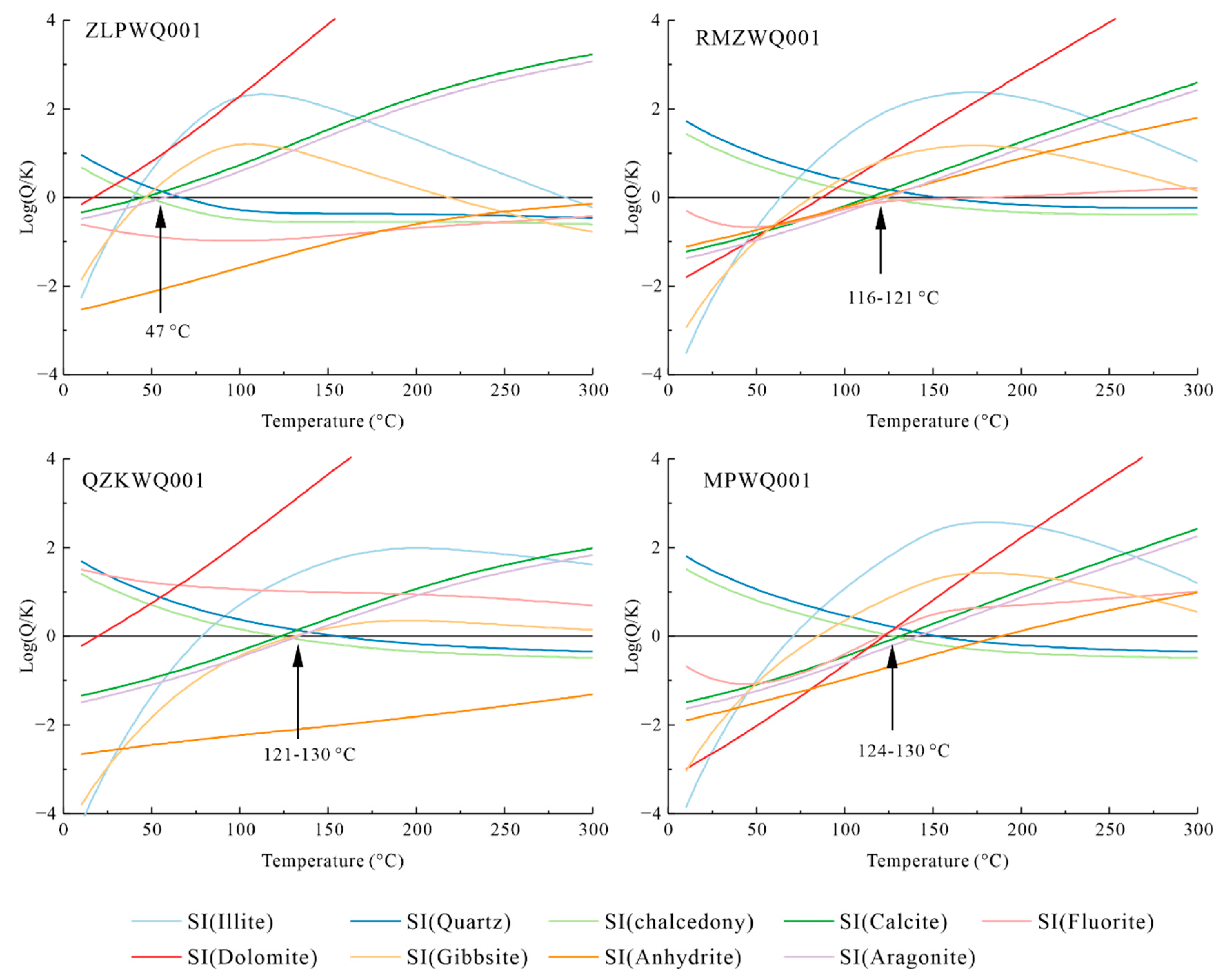
4.2.1. Mineral–Water Equilibrium Geothermometers
4.2.2. Chemical Geothermometers
Silica Geothermometers
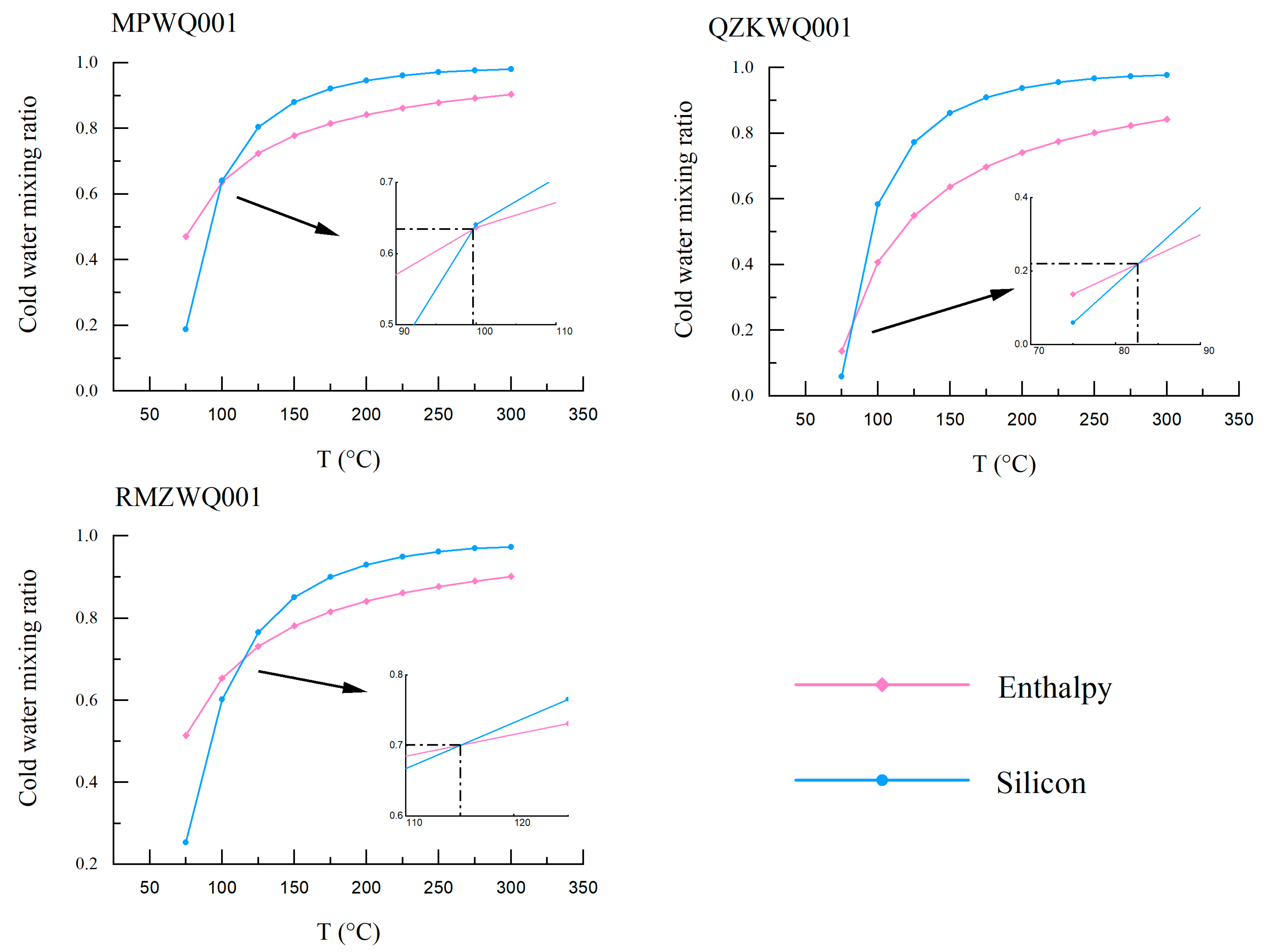
Silica-Enthalpy Model
Cation Geothermometers
4.3. Thermal Circulation Depth
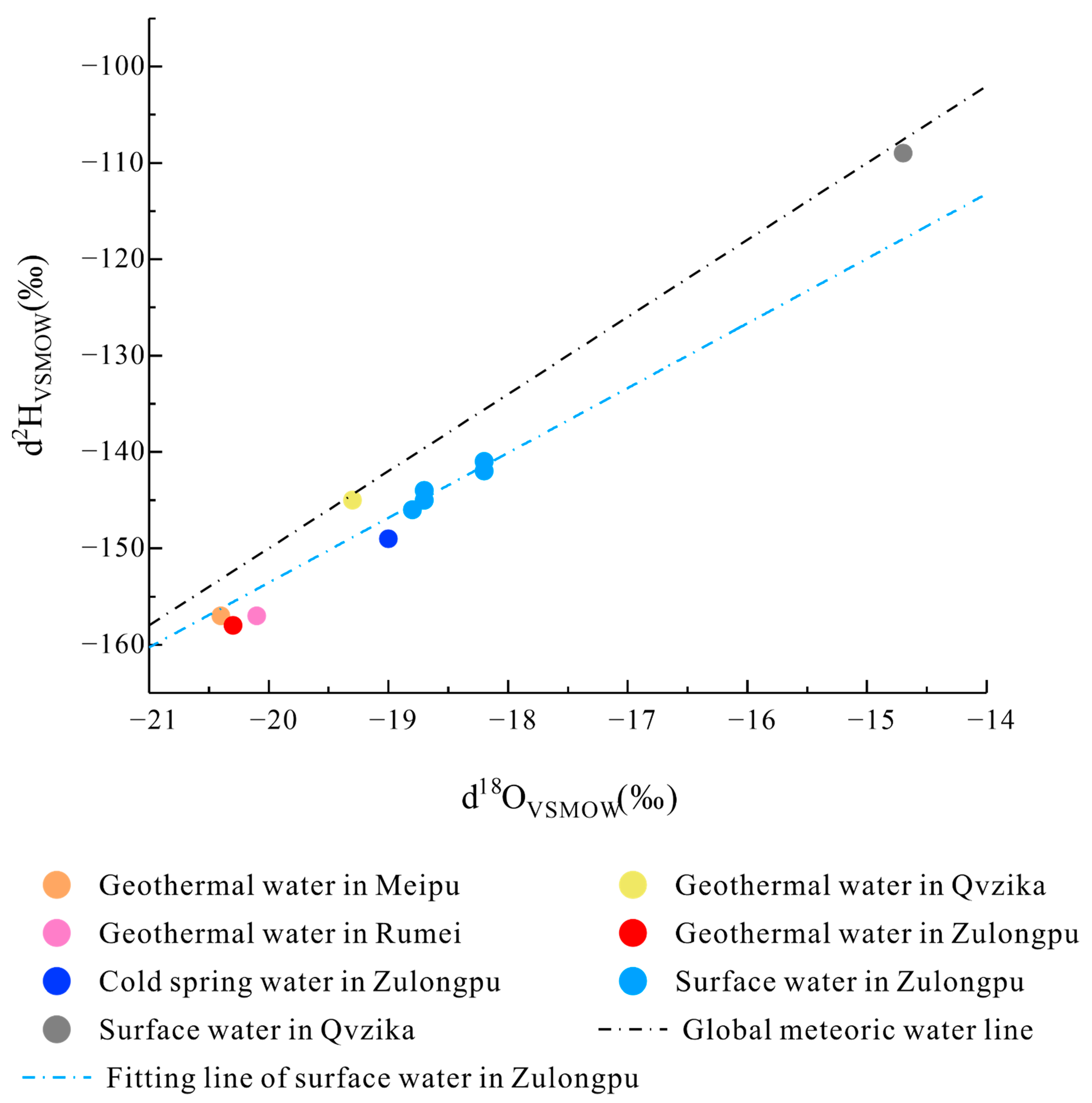
4.4. 2H and 18O Values
4.5. Water-Rock Interactions
4.6. Conceptual Model
5. Conclusions
- (1)
- The geothermal water in the Mangkang geothermal field has a geothermal type of HCO3·(Cl)-Na, while the surface water in the geothermal field is of the HCO3-Ca·Mg type. The high HBO2 and Na+ concentrations of the geothermal water indicate long water runoff and strong water–rock interactions.
- (2)
- Based on mineral–water solubility equilibrium, the hot springs in Meipu village, Qvzika village, Rumei town, and Zulongpu village have reservoir temperatures of 123–130 °C, 121–130 °C, 116–121 °C, and 47 °C, respectively. The silica-enthalpy model revealed that the geothermal water in Meipu village and Rumei town mixes with cold water, with mixing ratios of 63% and 70%, respectively.
- (3)
- As jointly determined by the geothermal gradients, annual temperature, and reservoir temperature, the circulation depth of the geothermal water in Meipu village, Qvzika village, Rumei town, and Zulongpu village is 3600–4300 m, 3900–4300 m, 3700–4000 m, and 1500 m, respectively.
- (4)
- The 2H and 18O isotopic analyses revealed the meteoric origin of the geothermal water. Based on the isotopic composition, the geothermal water in Meipu village, Rumei town, and Zulongpu village was found to share very similar recharge elevations (47000–4900 m), while the geothermal water in Qvzika village has the lowest recharge elevation (4400 m).
- (5)
- The hydrochemical composition of geothermal water varied with the precipitation of chalcedony and dolomite, the absorption of NaX, and the loss of CaX2.
- (6)
- This study comprehensively analyzed the hydrogeochemical characteristics, temperature, and depth of geothermal reservoirs and the water–rock interaction process in the Mangkang geothermal field in Tibet, China. The conceptual model established based on the analytical results can promote the understanding of the genesis of deep geothermal resources in Tibet. Moreover, this study may serve as a guide for future drilling in the Mangkang geothermal field.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, W.; Wang, G.; Gan, H.; Wang, A.; Yue, G.; Long, X. Heat Generation and Accumulation for Hot Dry Rock Resources in the Igneous Rock Distribution Areas of Southeastern China. Lithosphere 2022, 2021, 2039112. [Google Scholar] [CrossRef]
- Lin, W.; Wang, G.; Zhang, S.; Zhao, Z.; Xing, L.; Gan, H.; Tan, X. Heat Aggregation Mechanisms of Hot Dry Rocks Resources in the Gonghe Basin, Northeastern Tibetan Plateau. Acta Geol. Sin. 2021, 95, 1793–1804. [Google Scholar] [CrossRef]
- Liu, Y.; Long, X.; Liu, F. Tracer Test and Design Optimization of Doublet System of Carbonate Geothermal Reservoirs. Geothermics 2022, 105, 102533. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, Y.; Liu, F.; Wei, S.; Duan, H. Lithospheric Thermal Structure in Jinggangshan City: Implications for High Geothermal Background. Front. Earth Sci. 2022, 10, 854232. [Google Scholar] [CrossRef]
- Yu-fei, X.; Ya-bo, Z.; Yuen, D. Geothermal Structure Revealed by Curie Isothermal Surface under Guangdong Province, China. J. Groundw. Sci. Eng. 2021, 9, 114. [Google Scholar] [CrossRef]
- Wang, G.L.; Li, J.; Wu, A.M.; Zhang, W.; Hu, Q.Y. A Study of the Thermal Storage Characteristics of Gaoyuzhuang Formation, a New Layer System of Thermal Reservoir in Rongcheng Uplift Area, Hebei Province. Acta Geosci. Sin. 2018, 39, 533–541. [Google Scholar]
- Wang, G.L.; Lin, W.J. Main Hydro-Geothermal Systems and Their Genetic Models in China. Acta Geosci. Sin. 2020, 94, 1923–1937. [Google Scholar]
- Wang, G.L.; Zhang, W.; Liang, J.Y.; Lin, W.J.; Liu, Z.M.; Wang, W.L. Evaluation of Geothermal Resources Potential in China. Acta Geosci. Sin. 2017, 38, 449–459. [Google Scholar]
- Liu, F.; Wang, G.L.; Zhang, W.; Chen, Y.; Tao, L. Using Tough2 Numerical Simulation to Analyse the Geothermal Formation in Guide Basin, China. J. Groundw. Sci. 2020, 8, 328–337. [Google Scholar] [CrossRef]
- Feng, M.; Gui-ling, W.; Hong-li, S.; Zhan-xue, S. Indication of Hydrogen and Oxygen Stable Isotopes on the Characteristics and Circulation Patterns of Medium-Low Temperature Geothermal Resources in the Guanzhong Basin, China. J. Groundw. Sci. 2022, 10, 70–86. [Google Scholar] [CrossRef]
- Zhou, H.L.; Zhou, X.C.; Su, H.J.; Li, Y.; Liu, F.L.; Ouyang, S.P.; Yan, Y.C.; Bai, R.L. Hydrochemical Characteristics of Earthquake-Related Thermal Springs Along the Weixi-Qiaohou Fault, Southeast Tibet Plateau. Water 2022, 14, 132. [Google Scholar] [CrossRef]
- Qi, J.; Li, X.; Xu, M.; Yi, L.; Zhang, Q.; Qin, L.; Li, K. Origin of Saline Springs in Yanjing, Tibet: Hydrochemical and Isotopic Characteristics. Appl. Geochem. 2018, 96, 164–176. [Google Scholar] [CrossRef]
- Wang, S.Q.; Lu, C.; Nan, D.; Hu, X.C.; Shao, J.L. Geothermal Resources in Tibet of China: Current Status and Prospective Development. Environ. Earth Sci. 2017, 76, 239. [Google Scholar] [CrossRef]
- Zhu, B.; Zhu, L. Geochemical Exploration of Geothermal Fields; Geological Publishing House: Beijing, China, 1992. [Google Scholar]
- Guo, Q.H.; Wang, Y.X.; Liu, W. Hydrogeochemistry and Environmental Impact of Geothermal Waters from Yangyi of Tibet, China. J. Volcanol. Geotherm. Res. 2009, 180, 9–20. [Google Scholar] [CrossRef]
- Guo, Q.H. Hydrogeochemistry of High-Temperature Geothermal Systems in China: A Review. Appl. Geochem. 2012, 27, 1887–1898. [Google Scholar] [CrossRef]
- Tong, W. Geothermals beneath Xizang (Debit) Plateau; Science Press: Beijing, China, 1981. [Google Scholar]
- Wang, C.G.; Zheng, M.P. Hydrochemical Characteristics and Evolution of Hot Fluids in the Gudui Geothermal Field in Comei County, Himalayas. Geothermics 2019, 81, 243–258. [Google Scholar] [CrossRef]
- Tan, H.B.; Zhang, Y.F.; Zhang, W.J.; Kong, N.; Zhang, Q.; Huang, J.Z. Understanding the Circulation of Geothermal Waters in the Tibetan Plateau Using Oxygen and Hydrogen Stable Isotopes. Appl. Geochem. 2014, 51, 23–32. [Google Scholar] [CrossRef]
- Wang, Y.C.; Gu, H.Y.; Li, D.; Lyu, M.; Lu, L.H.; Zuo, Y.H.; Song, R.C. Hydrochemical Characteristics and Genesis Analysis of Geothermal Fluid in the Zhaxikang Geothermal Field in Cuona County, Southern Tibet. Environ. Earth Sci. 2021, 80, 415. [Google Scholar] [CrossRef]
- Wang, S. Hydrogeochemical Processes and Genesis Machenism of High-Temperature Geothermal System in Gudui, Tibet. Ph.D Thesis, China University of Geosciences (Beijing), Beijing, China, 2017. [Google Scholar]
- Qi, J.; Xv, M.; Zhang, Q.; Qing, L.; Jiang, K.; Qian, L. An Analysis of the Hydrogeochemical Characteristics for the Hot Underwater in Yanjing of Tibet, China. J. Chendu Univ. Technol. 2008, 35, 580–585. [Google Scholar]
- Qi, J.; Xu, M.; Zhang, Q.; Qin, L. Research on Circulation Mode for Salt Spring by Isotope Tracing in Yanjin, Tibet. Earth Environ. 2008, 36, 237–244. [Google Scholar]
- Wang, X.; Wang, G.L.; Gan, H.N.; Liu, Z.; Nan, D.W. Hydrochemical Characteristics and Evolution of Geothermal Fluids in the Chabu High-Temperature Geothermal System, Southern Tibet. Geofluids 2018, 2018, 8532840. [Google Scholar] [CrossRef] [Green Version]
- Özen, T.; Bülbül, A.; Tarcan, G. Reservoir and Hydrogeochemical Characterizations of Geothermal Fields in Salihli, Turkey. J. Asian Earth Sci. 2012, 60, 1–17. [Google Scholar] [CrossRef]
- Sun, Z.; Li, X. Studies of Geothermal Waters in Jiangxi Province Using Isotope Techniques. Sci. China Ser. E 2001, 44, 144–150. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, G.; Xing, L.; Li, T.; Zhao, J. Geochemical Response of Deep Geothermal Processes in the Litang Region, Western Sichuan. Energy Explor. Exploit. 2019, 37, 626–645. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, L.; Liu, P.; Li, C.; Zhou, Y.; Li, Y.; Xie, H.; Xiang, T. Hydrogeochemical Evolution Mechanism of Carbonate Geothermal Water in Southwest China. Arab. J. Geosci. 2021, 14, 1310. [Google Scholar] [CrossRef]
- Bozau, E.; Häußler, S.; van Berk, W. Hydrogeochemical Modelling of Corrosion Effects and Barite Scaling in Deep Geothermal Wells of the North German Basin Using Phreeqc and Phast. Geothermics 2015, 53, 540–547. [Google Scholar] [CrossRef]
- Alsemgeest, J.; Auqué, L.F.; Gimeno, M.J. Verification and Comparison of Two Thermodynamic Databases through Conversion to Phreeqc and Multicomponent Geothermometrical Calculations. Geothermics 2021, 91, 102036. [Google Scholar] [CrossRef]
- Zuo, Q.; Ye, T.; Feng, Y.; Ge, Z.; Wang, Y. Spatial Database of Serial Suite-Tectonic Map-Sheets of Mainland China (1:250,000). Geol. China 2018, 45, 1–26. [Google Scholar] [CrossRef]
- Tian, Y.; Yu, C.Q.; Luo, K.L.; Zha, X.J.; Wu, J.S.; Zhang, X.Z.; Ni, R.X. Hydrochemical Characteristics and Element Contents of Natural Waters in Tibet, China. J. Geogr. Sci. 2015, 25, 669–686. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, Y.; Liu, F.; Lu, C.; Zhao, G. Genetic Mechanism of Geothermal Resources in the Qutan and Reshuizhou Geothermal Fields, Jiangxi Province, China: Evidence from Hydrogeochemical Characteristics of Geothermal Water. Lithosphere 2022, 2021, 9778217. [Google Scholar] [CrossRef]
- Lang, X.; Lin, W.; Liu, Z.; Xing, L.; Wang, G. Hydrochemical Characteristics of Geothermal Wate in Guide Basin. Earth Sci. 2016, 41, 1723–1734. [Google Scholar]
- Mondal, N.C.; Singh, V.P.; Singh, V.S.; Saxena, V.K. Determining the Interaction between Groundwater and Saline Water through Groundwater Major Ions Chemistry. J. Hydrol. 2010, 388, 100–111. [Google Scholar] [CrossRef]
- Luo, B. The Chemical Characteristics and Geological Significance of the Formation Water of Mesozoic in Zhenjing Area of Ordos Basin. Inn. Mong. Petrochem. Ind. 2014, 5, 36–39. [Google Scholar]
- Hao, Y. Study on Chemistry Characteristic of Groundwater and Formation Mechanism in Akesu Area. Master’s Thesis, Tarim University, Aral Shehri, China, 2010. [Google Scholar]
- Pang, Z.-H.; Reed, M. Theoretical Chemical Thermometry on Geothermal Waters: Problems and Methods. Geochim. Cosmochim. Acta 1998, 62, 1083–1091. [Google Scholar] [CrossRef]
- Ren, Z. A Study of the Characteristics and Genesis of the Hot Spring and Salt Springs in the Langping Area of Yunan. Master’s Thesis, China Univerisity of Geosciences, Beijing, China, 2016. [Google Scholar]
- Spycher, N.; Peiffer, L.; Sonnenthal, E.L.; Saldi, G.; Reed, M.H.; Kennedy, B.M. Integrated Multicomponent Solute Geothermometry. Geothermics 2014, 51, 113–123. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, G.; Wang, X.; Qi, S.; Ma, F.; Zhang, W.; Zhang, H. Hydrogeochemical and Isotopic Analyses of Deep Geothermal Fluids in the Wumishan Formation in Xiong’an New Area, China. Lithosphere 2022, 2021, 2576752. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, W.; Zhang, H.; Qu, Z.; Li, M.; Yue, G. Hydrogeochemical Characteristics and Genesis of the Geothermal Fields in Batang of Sichuan. Hydrogeol. Engenerring Geol. 2019, 46, 81–89. [Google Scholar]
- Reed, M.; Spycher, N. Calculation of Ph and Mineral Equilibria in Hydrothermal Waters with Application to Geothermometry and Studies of Boiling and Dilution. Geochim. Cosmochim. Acta 1984, 48, 1479–1492. [Google Scholar] [CrossRef]
- Arnórsson, S. Isotopic and Chemical Techniques in Geothermal Exploration, Development and Use; International Atomic Energy Agency: Vienna, Austria, 2000; p. 351. [Google Scholar]
- Arnorsson, S. Application of Silica Geothermometer in Low-Temperature Hydrothermal Areas in Iceland. Am. J. Sci. 1975, 275. [Google Scholar] [CrossRef]
- Fournier, R.O.; Rowe, J.J. Estimation of Underground Temperatures from the Silica Content of Water from Hot Springs and Wet-Steam Wells. Amer. J. Sci. 1966, 264, 685–697. [Google Scholar] [CrossRef]
- Fournier, R.O.; Truesdell, A.H. Chemical Indicators of Subsurface Temperature Applied to Hot Spring Waters of Yellowstone National Park, Wyoming, U.S.A. Geothermics 1970, 2, 529–535. [Google Scholar] [CrossRef]
- Fournier, R.O.; Truesdell, A.H. An Empirical Na-K-Ca Geothermometer for Natural Waters. Geochim. Cosmochim. Acta 1973, 37, 1255–1275. [Google Scholar] [CrossRef]
- Fournier, R.O.; Truesdell, A.H. Geochemical Indicators of Subsurface Temperature. Part Ii. Estimation of Temperature and Fraction of Hot Water Mixed with Cold Water. J. Res. U.S. Geol. Surv. 1974, 2, 259–262. [Google Scholar]
- Schmitt, M.J.; Dehope, W.J.; Ferguson, P.E.; Hart, S.L.; Matranga, V.A. Improved Silica Geothermometer for Low Temperature Geothermal Resource Assessment. Monthly Progress Report, March–April 1983; Arizona Solar Energy Commission, Phoenix: Scottsdale, AZ, USA, 1983.
- Fournier, R.O. Chemical Geothermometers and Mixing Models for Geothermal Systems. Geothermics 1977, 5, 41–50. [Google Scholar] [CrossRef]
- Chimeddorj, B.; Munkhbat, D.; Altanbaatar, B.; Dolgorjav, O.; Oyuntsetseg, B. Hydrogeochemical Characteristics and Geothermometry of Hot Springs in the Altai Region, Mongolia. Geochem. Explor. Environ. Anal. 2021, 21, geochem2021-016. [Google Scholar] [CrossRef]
- Ahmad, M.; Akram, W.; Ahmad, N.; Tasneem, M.A.; Rafiq, M.; Latif, Z. Assessment of Reservoir Temperatures of Thermal Springs of the Northern Areas of Pakistan by Chemical and Isotope Geothermometry. Geothermics 2002, 31, 613–631. [Google Scholar] [CrossRef]
- Keenan, J.H.; Keyes, F.G.; Hill, P.G.; Moore, J.G. Steam Tables—Thermodynamic Properties of Water Including Vapor, Liquid, and Solid Phases (International Edition—Metric Units); Wiley: New York, NY, USA, 1969; p. 89. [Google Scholar]
- Truesdell, A.H.; Fournier, R.O. Procedure for Estimating the Temperature of a Hot-Water Component in a Mixed Water by Using a Plot of Dissolved Silica Versus Enthalpy. J. Res. U.S. Geol. Surv. 1977, 5, 49–52. [Google Scholar]
- Morey, G.; Fournier, R.; Rowe, J. The Solubility of Quartz in Water in the Temperature Interval from 25 to 300 °C. Geochim. Cosmochim. Acta 1962, 26, 1029–1043. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Geothermal Solute Equilibria. Derivation of Na-K-Mg-Ca Geoindicators. Geochim. Cosmochim. Acta 1988, 52, 2749–2765. [Google Scholar] [CrossRef]
- Das, P.; Maya, K.; Padmalal, D. Hydrochemistry, Geothermometry and Origin of the Low Temperature Thermal Springs of South Konkan Region, India. Geothermics 2020, 90, 101997. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, H.; Yu, F.; Liu, D. Oxygen Isotopic Composition of Meteoric Water in the Eastern Part of Xizang. Geochemica 1980, 2, 113–121. [Google Scholar] [CrossRef]
- Craig, H. Isotopic Variations in Meteoric Waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.W.; Ma, H.Z.; Zhang, X.Y.; Cheng, H.D.; Han, J.B.; Li, Y.S.; Miao, W.L.; Hai, Q.Y. Geochemical Constraints on the Origin and Evolution of Spring Waters in the Changdu-Lanping-Simao Basin, Southwestern China. Acta Geol. Sin. 2019, 93, 1097–1112. [Google Scholar] [CrossRef]
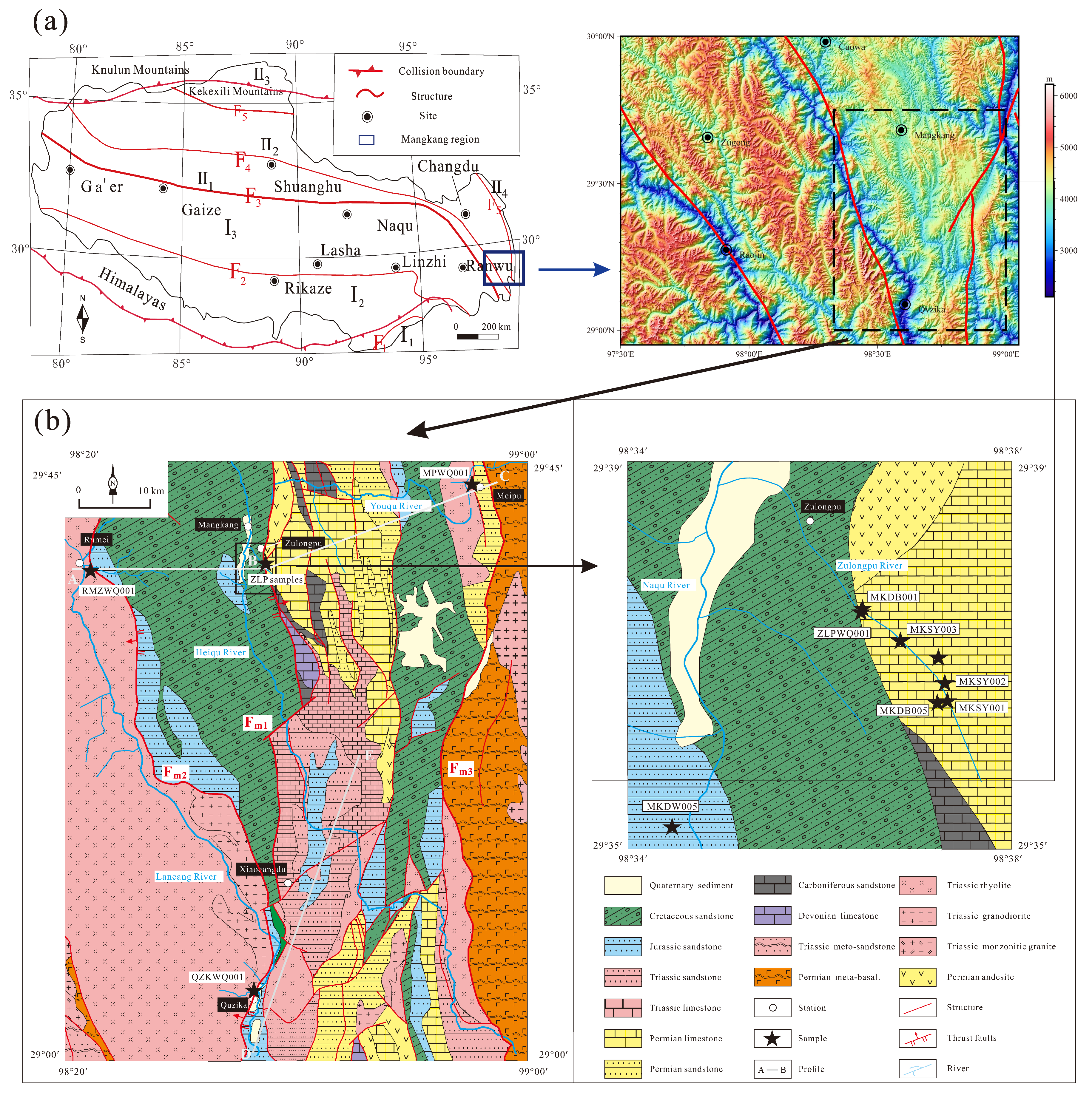
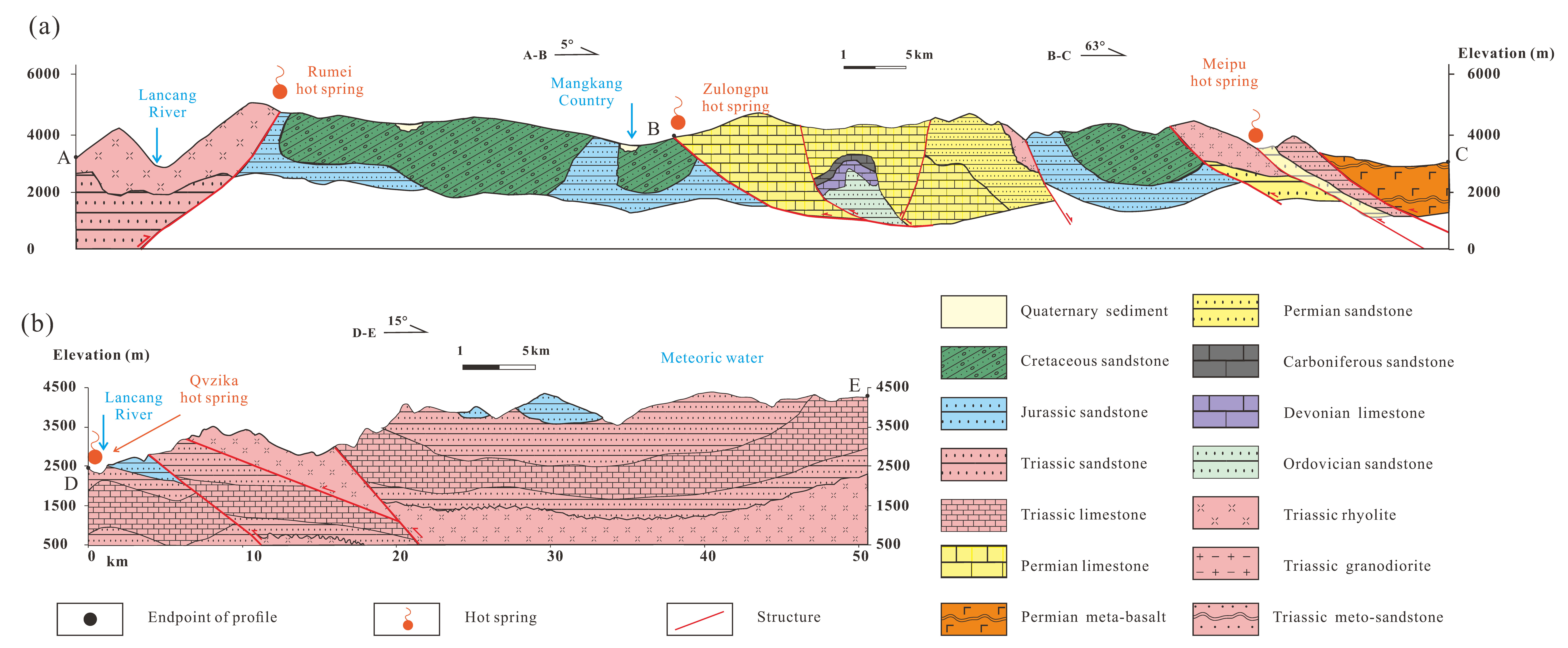
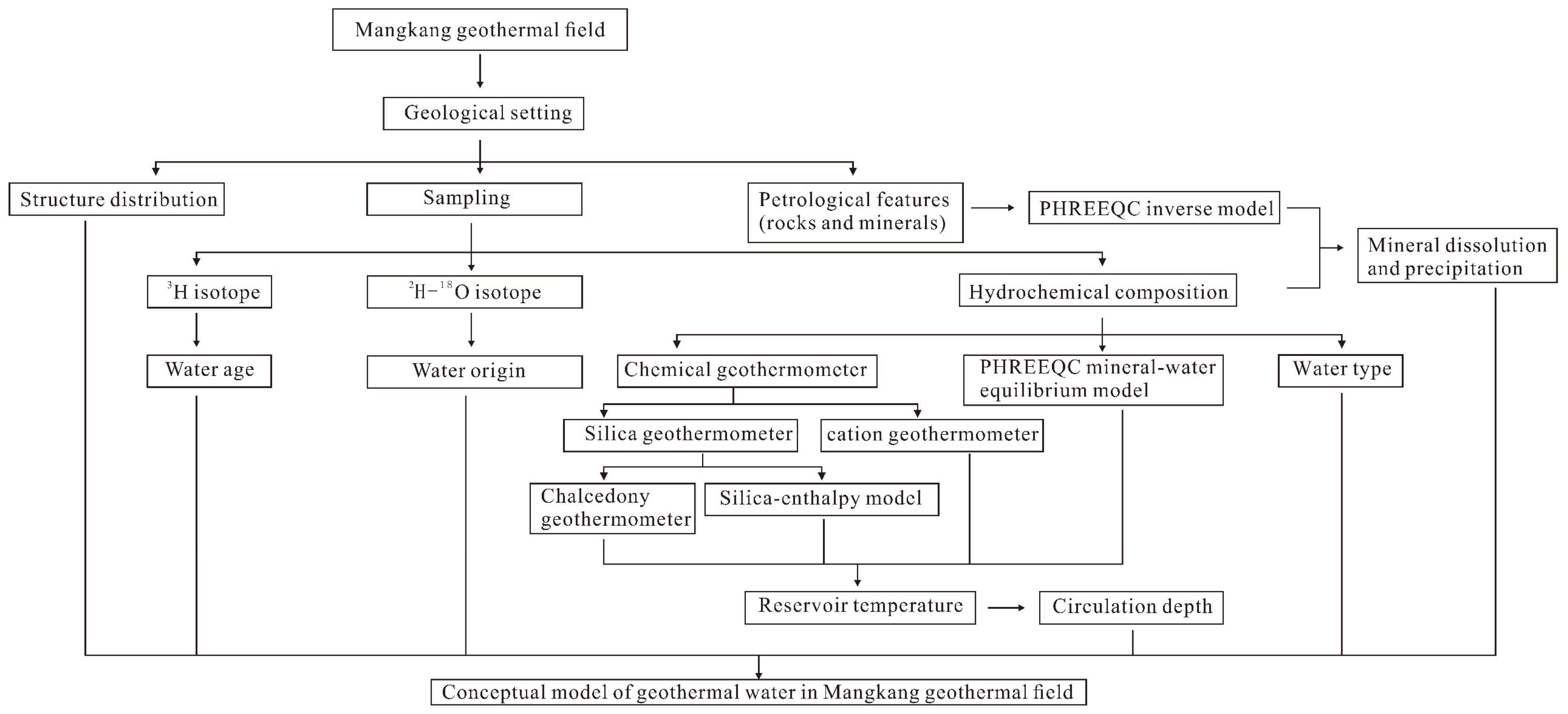

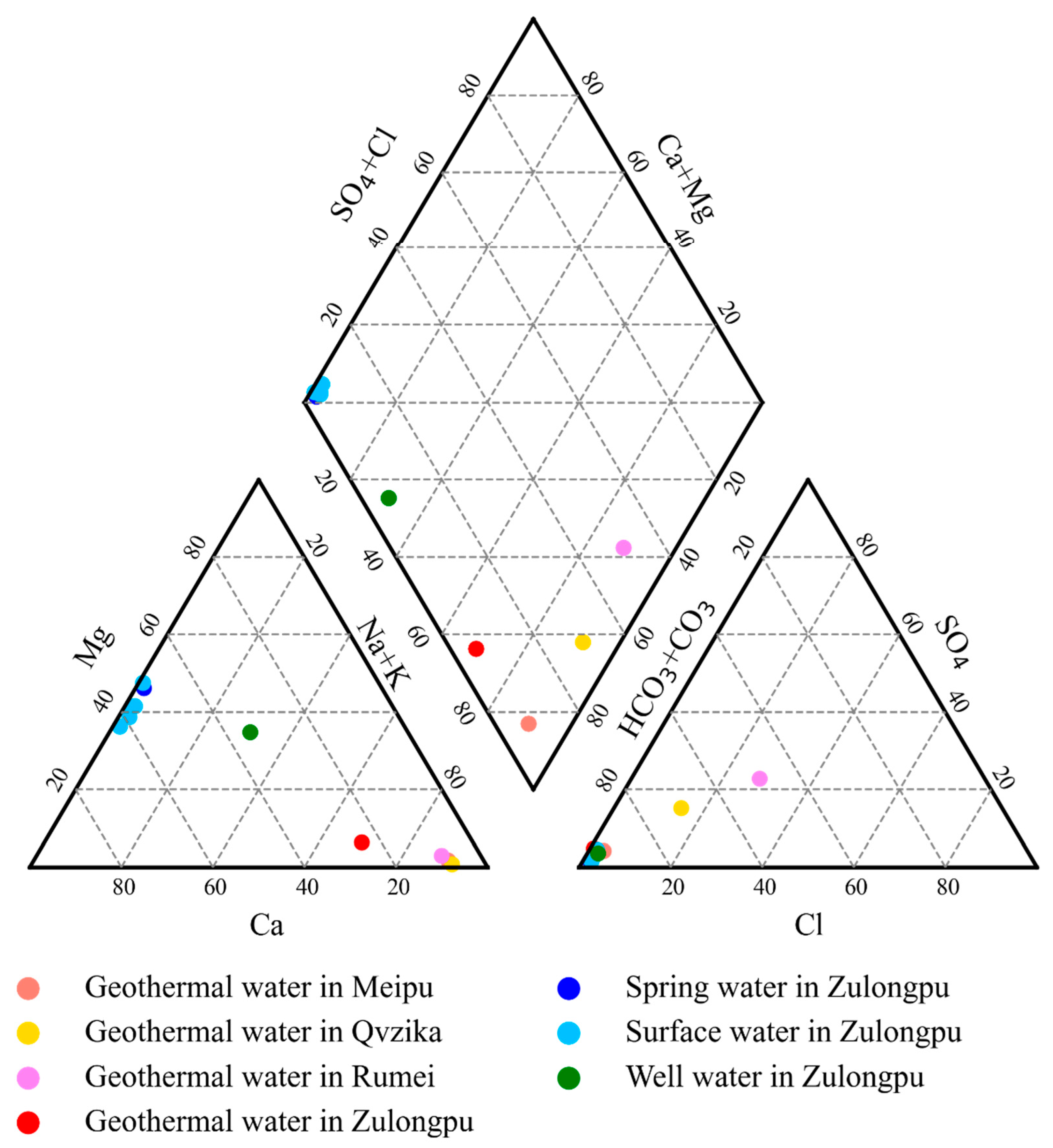

| Sample No. | Sample Property | Logtitude | Latitude | Altitude (m) |
|---|---|---|---|---|
| ZLPWQ001 | Geothermal water in Zulongpu | 98°37′04″ | 29°37′57″ | 3934 |
| RMZWQ001 | Geothermal water in Rumei | 98°22′12″ | 29°36′41″ | 3159 |
| QZKWQ001 | Geothermal water in Qvzika | 98°36′20″ | 29°05′33″ | 2533 |
| MPWQ001 | Geothermal water in Meipu | 98°55′03″ | 29°44′38″ | 2860 |
| MKQ001 | Spring water in Zulongpu | 98°37′55″ | 29°37′26″ | 3996 |
| MKDW001 | Well water in Zulongpu | 98°35′00″ | 29°35′35″ | 3641 |
| MKDB001 | Surface water in Zulongpu | 98°37′05″ | 29°37′58″ | 3932 |
| MKDB005 | 98°37′59″ | 29°37′00″ | 3970 | |
| MKSY-1 | 98°38′00″ | 29°37′00″ | 3986 | |
| MKSY-2 | 98°38′00″ | 29°37′12″ | 3981 | |
| MKSY-3 | 98°37′30″ | 29°37′38″ | 3981 |
| Sample | K | Na | Ca | Mg | HCO3− | SO42− | Cl− | F− | NO₃⁻ | CO₃2⁻ | HSiO3 |
| No. | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L |
| ZLPWQ001 | 9.33 | 159.3 | 50.26 | 7.95 | 572.7 | 23 | 2.8 | 1.86 | 1.29 | 0 | 59.72 |
| RMZWQ001 | 5.79 | 431.9 | 36.55 | 7.76 | 640.3 | 234.3 | 208.7 | 1.69 | 0.72 | 0 | 73.64 |
| QZKWQ001 | 5.89 | 112.7 | 8.21 | 0.49 | 175.8 | 30.02 | 21.01 | 14.3 | <0.20 | 17.8 | 92.46 |
| MPWQ001 | 10.55 | 213.3 | 16.35 | 2.25 | 598 | 21.48 | 11.9 | 3.29 | 0.41 | 0 | 84.41 |
| MKQ001 | 0.69 | 0.78 | 29.43 | 15.67 | 163.7 | 2.45 | 1.4 | 0.1 | 2.54 | 0 | 7.46 |
| MKDW001 | 3.53 | 59.26 | 59.88 | 36.29 | 499 | 15.08 | 7 | 0.5 | 3.44 | 0 | 9.94 |
| MKDB001 | 0.64 | 1.1 | 33.7 | 14.92 | 172.2 | 4.05 | 1.75 | 0.13 | 2.81 | 0 | 8.09 |
| MKDB005 | 0.45 | 0.67 | 34.93 | 12.24 | 137.7 | 5.14 | 1.4 | 0.11 | 2.26 | 11.9 | 7.55 |
| MKSY-1 | 0.81 | 1.38 | 40.85 | 16.11 | 200 | 4.78 | 1.75 | 0.14 | 2.54 | 0 | 9.04 |
| MKSY-2 | 0.3 | 0.42 | 29.64 | 16.39 | 163.7 | 2.18 | 1.75 | 0.11 | 3.23 | 0 | 5.92 |
| MKSY-3 | 0.48 | 0.8 | 33.29 | 14.51 | 168.5 | 3.96 | 1.75 | 0.12 | 2.49 | 0 | 8.26 |
| Sample | HBO2 | TDS | pH | T* | pH* | Eh* | CD* | TDS | E | CBE | WT |
| No. | mg/L | mg/L | mg/L | °C | mV | μS/cm | ppm | m | % | ||
| ZLPWQ001 | 6.04 | 583 | 7.64 | 18.10 | 7 | 1.00 | 788 | 393 | 3934 | 1.27 | HCO3-Na |
| RMZWQ001 | 5.59 | 1299 | 7.84 | 43.00 | 6.83 | 10 | 1652 | 827 | 3159 | 0.16 | HCO3·Cl-Na |
| QZKWQ001 | 3.02 | 375 | 8.33 | 67.50 | 7.56 | −38 | 493 | 253 | 2533 | 0.55 | HCO3-Na |
| MPWQ001 | 8.43 | 648 | 7.82 | 49.10 | 6.87 | 8 | 867 | 433 | 2860 | −0.98 | HCO3-Na |
| MKQ001 | <0.20 | 140 | 8.17 | 8.20 | 8.11 | −62 | 205 | 102 | 3996 | −0.18 | HCO3-Ca·Mg |
| MKDW001 | 1.42 | 449 | 7.86 | 10.80 | −33 | 613 | 306 | 3641 | 3641 | −0.75 | HCO3-Ca·Na |
| MKDB001 | <0.20 | 148 | 8.03 | 11.7 | 8.48 | −83 | 216 | 108 | 3932 | −0.50 | HCO3-Ca·Mg |
| MKDB005 | <0.20 | 141 | 8.33 | 14.20 | 8.52 | −86 | 202 | 101 | 3970 | −1.24 | HCO3-Ca·Mg |
| MKSY-1 | <0.20 | 173 | 8 | 12.20 | 8.07 | −60 | 238 | 120 | 3986 | −0.43 | HCO3-Ca·Mg |
| MKSY-2 | <0.20 | 139 | 8.06 | 7.90 | 8.01 | −55 | 201 | 100 | 3981 | 0.35 | HCO3-Ca·Mg |
| MKSY-3 | <0.20 | 145 | 8.13 | 17.80 | 8.43 | −83 | 210 | 105 | 3981 | −0.86 | HCO3-Ca·Mg |
| Sample | γNa/γCl | γCl/γHCO3 | γCl/γCa |
|---|---|---|---|
| MKWQ001 | 56.89 | 0.005 | 0.06 |
| RMZWQ001 | 2.07 | 0.326 | 5.71 |
| QZKWQ001 | 5.36 | 0.109 | 2.56 |
| MPWQ001 | 17.92 | 0.02 | 0.73 |
| Sample | Tf (°C) | Tm-w (°C) | Tc (°C) | TNa-k-Ca (°C) | Ts-e (°C) | Mixing Ratio (%) |
|---|---|---|---|---|---|---|
| MPWQ001 | 49.1 | 123–130 | 71.8 | 150.4 | 112 | 63 |
| QZKWQ001 | 67.5 | 121–130 | 76.5 | 148.4 | 85 | 22 |
| RMZWQ001 | 43 | 116–121 | 65.1 | 83.6 | 115 | 70 |
| ZLPWQ001 | 18.1 | 47 | 55.3 | 81 | / | / |
| Temperature | Pressure | Enthalpy | SiO2 Content |
|---|---|---|---|
| (°C) | (bars) | (cal/g) | (mg/L) |
| 50 | 0.12 | 50 | 13.5 |
| 75 | 0.39 | 75 | 26.6 |
| 100 | 1.01 | 100.1 | 48 |
| 125 | 2.32 | 125.4 | 80 |
| 150 | 4.76 | 151 | 125 |
| 175 | 8.92 | 177 | 185 |
| 200 | 15.54 | 203.6 | 265 |
| 225 | 25.48 | 230.9 | 365 |
| 250 | 39.73 | 259.2 | 486 |
| Sample | D (m) | t1 (°C) | t2 (°C) | G (°C/100 m) | h (m) |
|---|---|---|---|---|---|
| MPWQ001 | 3600–4300 | 112–130 | 3 | 3 | 20 |
| QZKWQ001 | 3900–4300 | 121–130 | 3 | 3 | 20 |
| RMZWQ001 | 3700–4000 | 115–121 | 3 | 3 | 20 |
| ZLPWQ001 | ~1500 | 47 | 3 | 3 | 20 |
| Sample | Sample Property | δ2HVSMOW (‰) | δ18OVSMOW (‰) | Elevation (m) | Recharge Elevation (m) | 3H (Tu) |
|---|---|---|---|---|---|---|
| MPWQ001 | Geothermal water in Meipu | −157 | −20.4 | 2860 | 4827 | <1.0 |
| QZKWQ001 | Geothermal water in Qvzika | −145 | −19.3 | 2533 | 4442 | <1.0 |
| RMZWQ001 | Geothermal water in Rumei | −157 | −20.1 | 3159 | 4750 | <1.0 |
| ZLPWQ001 | Geothermal water in Zulongpu | −158 | −20.3 | 3934 | 4865 | <1.0 |
| MKQ001 | Spring water in Zulongpu | −149 | −19 | 3996 | ||
| MKDB001 | Surface water in Zulongpu | −141 | −18.2 | 3932 | ||
| MKDB005 | Surface water in Zulongpu | −144 | −18.7 | 3970 | ||
| MKSY-1 | Surface water in Zulongpu | −142 | −18.2 | 3986 | ||
| MKSY-2 | Surface water in Zulongpu | −146 | −18.8 | 3981 | ||
| MKSY-3 | Surface water in Zulongpu | −145 | −18.7 | 3981 | ||
| 26 * | Surface water in Qvzika | −109 | −14.7 |
| Area | Meipu | Rumei | Zulongpu |
|---|---|---|---|
| Mole transfer of anhydrite | / | / | 1.6 × 10−4 |
| Mole transfer of calcite | / | / | 3.73 × 10−3 |
| Mole transfer of chalcedony | −7.91 × 10−4 | −4.76 × 10−3 | −5.45 × 10−4 |
| Mole transfer of dolomite | −2.83 × 10−3 | −5.72 × 10−2 | −8.39 × 10−4 |
| Mole transfer of fluorite | 6.34 × 10−5 | −2.48 × 10−4 | 4.25 × 10−5 |
| Mole transfer of gibbsite | −1.59 × 10−4 | 1.25 × 10−3 | −2.8 × 10−4 |
| Mole transfer of NaX | 8.51 × 10−3 | 1.53 × 10−2 | 6.84 × 10−3 |
| Mole transfer of CaX2 | −4.26 × 10−3 | −7.55 × 10−3 | −3.42 × 10−3 |
| Mole transfer of K-feldspar | 1.59 × 10−4 | −1.25 × 10−3 | 2.8 × 10−4 |
| Mole transfer of CO2 | / | −1.2 × 10−1 | / |
| Mole transfer of NH4X | / | −2.7 × 10−4 | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.; Liu, Y.; Wang, G.; Li, T.; Liu, F.; Wei, S.; Yan, X.; Gan, H.; Zhang, W. Genesis Mechanisms of Geothermal Resources in Mangkang Geothermal Field, Tibet, China: Evidence from Hydrochemical Characteristics of Geothermal Water. Water 2022, 14, 4041. https://doi.org/10.3390/w14244041
Liao Y, Liu Y, Wang G, Li T, Liu F, Wei S, Yan X, Gan H, Zhang W. Genesis Mechanisms of Geothermal Resources in Mangkang Geothermal Field, Tibet, China: Evidence from Hydrochemical Characteristics of Geothermal Water. Water. 2022; 14(24):4041. https://doi.org/10.3390/w14244041
Chicago/Turabian StyleLiao, Yuzhong, Yanguang Liu, Guiling Wang, Tingxin Li, Feng Liu, Shuaichao Wei, Xiaoxue Yan, Haonan Gan, and Wei Zhang. 2022. "Genesis Mechanisms of Geothermal Resources in Mangkang Geothermal Field, Tibet, China: Evidence from Hydrochemical Characteristics of Geothermal Water" Water 14, no. 24: 4041. https://doi.org/10.3390/w14244041
APA StyleLiao, Y., Liu, Y., Wang, G., Li, T., Liu, F., Wei, S., Yan, X., Gan, H., & Zhang, W. (2022). Genesis Mechanisms of Geothermal Resources in Mangkang Geothermal Field, Tibet, China: Evidence from Hydrochemical Characteristics of Geothermal Water. Water, 14(24), 4041. https://doi.org/10.3390/w14244041









