Abstract
Localized biogenic corrosion and extrication of annoying odors caused by hydrogen sulfide (H2S) have long been a big problem in the management of urban sewer systems. H2S emission control in sewers via chemically or biologically normal oxidation processes has also been investigated extensively and is costly. The objective of this work was to develop a new technology to mitigate the concentration of H2S in sewer pipes using conductive concrete. Experimental results after 66 days show that the concentration of hydrogen sulfide significantly decreased when conductive concrete was used as a microbial fuel cell. Both ordinary Portland cement and conductive concrete were utilized for the target experiment. Elemental sulfur was observed in the coating sludge of conductive concrete, whereas this trend was not observed for ordinary Portland cement. These observations demonstrate that conductive concrete provides an electron pathway from deposited sludge in the bottom of sewer pipes to oxygen dissolved in surface water electrons generated from hydrogen sulfide oxidation in an anaerobic environment via conductive concrete. Finally, regarding the mechanism responsible for hydrogen sulfide oxidation, chemical oxidation was the dominant process, and biological processes did not play a significant role.
1. Introduction
Traditionally, concrete corrosion is believed to cost billions of dollars annually in the remediation and replacement of corroding concrete sewers. Despite the complicated process of concrete corrosion, hydrogen sulfide (H2S) is formed as a product of anaerobic oxidation of sediments at the bottom of sewers under anaerobic conditions, which is the leading cause of microbial-induced concrete corrosion. Acid gases thrive in the sewer biofilm layer and cementation materials, mainly calcium and aluminum minerals, and contribute to reducing the load-bearing capacity of concrete structures. A large volume of published studies have presented solutions to controlling H2S in sewer systems, e.g., via the inhibition or elimination of H2S and other hazardous gases formed by chemical and biological mechanisms that cause high corrosion rates within wastewater infrastructure. Firer et al. [1] demonstrated that sulfide can be reduced to safe concentrations, around 0.1 mg S/L, by using ferric salts reacting with sulfide-containing wastewater to form elemental sulfur. Oxygen injection (15–25 mg O2/L per pump event) is considered as the biogenic production of hydrogen sulfide in sewers to control corrosive gas by 65% [2]. Additionally, according to Gutierrez et al. [3], their laboratory studies revealed the possibility of using pH shock treatment at 10.5 for 1–2 h to effectively control sulfide formation in sewers and showed that simultaneous treatment of nitrite at 0.26 mg-N/L and free nitrous acid could reduce the average level of sulfide production by >80% [4,5,6,7]. Recently, electrochemical oxidation of iron and alkalinity generation were shown to effectively control sulfide in sewers [8]. In summary, increases in pH and the addition of NaOH, Ca (OH)2 or biocides can inhibit H2S generation. The development of alternative electron acceptors, such as oxygen, nitrate, nitrite, etc., also reduces the activities of sulfate-reducing bacteria (SRB) and prevents production of H2S. Once H2S is formed, biological and chemical oxidation processes are necessary to eliminate H2S in sewers with the addition of chemical oxidants or iron salts. Difficulties arise, however, when the above attempts are made to remove hydrogen sulfide. Air injection is a good method to reduce H2S, BOD and nontoxicity in sewage. The only negative aspect is that it is operational only under high-pressure conditions, which may lead to fire threats. Using chemical oxidants or oxygen to control H2S in sewer systems is costly and high maintenance. More cost-effective and sustainable methods for hydrogen sulfide emission control should be considered to replace those currently practiced. In a study on H2S control in oil containers, Nemati et al. [9] reported that molybdate or formaldehyde, which is used mainly in the food industry, can be applied to inhibit microbial growth, but it is biodegrades easily in wastewater streams. Another way in which hydrogen sulfide formation can be eliminated for a long time is to slowly release oxygen in solid phases, such as MgO2 and CaO2, which react with water to produce Mg(OH)2 and Ca(OH)2, respectively [10,11]. Aguilar et al. [12] draws our attention to using anodes for solid oxide fuel cells (SOFCs) operating in H2S-containing fuels, which suggests H2S is preferentially oxidized. H2S is used as a fuel to generate electrical energy via electrochemical reactions with biochemical processes and is transformed into elemental sulfur in the presence of a fuel cell. The recommended approach to controlling hydrogen sulfide in sewer concrete is the application of microbial fuel cells (MFCs) as a sustainable and worthwhile solution. Questions have been raised about which material as a productive MFC could transform H2S in situ to electrical energy.
This paper attempts to show that conductive concrete can be used as an MFC to control hydrogen sulfide in sewer systems. To elucidate the inhibition effect of H2S, a conductive material was used as sewer concrete. First, we investigated the contribution of adsorption processes of conductive materials. Additionally, tests using conductive concrete and normal cement were conducted to verify the chemical or biological oxidation processes aiding in H2S reduction and to determine the mechanism by which conductive concrete transfers electrons to acceptors.
2. Materials and Methods
Experiments were carried out at room temperature at 24 ± 0.1 °C with distilled water (SA-2100A• A type, Tokyo Rika Kikai Co., Ltd., Tokyo, Japan).
2.1. Models and Materials
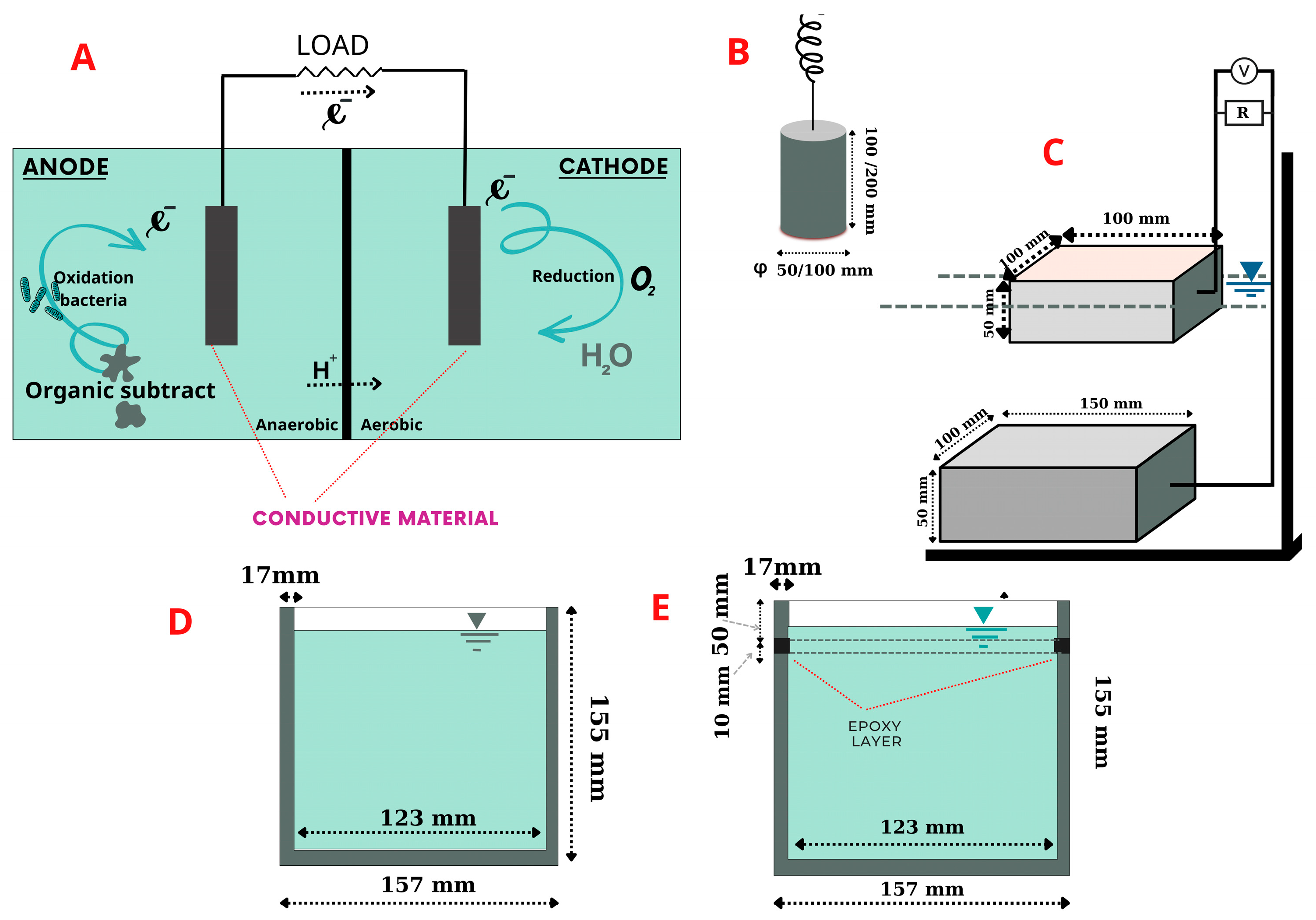
The operating principle of a microbial fuel cell is a biofilm model with two chambers (Figure 1A) based on the conduction mechanism of MFCs [13]; a bacterium in the anode area transfers electrons obtained as the outcome of biochemical reactions as electron donors to the anode electrode surface. Once electrons are generated, protons are also produced and migrate in mixed liquid–solid solution. The electrons flow from the anode through a conductive environment (an external resistance or load or conductive material in this study) to the cathode, where they reunify with protons in the final electron acceptor (oxygen).
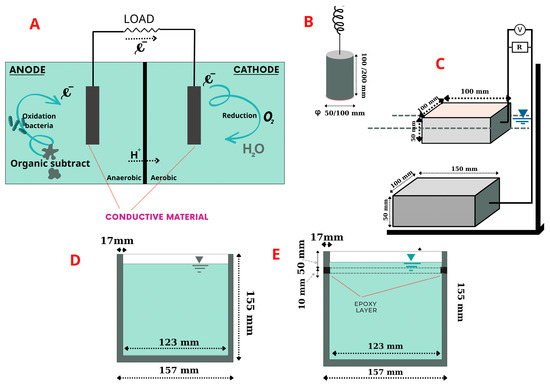
Figure 1.
Experimental apparatus and materials: (A) MFC model; (B) single-chamber MFC with air cathode; (C) plate specimens; (D) cup specimens; (E) cup specimens with epoxy-layer insulator.
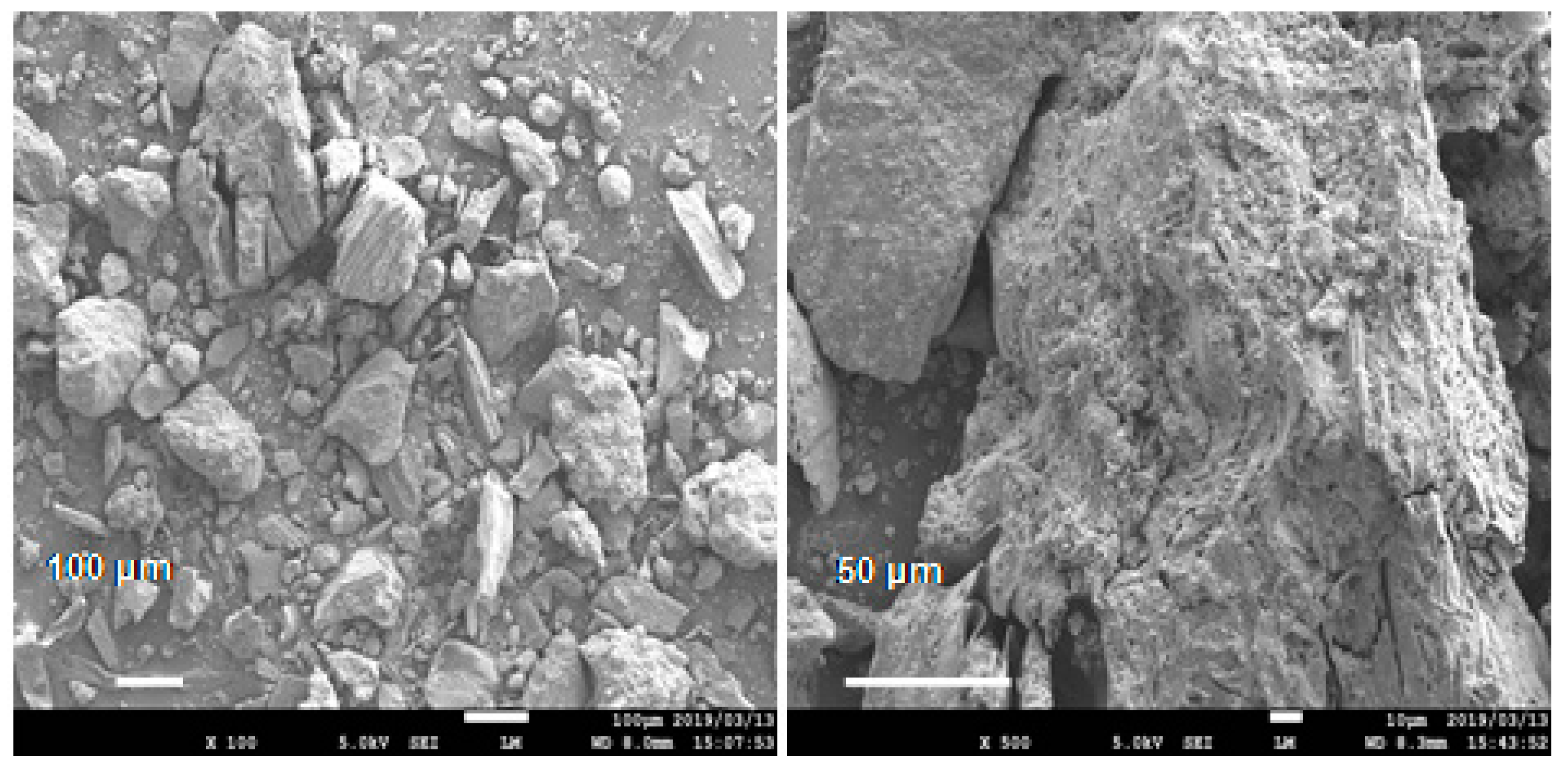
The conductive concrete used in this work was San-Earth M5C (hereinafter to as San-Earth) manufactured by Sankosha Co., Tokyo, Japan. San-Earth, having a specific gravity of approximately 1.285 g/cm3, was used and contains amorphous carbon produced from oil refining and is a gray, conductive material (Figure 2). San-Earth was selected as the conductive concrete due to having a higher carbon content and conductivity than other conductive and commercialized concretes. San-Earth has a maximum size of 0.3 mm and a surface area of 1.9 m2/g as measured by the BET method (Brunauer–Emmett–Teller method) using BELSORP-mini II (Mircrotrac Bell Co., Osaka, Japan), and a 50/100 proportion of amorphous carbon is achieved via separating conductive carbon from San-Earth in gently running water. The mixture of San-Earth is characterized by a water-to-cement (W/C) ratio of 0.4 and curing time of 27 days. San-Earth does not expand, shrink or crack when curing.
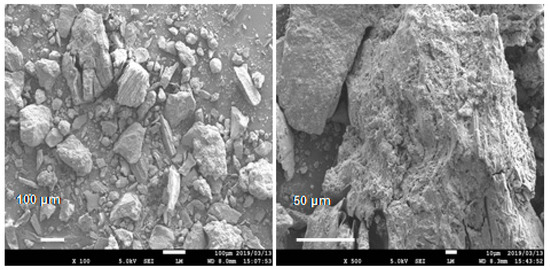
Figure 2.
SEM images of conductive material (amorphous carbon) of San-Earth.
Specimens of conductive concrete prepared in this study were classified as rod, plate and cup shapes depending on the target experiment. As shown in Figure 1B, rod electrodes were used for measurement of electrical resistivity, whereas the cup-shaped specimens in Figure 1D,E had a capacity of approximately 1.9 L with reinforcing bars. The conductive concretes in Figure 1D,E were the same volume, but one had a 10 mm epoxy layer inserted 50 mm from the top edge (Figure 1E) to prevent currents from passing through. As shown in Figure 1C, a couple of plate-shaped specimens, with dimensions of 100 mm × 100 mm × 50 mm and 150 mm× 100 mm × 50 mm, respectively, were set up to conduct an experiment that involved an external resistance of 100 Ω. These plate specimens were remolded and treated with an alum-based scour-removing agent with no inside aggregate.
For verification of the San-Earth material, ordinary Portland cement (OPC) was used for the experiment. Portland cement is a commonly used building material, manufactured by Sumitomo Osaka cement Co. Ltd., Tokyo, Japan. OPC has a specific gravity of 3.15 g/cm3 and specific surface area of 0.3350 m2/g (San-Earth, up to 1.9 m2/g). Since San-Earth contains amorphous carbon as a conductive material, it has a higher water absorption rate than OPC. Therefore, regardless of the shape of the specimen, the water/cement ratio of OPC was set to 30% to maintain the same workability (easy handling).
2.2. Electrical Resistivity
The electrical resistivity of conductive concrete was measured by a chemical impedance analyzer (model: IM3590) manufactured by Hioki Co., Ltd., Nagano, Japan, using the four-electrode method. The specimens were rod electrodes of φ 50 × 100 mm2 or φ 100 × 200 mm2 and were cured in water because the real sewage concrete operates in a water-wet condition. The voltage applied during measurement was set to a relatively low 1.0 V, as the voltage obtained in this study was around 100 mV, and the power supply frequency was 50–80,000 Hz. The electrical resistivity of concrete changes with the period of application time because of its high electrolyte content. The electrical resistivity reported in this study was the value recorded after 30 min, when preliminary experiments confirmed that the value was stable even at low frequencies, for which it is said to take a long time to converge to a constant value.
2.3. Sample Preparation
For this study, artificial wastewater and an admixture of anaerobic and aerobic sludge with a volume fraction of 1:1 collected from the Ube City Wastewater Treatment Plant (Ube WWTP) were used to conduct an effective assessment of conductive concrete for inhibiting hydrogen sulfide. The artificial wastewater was synthetized from dechlorinated tap water and chemical components as listed in Table 1.

Table 1.
Substrate compositions used in the artificial effluent (per 1 L) [14].
This work used synthetic wastewater of hydrogen sulfide to evaluate the adsorption of hydrogen sulfide by amorphous carbon in conductive concrete. The concentration of sulfide ions was adjusted to approximately 10 mg S/L by adding a small amount of a stock solution of sodium sulfide to 1.4 L of distilled water. The sulfide concentration was examined over time by maintaining the pH in the range of 7.0 ± 0.1 by adding hydrochloric acid solution as required. Given that the acid dissociation constant kPa of hydrogen sulfide is 6.9, approximately 50% of the hydrogen sulfide was present as volatile H2S at pH 7.0 ± 0.1, the value at which the experiments were conducted.
2.4. Experimental Procedure and Mechanism Assessment
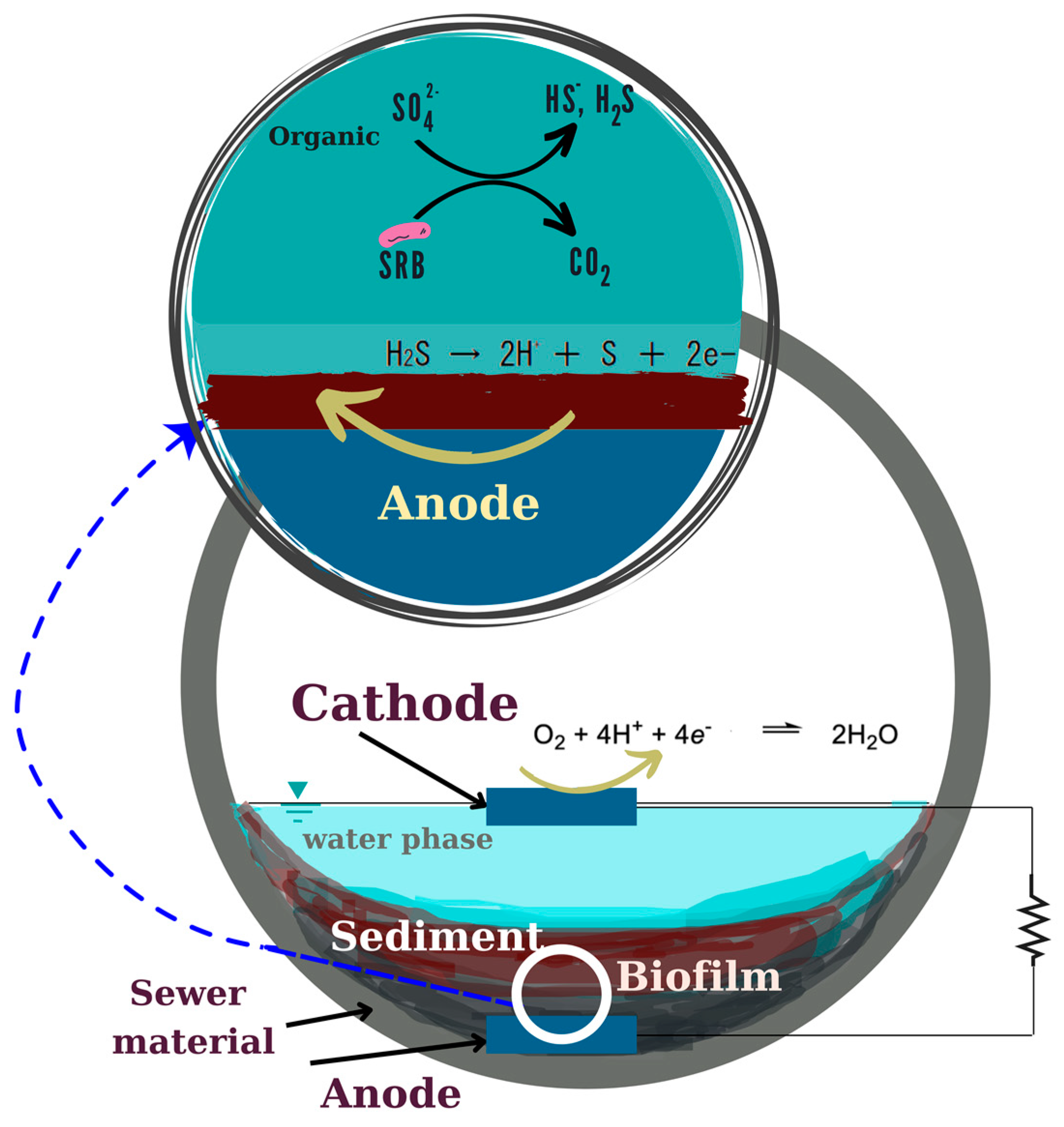
In this study, which focused on H2S inhibition by conductive material from San-Earth, it was assumed that an electron pathway exists between anaerobic and aerobic areas (Figure 3). The utilization of conductive material containing amorphous carbon was considered as a possible reason why conductive concrete is effective in suppressing hydrogen sulfide, working in 3 ways: (1) adsorption of hydrogen sulfide by conductive carbon; (2) chemical oxidation of hydrogen sulfide by oxygen and conductive materials; (3) oxidation of hydrogen sulfide by electron-producing bacteria (EPB). To elucidate this, an experimental series was fulfilled as follows.
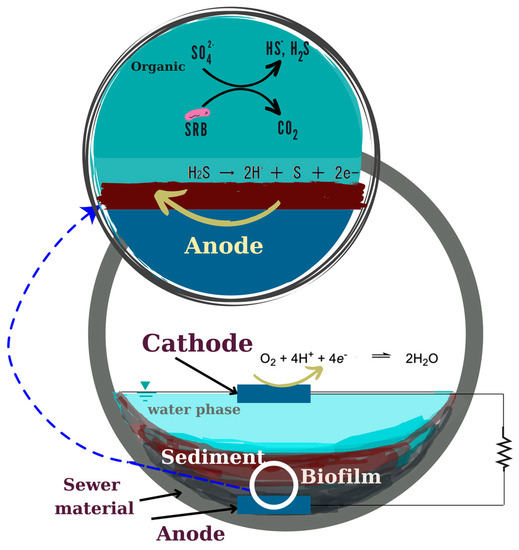
Figure 3.
Inhibition mechanism of sulfide by MFC strain.
2.4.1. Adsorption Assessment of Conductive Material regarding H2S
The experimental model of a cup-shaped specimen as shown in Figure 1D was developed to clarify the adsorptive capacity of the material. To determine this, a volume of 1.4 L of synthetic wastewater of sole hydrogen sulfide was prepared, and the dissolved oxygen concentration was kept below 0.1 mg/L in anaerobic conditions by purging the synthetic wastewater with pure-nitrogen bubbles. Multiple layers of plastic film were placed over the water surface to prevent dissolved oxygen from oxidizing H2S and diffusing H2S from inside. The purpose of the experiment was to allow the concrete material to become the only way to eliminate H2S. The concentration of sulfide ions was adjusted to approximately 10 mg S/L as per the above instructions, and the pH was maintained in the range of 7.0 ± 0.1. Both the conductive concrete made of San-Earth and the OPC material were evaluated to scrutinize the adsorption capacity.
2.4.2. Voltage Measurement Experiments Using Plate-Shaped Specimens
The model in Figure 1C was established to elucidate whether concrete material can transfer electrons via a pathway or not. This is necessary for the chemical or biological oxidation processes of hydrogen sulfide to be able to accept oxygen from the air–liquid surface. The insulated vessel made of polypropylene was filled with 0.8 L of mixed sludge, called Mix 1 (see Table 2 for details), which covered the anode area, and 3.2 L of the artificial wastewater (see Table 1 for details). The 5 cm-thick cathode area was placed into the vessel in such a way that 3 cm of the cathode was immersed in liquid and 2 cm was above the water. The anode and cathode were connected via a 100-Ω external resistor, and the voltage across the resistive section of the closed circuit was measured with a data logger using midi LOGGER GL-240 (GRAPHTEC, Graphtec Corporation, Yokohama, Japan). San-Earth and OPC were used to assess the electron transfer.

Table 2.
Composition of sludge of Ube WTTP.
2.4.3. Hydrogen Sulfide Suppression Experiment
Testing was carried out to evaluate the capacity of San-Earth as a conductive concrete to control H2S. The cup-shaped specimen (Figure 1D) was used and filled with 0.14 L of mixed sludge, No. 2, wholly mingled with 1.12 L of the artificial wastewater (see Table 1 and Table 2 for details). The water surface was not covered by a plastic layer, referred to as 4.a, to maintain its natural state proceeding the evaluation of the H2S inhibition effect, which was to be tested via the three ways mentioned above: adsorption, chemical oxidation and oxidation of hydrogen sulfide by sludge-residing EPB. The water samplings 5 cm from the surface were measured by pH, sulfate and sulfide ion concentrations.
The experimental period was observed for 66 days. Once outcomes confirmed that sulfate was nearly completely consumed by SRB, a substrate of glucose (100 mg/L) and magnesium sulfate (33 mg S/L) was instantaneously added.
2.4.4. Hydrogen Sulfide Suppression with Epoxy-Layer Cup-Shaped Specimen
The aim of this experiment using cup-shaped specimens (Figure 1D,E) was to verify whether chemical or biological oxidation reactions contribute to the suppression of hydrogen sulfide. First, dissolved oxygen concentrations were measured with a dissolved oxygen meter OM-71-2 (HORIBA Advanced Techno Co., Ltd., Kyoto, Japan) at different depths in a preliminary experiment using cup-shaped specimens (Figure 1D). The results show that dissolved oxygen was present only in the surface layer at a depth of nearly 1 cm and was almost 0 mg/L under the 1 cm layer. Furthermore, if hydrogen sulfide was oxidized chemically or biologically in the electron transfer pathway, as assumed in this study, then the use of a cup-shaped specimen, as shown in Figure 1E, with a nonconductive epoxy resin surrounding the inside of the concrete material at approximately 5 cm below the water surface would result in the prevention of electron transfer through the epoxy resin. Therefore, if electrons were not transferred to be close to the surface layer containing dissolved oxygen or into the air, the oxidation reaction of hydrogen sulfide would not proceed due to the absence of electron acceptors. Otherwise, if adsorption of the conductive carbon was the main mechanism for the suppression of hydrogen sulfide, the effect would not be lost regardless of whether epoxy resin was inserted or not. The experiments were accomplished, as shown in Figure 1D,E, by using mixed sludge Mix 3 (see Table 2 for details) and artificial wastewater for 18 days under the same conditions.
2.5. Analysis Instructions
Physical analyses were required to ensure and control the biochemical processes. Measurements of pH and dissolved oxygen were performed using a pH meter D-72 and DO meter OM-71-2, respectively (HORIBA Advanced Techno Co., Ltd., Kyoto, Japan). Sulfate concentration in water samples was measured using the barium sulfate turbidimetric method according to the USEPA method (375.4) after filtration through a 0.45 µm membrane filter. Sulfide was quantified using the methylene blue method according to the USEPA method (376.2).
Sulfur analysis: sludge sediment deposited at the bottom of the specimen after 66 days was scraped off a few millimeters from the surface and dried under a nitrogen atmosphere, and the concentration of sulfur in the sludge sediment was determined by X-ray photoelectron spectroscopy (XPS) using a K-Alpha™ XPS system (Thermo Fisher Scientific, Waltham, MA, USA), and the energy shift at the 2p 3/2 level provided insight into the oxidation number of sulfur.
PCR and DGGE analysis: genomic DNA isolated from sludge sediments was analyzed by PCR-DGGE (polymerase chain reaction–denaturing gel gradient electrophoresis) to determine whether there were differences in microbial communities between ordinary Portland cement and San-Earth. If EPB contributed significantly to the oxidation of hydrogen sulfide as assumed, then the microbial community should have been observed to differ because of the accumulation of EPB in San-Earth. DNA extraction was carried out using a MORA-EXTRACT kit (Kyokuto Pharmaceutical Industrial Co., Ltd., Tokyo, Japan) according to the manufacturer’s instructions, and PCR was performed with the touch-down method [15] using primer GC-341f and 518 r for amplification of 200 bp 16S rDNA [16]. DGGE was performed using the D-code system apparatus (Bio-Rad), and the PCR product was applied to 8% (w/v) polyacrylamide gel (denaturant gradient range of the gel: 25–65%). Electrophoresis was run for 12 h at 60 °C at 100 V.
3. Results
3.1. Measurement of Electrical Resistivity
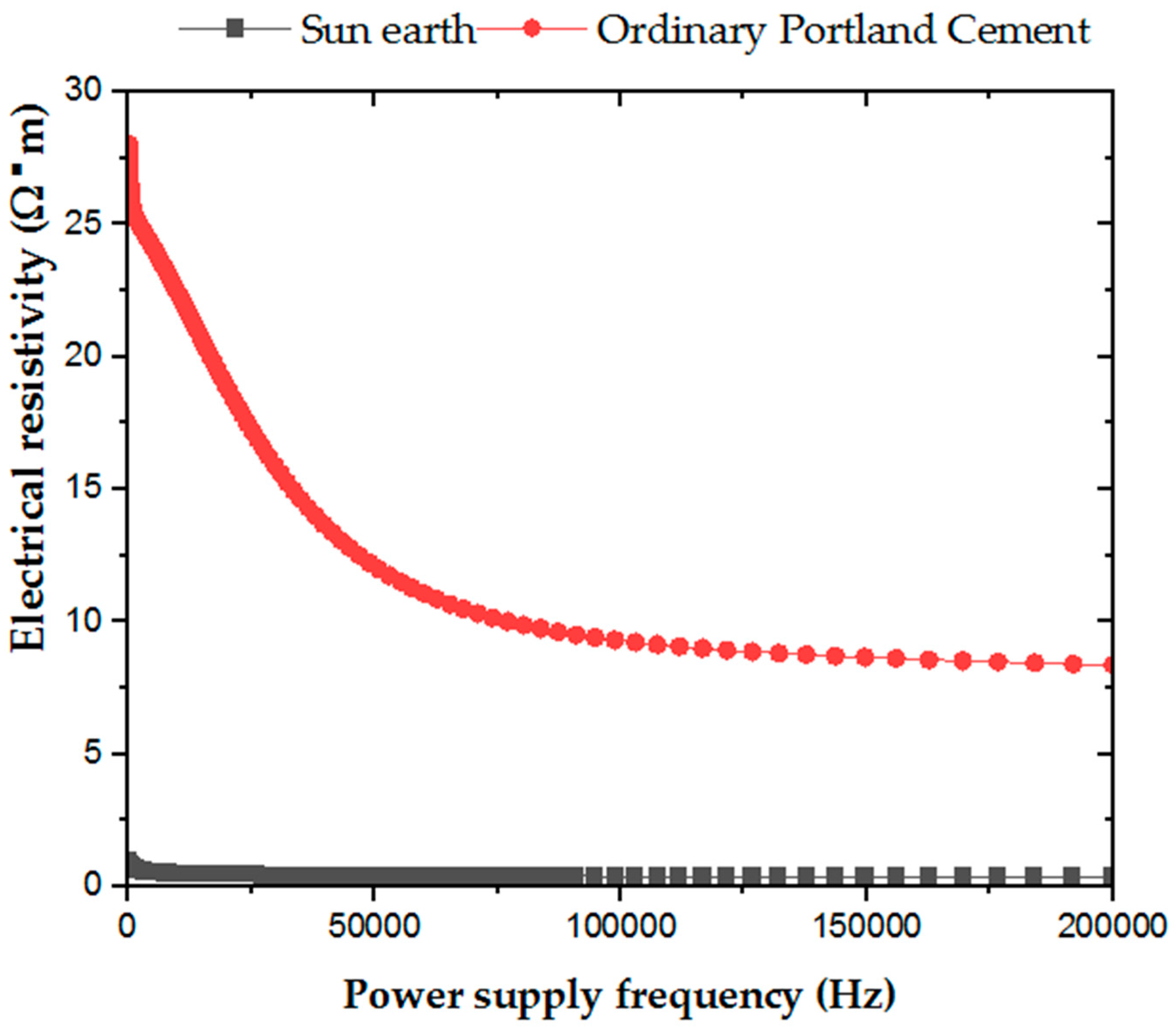
In determining the electrical resistivity of ordinary Portland cement and San-Earth, as shown in Figure 4, a tendency of the preliminary measurement of electricity resistivity to decrease as the power frequency increased was seen. However, at all power supply frequencies, the electrical resistivity of San-Earth was significantly lower than that of ordinary Portland cement, varying between 3 and 28 times. These results suggest that the specimens prepared using San-Earth had low resistance to the flow of electrons and that the electrons supplied by the oxidation of hydrogen sulfide could move easily from the anode to the cathode.
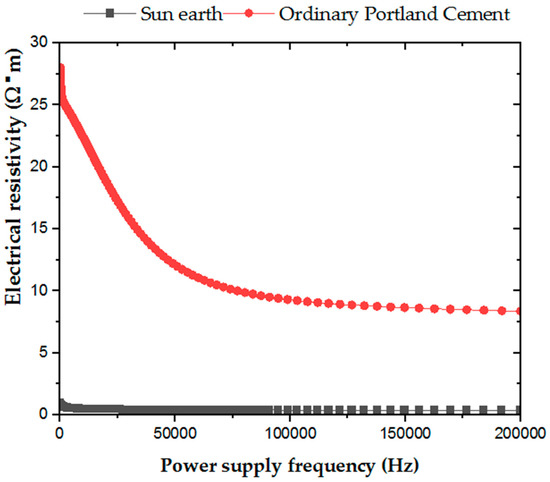
Figure 4.
Resistivity versus frequency graph for sample OPC and San-Earth.
3.2. Results of Adsorption Experiment with Hydrogen Sulfide
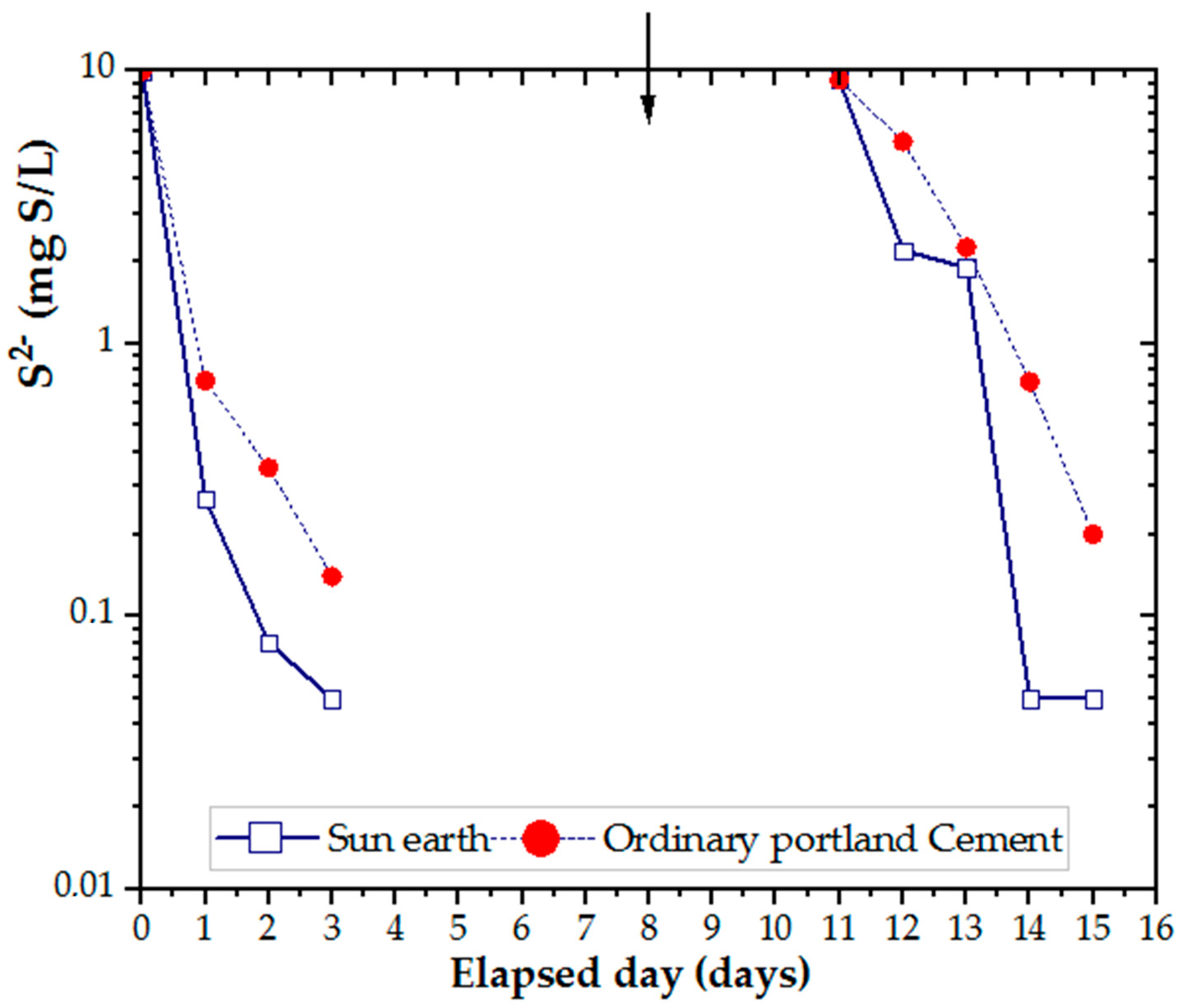
Plate specimens, as shown in Figure 1D, were used to evaluate the adsorption capacity of different concretes. From the data in Figure 5, it is apparent that the concentration of hydrogen sulfide was reduced to less than 1/10 after three days by adsorption of concrete. To evaluate the reduction in H2S, sodium sulfide was added as described above. In comparing the two results, concrete made from San-Earth was found to have a higher rate of H2S reduction than OPC.
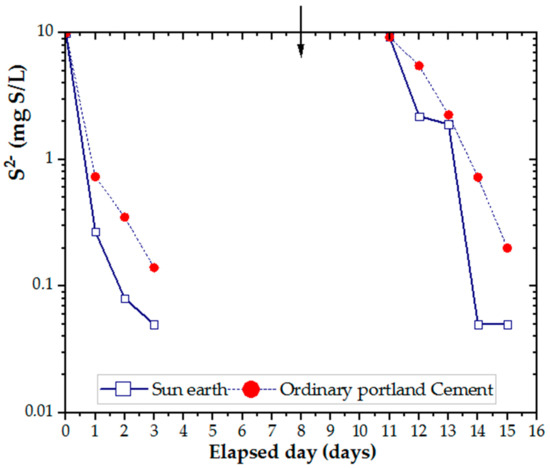
Figure 5.
Efficiency of OPC and San-Earth materials in H2S adsorption. Arrow (↓) indicates the addition of sodium sulfide.
The most striking result to emerge from the data is that hydrogen sulfide in water was removed by the adsorption of amorphous carbon contained in San-Earth, which is approximately 50 wt.%.
3.3. Voltage Measurement Experiments Using Plate-Shaped Specimens
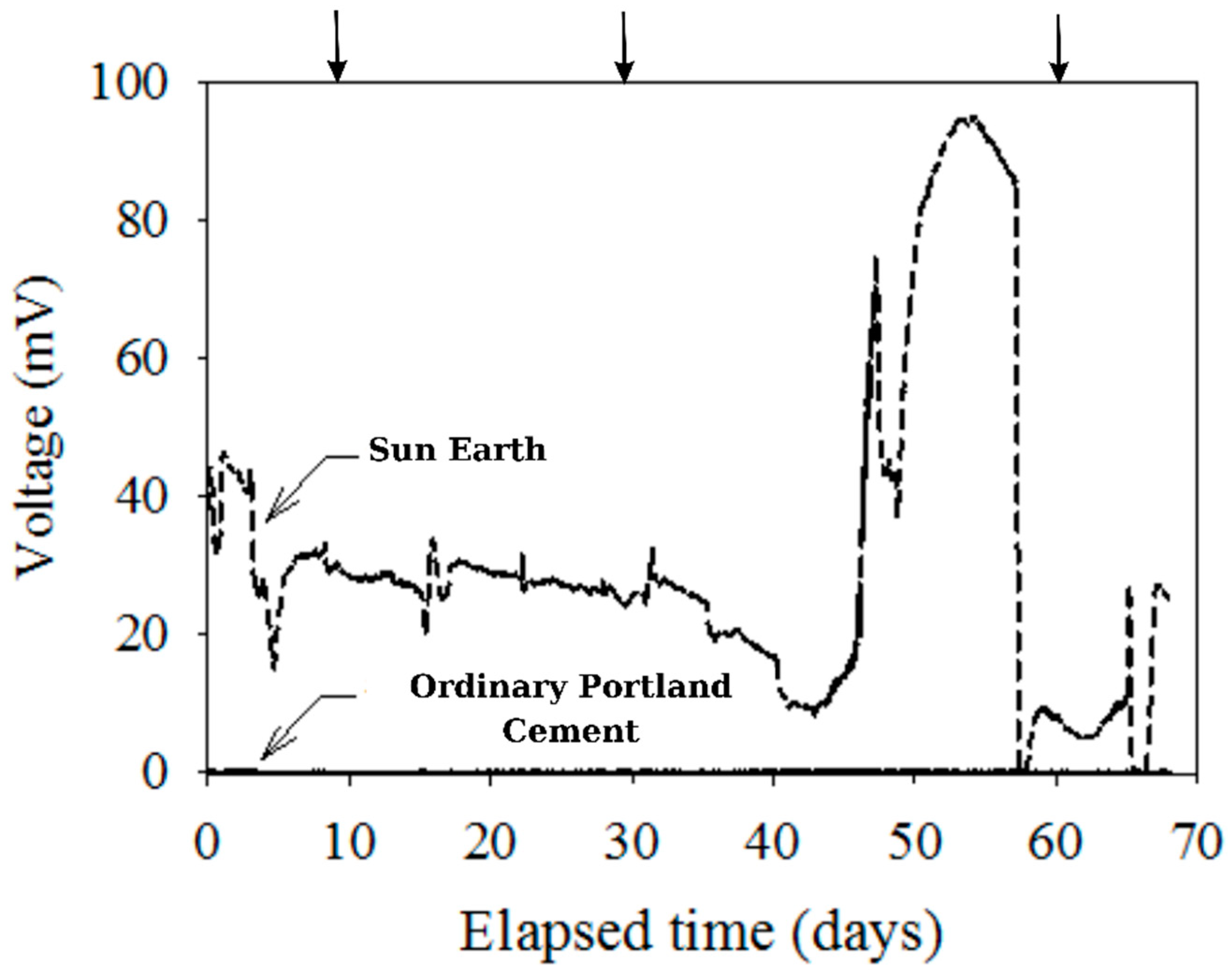
A continuous diagram visualizing time and voltage in regard to electrode materials’ correlation was obtained to determine whether there was an electron pathway from the anode to cathode or not (in Figure 6). As described in the above section detailing methods, plate-shaped specimens on an insulated container were used, and the arrows in Figure 6 present the addition of glucose and sodium sulfate to maintain the environmental substrate. What is interesting about these data is that voltage values of the OPC sample were almost 0 mV, whereas the San-Earth sample recorded the maximum voltage at 100 mV. There was a significant difference between the electrode materials. San-Earth had a lower electrical resistivity than OPC due to the presence of conductive carbon, which facilitates the transfer of electrons through the material. Additionally, the data shown in Figure 4 suggest the low resistance and high conductivity of San-Earth make it a flexible framework for attracting generated electrons from the anaerobic area at the vessel’s bottom to the surface with the presence of oxygen as a target acceptor. This finding indicates San-Earth’s efficacy as a conductive concrete.
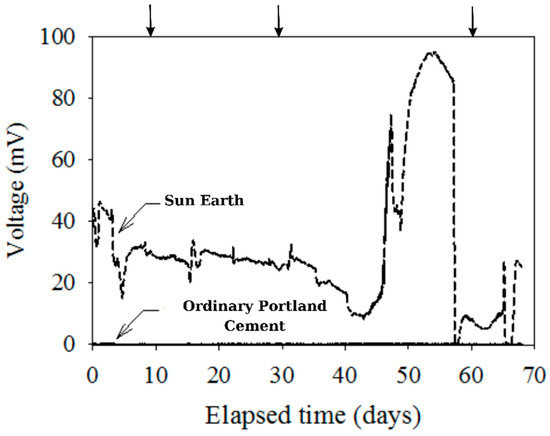
Figure 6.
Experimental results of voltage measurement by time. Arrow (↓) indicates the addition of glucose (100 mg/L) and magnesium sulfate (33 mg S/L).
3.4. Hydrogen Sulfide Suppression Experiment Using Cup-Shaped Specimen
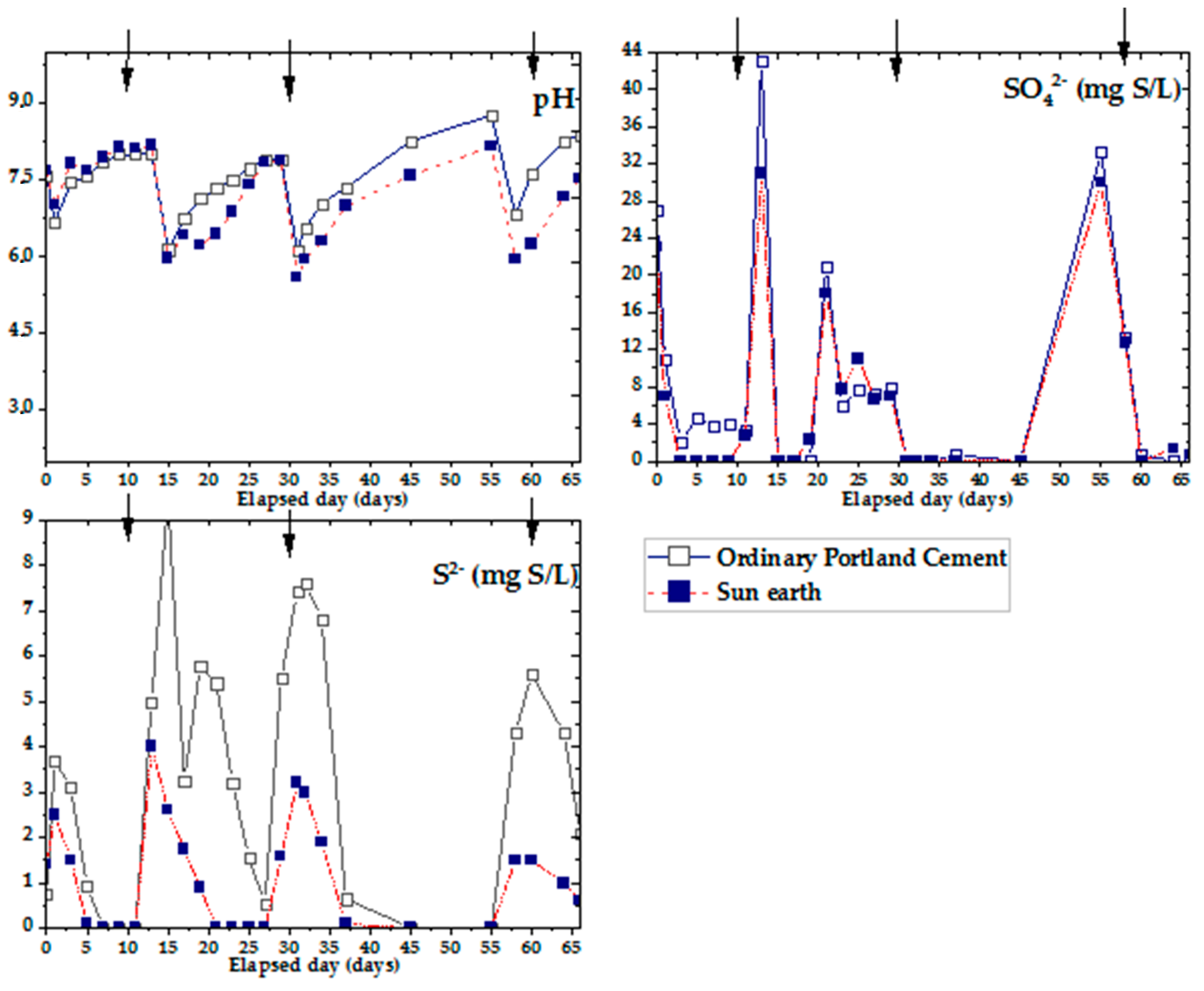
Figure 7 shows the results of changes in pH, sulfate ions and sulfide ions over time using OPC and San-Earth materials for cup-shaped specimens. Arrows appearing in Figure 7 indicate the same conditions as those in Figure 6. The results obtained from the preliminary analysis of pH are shown in Figure 7. After every addition of glucose and sodium sulfate, the pH of both specimens decreased to 5.5, showing a clear trend of organic acid formation that increased over a few days. This was due to volatilization and decomposition of organic acids in the liquid mix. Turning now to the experimental evidence on changes in sulfate ions among both specimen samples, after each stage of adding substrates, the number of sulfate ions fell significantly to approximately 0 mg S/L within only a few days. No significant differences were found between samples of OPC and San-Earth even though there was a slightly higher level of sulfate ions in OPC than in San-Earth.
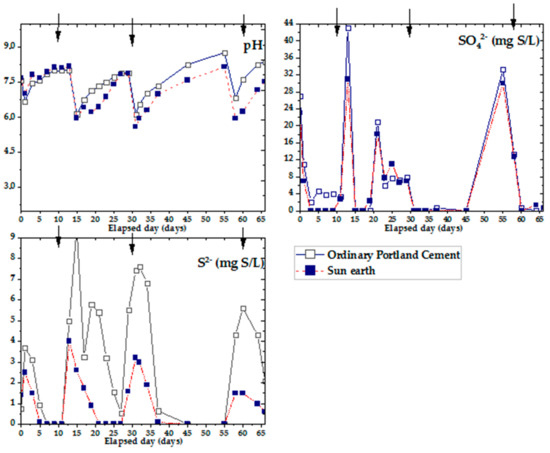
Figure 7.
Experimental results of hydrogen sulfide inhibition effect by OPC and San-Earth over time. Arrow (↓) indicates the addition of glucose (100 mg/L) and magnesium sulfate (33 mg S/L).
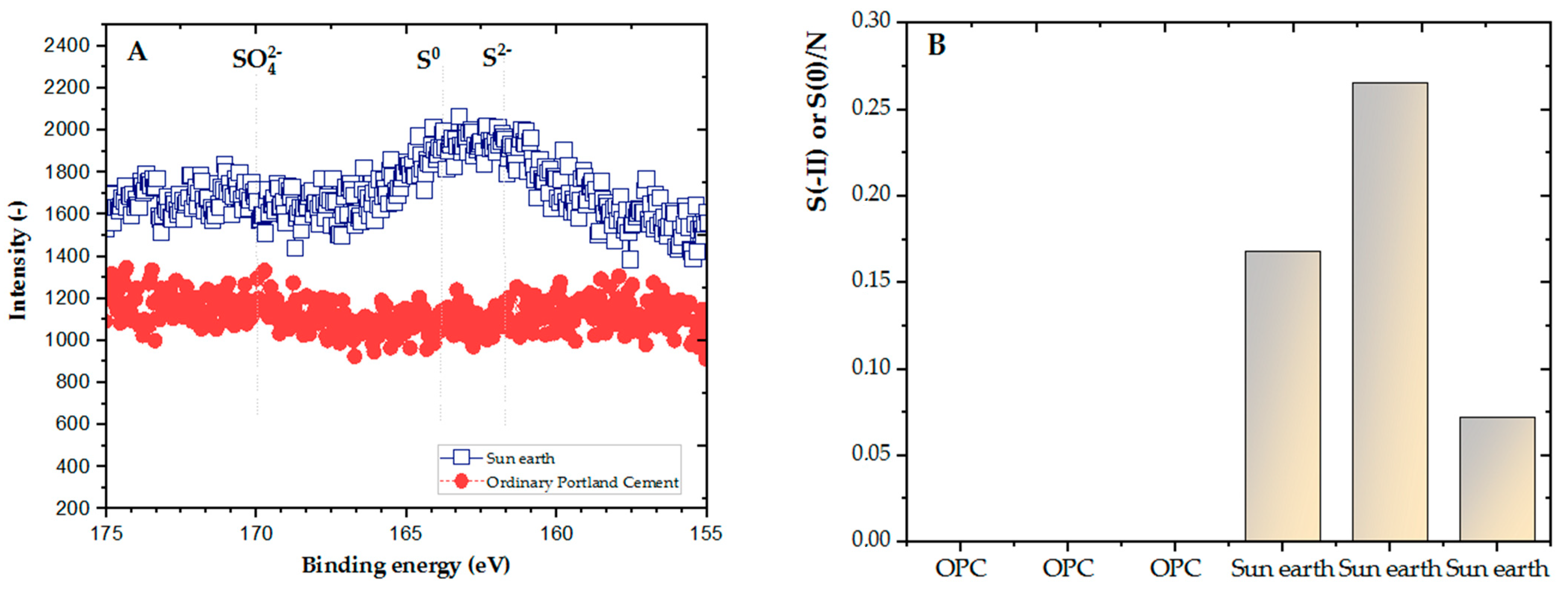
In comparing the results of hydrogen sulfide changes in OPC and San-Earth, the concentration of H2S was observed to be significantly reduced by San-Earth. At every stage, San-Earth was shown to have stable H2S inhibition, reducing H2S to a range of 0–4 mg S/L, whereas when OPC was used, the level of H2S increased to approximately 10 mg S/L. To clarify the cause of H2S reduction in the sample of San-Earth, other results from XPS spectra and analysis of the S/N ratio were evaluated. The S/N ratios of the sludge sediments collected after 66 days from OPC and San-Earth are shown in Figure 8. First, sulfur was almost undetectable in the sludge sediment collected from ordinary Portland cement, being below the limit of quantification (≈0.4 atom.%) in all three samples measured. Conversely, high concentrations of sulfur with S/N ratios of 0.072–0.17 were detected in the sludge sediments collected from San-Earth, and energy shifts indicated that the sulfur in the sludge sediments was elemental sulfur (S0) or sulfide ions (S2−). These two different oxidation numbers of sulfur are difficult to quantitatively separate due to the small difference in energy shift; it is unlikely that S2− accumulated at high concentrations in the sludge only in the case of San-Earth. In other words, the results shown in Figure 8 can be interpreted as the accumulation of hydrogen sulfide after it was oxidized to elemental sulfur in the sludge.
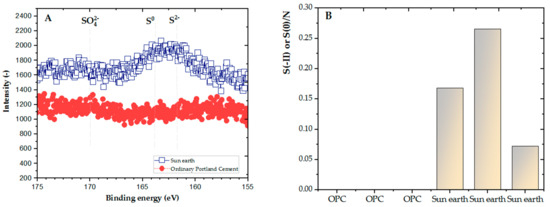
Figure 8.
(A) Result of 2p3/2 spectrum of sulfur in the layer of sediment sludge after 66 days, (B) S/N ratio.
As shown in Figure 8, the reduction and accumulation of hydrogen sulfide in the sludge from San-Earth compared to that in the sludge from OPC revealed interesting outcomes. To gain insight into the mechanism, PCR-DGGE analysis was carried out to evaluate the oxidation of hydrogen sulfide by electron-producing bacteria, which was assumed to be one of the possibilities in this work. The results show that the microbial community structure in the sludge after 66 days of experimentation was different from that in the initial sludge, but no significant differences between OPC and San-Earth were observed. In other words, the accumulation of EPB in San-Earth samples could not be confirmed. This result suggests that the oxidation of hydrogen sulfide by EPB as described did not contribute significantly to the suppression of hydrogen sulfide under the conditions of this study.
3.5. Hydrogen Sulfide Suppression Experiments Using Cup-Shaped Specimens of Sun Earth Sample
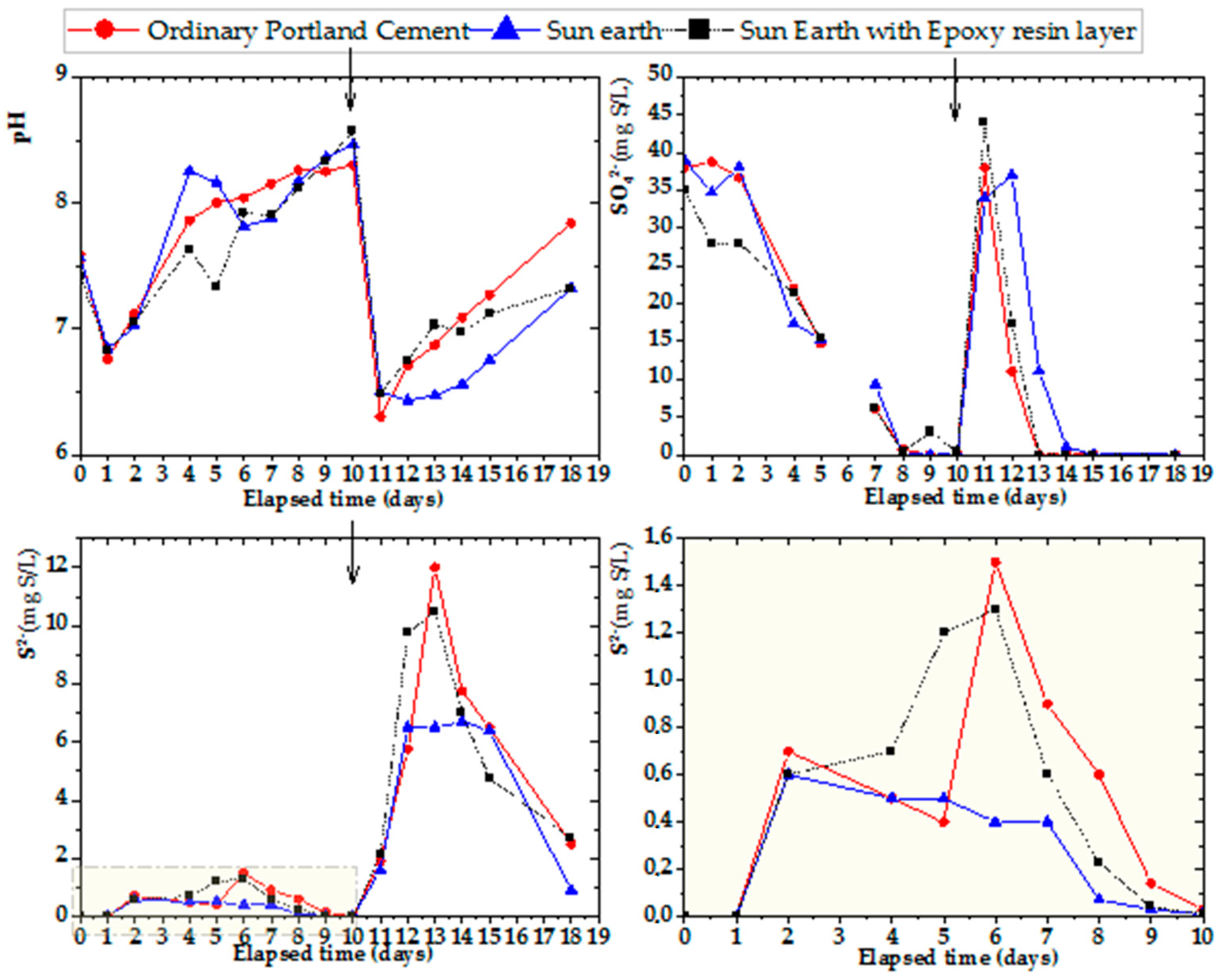
Experiments using cup-shaped specimens for three samples of OPC, San-Earth and San-Earth with an epoxy rinse layer, as presented in Figure 9, were conducted to explore the role of conductive concrete made from San-Earth in the reduction in hydrogen sulfide. As for the previous figures, the arrows in the figure indicate the addition of glucose (100 mg/L) and magnesium sulfate (33 mg S/L). Comparisons between the two stages, the first nine days and the four days after adding the substrates, showed changes in the pH and sulfate ions. At the first stage, the original concentration of sulfate reached 40 mg S/l, and the pH remained at the neutral range of around 7–8.5, which SRB communities prefer, and the complete reduction in SO42− ions took nearly 9 days. Meanwhile, it took only four days to reduce the concentration of sulfate from nearly 45 mg S/L to 0 after the tenth day due to the remarkably low pH. To assess the inhibition effect of sulfide ion, a conductive material was added to the cup-type specimen with an epoxy resin layer. From the graph above in Figure 9, we can see that the outcomes of OPC and San-Earth with epoxy are similar, and almost no inhibitory effect on hydrogen sulfide was observed. In comparison, the specimen without epoxy resin showed a clear inhibitory effect on hydrogen sulfide. In other words, the insertion of the epoxy resin layer led to the loss of the hydrogen sulfide suppression effect of San-Earth.
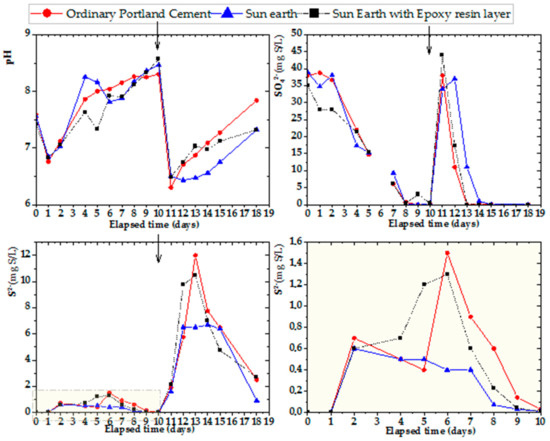
Figure 9.
Experimental results for hydrogen sulfide inhibition effect of San-Earth/San-Earth with epoxy by time. Arrow (↓) indicates the addition of glucose (100 mg/L) and magnesium sulfate (33 mg S/L).
4. Discussion
This study set out with the aim of assessing the importance of conductive concrete in connecting an electron transit for inhibition of H2S in sewer systems. The present work was designed to determine the effect of material made of San-Earth, a commercially available conductive concrete, in comparison with ordinary Portland cement. It was hypothesized that the main mechanisms of hydrogen sulfide suppression are expressed via three possible capacities: first, adsorption of hydrogen sulfide by conductive carbon; second, chemical oxidation of hydrogen sulfide adsorbed on conductive carbon; and third, biological oxidation of H2S by electron-producing bacteria. Each finding clarifies the way in which conductive material made of San-Earth contributes to remove hydrogen sulfur.
Regarding the adsorption of hydrogen sulfide by conductive carbon, San-Earth was found to absorb H2S better than OPC, as shown in Figure 5. The adsorptive capacity of carbon in San-Earth and OPC in this study is corroborated by these earlier findings. Alkaline-activated carbon was found to be effective in removing hydrogen sulfur from the gas phase, and the mechanism and surface area of the micropores in relation to the adsorption capacity have been widely discussed [16,17,18]. One of the issues that emerges from these findings is chemisorption on the surface of activated carbon, which occurs faster than physisorption in the pores present in the interior. This enables amorphous carbon in San-Earth to suppress H2S in the gas phase or its evaporation in the liquid phase and minimizes sulfuric acid formation. However, this study was unable to demonstrate that the adsorption capacity of conductive carbon is the main mechanism by which H2S is inhibited. As shown by the results in Figure 9, inserting an epoxy rinse layer completely isolated electrons from inside conductive concrete made of San-Earth, and the OPC and San-Earth material with an epoxy layer exhibited less H2S suppression than the cup specimen made of only San-Earth material. Therefore, the possibility of a biological or chemical oxidation mechanism is indicated. Hydrogen sulfur could oxidize to elemental sulfur (S0) by oxygen after adsorption [18,19]. Iron oxides in the available concrete used as part of the raw materials contribute as a catalyst to the oxidation of hydrogen sulfide with coexisting oxygen [20]. In this study, hydrogen sulfur was generated from an anaerobic environment in a sludge sediment layer without the presence of oxygen. This combination of findings provides some support for the conceptual premise that there is an electron pathway allowing conductive concrete to enable biological or chemical oxidation of H2S. In other words, oxygen at the surface as an electron acceptor oxidized hydrogen sulfur at the anode area.
It can thus be suggested that EPB are released to generate electrons when organic matter and hydrogen sulfide are oxidized. This also agrees with earlier studies, in which EPB communities were investigated as microbial fuel cells for biological energy [20,21,22]. To gain energy from a microbial fuel cell, an anode electrode made from conductive materials that collects the electrons discharged by EPB and an electron acceptor that receives the electrons on the surface of the cathode electrode are required [23]. Herein, it was shown amorphous carbon contained in San-Earth plays a role as an anode electrode to act as a bridge from the electrons to the acceptor. PCR-DGGE was used to analyze the relationship between microbial communities of OPC and San-Earth. No significant differences were found between these samples. The various performances shown in Figure 9 indicate that the contribution of EPB in the oxidation of hydrogen sulfide is relatively small or no significant reduction in H2S was induced by biological oxidation. The single most striking observation to emerge from the data comparison for chemical oxidation of hydrogen sulfide adsorbed on conductive carbon is the main mechanism of hydrogen sulfide suppression.
5. Conclusions
This work presents the noteworthy discovery that San-Earth, a commercial conductive concrete, may be used to control H2S in sewer systems. The present study was designed to determine the effect of conductive concrete to demonstrate its suppressive capacity for hydrogen sulfide production and its mechanism. One of the more significant findings to emerge from the experimental results is that, even in an anaerobic environment, conductive concrete is an effective new solution for controlling hydrogen sulfide; taken together, these results suggest that conductive concrete acts as an electron transfer pathway and chemically oxidizes hydrogen sulfide using oxygen near the water surface as an electron acceptor. The current research was not specifically designed to evaluate factors related to the measurement of the concentration of hydrogen sulfide in the gas phase and the pH of the concrete surface or to conduct such a long experiment; further investigation and experimentation regarding San-Earth is strongly recommended to achieve a clearer understanding of the mechanism of hydrogen sulfide suppression, which is expected to change over time. Therefore, the goal of further research is to develop a new conductive concrete specifically designed for the control of hydrogen sulfide.
Author Contributions
Conceptualization and writing—original draft preparation, H.T.V.; methodology, validation and supervision, T.I.; methodology, visualization and software, M.F.; formal analysis, M.F. and T.S.; investigation and project administration, T.I., H.S. and T.H.; writing—review and editing, Y.-T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded a part by JSPS KAKENHI (20K04749); GAIA Project, Ministry of Land, Infrastructure, Transport and Tourism (No. 4).
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to Shuji Tanaka, a consultant for TNK Waterworks Co., Ltd., for his useful comments and advice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Firer, D.; Friedler, E.; Lahav, O. Control of sulfide in sewer systems by dosage of iron salts: Comparison between theoretical and experimental results, and practical implications. Sci. Total Environ. 2008, 392, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, O.; Mohanakrishnan, J.; Sharma, K.R.; Meyer, R.L.; Keller, J.; Yuan, Z. Evaluation of oxygen injection as a means of controlling sulfide production in a sewer system. Water Res. 2008, 42, 4549–4561. [Google Scholar] [CrossRef]
- Gutierrez, O.; Sudarjanto, G.; Ren, G.; Ganigué, R.; Jiang, G.; Yuan, Z. Assessment of pH shock as a method for controlling sulfide and methane formation in pressure main sewer systems. Water Res. 2014, 48, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Gutierrez, O.; Sharma, K.R.; Keller, J.; Yuan, Z. Optimization of intermittent, simultaneous dosage of nitrite and hydrochloric acid to control sulfide and methane productions in sewers. Water Res. 2011, 45, 6163–6172. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Keating, A.; Corrie, S.; O’halloran, K.; Nguyen, L.; Yuan, Z. Dosing free nitrous acid for sulfide control in sewers: Results of field trials in Australia. Water Res. 2013, 47, 4331–4339. [Google Scholar] [CrossRef]
- Sun, X.; Jiang, G.; Bond, P.L.; Keller, J.; Yuan, Z. A novel and simple treatment for control of sulfide induced sewer concrete corrosion using free nitrous acid. Water Res. 2015, 70, 279–287. [Google Scholar] [CrossRef]
- Li, X.; Jiang, G. Mitigation of Microbially Influenced Corrosion of Concrete Sewers Using Nitrite. In Biotechnological Innovations for Environmental Bioremediation; Arora, S., Kumar, A., Ogita, S., Yau, Y.-Y., Eds.; Springer: Singapore, 2022; pp. 119–135. ISBN 978-981-16-9001-3. [Google Scholar]
- Pikaar, I.; Flugen, M.; Lin, H.W.; Salehin, S.; Li, J.; Donose, B.C.; Dennis, P.G.; Bethke, L.; Johnson, I.; Rabaey, K.; et al. Full-scale investigation of in-situ iron and alkalinity generation for efficient sulfide control. Water Res. 2019, 167, 115032. [Google Scholar] [CrossRef]
- Nemati, M.; Jenneman, G.E.; Voordouw, G. Mechanistic study of microbial control of hydrogen sulfide production in oil reservoirs. Biotechnol. Bioeng. 2001, 74, 424–434. [Google Scholar] [CrossRef]
- Lee, E.K.; Jung, K.D.; Joo, O.S.; Shul, Y.G. Liquid-phase oxidation of hydrogen sulfide to sulfur over CuO/MgO catalyst. React. Kinet. Catal. Lett. 2005, 87, 115–120. [Google Scholar] [CrossRef]
- Chang, Y.J.; Chang, Y.T.; Chen, H.J. A method for controlling hydrogen sulfide in water by adding solid phase oxygen. Bioresour. Technol. 2007, 98, 478–483. [Google Scholar] [CrossRef]
- Aguilar, L.; Zha, S.; Cheng, Z.; Winnick, J.; Liu, M. A solid oxide fuel cell operating on hydrogen sulfide (H2S) and sulfur-containing fuels. J. Power Sources 2004, 135, 17–24. [Google Scholar] [CrossRef]
- Marcus, A.K.; Torres, C.I.; Rittmann, B.E. Conduction-based modeling of the biofilm anode of a microbial fuel cell. Biotechnol. Bioeng. 2007, 98, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Imai, T.; Ukita, M.; Sekine, M.; Higuchi, T. Triggering forces for anaerobic granulation in UASB reactors. Process. Biochem. 2006, 41, 36–43. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; De Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Kubota, M.; Matsuda, H.; D’Elia Camacho, L.F. Adsorption behaviors of ammonia and hydrogen sulfide on activated carbon prepared from petroleum coke by KOH chemical activation. Fuel Process. Technol. 2016, 144, 164–169. [Google Scholar] [CrossRef]
- Shi, L.; Yang, K.; Zhao, Q.; Wang, H.; Cui, Q. Characterization and Mechanisms of H2S and SO2 Adsorption by Activated Carbon. Energy Fuels 2015, 29, 6678–6685. [Google Scholar] [CrossRef]
- Katoh, H.; Kuniyoshi, I.; Hirai, M.; Shoda, M. Studies of the oxidation mechanism of sulphur-containing gases on wet activated carbon fibre. Appl. Catal. B Environ. 1995, 6, 255–262. [Google Scholar] [CrossRef]
- Yan, R.; Liang, D.T.; Tsen, L.; Tay, J.H. Kinetics and mechanisms of H2S adsorption by alkaline activated carbon. Environ. Sci. Technol. 2002, 36, 4460–4466. [Google Scholar] [CrossRef]
- Rabaey, K.; Van De Sompel, K.; Maignien, L.; Boon, N.; Aelterman, P.; Clauwaert, P.; De Schamphelaire, L.; Pham, H.T.; Vermeulen, J.; Verhaege, M.; et al. Microbial fuel cells for sulfide removal. Environ. Sci. Technol. 2006, 40, 5218–5224. [Google Scholar] [CrossRef]
- Liu, S.H.; Lai, Y.C.; Lin, C.W. Enhancement of power generation by microbial fuel cells in treating toluene-contaminated groundwater: Developments of composite anodes with various compositions. Appl. Energy 2019, 233–234, 922–929. [Google Scholar] [CrossRef]
- González, T.; Ureta-Zañartu, M.S.; Marco, J.F.; Vidal, G. Effect of Zeolite-Fe on graphite anode in electroactive biofilm development for application in microbial fuel cells. Appl. Surf. Sci. 2019, 467–468, 851–859. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).