A Review on Cadmium and Lead Contamination: Sources, Fate, Mechanism, Health Effects and Remediation Methods
Abstract
1. Introduction
- i.
- The source of cadmium and lead in soil and food plants.
- ii.
- The accumulation mechanism and fate of cadmium and lead in soil and plant systems.
- iii.
- The human health risks resulting from cadmium and lead accumulation and exposure.
- iv.
- The possible remediation technologies.
2. Methods and Scope of Study
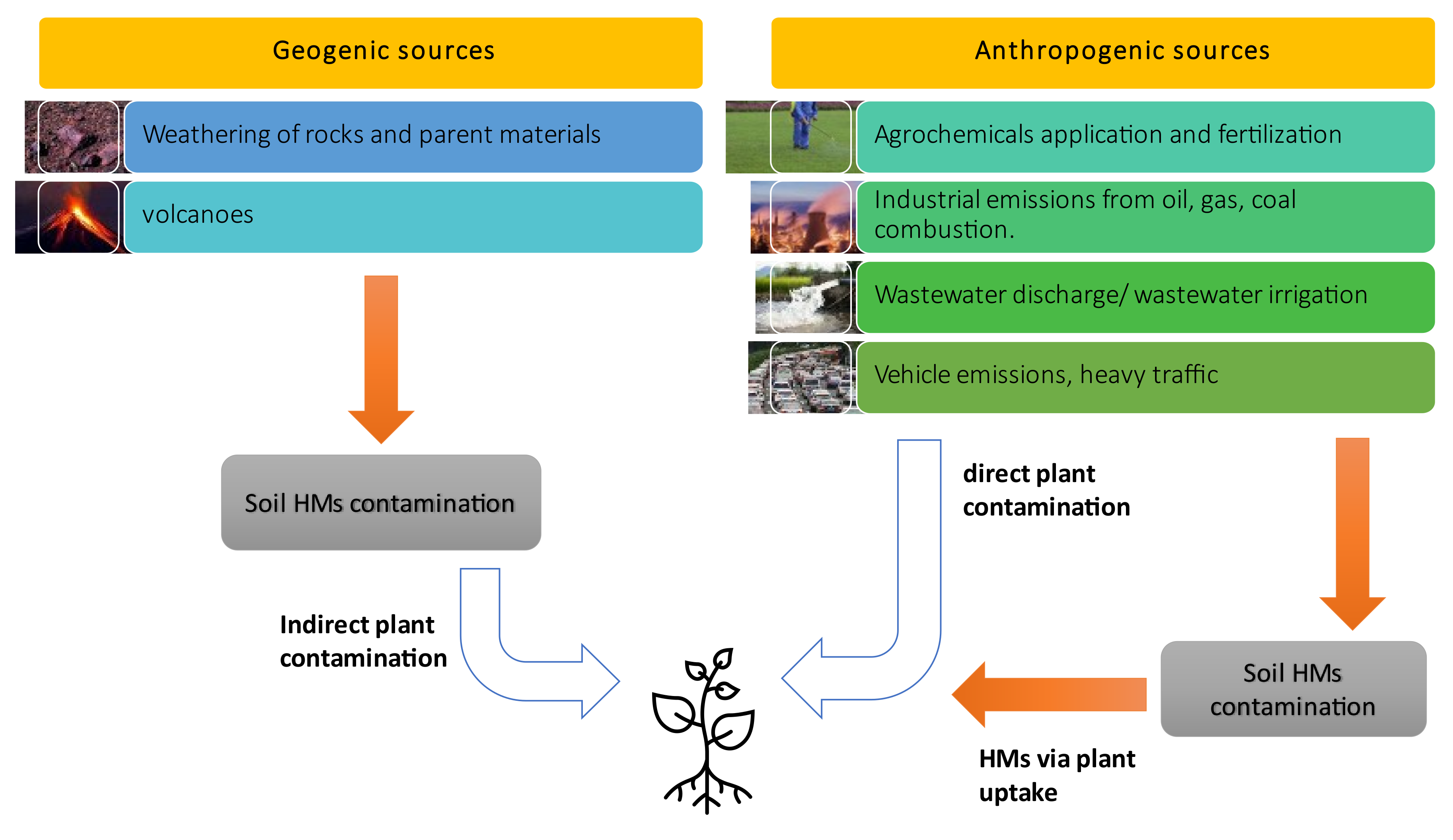
3. Sources of Cadmium and Lead in Both Soil and Plants
| Plants | Soil | Origin of Cd/Pb in That Region | Summary and Main Finding | Reference |
|---|---|---|---|---|
| Elytrigia repens Galium verum Phragmites australis Wildflower | Composite soil from surface and subsoil layer | Estuarine floodplain pollution (human factors) | The study revealed the anthropogenic source of heavy metals in the surface soil, and it was found that heavy metals can be mobilised by root exudates and become phyto-available, as shown by the increased plant uptake of HMs due to their non-soluble nature. | [40] |
| Rice, Chinese cabbage, Spinach, Cowpea | Paddy fields, vegetable field, dumping site, burning site, and acid leaching site | Abandoned e-waste recycling site | The study showed that pond water was utilized for irrigation, crops on agricultural fields can be polluted, which would have a negative impact on human health after being consumed. Aquatic life in the pond had become highly acidified and polluted with heavy metals due to previous recycling activities. | [41] |
| Bok choy Water spinach Shanghai green cabbage Leaf lettuce | Agriculture soils and road dust | Coal-fired plants, Stationary industrial emissions, Municipal waste incineration emissions. | The finding of this study highlighted that the amount of heavy metal pollution varies with metal species, location, and environmental medium. | [23] |
| Woody, shrubby, and herbaceous plants species | Non-farmland soil (Pb smelter) | Heavy metals emissions from Pb smelter | The finding showed that plants with lower BCF values may be able to be reproduced and seeded in polluted areas, which might minimise heavy metal build-up in the food chain. | [42] |
| Rice grain, corn kernels, and vegetables | Soil (rhizosphere part) | Smelting-mining. Vehicle emissions from diesel fuels. Atmospheric dust fall. | The study revealed that the amounts of HMs found in the soil-dust-fall plant system in the investigated area varied depending on the metal type and the environment. Compared to corn kernels, rice grains have a greater potential to enrich HMs. | [29] |
| (Wheat, rice, maize) grains, mustard seeds | Soil from agriculture fields | Thermal power plant (fly ash deposition). Agrochemicals application. Cement factory. | An investigation of HMs concentration in crop fields near to a thermal power plant; the final finding shows that HMs in that area cause a non-cancer health risk to the residents. | [22] |
| Brown rice | Soil near three mine areas | Nonferrous metal industry Mining and smelting | The investigation revealed the hyperaccumulation tendency of HMs in rice grains particularly in mining areas; rice has been identified as a major source of heavy metal exposure. | [43] |
| - | Soil from different land use (aquacultural pond, barren land, built-up land, dry land, inland halophyte, intertidal flats, open water, reed marsh and rice paddy) | Industrial and agricultural wastewater discharge, domestic sewage discharge, atmospheric deposition. | As a result of these findings, heavy metal concentrations in soil and sediment of a long-term reclaimed region might be affected by land-use intensity differences. It was shown that soil characteristics such as soil organic carbon and grain size had a significant impact on the dispersion of heavy metals. | [27] |
| Rice grain (Oryza sativa L.) | Agricultural soil/agrochemicals | Agrochemicals | The finding demonstrated the correlation between cadmium concentration and the soil pH when the increased level of cadmium is related to the decreased soil pH. Furthermore, some traditional cultivars, including Pachcha Perumal, consistently shown very high resistance to cadmium absorption in both seasons. When compared to other farmed types, the Madathawalu and Kuruluthuda cultivars were relatively resistant. | [44] |
| - | Chinese Natural ecosystems (agricultural, aquatic, desert, forest, grassland, Karst ecosystems) | Atmospheric deposition from oil and coal consumption. Number of vehicles | In this study, the wet deposition of lead and cadmium was shown to be positively linked to cadmium and lead soil concentration which confirms the atmospheric deposition origin of HMs in that region. | [30] |
| - | Surface soil | Atmospheric deposition from coal combustion, chemical factories, iron and steel smelting, heavy traffic. | The results showed that cadmium air deposition fluxes were highest in a coal mining area and significantly lower in a rural area. Coal combustion connected to chemical and metallurgical industries was identified as the primary cause of cadmium pollution in Lianyuan city’s atmosphere. | [32] |
| - | Peri-urban agricultural soils | Fertilization and atmospheric deposition | The investigation revealed that the intensive agricultural output led to the build-up of heavy metals in the soil, a reduction in soil pH, and an increase in soil organic matter content (SOM) and according to the results of the lead isotope ratio analysis IRA, input fluxes analysis (IFA), and positive matrix factorization (PMF) models, air deposition and fertilization were the primary causes of heavy metal accumulation in soils. | [28] |
| Pakchoi (Brassica chinensis L.) | Soil reciprocal (designed for the experiment) | Atmospheric deposition from copper smelter | The study showed that the substantial proportion (20–85%) of cadmium, and lead discovered in the edible shoot of pakchoi near the copper smelter was caused by freshly deposited metals in the atmosphere, which also resulted in a higher health risk of pakchoi intake. These findings demonstrated that the function of freshly deposited heavy metals was critical in the risk management of heavy metal pollution in soil-vegetation systems. | [33] |
| - | Urban road surface | Direct deposition of lead on the urban road | The results show that lead contributed to road build-up and the atmosphere by the soil along the road that has been disturbed by natural and traffic-induced wind. In comparison to atmospheric deposition, direct deposition is the most common route for lead to reach the roadways. | [45] |
| - | Agricultural soils | Agrochemicals, atmospheric deposition and industrial emissions, sewage irrigation, leather tanning industry. | This resource-based region’s agricultural soils were extensively contaminated with lead and cadmium. The primary causes of contamination were human activities such as agriculture and sewage irrigation, as well as industrial and atmospheric pollutants. | [46] |
| Vegetables and grains | Agricultural soils | Weathering of parent materials, phosphorus fertilizers | The overall finding showed that heavy metal contents in soil are naturally occurring and due to basaltic parent material, that forms the soil, and it increases with the increase of the weathering duration. In addition, the usage of phosphorus fertilizers may have altered the cadmium content of the soil. | [38] |
| - | Agricultural soil | Agrochemicals and fertilization | The various accumulation patterns observed in wheat field soil revealed that the accumulation level of HMs and REEs in the soil is connected to the continuous application of fertilizers, in addition to pesticides and herbicides, over time. | [37] |
| - | Soil from shallot fields | Long-term fertilization, pesticides, organic manure. Parent materials. | In this study, cadmium concentrations are induced by agricultural activities such as long-term applications of animal manure, insecticides, and phosphorus fertilizers. The lead concentration of the agricultural soils is thought to be controlled by natural sources such as lithogenic factors and parent materials. | [39] |
| - | Agricultural soil | Agrochemicals, atmospheric dust, traffic density Fossil fuel combustion from gas and oil fields nearby, transported by dust storms. Non-crustal sources. | According to the results obtained from PERI, cadmium contributed 97.2% to the total potential ecological risk in the soils. Cadmium concentrations in soil samples above background values for continental crust and average global soils. | [47] |
| Carrot and cabbage | Carrot and cabbage soil | Wastewater irrigation, fertilizer application | The results revealed that both the cabbage and carrots, as well as the soils, had heavy metal levels over the threshold standards specified by international organizations regulating food safety. The intensive application of fertilizers resulted a decrease in pH and organic matter production rates in soil. | [48] |
4. Cadmium and Lead Contamination Levels in Different Types of Soil and Plant
4.1. Cadmium and Lead Contamination in the Soil Ecosystem
4.2. Cadmium and Lead Contamination in Plants
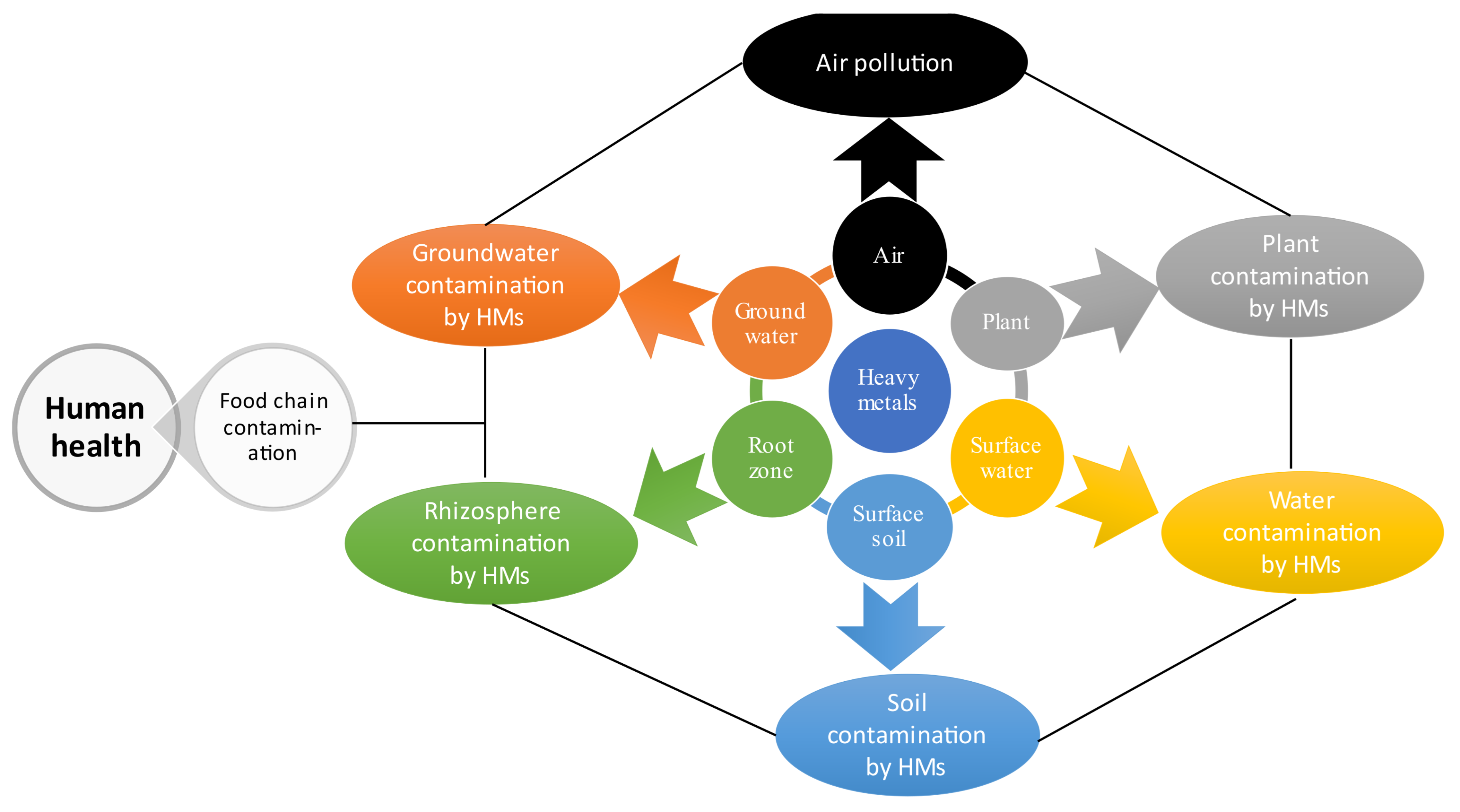
5. Fate and Accumulation Mechanisms of Cadmium and Lead in Soil-Plant System
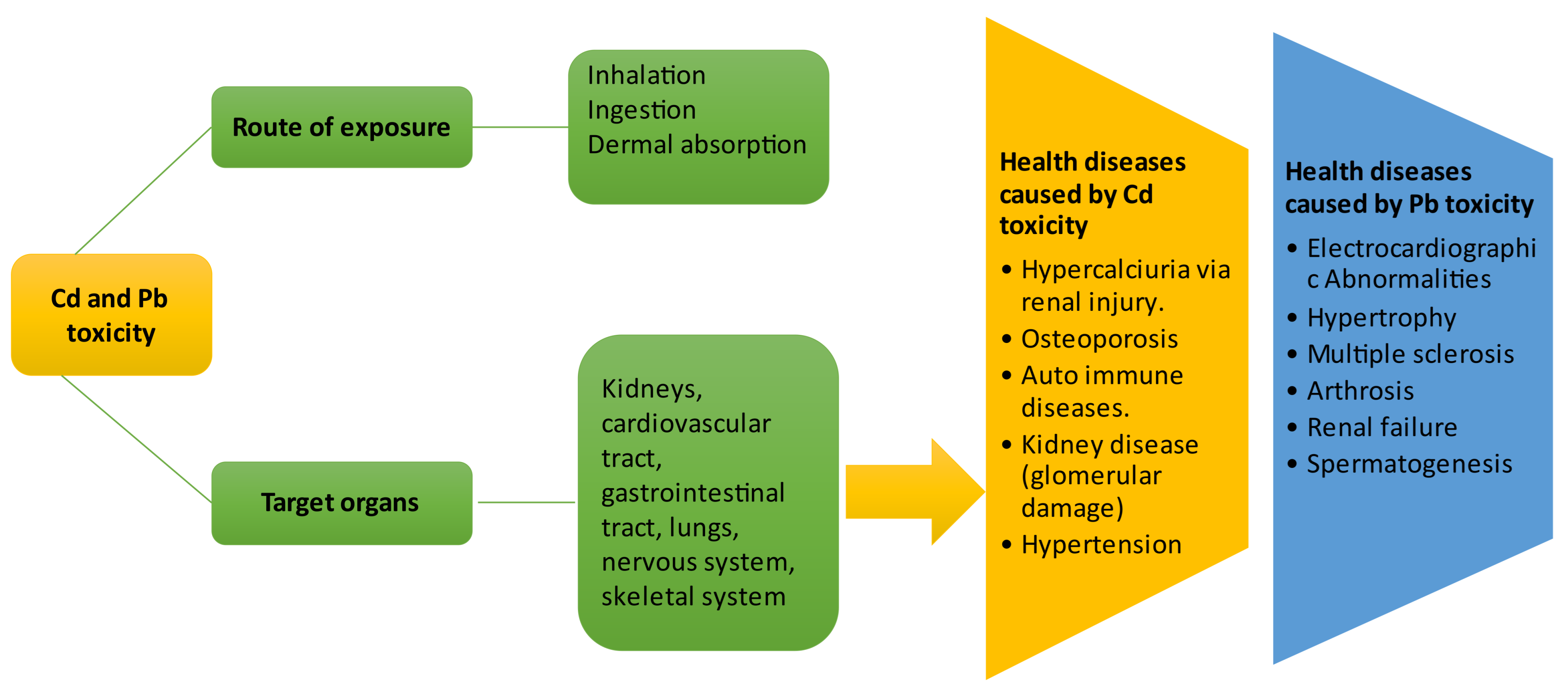
6. Health Effects of Cadmium and Lead Exposure
7. The Remediation and Mitigation Techniques and Strategies
| Contaminant | Reagent/Plant Species Used | Main Finding and Summary | Advantages | Limitations | Reference |
|---|---|---|---|---|---|
| Cadmium from agricultural soil | fulvic acid-aided hydroxyapatite nanofluid. Nanotechnology | The findings show that nHAP nanofluid has a high potential for removing cadmium from polluted soils, and that organic acids play a critical role in assisting the process provided proper subsurface drainage and leachate collecting systems are present on-site. | Environment friendly. cost-effective nanosuspension. | Doubt in separation of the nanomaterials from soil particles. | [110] |
| Lead from agricultural soil | biodegradable chelators (N, N-dicarboxymethyl glutamic acid tetrasodium salt (GLDA), ascorbic acid and citric acid). Soil washing | Pb removal might be considerably improved by combining GLDA with CA (citric acid) and ASC (ascorbic acid). The findings of this study imply that a GLDA-ASC combination might be a viable alternative for Pb elimination. | Permanent results Low-cost method Does not take a long time The used chelating agents are biodegradable. GLDA has a low ecological footprint. Applicable for soils with high HMs contamination rates. | Lab based experiment. | [111] |
| Cadmium | Sorghum bicolor Phytoremediation | Cadmium at low levels might enhance the development of S. bicolor, while S. bicolor could not accumulate cadmium at high levels. Many variables impact cadmium absorption by plants, including soil quality, plant species, soil microorganisms, and cadmium species in soil. Under cadmium stress, a high level of microbial diversity was discovered, which influenced plant growth. | High biomass yield and rapid growth. High tolerance to adverse environment and ability to produce bioenergy. Effective, economic, and environment friendly method | The S. bicolor cannot accumulate Cd in high levels. Lab-based experiment. | [112] |
| Cadmium | Two ecotypes of Bidens pilosa L. Phytoextraction | The net photosynthetic rate, transpiration rate, SOD activity, and extractable Cd content were all greater after HAE (Hanzhong ecotype of Bidens pilosa L.) treatment than after SHE (Shenyang ecotype of Bidens pilosa L.) treatment. These characteristics may be partly responsible for HAE’s increased cadmium accumulation from soil, indicating a genetic foundation for this hyperaccumulator’s enhanced tolerance and accumulation. | Cost-effective and environment friendly technology. | Small scale only. | [113] |
| Lead | Ethylene-diamine-teraacetic acid disodium salt (EDTA-2Na) combined with diluted deep eutectic solvent (DES). Soil washing | The treated soil showed no corrosion or mineralogical alterations because of the chemical washing. The washing process had no discernible effect on the mineral phase of the soil or the functional groups of CEEN. As a result, this method may be used to treat lead-contaminated soil. | Rapid and efficient remediation method. Environmentally friendly, non-toxic, and affordable. | High viscosity of the reagents makes recycling the soil after remediation challenging, and a dilution of the reagents is required. | [114] |
| Cadmium | Amaranthus hypochondriacus L. Phytoremediation | The level of soil cadmium contamination and soil CEC both influenced grain amaranth growth and cadmium accumulation. The results indicated that low soil CEC is a significant limiting factor impacting the phytoremediation efficacy of grain amaranth. | Cost-effective and eco-friendly method. | Small-scale. | [115] |
| Lead and cadmium | Extracted water from Fagopyrum esculentum and Fordiophyton faberi Soil washing | The study showed the capacity of plant solutions in soil washing, and results revealed the ability of the prepared solutions to extract lead and cadmium from soil, where F. esculentum has shown higher extraction levels than F. faberi. | When compared to EDTA, plant washing agents perform better in terms of lowering metal hazards and mitigating the impact of washing on soil chemical characteristics. | Small-scale only. | [116] |
| Cadmium | Biosurfactant: rhamnolipid (RL) and saponin (SP) Soil amendment | The findings indicate that using biosurfactants can help in soil remediation by corn where the results reveal that raising the biosurfactant concentration from 1 to 5 mmol kg−1 influenced the amount of Cd leached out of the soil samples. | It does not need a disposal site. The biosurfactants can increase the corn biomass. The produced biomass can be used as bioenergy. | It requires a suitable place/plant. | [117] |
| Lead | Biochar (magnetic biochar MBC) Soil amendment | Results revealed that MBC can efficiently remediate lead contaminated soils. The key parameters influencing Pb removal effectiveness from soils were Pb sorption capacity by magnetic biochar, magnetic biochar recovery efficiency, and chemical forms of lead in soils. | Feasible approach for lead contaminated soils. | Soil type and magnetic biochar can influence the efficiency of the remediation. | [118] |
| Lead and Cadmium | Adsorbent (bentonite) Soil immobilization | Bentonite additions lowered the exchangeable proportion of cadmium and lead, the majority of which was transformed into inaccessible forms. Cadmium and lead translocation from soil to aerial portions of Oryza sativa L. was inhibited by bentonite treatments. | Short cycle, cost-effective. Easy to implement. High efficiency. | The immobilised heavy metals can still be present in soils and may be released back into water if soil conditions alter. | [119] |
| Lead and cadmium | Newly modified material fly ash (NA), zeolite (ZE), and fly ash (FA). Soil amendment | The use of NA and ZE lowered the concentration of accessible metal ions and reduced cadmium/lead accumulation and capability for sequestration, resulting in a decrease in the acid-exchangeable fraction of cadmium/lead and an increase in the oxidizable and residual fractions. | Can be applicable in field. Sustainable treatment. | Secondary pollution may be caused. Side effects of the fly ash. Cannot be used to remediate a mixed heavy metal pollution. | [120] |
| Lead and cadmium | Thiol-modified rice straw biochar Soil immobilization | These findings imply that RS (rice straw) might be a viable remediation solution for heavy metal pollution in water and soil. | Effective method for cadmium and lead remediation. | It may influence the microbial activity. Difficult to separate from soil after treatment. | [121] |
| Cadmium | Non-magnetic silicate bonded biochar (SBC) and magnetic silicate bonded biochar (MSBC) Soil amendment | The biochar substance has magnetic characteristics and can be separated by magnetic field force, making it ideal for recycling and secondary use. | The ability to reduce cadmium bioavailability in soil. Good adsorption and passivation performance. The treated soil with silicate bonded biochar helps in the plant growth. | It requires more than one cycle. In-situ passivation cannot completely remove Cd from soil. | [122] |
| Cadmium | Composite (biochar-supported iron phosphate nanoparticles, sodium carboxymethyl cellulose) Soil immobilization | The findings of consecutive extraction techniques revealed that the decrease in Cd bioavailability in soils was caused by the change of more conveniently extractable Cd to the least accessible form. Experiments on plant development showed that the composite may prevent Cd absorption to both the belowground and above-ground parts of the plant. | The method can immobilize cadmium in soil by reducing its bio accessibility. Short term method. The composite had the ability to successfully change a more bioavailable cadmium speciation into a significantly less bioavailable speciation. It helps in promoting the plant growth. | It may influence the soil fertility. It needs more field investigations. Secondary pollution. Chemical immobilisation might not reduce heavy metal concentrations in the long-term. | [123] |
| Cadmium | Sunflower (Helianthus annuus L.) + chelating agents (citric acid (CA), oxalic acid (OA) and ethylenediamine disuccinate (EDDS). Phytoremediation | According to the findings, chelating chemicals exacerbated the negative effects of Cd combined stress on the sunflower by lowering plant biomass and limiting photosynthesis, while boosting sunflowers’ ability to absorb and transport Cd to variable degrees. | Phytoremediation potential can be increased by adding chelating agents (chelating agents can stimulate the metals uptake by plants). Cost-effective and eco-friendly method. High biodegradability of the chelating agents. Sunflower has high bioaccumulation capacity, strong stress tolerance and short growth cycle. | Enhanced plant stress, decreasing biomass, inhibiting photosynthesis, and increasing malondialdehyde and H2O2 levels. Cannot be used in highly contaminated regions. | [124] |
| Cadmium | Electrokinetic remediation | The cross-impact of factors affects Cd migration in soil, and effective in-situ removal of Cd from soil may be obtained by adjusting parameters appropriately. | Minimal soil disruption. Suitable for low permeability soil. High removal efficiency. | The degree of solubilization and desorption of the metal may impact the removal efficiency. | [125] |
| Lead | Ammonium-based deep eutectic solvents with saponin (DESs) Soil washing | This study indicates the appropriateness of employing DESs in conjunction with saponin for soil washing. The DESs and saponin worked better when used together rather than separately, showing a synergistic behaviour in which they both contribute to Pb2+ removal from soil. | Biodegradable and low-cost solvents. Sustainable technique. | Need further investigations. | [126] |
| Lead | Helianthus annuus L. Phytoextraction | The study revealed the ability of five varieties of Helianthus annuus L. to extract Pb from contaminated soil. The potential of Phule Bhaskar to accumulate Pb is larger than that of the other varieties. (it took 60 days) | Green technology, cost-effective. | It requires a long time for plant growth and metals uptake. Long life cycle. Low removal efficiency. | [127] |
| Cadmium | Cation exchange resin (CER), biochar (BC), and steel slag (SS). Soil amendment | The results illustrated that the used device with the chosen amendments (CER, BC, SS) could eliminate Cd in soil, and reduce Cd levels in rice grain as well. | Easy implementation. Cost-effective. Reusable in-situ technique. Stimulates rice development. Improves crop photosynthesis. Minimizes oxidative damage. Diversifies the microbial population. Lower rice grain HRI. | [128] | |
| Lead and cadmium | Statice (Limonium sinuatum (L.) Mill) Phytostabilization | The study revealed that mycorrhizal plants produced more biomass at the highest Cd or Pb doses. Thus, Statice plant is a viable alternative for revegetating lead or cadmium-polluted industrial sites or urban landscapes, especially following mycorrhizal inoculation. | Plant roots have higher capability to accumulate the metals than other plant parts. | Pb and Cd could inhibit the plant growth and lead to metal stress. | [129] |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Nkwunonwo, U.C.; Odika, P.O.; Onyia, N.I. A review of the health implications of heavy metals in food Chain in Nigeria. Sci. World J. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zarcinas, B.A.; Ishak, C.F.; McLaughlin, M.J.; Cozens, G. Heavy metals in soils and crops in southeast Asia. 1. Peninsular Malaysia. Environ. Geochem. Health 2004, 26, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Budianta, W. Lead contamination in soil of Yogyakarta city, Indonesia. J. Appl. Geol. 2015, 4, 90–98. [Google Scholar] [CrossRef]
- Gottesfeld, P.; Were, F.H.; Adogame, L.; Gharbi, S.; San, D.; Nota, M.M.; Kuepouo, G. Soil contamination from lead battery manufacturing and recycling in seven African countries. Environ. Res. 2018, 161, 609–614. [Google Scholar] [CrossRef]
- Ericson, B.; Otieno, V.O.; Nganga, C.; St Fort, J.; Taylor, M.P. Assessment of the presence of soil lead contamination near a former lead smelter in Mombasa, Kenya. J. Health Pollut. 2019, 9, 190307. [Google Scholar] [CrossRef]
- Peng, T.; O’Connor, D.; Zhao, B.; Jin, Y.; Zhang, Y.; Tian, L.; Zheng, N.; Li, X.; Hou, D. Spatial distribution of lead contamination in soil and equipment dust at children’s playgrounds in Beijing, China. Environ. Pollut. 2019, 245, 363–370. [Google Scholar] [CrossRef]
- Amphalop, N.; Suwantarat, N.; Prueksasit, T.; Yachusri, C.; Srithongouthai, S. Ecological risk assessment of arsenic, cadmium, copper, and lead contamination in soil in e-waste separating household area, Buriram province, Thailand. Environ. Sci. Pollut. Res. 2020, 27, 44396–44411. [Google Scholar] [CrossRef]
- Khan, A.Z.; Khan, S.; Muhammad, S.; Baig, S.A.; Khan, A.; Nasir, M.J.; Azhar, M.; Naz, A. Lead contamination in shooting range soils and its phytoremediation in Pakistan: A greenhouse experiment. Arab. J. Geosci. 2021, 14, 1–7. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhao, K.L.; Fu, W.J. Spatial Distribution Characteristics and Risk Assessment of Cadmium Pollution in Soil-crops system of an E-waste Dismantling Area. Environ. Sci. 2021, 42, 4432–4440. [Google Scholar] [CrossRef]
- Khan, N.H.; Nafees, M.; Bashir, A. Study of heavy metals in soil and wheat crop and their transfer to food chain. Sarhad J. Agric. 2016, 32, 70–79. [Google Scholar] [CrossRef]
- Tighe, M.; Beidinger, H.; Knaub, C.; Sisk, M.; Peaslee, G.F.; Lieberman, M. Risky bismuth: Distinguishing between lead contamination sources in soils. Chemosphere 2019, 234, 297–301. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, D.; O’Connor, D.; Shen, Z.; Shi, P.; Ok, Y.S.; Tsang, D.C.W.; Wen, Y.; Luo, M. Lead contamination in Chinese surface soils: Source identification, spatial-temporal distribution and associated health risks. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1386–1423. [Google Scholar] [CrossRef]
- Yan, K.; Dong, Z.; Wijayawardena, M.A.A.; Liu, Y.; Li, Y.; Naidu, R. The source of lead determines the relationship between soil properties and lead bioaccessibility. Environ. Pollut. 2019, 246, 53–59. [Google Scholar] [CrossRef]
- Scaccabarozzi, D.; Castillo, L.; Aromatisi, A.; Milne, L.; Castillo, A.B.; Muñoz-Rojas, M. Soil, site, and management factors affecting cadmium concentrations in cacao-growing soils. Agronomy 2020, 10, 806. [Google Scholar] [CrossRef]
- Carne, G.; Leconte, S.; Sirot, V.; Breysse, N.; Badot, P.M.; Deportes, I.Z.; Dumat, C.; Rivière, G.; Crépet, A. Mass balance approach to assess the impact of cadmium decrease in mineral phosphate fertilizers on health risk: The case-study of French agricultural soils. Sci. Total Environ. 2021, 760, 143374. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Huang, B.; Luo, N.; Huang, M.; Zhang, Q.; Zeng, G. Remediation of multiple heavy metal-contaminated soil through the combination of soil washing and in situ immobilization. Sci. Total Environ. 2018, 635, 92–99. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Z.; Li, A.; Cui, C. Enhanced electrokinetic remediation of heavy metals contaminated soil by biodegradable complexing agents. Environ. Pollut. 2021, 283, 117111. [Google Scholar] [CrossRef]
- Lin, H.; Liu, C.; Li, B.; Dong, Y. Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms. J. Hazard. Mater. 2021, 402, 123829. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Nabil, A.; Awangku Metosen, S.; Nori, H. Uptake of heavy metals from palm oil mill effluent sludge amended soils in water spinach. J. Sustain. Sci. Manag. 2016, 11, 113–120. [Google Scholar]
- Yuan, Z.; Luo, T.; Liu, X.; Hua, H.; Zhuang, Y.; Zhang, X.; Zhang, L.; Zhang, Y.; Xu, W.; Ren, J. Tracing anthropogenic cadmium emissions: From sources to pollution. Sci. Total Environ. 2019, 676, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Nagpal, A.K.; Kaur, I. Heavy metal contamination in soil, food crops and associated health risks for residents of Ropar wetland, Punjab, India and its environs. Food Chem. 2018, 255, 15–22. [Google Scholar] [CrossRef]
- Bi, C.; Zhou, Y.; Chen, Z.; Jia, J.; Bao, X. Heavy metals and lead isotopes in soils, road dust and leafy vegetables and health risks via vegetable consumption in the industrial areas of Shanghai, China. Sci. Total Environ. 2018, 619–620, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Kicińska, A. Physical and chemical characteristics of slag produced during Pb refining and the environmental risk associated with the storage of slag. Environ. Geochem. Health 2021, 43, 2723–2741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, C.; Cheng, X.; Ma, H.; He, L. Ecological assessment, spatial analysis, and potential sources of Heavy Metals (HMs) in soils with high background values in the lead-zinc mine, Hezhang County, Southwestern China. Water 2022, 14, 783. [Google Scholar] [CrossRef]
- Shareef, R.S.; Mamat, A.S.; Al-Shaheen, M.R.; Aslam, M.S. Study of heavy metals in Mango (Mangifera indica L.) in Perlis, Malaysia. Indian Res. J. Pharm. Sci. 2015, 2, 299–303. [Google Scholar]
- Yan, X.; Liu, M.; Zhong, J.; Guo, J.; Wu, W. How human activities affect heavy metal contamination of soil and sediment in a long-term reclaimed area of the Liaohe River Delta, North China. Sustainability 2018, 10, 338. [Google Scholar] [CrossRef]
- Wenyou, H.; Huifeng, W.; Lurui, D.; Biao, H.; Borggaard, O.K.; Hansen, H.C.B.; He, Y.; Holm, P.E. Source identification of heavy metals in peri-urban agricultural soils of southeast China: An integrated approach. Environ. Pollut. 2018, 237, 650–661. [Google Scholar] [CrossRef]
- Wang, J.; Su, J.; Li, Z.; Liu, B. Source apportionment of heavy metal and their health risks in soil-dustfall-plant system nearby a typical non-ferrous metal mining area of Tongling, Eastern China. Environ. Pollut. 2019, 254, 113089. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Q.; Yu, H.; Li, M.; He, N. Heavy metal deposition through rainfall in Chinese natural terrestrial ecosystems: Evidences from national-scale network monitoring. Chemosphere 2016, 164, 128–133. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.; Wang, C.; Fu, X.; Wu, G. Impact of coal power generation on the characteristics and risk of heavy metal pollution in nearby soil. Ecosyst. Health Sustain. 2020, 6, 1787092. [Google Scholar] [CrossRef]
- Liang, J.; Feng, C.; Zeng, G.; Zhong, M.; Gao, X.; Li, X.; He, X.; Li, X.; Fang, Y.; Mo, D. Atmospheric deposition of mercury and cadmium impacts on topsoil in a typical coal mine city, Lianyuan, China. Chemosphere 2017, 189, 198–205. [Google Scholar] [CrossRef]
- Liu, H.L.; Zhou, J.; Li, M.; Hu, Y.M.; Liu, X.; Zhou, J. Study of the bioavailability of heavy metals from atmospheric deposition on the soil-pakchoi (Brassica chinensis L.) system. J. Hazard. Mater. 2018, 362, 9–16. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, B.; Asad, N.; Mian, I.A.; Jamil, M. Traffic-related lead pollution in roadside soils and plants in Khyber Pakhtunkhwa, Pakistan: Implications for human health. Int. J. Environ. Sci. Technol. 2019, 16, 8015–8022. [Google Scholar] [CrossRef]
- Jin, Y.; O’Connor, D.; Ok, Y.S.; Tsang, D.C.W.; Liu, A.; Hou, D. Assessment of sources of heavy metals in soil and dust at children’s playgrounds in Beijing using GIS and multivariate statistical analysis. Environ. Int. 2019, 124, 320–328. [Google Scholar] [CrossRef]
- Sisay, B.; Debebe, E.; Meresa, A.; Abera, T. Analysis of cadmium and lead using atomic absorption spectrophotometer in roadside soils of Jimma town. J. Anal. Pharm. Res. 2019, 8, 144–147. [Google Scholar] [CrossRef]
- Naccarato, A.; Tassone, A.; Cavaliere, F.; Elliani, R.; Pirrone, N.; Sprovieri, F.; Tagarelli, A.; Giglio, A. Agrochemical treatments as a source of heavy metals and rare earth elements in agricultural soils and bioaccumulation in ground beetles. Sci. Total Environ. 2020, 749, 141438. [Google Scholar] [CrossRef]
- Dinter, T.C.; Gerzabek, M.H.; Puschenreiter, M.; Strobel, B.W.; Couenberg, P.M.; Zehetner, F. Heavy metal contents, mobility and origin in agricultural topsoils of the Galápagos Islands. Chemosphere 2021, 272, 129821. [Google Scholar] [CrossRef]
- Dewi, T.; Martono, E.; Hanudin, E.; Harini, R. Source identification and spatial distribution of heavy metal concentrations in shallot fields in Brebes Regency, Central Java, Indonesia. Appl. Environ. Soil Sci. 2021, 2021, 3197361. [Google Scholar] [CrossRef]
- Enya, O.; Lin, C.; Qin, J. Heavy metal contamination status in soil-plant system in the Upper Mersey Estuarine Floodplain, Northwest England. Mar. Pollut. Bull. 2019, 146, 292–304. [Google Scholar] [CrossRef]
- Wu, Q.; Leung, J.Y.S.; Geng, X.; Chen, S.; Huang, X.; Li, H.; Huang, Z.; Zhu, L.; Chen, J.; Lu, Y. Heavy metal contamination of soil and water in the vicinity of an abandoned e-waste recycling site: Implications for dissemination of heavy metals. Sci. Total Environ. 2015, 506–507, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Liu, H.; Banet, T.; Wang, H.; Ippolito, J.A.; Li, L. Ecotoxicology and environmental safety cadmium, copper, lead and zinc accumulation in wild plant species near a lead smelter. Ecotoxicol. Environ. Saf. 2020, 198, 110683. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhu, T.; Li, M.; He, J.; Huang, R. Heavy Metal Contamination in Soil and Brown Rice and Human Health Risk Assessment near Three Mining Areas in Central China. J. Healthc. Eng. 2017, 2017, 4124302. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Mlsna, T. Groundwater for Sustainable Development Intrusion of heavy metals/metalloids into rice (Oryza sativa L.) in relation to their status in two different agricultural management systems in Sri Lanka. Groundw. Sustain. Dev. 2021, 14, 100619. [Google Scholar] [CrossRef]
- Gunawardena, J.; Ziyath, A.M.; Egodawatta, P.; Ayoko, G.A.; Goonetilleke, A. Sources and transport pathways of common heavy metals to urban road surfaces. Ecol. Eng. 2015, 77, 98–102. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.H.; Karmaker, S.C.; Bodrud-Doza, M.; Rakib, M.A.; Saha, B.B. Enrichment, sources and ecological risk mapping of heavy metals in agricultural soils of dhaka district employing SOM, PMF and GIS methods. Chemosphere 2021, 263, 128339. [Google Scholar] [CrossRef]
- Al-Taani, A.; Nazzal, Y.; Howari, F.; Iqbal, J.; Orm, N.B.; Xavier, C.; Bărbulescu, A.; Sharma, M.; Dumitriu, C.-S. Contamination assessment of heavy metals in agricultural soil, in the liwa area (UAE). Toxics 2021, 9, 53. [Google Scholar] [CrossRef]
- Fonge, B.A.; Larissa, M.T.; Egbe, A.M.; Afanga, Y.A.; Fru, N.G.; Ngole-Jeme, V.M. An assessment of heavy metal exposure risk associated with consumption of cabbage and carrot grown in a tropical Savannah region. Sustain. Environ. 2021, 7, 1909860. [Google Scholar] [CrossRef]
- Norton, G.J.; Deacon, C.M.; Mestrot, A.; Feldmann, J.; Jenkins, P.; Baskaran, C.; Meharg, A.A. Cadmium and lead in vegetable and fruit produce selected from specific regional areas of the UK. Sci. Total Environ. 2015, 533, 520–527. [Google Scholar] [CrossRef]
- Lawal, N.S.; Agbo, O.; Usman, A. Health risk assessment of heavy metals in soil, irrigation water and vegetables grown around Kubanni River, Nigeria. J. Phys. Sci. 2017, 28, 49–59. [Google Scholar] [CrossRef][Green Version]
- Wang, M.; Chen, W.; Peng, C. Risk assessment of Cd polluted paddy soils in the industrial and township areas in Hunan, Southern China. Chemosphere 2016, 144, 346–351. [Google Scholar] [CrossRef]
- Xie, L.; Tang, S.; Wei, X.; Shao, G.; Jiao, G.; Sheng, Z.; Luo, J.; Hu, P. The cadmium and lead content of the grain produced by leading Chinese rice cultivars. Food Chem. 2017, 217, 217–224. [Google Scholar] [CrossRef]
- Department of Environment, Ministry of Environment and Water. Contaminated Land Management and Control Guidelines No. 1: Malaysian Recommended Site Screening Levels for Contaminated Land; Federal Government Administrative Centre: Putrajaya, Malaysia, 2009.
- Hussein, M.; Yoneda, K.; Mohd-Zaki, Z.; Amir, A.; Othman, N. Heavy metals in leachate, impacted soils and natural soils of different landfills in Malaysia: An alarming threat. Chemosphere 2021, 267, 128874. [Google Scholar] [CrossRef]
- Wieczorek, J.; Baran, A.; Urbański, K.; Mazurek, R.; Klimowicz-Pawlas, A. Assessment of the pollution and ecological risk of lead and cadmium in soils. Environ. Geochem. Health 2018, 40, 2325–2342. [Google Scholar] [CrossRef]
- Ismail, S.N.S.; Abidin, E.Z.; Praveena, S.M.; Rasdi, I.; Mohamad, S.; Ismail, W.M.I.W. Heavy metals in soil of the tropical climate bauxite mining area in Malaysia. J. Phys. Sci. 2018, 29, 7–14. [Google Scholar] [CrossRef]
- Diami, S.M.; Kusin, F.M.; Madzin, Z. Potential ecological and human health risks of heavy metals in surface soils associated with iron ore mining in Pahang, Malaysia. Environ. Sci. Pollut. Res. 2016, 23, 21086–21097. [Google Scholar] [CrossRef]
- Praveena, S.M.; Pradhan, B.; Syed, S.N. Human and Ecological Risk Assessment: An International Spatial Assessment of Heavy Metals in Surface Soil from Klang District (Malaysia): An Example from a Tropical Environment. Hum. Ecol. Risk Assess. Int. J. 2015, 21, 1980–2003. [Google Scholar] [CrossRef]
- Rahman, H.A.; Zaim, F.A. Concentration level of heavy metals in soil at vegetables areas in Kota Bharu, Kelantan, Malaysia. Int. J. Environ. Sci. Dev. 2015, 6, 843–848. [Google Scholar] [CrossRef]
- Ismail, S.; Ishak, C.; Samah, M.; Hatta, E.; Wahab, A. Soil contamination from non-sanitary waste landfill in Langat Water Catchment Area, Malaysia. J. Sci. Res. Rep. 2015, 7, 480–493. [Google Scholar] [CrossRef]
- Aziz, R.A.; Rahim, S.A.; Sahid, I.; Idris, W.M.R.; Bhuiyan, M.A.R. Determination of heavy metals uptake in soil and paddy plants. Am. J. Agric. Environ. Sci. 2015, 15, 161–164. [Google Scholar]
- Ong, G.H.; Wong, L.S.; Tan, A.L.; Yap, C.K. Effects of metal-contaminated soils on the accumulation of heavy metals in gotu kola (Centella asiatica) and the potential health risks: A study in Peninsular Malaysia. Environ. Monit. Assess. 2016, 188, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kamarudzaman, A.N.; Woo, Y.S.; Jalil, M.F.A. Distribution and analysis of heavy metals contamination in soil, Perlis, Malaysia. E3S Web Conf. 2018, 34, 1–5. [Google Scholar] [CrossRef]
- Sankhla, M.S.; Kumari, M.; Nandan, M.; Kumar, R.; Agrawal, P.; Kaur, M.; Kumar, A.; Mehra, R.; Mishra, R.; Balkhair, K.S. Heavy Metals Contamination in Paddy Soil and Water and Associated Dermal Health Risk Among Farmers. Pure Appl. Biol. 2016, 23, 2–10. [Google Scholar]
- Sulaiman, F.; Hamzah, H. Heavy metals accumulation in suburban roadside plants of a tropical area (Jengka, Malaysia). Ecol. Process. 2018, 7, 28. [Google Scholar] [CrossRef]
- Zulkafflee, N.S.; Redzuan, N.A.M.; Selamat, J.; Ismail, M.R.; Praveena, S.M.; Razis, A.F.A. Evaluation of Heavy Metal Contamination in Paddy Plants at the Northern Region of Malaysia Using ICPMS and Its Risk Assessment. Plants 2021, 10, 3. [Google Scholar] [CrossRef]
- Mu, T.; Wu, T.; Zhou, T.; Li, Z.; Ouyang, Y.; Jiang, J.; Zhu, D.; Hou, J.; Wang, Z.; Luo, Y. Geographical variation in arsenic, cadmium, and lead of soils and rice in the major rice producing regions of China. Sci. Total Environ. 2019, 677, 373–381. [Google Scholar] [CrossRef]
- Ochoa, M.; Tierra, W.; Tupuna-Yerovi, D.S.; Guanoluisa, D.; Otero, X.L.; Ruales, J. Assessment of cadmium and lead contamination in rice farming soils and rice (Oryza sativa L.) from Guayas province in Ecuador. Environ. Pollut. 2020, 260, 114050. [Google Scholar] [CrossRef]
- Song, W.-E.; Chen, S.-B.; Liu, J.-F.; Chen, L.; Song, N.-N.; Li, N.; Liu, B. Variation of Cd concentration in various rice cultivars and derivation of cadmium toxicity thresholds for paddy soil by species-sensitivity distribution. J. Integr. Agric. 2015, 14, 1845–1854. [Google Scholar] [CrossRef]
- Yaacob, A.; Yap, C.K.; Nulit, R.; Omar, H.; Al-Shami, S.A.; Bakhtiari, A.R. Assessment of health risks of the toxic Cd and Pb between leafy and fruit vegetables collected from selected farming areas of Peninsular Malaysia. Integr. Food Nutr. Metab. 2018, 5, 1–9. [Google Scholar] [CrossRef]
- Sulaiman, F.R.; Ibrahim, N.H.; Ismail, S.N.S. Heavy metal (As, Cd, and Pb) concentration in selected leafy vegetables from Jengka, Malaysia, and potential health risks. SN Appl. Sci. 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Yap, C.K.; Yaacob, A.; Ibrahim, M.H.; Nulit, R.; Leow, C.S. Heavy metals in bitter gourd (Momordica charantia): Human health risk assessment. ARC J. Nutr. Growth 2019, 5, 1–5. [Google Scholar] [CrossRef]
- Ismail, N.F.N.; Anua, S.M.; Samad, N.I.A.; Hamzah, N.A.; Mazlan, N. Heavy metals in soil and vegetables at agricultural areas in Kota Bharu and bachok districts of Kelantan, Malaysia. Malays. J. Med. Health Sci. 2020, 16, 159–165. [Google Scholar]
- Abt, E.; Sam, J.F.; Gray, P.; Robin, L.P. Cadmium and lead in cocoa powder and chocolate products in the US Market. Food Addit. Contam. Part B Surveill. 2018, 11, 92–102. [Google Scholar] [CrossRef]
- Corradini, F.; Correa, A.; Moyano, M.S.; Sepúlveda, P.; Quiroz, C. Nitrate, arsenic, cadmium, and lead concentrations in leafy vegetables: Expected average values for productive regions of Chile. Arch. Agron. Soil Sci. 2018, 64, 299–317. [Google Scholar] [CrossRef]
- Vega, F.A.; Covelo, E.F.; Andrade, M.L.; Marcet, P. Relationships between heavy metals content and soil properties in minesoils. Anal. Chim. Acta 2004, 524, 141–150. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Cobb, G.P.; Sands, K.; Waters, M.; Wixson, B.G.; Dorward-King, E. Accumulation of heavy metals by vegetables grown in mine wastes. Environ. Toxicol. Chem. 2000, 19, 600–607. [Google Scholar] [CrossRef]
- Krzesłowska, M. The cell wall in plant cell response to trace metals: Polysaccharide remodeling and its role in defense strategy. Acta Physiol. Plant. 2011, 33, 35–51. [Google Scholar] [CrossRef]
- Filippini, T.; Cilloni, S.; Malavolti, M.; Violi, F.; Malagoli, C.; Tesauro, M.; Bottecchi, I.; Ferrari, A.; Vescovi, L.; Vinceti, M. Dietary intake of cadmium, chromium, copper, manganese, selenium and zinc in a Northern Italy community. J. Trace Elem. Med. Biol. 2018, 50, 508–517. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Wang, P.; Wang, Z.; Li, M.; Zhao, F.J. Dietary cadmium intake from rice and vegetables and potential health risk: A case study in Xiangtan, southern China. Sci. Total Environ. 2018, 639, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Buyun, D.; Jun, Z.; Binxgin, L.; Chen, Z.; Demin, L.; Jing, Z.; Shaojuin, J.; Keqiang, Z.; Houhu, Z. Environmental and human health risks from cadmium exposure near an active lead-zinc mine and a copper smelter, China. Sci. Total Environ. 2020, 720, 137585. [Google Scholar] [CrossRef]
- Yang, Y.; Chang, A.C.; Wang, M.; Chen, W.; Peng, C. Assessing cadmium exposure risks of vegetables with plant uptake factor and soil property. Environ. Pollut. 2018, 238, 263–269. [Google Scholar] [CrossRef]
- Pan, L.; Wang, Z.; Peng, Z.; Liu, G.; Zhang, H.; Zhang, J.; Jiang, J.; Pathiraja, N.; Xiao, Y.; Jiao, R. Dietary exposure to lead of adults in Shenzhen city, China. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 1200–1206. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.; Mao, W.; Sui, H.; Yong, L.; Yang, D.; Jiang, D.; Zhang, L.; Gong, Y. Dietary cadmium exposure assessment among the Chinese population. PLoS ONE 2017, 12, e0177978. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Li, W.; Wang, L.; Jiao, Y.; Wang, Y.; Jiang, D.; Gao, X. Occurrence and dietary exposure of heavy metals in marketed vegetables and fruits of Shandong Province, China. Food Sci. Nutr. 2021, 9, 5166–5173. [Google Scholar] [CrossRef]
- Pan, X.D.; Wu, P.G.; Jiang, X.G. Levels and potential health risk of heavy metals in marketed vegetables in Zhejiang, China. Sci. Rep. 2016, 6, 20317. [Google Scholar] [CrossRef]
- Yuswir, N.S.; Praveena, S.M.; Aris, A.Z.; Ismail, S.N.S.; Hashim, Z. Health risk assessment of heavy metal in urban surface soil (Klang District, Malaysia). Bull. Environ. Contam. Toxicol. 2015, 95, 80–89. [Google Scholar] [CrossRef]
- Chen, C.; Xun, P.; Nishijo, M.; Carter, S.; He, K. Cadmium exposure and risk of prostate cancer: A meta-analysis of cohort and case-control studies among the general and occupational populations. Sci. Rep. 2016, 6, 25814. [Google Scholar] [CrossRef]
- Chen, C.; Xun, P.; Nishijo, M.; He, K. Cadmium exposure and risk of lung cancer: A meta-analysis of cohort and case-control studies among general and occupational populations. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.K.; Hassan, Z.; Shamsudin, Z.; Nulit, R.; Nallappan, M.; Cheng, W.; Hao, S.; Peng, T.; Yap, C. A preliminary study on heavy metals in green mustard Brassica juncea: A human health risk assessment. Eco. Environ. Cons. 2021, 27, 371–375. [Google Scholar]
- Browar, A.W.; Koufos, E.B.; Wei, Y.; Leavitt, L.L.; Prozialeck, W.C.; Edwards, J.R. Cadmium exposure disrupts periodontal bone in experimental animals: Implications for periodontal disease in humans. Toxics 2018, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Suhani, I.; Sahab, S.; Srivastava, V.; Singh, R.P. Impact of cadmium pollution on food safety and human health. Curr. Opin. Toxicol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Betteridge, D.J. What is oxidative stress? Metab. Clin. Exp. 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Boskabady, M.; Marefati, N.; Farkhondeh, T.; Shakeri, F.; Farshbaf, A.; Boskabady, M.H. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ. Int. 2018, 120, 404–420. [Google Scholar] [CrossRef]
- Hazrati, S.; Farahbakhsh, M.; Heydarpoor, G.; Besalatpour, A.A. Mitigation in availability and toxicity of multi-metal contaminated soil by combining soil washing and organic amendments stabilization. Ecotoxicol. Environ. Saf. 2020, 201, 110807. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Wang, H.; Li, Q.; Li, Y. Effect of soil washing on heavy metal removal and soil quality: A two-sided coin. Ecotoxicol. Environ. Saf. 2020, 203, 110981. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, J.; Liu, X.; Yuan, L.; Liu, X.; Deng, H. Comparative study on washing effects of different washing agents and conditions on heavy metal contaminated soil. Surf. Interfaces 2021, 27, 101563. [Google Scholar] [CrossRef]
- Lee, D.; Son, Y. Ultrasound-assisted soil washing processes using organic solvents for the remediation of PCBs-contaminated soils. Ultrason. Sonochem. 2021, 80, 105825. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, S.; Zhong, Q.; Wang, G.; Feng, C.; Xu, X.; Pu, Y.; Guo, X. Removal of heavy metals from abandoned smelter contaminated soil with poly-phosphonic acid: Two-objective optimization based on washing efficiency and risk assessment. Chem. Eng. J. 2021, 421, 129882. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, S.; Zhong, Q.; Wang, G.; Pan, X.; Xu, X.; Zhou, W.; Li, T.; Luo, L.; Zhang, Y. Soil washing remediation of heavy metal from contaminated soil with EDTMP and PAA: Properties, optimization, and risk assessment. J. Hazard. Mater. 2020, 381, 120997. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Zhang, S.; Cao, Y.; Zhong, Q.; Wang, G.; Li, T.; Xu, X. Remediation of cadmium, lead and zinc in contaminated soil with CETSA and MA/AA. J. Hazard. Mater. 2019, 366, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.C.; Boyce, A.N.; Rahman, M.M.; Abas, M.R. Tolerance threshold and phyto-assessment of cadmium and lead in Vetiver grass, Vetiveria zizanioides (Linn.) Nash. Chiang Mai J. Sci. 2017, 44, 1367–1378. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Manan, F.A.; Chai, T.T.; Samad, A.A.; Mamat, D.D. Evaluation of the phytoremediation potential of two medicinal plants. Sains Malays. 2015, 44, 503–509. [Google Scholar] [CrossRef]
- Ismail, A.; Melissa Muharam, F. Phytoremediation Studies on Soils Contaminated with Heavy Metals in Malaysia: A Review Article. Am. Eurasian J. Agric. Environ. Sci. 2016, 8, 1504–1514. [Google Scholar] [CrossRef]
- Saad, F.N.M.; Lim, F.J.; Izhar, T.N.T.; Odli, Z.S.M. Evaluation of phytoremediation in removing Pb, Cd and Zn from contaminated soil using Ipomoea aquatica and Spinacia oleracea. IOP Conf. Ser. Earth Environ. Sci. 2020, 476, 012142. [Google Scholar] [CrossRef]
- Moktar, K.A.; Mohd Tajuddin, R. Phytoremediation of heavy metal from leachate using imperata cylindrica. MATEC Web Conf. 2019, 258, 01021. [Google Scholar] [CrossRef]
- Li, Q.; Chen, X.; Chen, X.; Jin, Y.; Zhuang, J. Cadmium removal from soil by fulvic acid-aided hydroxyapatite nanofluid. Chemosphere 2019, 215, 227–233. [Google Scholar] [CrossRef]
- Thinh, N.V.; Osanai, Y.; Adachi, T.; Vuong, B.T.S.; Kitano, I.; Chung, N.T.; Thai, P.K. Removal of lead and other toxic metals in heavily contaminated soil using biodegradable chelators: GLDA, citric acid and ascorbic acid. Chemosphere 2021, 263, 127912. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Wang, J. Phytoremediation of cadmium-contaminated soil by Sorghum bicolor and the variation of microbial community. Chemosphere 2019, 235, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wei, S.; Skuza, L.; Zhang, Q. Phytoremediation of two ecotypes cadmium hyperaccumulator Bidens pilosa L. sourced from clean soils. Chemosphere 2021, 273, 129652. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Shen, Y.; Wang, X.; Song, X.; Yuan, W.; Xie, J.; Wang, S.; Bai, J.; Wang, J. Choline-based deep eutectic solvent combined with EDTA-2Na as novel soil washing agent for lead removal in contaminated soil. Chemosphere 2021, 279, 130568. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Mao, P.; Sun, S.; Huang, R.; Fan, Y.; Li, Y.; Li, Y.; Zhuang, P.; Li, Z. Phytoremediation of cadmium contaminated soils by Amaranthus hypochondriacus L.: The effects of soil properties highlighting cation exchange capacity. Chemosphere 2021, 283, 131067. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Chen, Y.; Zhang, S.; Wang, G.; Zhong, Q.; Zhou, W.; Xu, X.; Li, T. Removal of lead, zinc and cadmium from contaminated soils with two plant extracts: Mechanism and potential risks. Ecotoxicol. Environ. Saf. 2020, 187, 109829. [Google Scholar] [CrossRef]
- Mekwichai, P.; Tongcumpou, C.; Kittipongvises, S.; Tuntiwiwattanapun, N. Simultaneous biosurfactant-assisted remediation and corn cultivation on cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 192, 110298. [Google Scholar] [CrossRef]
- Gong, H.; Chi, J.; Ding, Z.; Zhang, F.; Huang, J. Removal of lead from two polluted soils by magnetic wheat straw biochars. Ecotoxicol. Environ. Saf. 2020, 205, 111132. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Xu, Y.; Liang, X.; Wang, L. In situ stabilization remediation of cadmium (Cd) and lead (Pb) co-contaminated paddy soil using bentonite. Appl. Clay Sci. 2015, 105–106, 200–206. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, X.; Liu, F.; Hu, X.; Zhao, X.; Wang, L.; Gao, P.; Li, X.; Ji, P. Potential of using a new aluminosilicate amendment for the remediation of paddy soil co-contaminated with Cd and Pb. Environ. Pollut. 2021, 269, 116198. [Google Scholar] [CrossRef]
- Fan, J.; Cai, C.; Chi, H.; Reid, B.J.; Coulon, F.; Zhang, Y.; Hou, Y. Remediation of cadmium and lead polluted soil using thiol-modified biochar. J. Hazard. Mater. 2020, 388, 122037. [Google Scholar] [CrossRef]
- Gong, H.; Tan, Z.; Huang, K.; Zhou, Y.; Yu, J.; Huang, Q. Mechanism of cadmium removal from soil by silicate composite biochar and its recycling. J. Hazard. Mater. 2021, 409, 125022. [Google Scholar] [CrossRef]
- Qiao, Y.; Wu, J.; Xu, Y.; Fang, Z.; Zheng, L.; Cheng, W.; Tsang, E.P.; Fang, J.; Zhao, D. Remediation of cadmium in soil by biochar-supported iron phosphate nanoparticles. Ecol. Eng. 2017, 106, 515–522. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.Y.; Wang, D. Phytoremediation of uranium and cadmium contaminated soils by sunflower (Helianthus annuus L.) enhanced with biodegradable chelating agents. J. Clean. Prod. 2020, 263, 121491. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; He, X.; Liu, Y. Optimization analysis and mechanism exploration on the removal of cadmium from contaminated soil by electrokinetic remediation. Sep. Purif. Technol. 2020, 250, 117180. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Mukherjee, S.; Adnan, N.F.; Hayyan, A.; Hayyan, M.; Hashim, M.A.; Sen Gupta, B. Ammonium-based deep eutectic solvents as novel soil washing agent for lead removal. Chem. Eng. J. 2016, 294, 316–322. [Google Scholar] [CrossRef]
- Chauhan, P.; Rajguru, A.B.; Dudhe, M.Y.; Mathur, J. Efficacy of lead (Pb) phytoextraction of five varieties of Helianthus annuus L. from contaminated soil. Environ. Technol. Innov. 2020, 18, 100718. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, H.; Dong, X.; Huang, H.; Zheng, Q.; Dai, Z.; Zhang, Z.; Li, Z.; Feng, Q.; Xiong, S.; et al. In situ cadmium removal from paddy soils by a reusable remediation device and its health risk assessment in rice. Environ. Technol. Innov. 2021, 23, 101713. [Google Scholar] [CrossRef]
- Sheikh-Assadi, M.; Khandan-Mirkohi, A.; Alemardan, A.; Moreno-Jiménez, E. Mycorrhizal Limonium sinuatum (L.) Mill. Enhances Accumulation of Lead and Cadmium. Int. J. Phytoremediation 2015, 17, 556–562. [Google Scholar] [CrossRef]


| Organizations | Soil/Plant Types | Cd and Pb Standards | References | |
|---|---|---|---|---|
| EU a | Soil | - | Cd 0.2 mg/kg | [49] |
| Plant | Leafy vegetables, fresh herbs, celeriac, cultivated fungi | Cd 0.2 mg/kg | ||
| Stem vegetables, root vegetables, and potatoes | Cd 0.1 mg/kg | |||
| Vegetables and fruits (excluding leafy vegetables) and other products | Cd 0.05 mg/kg | |||
| Brassicas, leaf vegetables | Pb 0.3 mg/kg | |||
| Vegetables (including peeled vegetables) | Pb 0.1 mg/kg | |||
| FAO/ WHO b (2007) | Soil | - | Cd 0.07 mg/kg, Pb 10 mg/kg | [48] |
| Plant | - | Cd 0.2 mg/kg, Pb 5 mg/kg | [50] | |
| SEQS c | Soil | - | Cd 0.3 mg/kg | [51] |
| AQSIQ d | Plant | Rice | Cd, Pb 0.2000 mg/kg | [52] |
| DOE e (2009) | Soil | Residential soil | Cd 7.0 mg/kg, Pb 4.0 mg/kg | [53] |
| Industrial soil | Cd 8.1 mg/kg, Pb 8.0 mg/kg | |||
| No. | Location | Cadmium/Lead Concentration Range (mg/kg) | Land Use | References |
|---|---|---|---|---|
| From Malaysian lands | ||||
| 01 | Klang district, Selangor | Cd (0.77 mg/kg) Pb (52.73 mg/kg) | Urban soil | [58] |
| 02 | (Kg. kubang and Kg. tok kambing) Kota bharu, Kelantan | Pb (7.396–11.30 mg/kg) | Soil from an agricultural area (cucumber crop) | [59] |
| 03 | Selangor | Pb (24.3–37.9 mg/kg) in landfill area Pb (14.5–27.9 mg/kg) in residential area Cd in landfill area (0.6–4.61 mg/kg) Cd in agricultural area (1.3–4.99 mg/kg) | Soil from Langat water catchment area (landfill, agricultural, industrial, residential) | [60] |
| 04 | Perlis | Pb (0.4 mg/kg) Cd (0.98 mg/kg) | Soil from the mango plantation area | [26] |
| 05 | Ranau Valley, Ranau, Sabah | Cdmax (2.83 mg/kg) | Soil from paddy field | [61] |
| 06 | Different sites from Peninsular Malaysia | Pb (0.05–0.09 mg/kg) Cd (7.8 × 10−4–1.9 × 10−3 mg/kg) | Soil from Gotu Kola plantation | [62] |
| 07 | Kuala Lipis, Pahang | Pb (63.5–72.5 mg/kg) | Surface soils from active and abandoned iron ore mining sites | [57] |
| 08 | Kuantan Port and bukit Goh, Pahang | Pb (44.76 mg/kg) Cd (3.63 mg/kg) in Bukit Goh Cd (4.61 mg/kg) in Kuantan Port | The soil of bauxite mining area | [56] |
| 09 | Perlis | Cd (0.075 mg/kg) | Soil from 5 different sites (Jejawi, Kangar, chuping, kuala perlis, Beseri) | [63] |
| 10 | KKampung Sawah Sempadan, Tanjung Kanany, Selangor | Pb (6.64 mg/kg) Cd (6.00 × 10−2 mg/kg) | Paddy soil | [64] |
| 11 | Jengka, Pahang | Pb (0.03–4.60 mg/kg) Cd (0.01–0.32 mg/kg) | Soil from plantation area | [65] |
| 12 | Malaka and Negeri Sembilan | Pb (1.88 mg/kg) Cd (171.72 mg/kg) In non-sanitary sites | Soils from sanitary and non-sanitary sites | [54] |
| 13 | Yan, Kubang Pasu, and Pendang, Kedah | Pb (0.026–1.063 mg/kg) in Pendang area Pb (0.023–0.858 mg/kg) in Yan area Pb in Kubang Pasu (0.023–1.107 mg/kg) Cd in Pendang: (0.024–0.77 mg/kg) Cd (0.0018–0.013 mg/kg) in Yan Cd in Kubang Pasu: (0.001–0.152 mg/kg) | Paddy soils | [66] |
| Lands from other countries | ||||
| 14 | Hunan, Southern China | Cd in soil (0.228–1.91 mg/kg) | Paddy soils | [51] |
| 15 | Northeast, Central and West, South, Yangtze Delta, China | Cd 0.45 mg/kg Pb 25.7 mg/kg | Paddy soils | [67] |
| 16 | Guayas province, Ecuador | Cd (0.26 ± 0.15 mg/kg) and Pb (13.52 ± 8.46 mg/kg) | Paddy soils | [68] |
| 17 | Zhejiang Province, China | Cd 0.126 mg/kg (background concentration, before adding Cd to experimental pots) | Paddy soils | [69] |
| 18 | Malopolska, Poland | Cd 0.01 to 16.9 mg/kg Pb 3 to 586 mg/kg (in topsoil) | Grassland, arable land, forest, wasteland | [55] |
| No | Location | Cadmium/Lead Concentration Range (mg/kg) | Food Plants | References |
|---|---|---|---|---|
| In Malaysian regions | ||||
| 01 | Different sites from peninsular Malaysia | Pb-roots (0.022–0.037 mg/kg) Pb-shoots (0.016–0.025 mg/kg) Cd-roots (1 × 10−3–1.7 × 10−3 mg/kg) Cd-shoots (4.1 × 10−4–9.3 × 10−4 mg/kg) | Gotu Kola (Centella asiatica) | [62] |
| 02 | Ranau Valley, Ranau, Sabah | Cd (3.92 mg/kg) | Rice grain | [61] |
| 03 | Penang, Kedah and Perak | Cd-fruit vegetables (0.17–1.32 mg/kg) Cd-leafy vegetables (0.74–2.17 mg/kg) Pb-fruit vegetables (0.62–1.85 mg/kg) Pb-leafy vegetables (1.23–2.74 mg/kg) dw | Vegetables (leaves and fruits) | [70] |
| 04 | Jengka, Pahang | Pb-roots (0.16–3.37 mg/kg) Cd-roots (0–0.11 mg/kg) | (Athyrium esculentum) (Chromolaena odorata) (Lantana camara) | [65] |
| 05 | Pahang | Pb (0.79–1.46 mg/kg) | Bitter Gourd (Momordica charantia) | [72] |
| 06 | Jengka, Pahang | Cd (0.15–0.54 mg/kg) Pb (0.03–0.05 mg/kg) | Leafy vegetables Pak choi (Brassica chinensis L.) Amaranth (Amaranthus gangeticus) Caisim (Brassica rapa var. parachinensis). | [71] |
| 07 | Kampung Binjiai Manis, Kampung Aman and Bachok, Kelantan | Kampung Binjiai Manis: Pb in eggplant (3.44 mg/kg) Cd in Luffa (0.93 mg/kg) Kampung Aman: Pb in Eggplant (0.82 mg/kg) Cd in Luffa (1.12 mg/kg) | Eggplant (Solanum melongena) Chili (Capsium annum) Luffa (Luffa acutangular) | [73] |
| In other countries | ||||
| 08 | (Devon, Cornwall, Aberdeenshire) Britain | High Cd in Spinach 0.04 mg/kg High Pb in blackcurrant 0.16 mg/kg | Fruits and vegetables | [49] |
| 09 | China | Cd (0.0025 to 0.2530) mg/kg Pb (0.0250–0.3830) mg/kg | Rice grains (indica and japonica) | [52] |
| 10 | United States (US) | Cd 3.15 mg/kg Pb 0.38 mg/ kg | Products originated from cocoa beans (cocoa powder, dark chocolate, milk chocolate, and cocoa nibs). | [74] |
| 11 | Zhejiang province, China | Cd 0.128 to 0.806 mg/kg | Rice cultivars | [69] |
| 12 | Chile | Lettuce (Cd 0.057, Pb 0.208 mg/kg)max Spinach (Cd 0.247, Pb < 0.263 mg/kg)max Chard (Cd 0.116, Pb < 0.238 mg/kg) | Lettuce, spinach, chard | [75] |
| Location | Trace Element | Analytical Method | Main Food Contributor to TE Exposure | Dietary Daily Intake (μg/kg/BW/day) | References |
|---|---|---|---|---|---|
| Italy | Cadmium | ICP-MS (7500 Agilent) | Cereals, vegetables | 0.0714 μg/kg BW/day | [80] |
| China | Cadmium | ICP-MS | Rice, vegetables | 2.37–6.93 μg/kg BW/day | [81] |
| China | Cadmium | (ICP-MS, Perkin Elmer, Waltham, MA, USA) | Rice, vegetables | Adult 3.4–6.5 Children 4.1–7.9 μg/kg BW/day | [82] |
| China | Cadmium | GFAAS | Vegetables (loofah, carrot, radish, bok choy, cabbage, celery, Chinese cabbage, lettuce, mustard) | 100 μg/BW kg/day | [83] |
| China | Lead | ICP-MS | Vegetables and their products | 0.0938–0.1210 μg/kg bw/day/ | [84] |
| China | Cadmium | GFAAS | Rice leafy vegetables and wheat flour | 0.17 μg/kg bw/day | [85] |
| China | Lead Cadmium | ICP-MS | Vegetables (stem, leafy, and fruit vegetables) | 0.052 µg/kg bw/day 0.038 µg/kg bw/day | [86] |
| China | Lead | GFAAS (Thermo SOLAAR model iCE3000) | Marketed vegetables | 0.459 µg/kg bw/day | [87] |
| China | Lead Cadmium | AAS (AANALYST800, Perkin-Elmer) | Leafy vegetables | 0.219 ug/kg/day 0.013 ug/kg/day | [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouida, L.; Rafatullah, M.; Kerrouche, A.; Qutob, M.; Alosaimi, A.M.; Alorfi, H.S.; Hussein, M.A. A Review on Cadmium and Lead Contamination: Sources, Fate, Mechanism, Health Effects and Remediation Methods. Water 2022, 14, 3432. https://doi.org/10.3390/w14213432
Bouida L, Rafatullah M, Kerrouche A, Qutob M, Alosaimi AM, Alorfi HS, Hussein MA. A Review on Cadmium and Lead Contamination: Sources, Fate, Mechanism, Health Effects and Remediation Methods. Water. 2022; 14(21):3432. https://doi.org/10.3390/w14213432
Chicago/Turabian StyleBouida, Leila, Mohd Rafatullah, Abdelfateh Kerrouche, Mohammad Qutob, Abeer M. Alosaimi, Hajer S. Alorfi, and Mahmoud A. Hussein. 2022. "A Review on Cadmium and Lead Contamination: Sources, Fate, Mechanism, Health Effects and Remediation Methods" Water 14, no. 21: 3432. https://doi.org/10.3390/w14213432
APA StyleBouida, L., Rafatullah, M., Kerrouche, A., Qutob, M., Alosaimi, A. M., Alorfi, H. S., & Hussein, M. A. (2022). A Review on Cadmium and Lead Contamination: Sources, Fate, Mechanism, Health Effects and Remediation Methods. Water, 14(21), 3432. https://doi.org/10.3390/w14213432









