Mechanism and Reactive Species in a Fountain-Strip DBD Plasma for Degrading Perfluorooctanoic Acid (PFOA)
Abstract
1. Introduction
2. Experiments
2.1. Materials
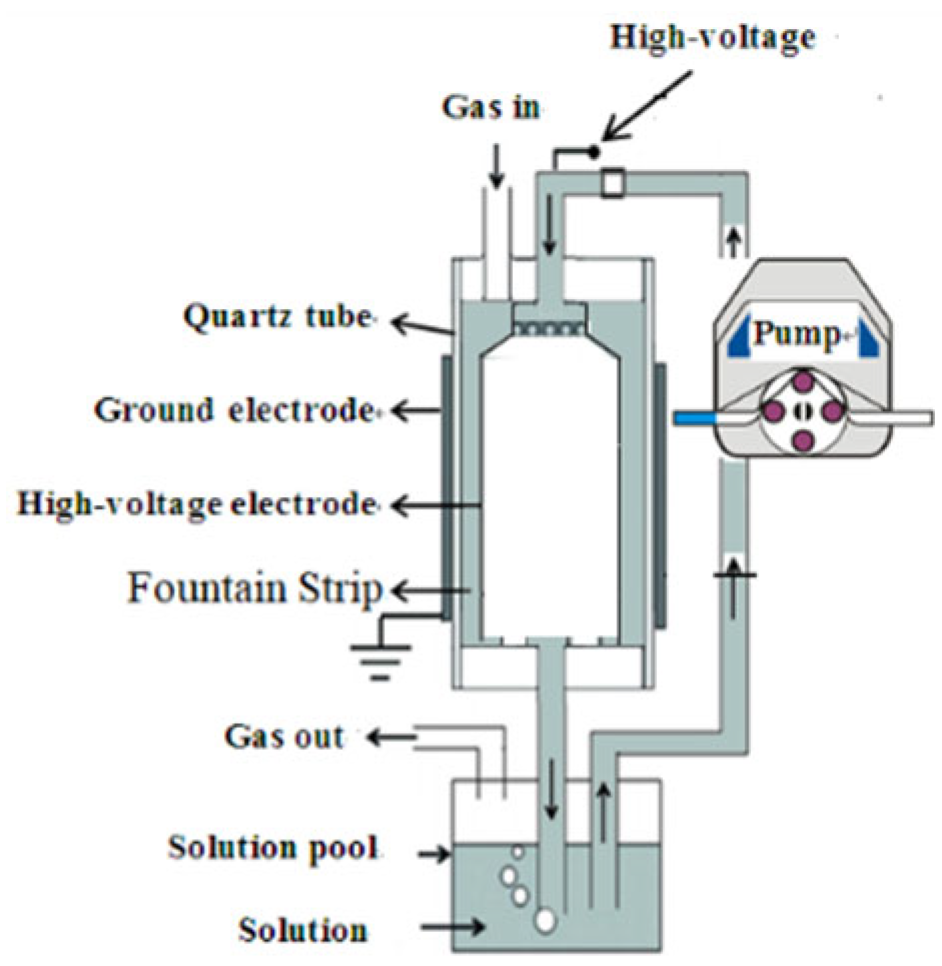
2.2. Experimental Setup
2.3. Analytical Methods
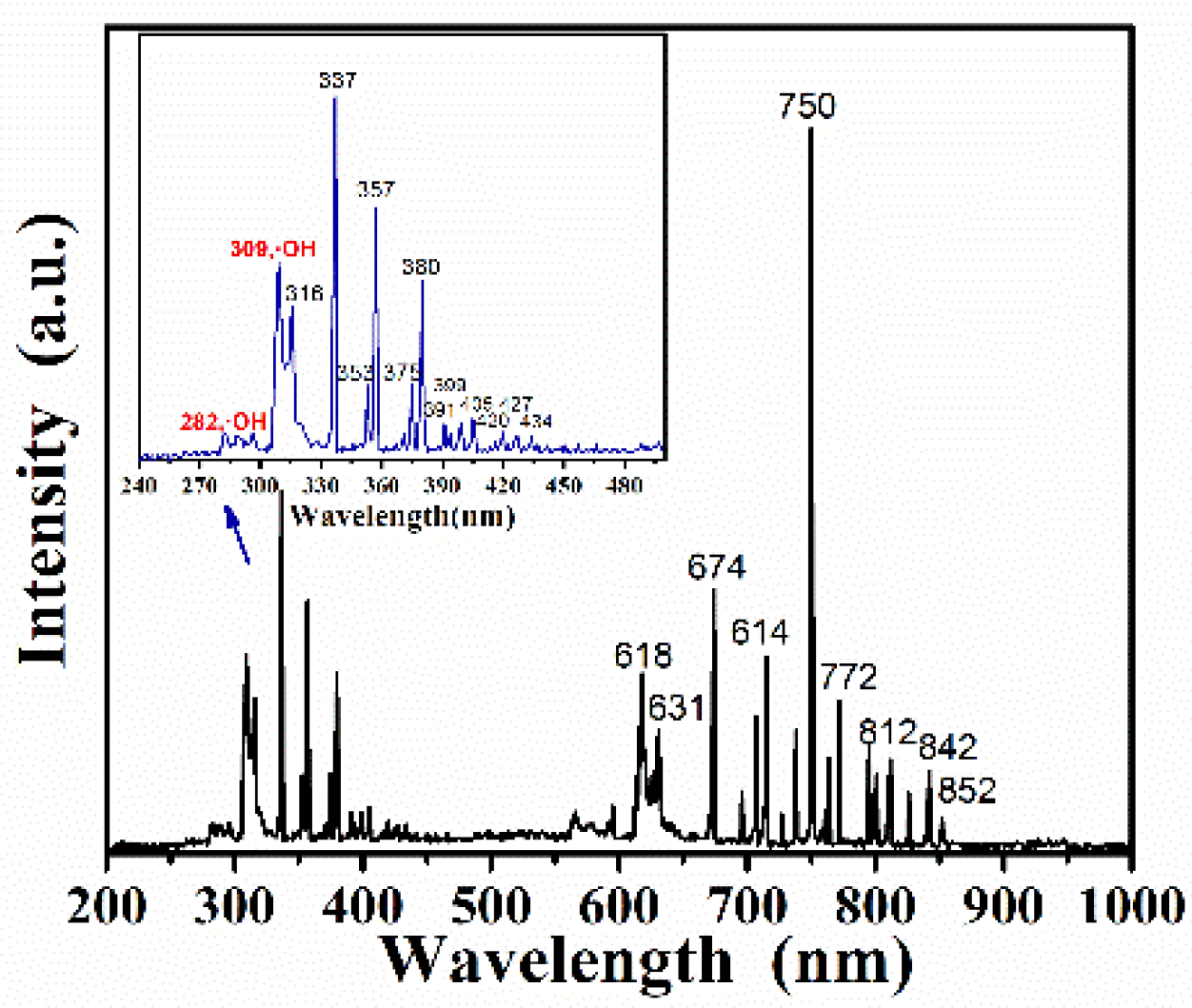
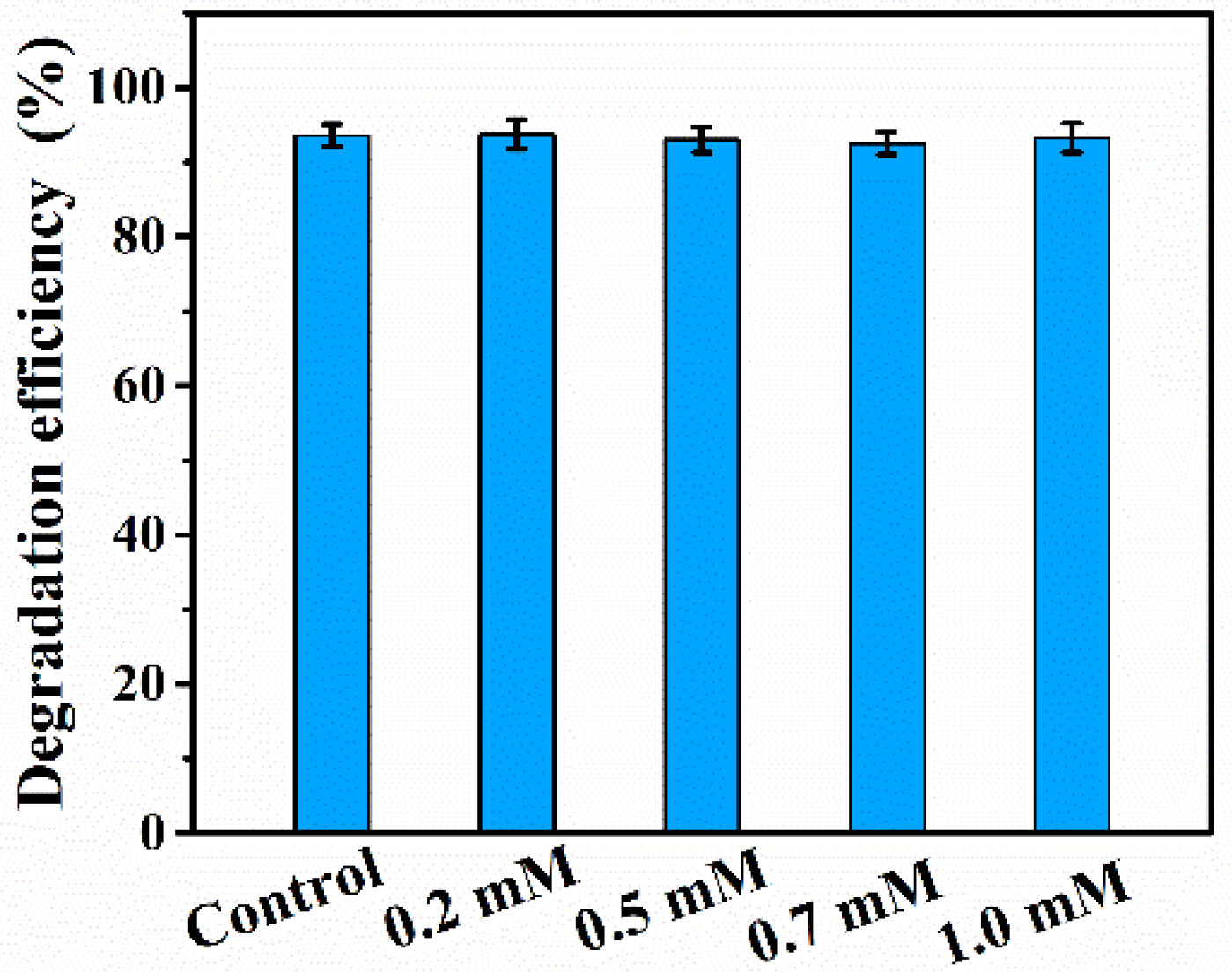
3. Results and Discussion
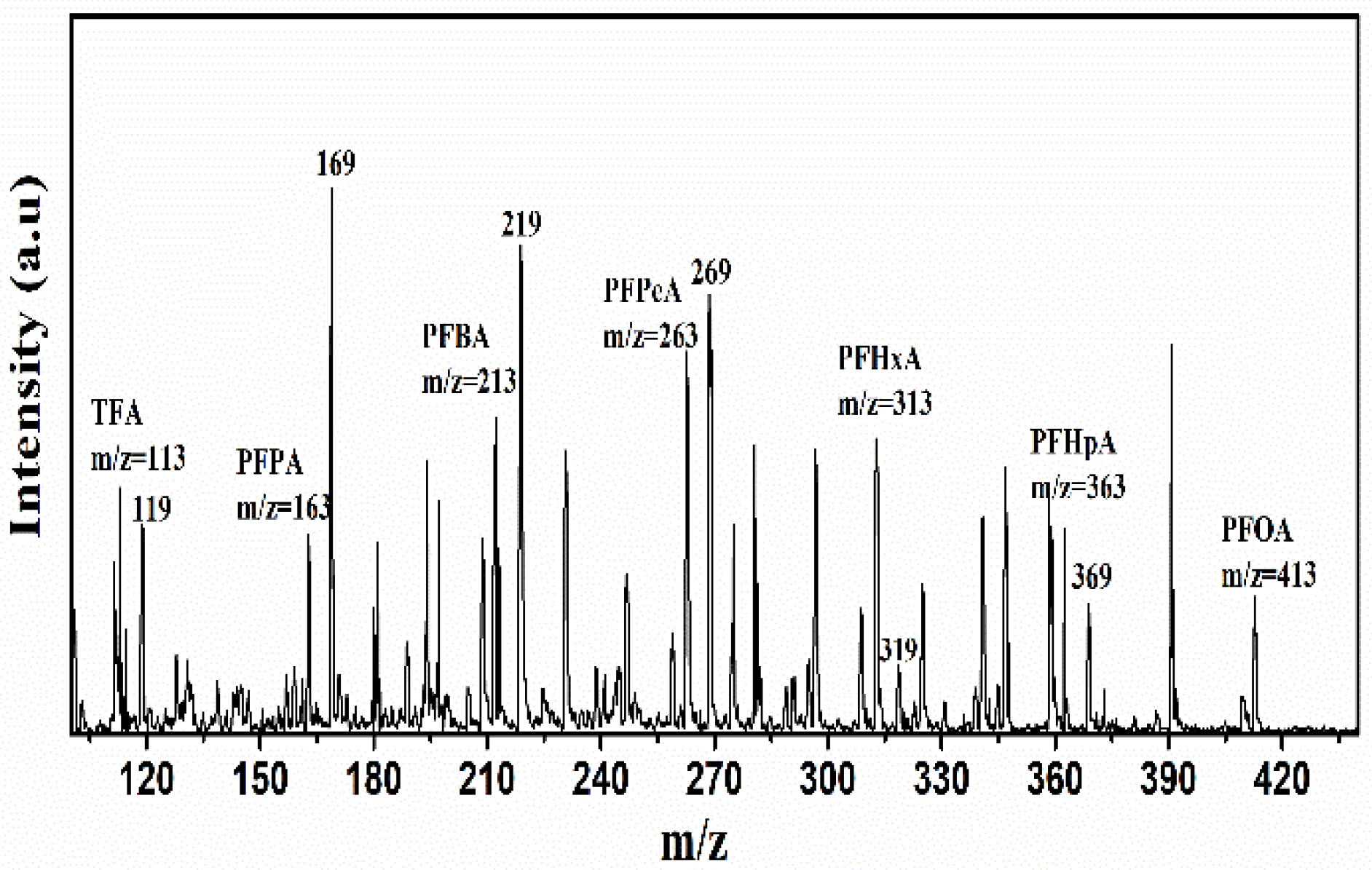
Decomposition Mechanism Analyzed from the Intermediates/Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, M.J.; Jovicic, V.; Zbogar-Rasic, A.; Poser, A.; Freichels, K.; Delgado, A. Effectiveness of non-thermal plasma induced degradation of per- and polyfluoroalkyl substances from water. Water 2022, 14, 1408. [Google Scholar] [CrossRef]
- Wan, H.; Miills, R.; Qu, K.; Hower, J.C.; Mottaleb, M.A.; Bhattacharyya, D.; Xu, Z. Rapid removal of PFOA and PFOS via modified industrial solid waste: Mechanisms and influences of water matrices. Chem. Eng. J. 2022, 433, 133271. [Google Scholar] [CrossRef]
- Zhang, L.H.; Cheng, J.H.; You, X.; Hu, Y. Photochemical defluorination of aqueous perfluorooctanoic acid (PFOA) by Fe0/GAC micro-electrolysis and VUV-Fenton photolysis. Environ. Sci. Pollut. Res. 2016, 23, 13531–13542. [Google Scholar] [CrossRef]
- Maisonet, M.; Terrell, M.L.; McGeehin, M.A.; Christensen, K.Y.; Holmes, A.; Calafat, A.M.; Marcus, M. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ. Health Perspect. 2012, 120, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Wang, X.; Pan, Z.Q.; Xukai Li Yu Ling Laisheng, L.i. Efficient degradation of perfluorooctanoic acid (PFOA) by photocatalytic ozonation. Chem. Eng. J. 2016, 296, 329–334. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chen, M.J.; Huang, C.P.; Kuoc, J.; Lo, S.-L. Efficient sonochemical degradation of perfluorooctanoic acid using periodate. Ultrason. Sonochem 2016, 31, 499–505. [Google Scholar] [CrossRef]
- Yasuoka, K.; Sasaki, K.; Hayashi, R. An energy-efficient process for decomposing perfluorooctanoic and perfluorooctane sulfonic acids using dc plasmas generated within gas bubbles. Plasma Sources Sci. Technol. 2011, 20, 034009. [Google Scholar] [CrossRef]
- Mhadhbi, L.; Rial, D.; Pérez, S.; Beiras, R. Ecological risk assessment of perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in marine environment using Isochrysis galbana, Paracentrotus lividus, Siriella armata and Psetta maxima. J. Environ. Monit. 2012, 14, 1375–1382. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, C.; Li, F.; Bo, X.; Liu, G.; Zhou, Q. Equilibrium and kinetics study on the adsorption of perfluorooctanoic acid from aqueous solution onto powdered activated carbon. J. Hazard. Mater. 2009, 69, 146–152. [Google Scholar] [CrossRef]
- Lampert, D.J.; Frisch, M.A.; Speitel, G.E. Removal of Perfluorooctanoic Acid and Perfluorooctane Sulfonate from Wastewater by Ion Exchange. Pract. Period Hazard Toxic Radioact. Waste Manag. 2007, 11, 60–68. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Bartosiewicz, I.; Kulisa, K. Advanced oxidation/reduction processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS)–a review of recent advances. Chem. Eng. J. 2018, 336, 170–199. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, P.Y.; Liu, J. Photodegradation of perfluorooctanoic acid by 185 nm vacuum ultraviolet light. J. Environ. Sci. 2007, 19, 387–390. [Google Scholar] [CrossRef]
- Moriwaki, H.; Takagi, Y.; Tanaka, M.; Tsuruho, K.; Okitsu, K.; Maeda, Y. Sonochemical decomposition of perfluorooctane sulfonate and perfluorooctanoic acid. Environ. Sci. Technol. 2005, 39, 3388–3392. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lo, S.L.; Chiueh, P.T.; Liou, Y.H.; Chen, M.L. Microwave-hydrothermal decomposition of perfluorooctanoic acid in water by iron-activated persulfate oxidation. Water Res. 2010, 44, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Niu, J.F.; Ding, S.Y.; Zhang, L.L. Electrochemical degradation of perfluorooctanoic acid (PFOA) by Ti/SnO2–Sb, Ti/SnO2–Sb/PbO2 and Ti/SnO2–Sb/MnO2 anodes. Water Res. 2012, 46, 2281–2289. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Bartosiewicz, I.; Bojanowska-Czajka, A.; Kulisa, K.; Szreder, T.; Bobrowski, K.; Nichipor, H.; Garcia-Reyes, J.F.; Nałęcz-Jawecki, G.; Męczyńska-Wielgosz, S. Application of ionizing radiation in decomposition of perfluorooctanoate (PFOA) in waters. Chem. Eng. J. 2019, 357, 698–714. [Google Scholar] [CrossRef]
- Singh, R.K.; Fernando, S.; Baygi, S.F.; Multari, N.; Thagard, S.M.; Holsen, T.M. Breakdown products from perfluorinated alkyl substances (PFAS) degradation in a plasma-based water treatment process. Environ. Sci. Technol. 2019, 53, 2731–2738. [Google Scholar] [CrossRef]
- Hayashi, R.; Obo, H.; Takeuchi, N.; Yasuoka, K. Decomposition of perfluorinated compounds in water by DC plasma within oxygen bubbles. Electr. Eng. Jpn. 2015, 190, 9–16. [Google Scholar] [CrossRef]
- Stratton, G.R.; Dai, F.; Bellona, C.L.; Holsen, T.M.; Dickenson, E.R.; Thagard, M.S. Plasma-based water treatment: Efficient transformation of perfluoroalkyl substances in prepared solutions and contaminated groundwater. Environ. Sci. Technol. 2017, 51, 1643–1648. [Google Scholar] [CrossRef]
- Cheng, J.S.; Fan, Y.Y.; Pei, X.Y.; Tian, D.; Liu, Z.; Yang, L.; Feng, E.; Ji, H.-F.; Chen, Q. An Energy Efficient Process for Degrading Perfluorooctanoic Acid (PFOA) Using Strip Fountain Dielectric Barrier Discharge Plasma. Water 2022, 14, 2420. [Google Scholar] [CrossRef]
- Takeuchi, N.; Kitagawa, Y.; Kosugi, A.; Tachibana, K.; Obo, H.; Yasuoka, K. Plasma–liquid interfacial reaction in decomposition of perfluoro surfactants. J. Phys. D Appl. Phys. 2013, 47, 045203. [Google Scholar] [CrossRef]
- Panchangam, S.C.; Lin, A.-Y.C.; Shaik, K.L.; Lin, C.F. Decomposition of perfluorocarboxylic acids (PFCAs) by heterogeneous photocatalysis in acidic aqueous medium. Chemosphere 2009, 77, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Sato, S.; Abe, M.; Serpone, N. A novel liquid plasma AOP device integrating microwaves and ultrasounds and its evaluation in defluorinating perfluorooctanoic acid in aqueous media. Ultrason. Sonochem. 2011, 18, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Vanraes, P.; Willems, G.; Nikiforov, A.; Surmont, P.; Lynen, F.; Vandamme, J.; Van Durme, J.; Verheust, Y.P.; Van Hulle, S.W.; Dumoulin, A.; et al. Removal of atrazine in water by combination of activated carbon and dielectric barrier discharge. J. Hazard. Mater. 2015, 299, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Nohara, K.; Toma, M.; Kutsuna, S.; Takeuchi, K.; Ibusuki, T. Cl atom-initiated oxidation of three homologous methyl perfluoroalkyl ethers. Environ. Sci. Technol. 2001, 35, 114–120. [Google Scholar] [CrossRef]
- Wallington, T.J.; Hurley, M.D.; Fracheboud, J.M.; Orlando, A.J.J.; Tyndall, G.S.; Sehested, J.; Møgelberg, T.E.; Nielsen, O.J. Role of excited CF3CFHO radicals in the atmospheric chemistry of HFC-134a. J. Phys. Chem. 1996, 100, 18116–18122. [Google Scholar] [CrossRef]
- Jorfi, S.; Kakavandi, B.; Motlagh, H.R.; Ahmadi, M.; Jaafarzadeh, N. A novel combination of oxidative degradation for benzotriazole removal using TiO2 loaded on FeIIFe2IIIO4@C as an efficient activator of peroxymonosulfate. Appl. Catal. B Environ. 2017, 219, 216–230. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, F.; Xue, T.S.; Liu, C.; Yuan, D.; Qi, F.; Xu, B. Heterogeneous activation of peroxymonosulfate by hierarchical CuBi2O4 to generate reactive oxygen species for refractory organic compounds degradation: Morphology and surface chemistry derived reaction and its mechanism. Environ. Sci. Pollut. Res. Int. 2018, 25, 4419–4434. [Google Scholar] [CrossRef]
- Liu, Z.C.; Liu, D.X.; Chen, C.; Li, D.; Yang, A.J.; Rong, M.Z.; Chen, H.L.; Kong, M.G. Physicochemical processes in the indirect interaction between surface air plasma and deionized water. J. Phys. D Appl. Phys. 2015, 48, 495201. [Google Scholar] [CrossRef]
- Peng, J.W.; Lee, S. Atmospheric Pressure Plasma Degradation of Azo Dyes in Water: pH and Structural Effects. Plasma Chem. Plasma Process. 2013, 33, 1063–1072. [Google Scholar] [CrossRef]
- Hong, Y.J.; Nam, C.J.; Song, K.B.; Cho, G.S.; Uhm, H.S.; Choi, D.I.; Choi, H. Measurement of hydroxyl radical density generated from the atmospheric pressure bioplasma jet. J. Instrum. 2012, 7, C03046. [Google Scholar] [CrossRef]
- Durantini, A.M.; Greene, L.E.; Lincoln, R.; Martinez, S.R.; Cosa, G. Reactive oxygen species mediated activation of a dormant singlet oxygen photosensitizer: From auto- catalytic singlet oxygen amplification to chemicontrolled photodynamic therapy. J. Am. Chem. Soc. 2016, 138, 1215–1225. [Google Scholar] [CrossRef]
- Mahyar, A.; Miessner, H.; Mueller, S.; Aziz, K.H.H.; Kalass, D.; Moeller, D.; Kretschmer, K.; Manuel, S.R.; Noack, J. Development and application of different non-thermal plasma reactors for the removal of perfluorosurfactants in water: A comparative study. Plasma Chem. Plasma Process. 2019, 39, 531–544. [Google Scholar] [CrossRef]
- Kiwi, J.; Lopez, A.; Nadtochenko, V. Mechanism and kinetics of the OH-radical intervention during Fenton oxidation in the presence of a significant amount of radical scavenger (Cl-). Environ. Sci. Technol. 2000, 34, 2162–2168. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma–liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Song, Z.; Tang, H.Q.; Wang, N.; Zhu, L.H. Reductive defluorination of perfluorooctanoic acid by hydrated electrons in a sulfite-mediated UV photochemical system. J. Hazard. Mater. 2013, 262, 332–338. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Liu, G.; Fang, J.; Yue, S.; Guan, Y.; Chen, L.; Liu, X. Efficient reductive dechlorination of monochloroacetic acid by sulfite/UV process. Environ. Sci. Technol. 2012, 46, 7342–7349. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/⋅O− in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Rumbach, P.; Bartels, D.M.; Sankaran, R.M.; Go, D.B. The solvation of electrons by an atmospheric-pressure plasma. Nat. Commun. 2015, 6, 7248. [Google Scholar] [CrossRef]
- Wang, Y.; Craven, M.; Yu, X.; Ding, J.; Bryant, P.; Hualng, J.; Tu, X. Plasma-Enhanced Catalytic Synthesis of Ammonia over a Ni/Al2O3 Catalyst at Near-Room Temperature: Insights into the Importance of the Catalyst Surface on the Reaction Mechanism. ACS Catal. 2019, 9, 10780–10793. [Google Scholar] [CrossRef] [PubMed]

| C Number | Compound | Chemical Formula |
|---|---|---|
| 6 | Perfluoroheptanoic acid | C6F13COOH |
| 5 | Perfluorohexanoic acid, | C5F11COOH |
| 4 | Perfluoropentanoic acid | C4F9COOH |
| 3 | Perfluorobutanoic acid | C3F7COOH |
| 2 | Perfluoropropionic acid | C2F5COOH |
| 1 | Trifluoroacetic acid | CF3COOH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Fan, Y.; Pei, X.; Tian, D.; Liu, Z.; Wei, Z.Z.; Ji, H.-f.; Chen, Q. Mechanism and Reactive Species in a Fountain-Strip DBD Plasma for Degrading Perfluorooctanoic Acid (PFOA). Water 2022, 14, 3384. https://doi.org/10.3390/w14213384
Cheng J, Fan Y, Pei X, Tian D, Liu Z, Wei ZZ, Ji H-f, Chen Q. Mechanism and Reactive Species in a Fountain-Strip DBD Plasma for Degrading Perfluorooctanoic Acid (PFOA). Water. 2022; 14(21):3384. https://doi.org/10.3390/w14213384
Chicago/Turabian StyleCheng, Jiushan, Yangyang Fan, Xueyun Pei, Di Tian, Zhongwei Liu, Zachary Z. Wei, Hai-feng Ji, and Qiang Chen. 2022. "Mechanism and Reactive Species in a Fountain-Strip DBD Plasma for Degrading Perfluorooctanoic Acid (PFOA)" Water 14, no. 21: 3384. https://doi.org/10.3390/w14213384
APA StyleCheng, J., Fan, Y., Pei, X., Tian, D., Liu, Z., Wei, Z. Z., Ji, H.-f., & Chen, Q. (2022). Mechanism and Reactive Species in a Fountain-Strip DBD Plasma for Degrading Perfluorooctanoic Acid (PFOA). Water, 14(21), 3384. https://doi.org/10.3390/w14213384







