Adsorption Behavior of Nonylphenol on Polystyrene Microplastics and Their Cytotoxicity in Human Caco-2 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Characterization of PS-MPs and Detection of NP
2.3. Adsorption and Desorption Experiments
2.4. The Factors That Influence the Adsorption Behavior of NP on PS-MPs
2.5. Adsorption Kinetic and Isotherm Equations
2.6. Cell Culture
2.7. Cytotoxicity Assays
2.7.1. Cell Viability
2.7.2. Cell Cycle
2.7.3. Apoptosis
2.7.4. Mitochondrial Membrane Potential (MMP)
2.7.5. Reactive Oxygen Species (ROS)
2.8. Statistical Analysis
3. Results
3.1. Characterization of PS-MPs
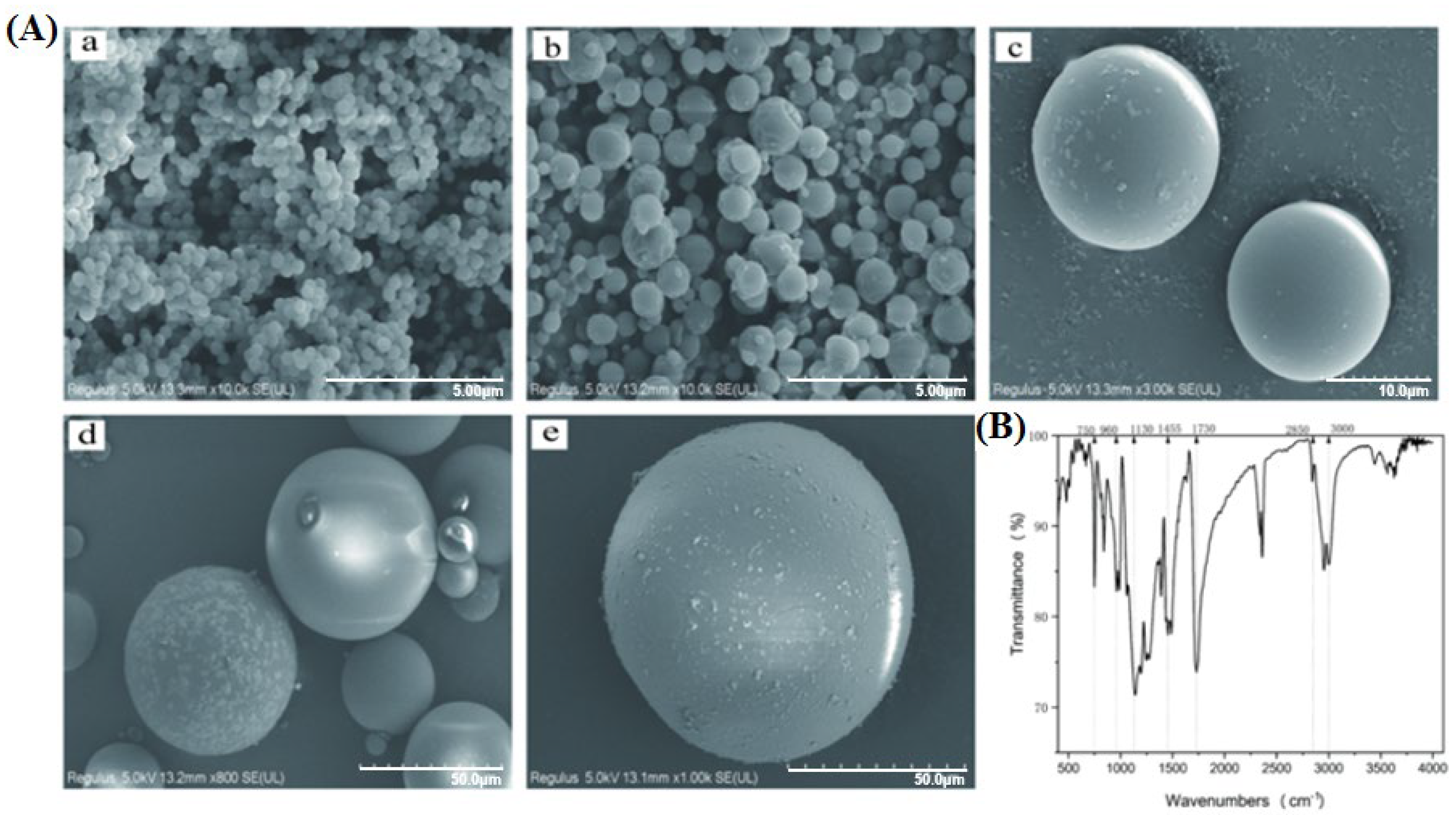
| Particle Size (μm) | 0.1 | 1 | 10 | 50 | 100 |
|---|---|---|---|---|---|
| Surface area (m2/g) | 62.248 | 38.193 | 16.887 | 3.824 | 3.351 |
| Total pore volume (cc/g) | 0.336 | 0.476 | 0.043 | 0.007 | 0.004 |
| Average pore diameter (nm) | 19.970 | 9.818 | 10.013 | 7.082 | 5.096 |
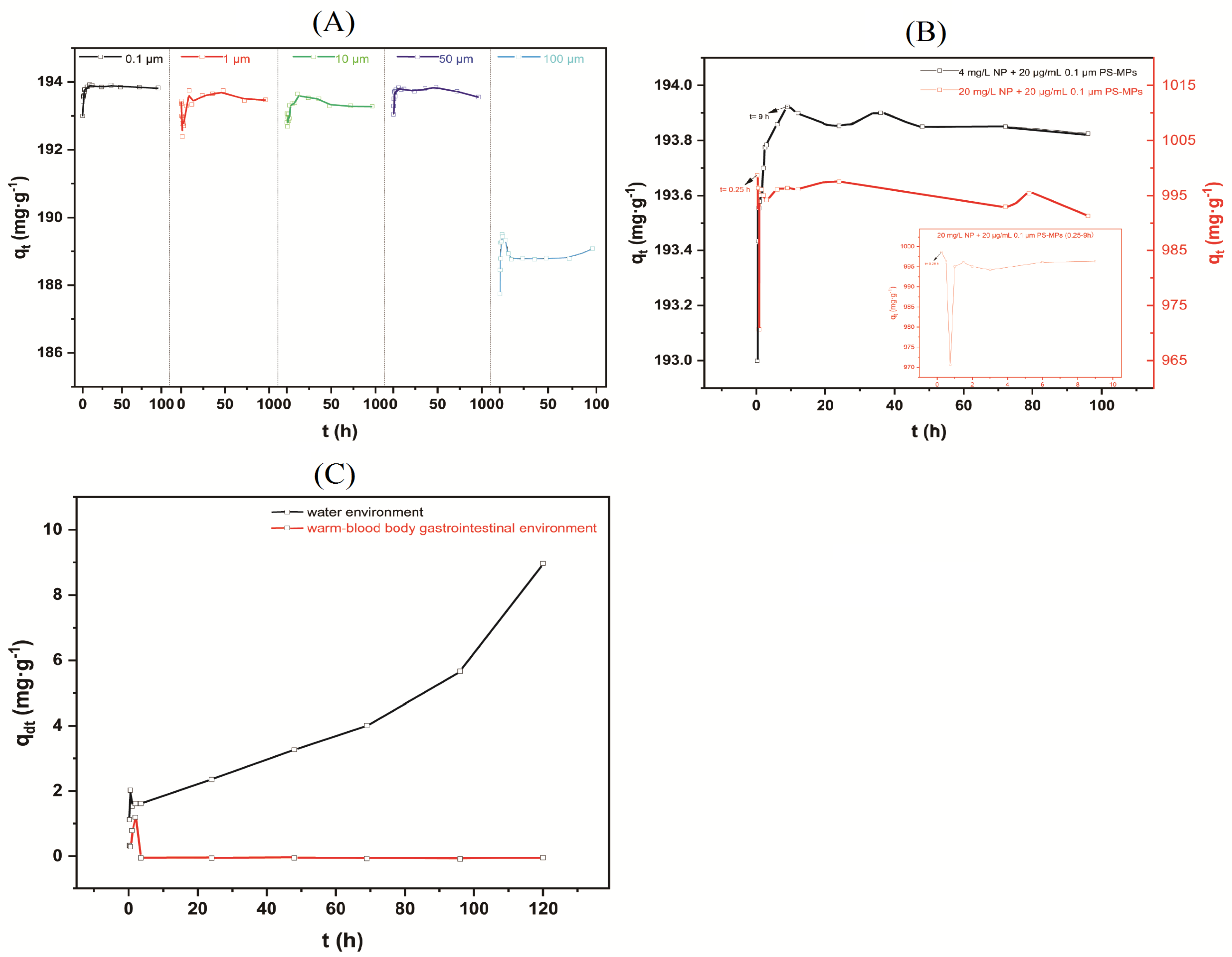
3.2. Effect of Reaction Time on Adsorption and Desorption of NP on PS-MPs
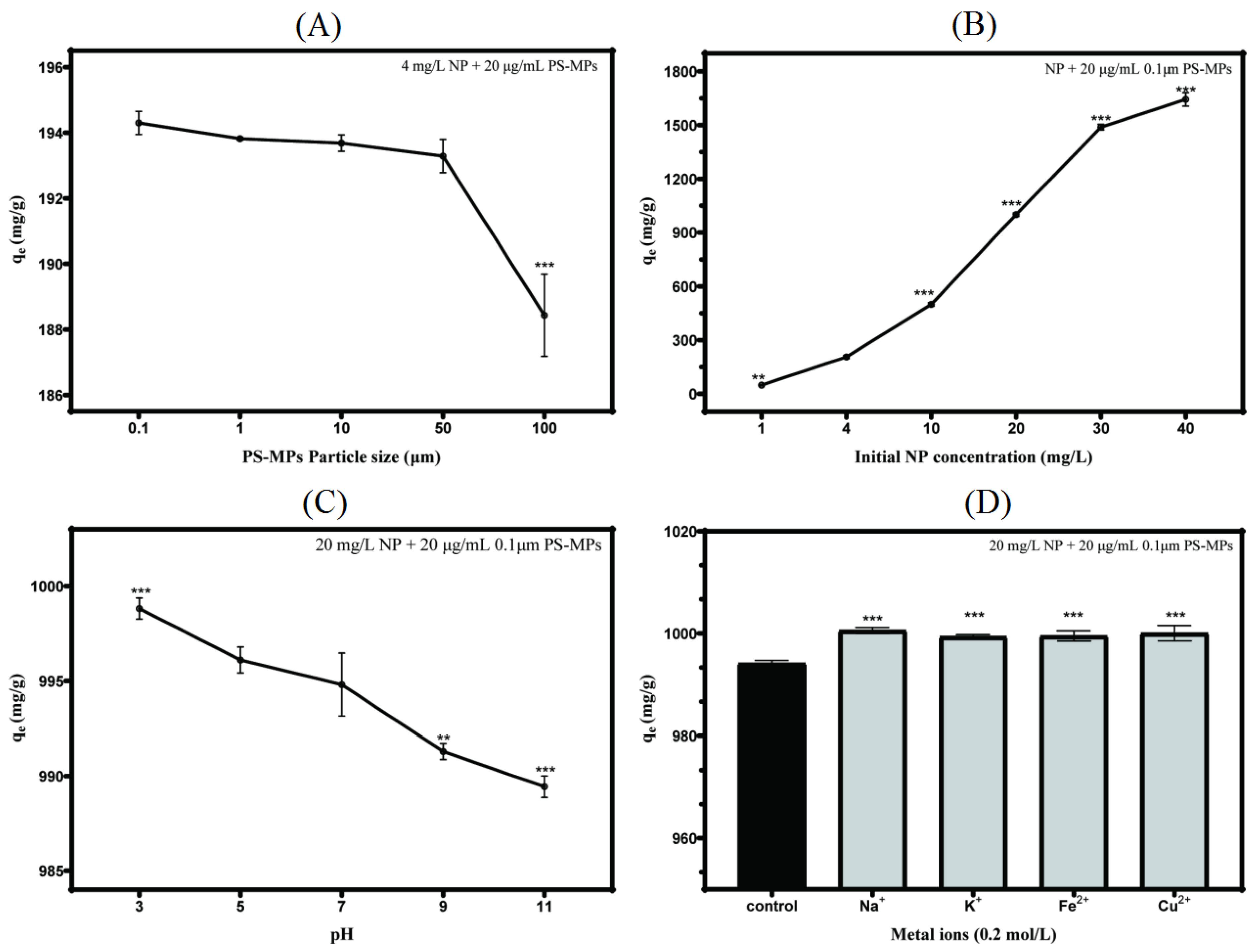
3.3. Intrinsic and Extrinsic Factors That Influence NP Adsorption to PS-MPs
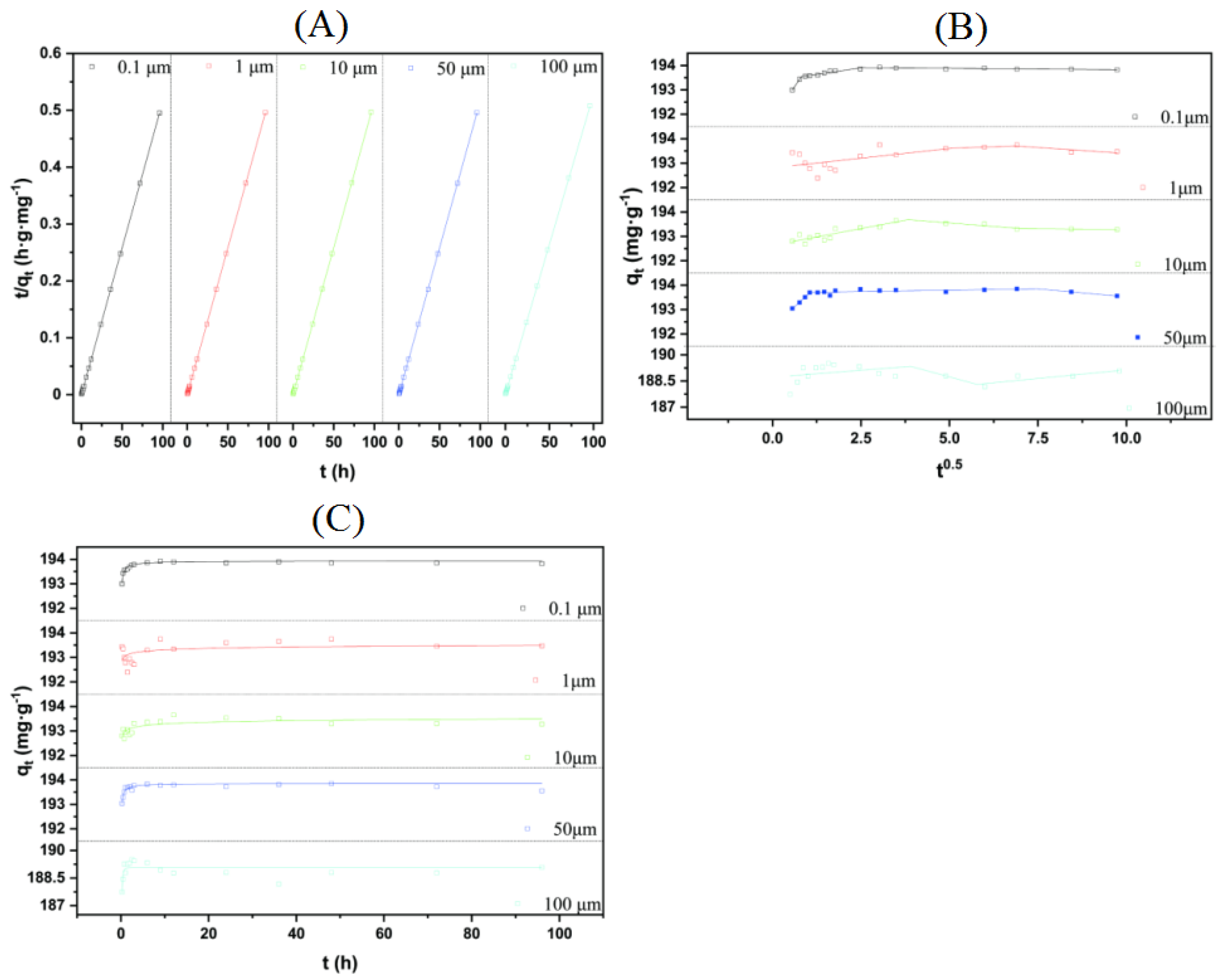
3.4. Adsorption Kinetics
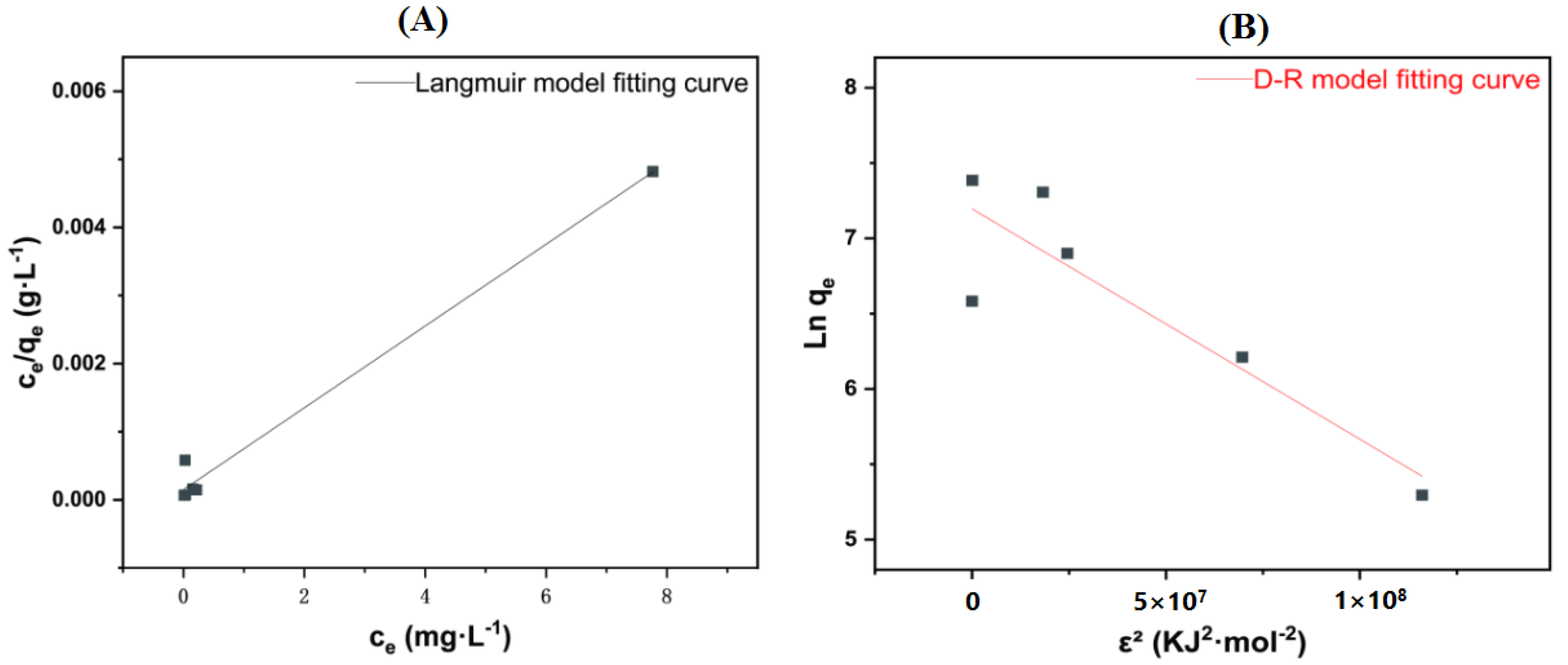
3.5. Adsorption Isotherm
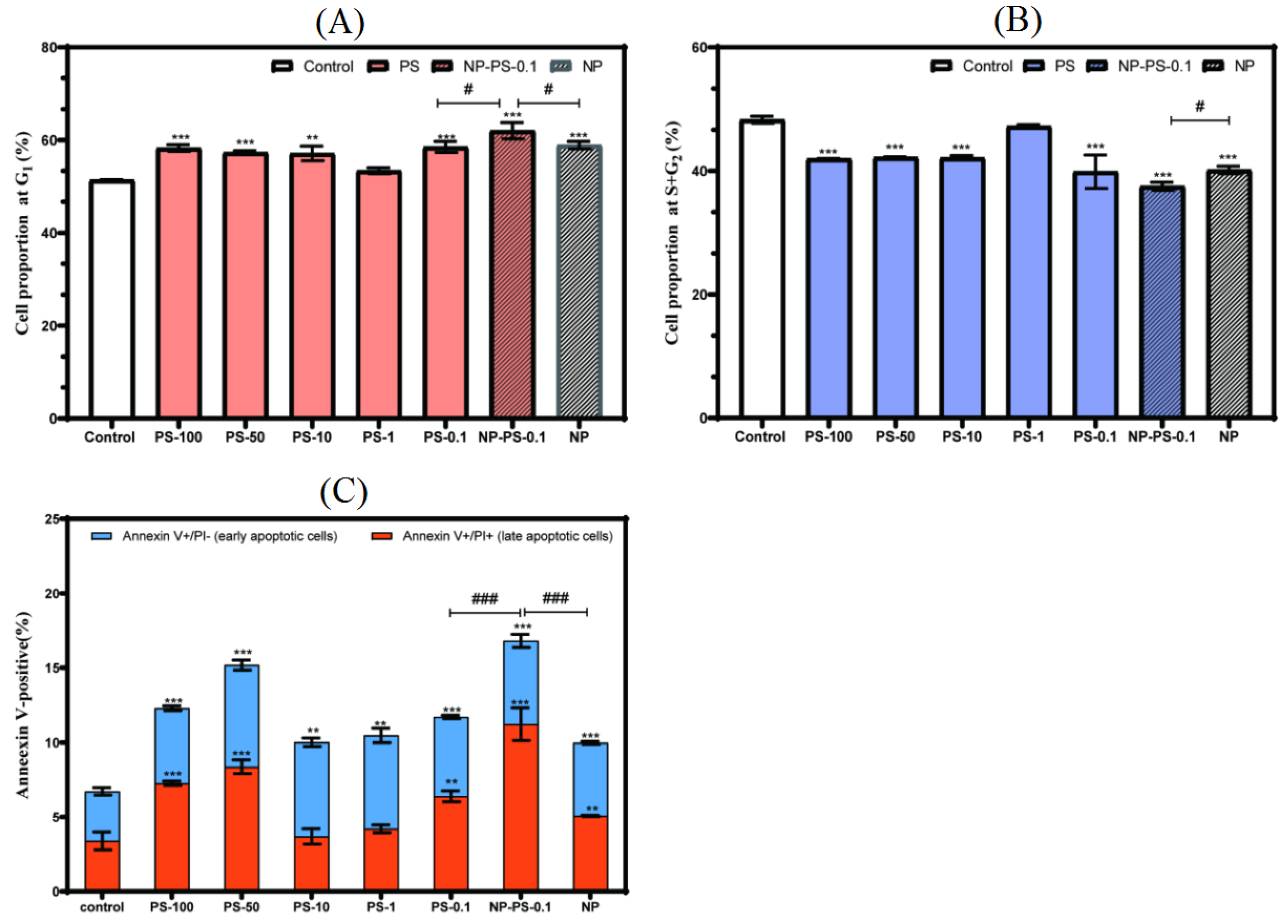
3.6. Cell Proliferation and Apoptosis
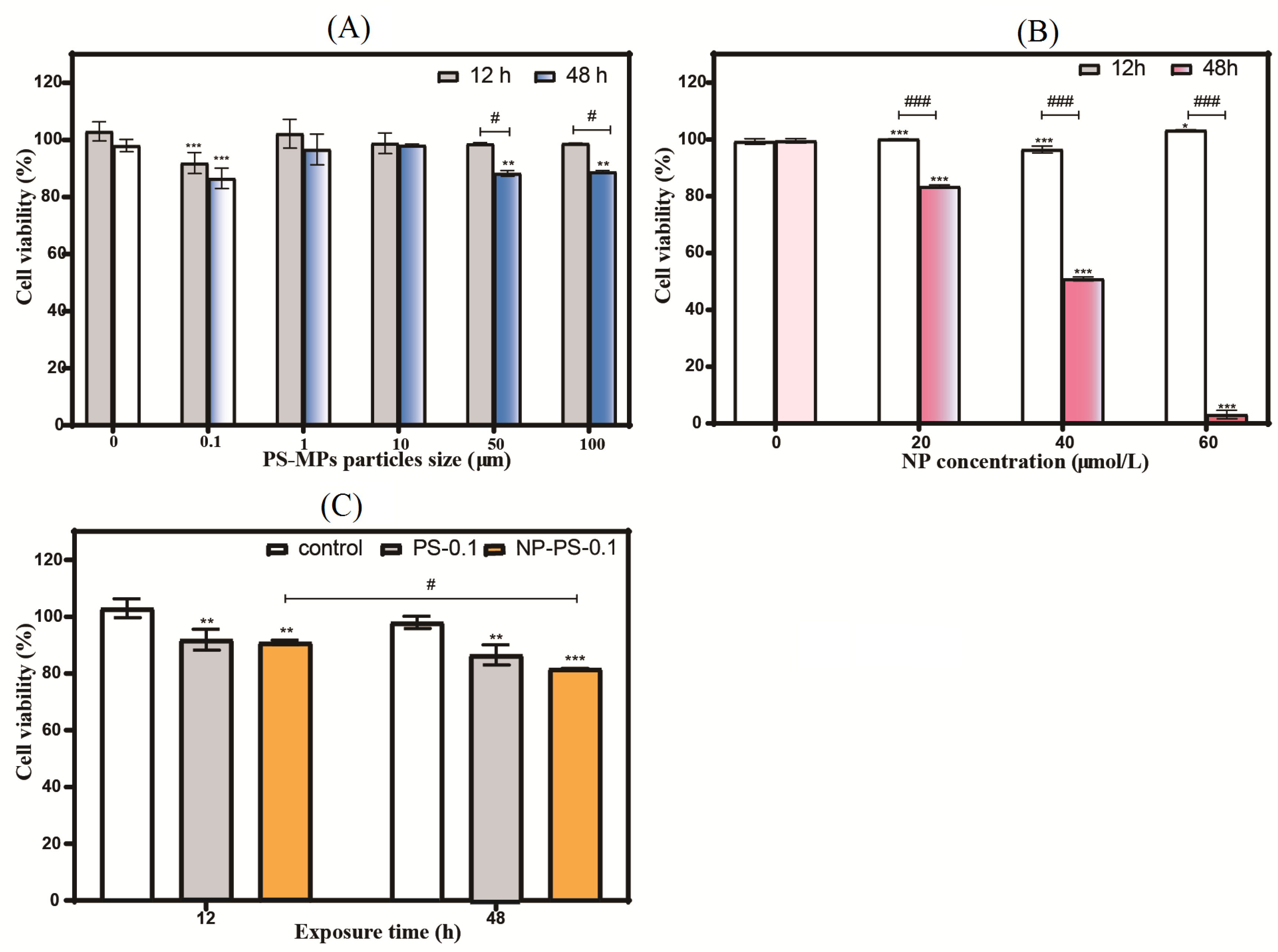
3.6.1. Cell Viability
3.6.2. Cell Cycle
3.6.3. Apoptosis
3.7. Cell Function
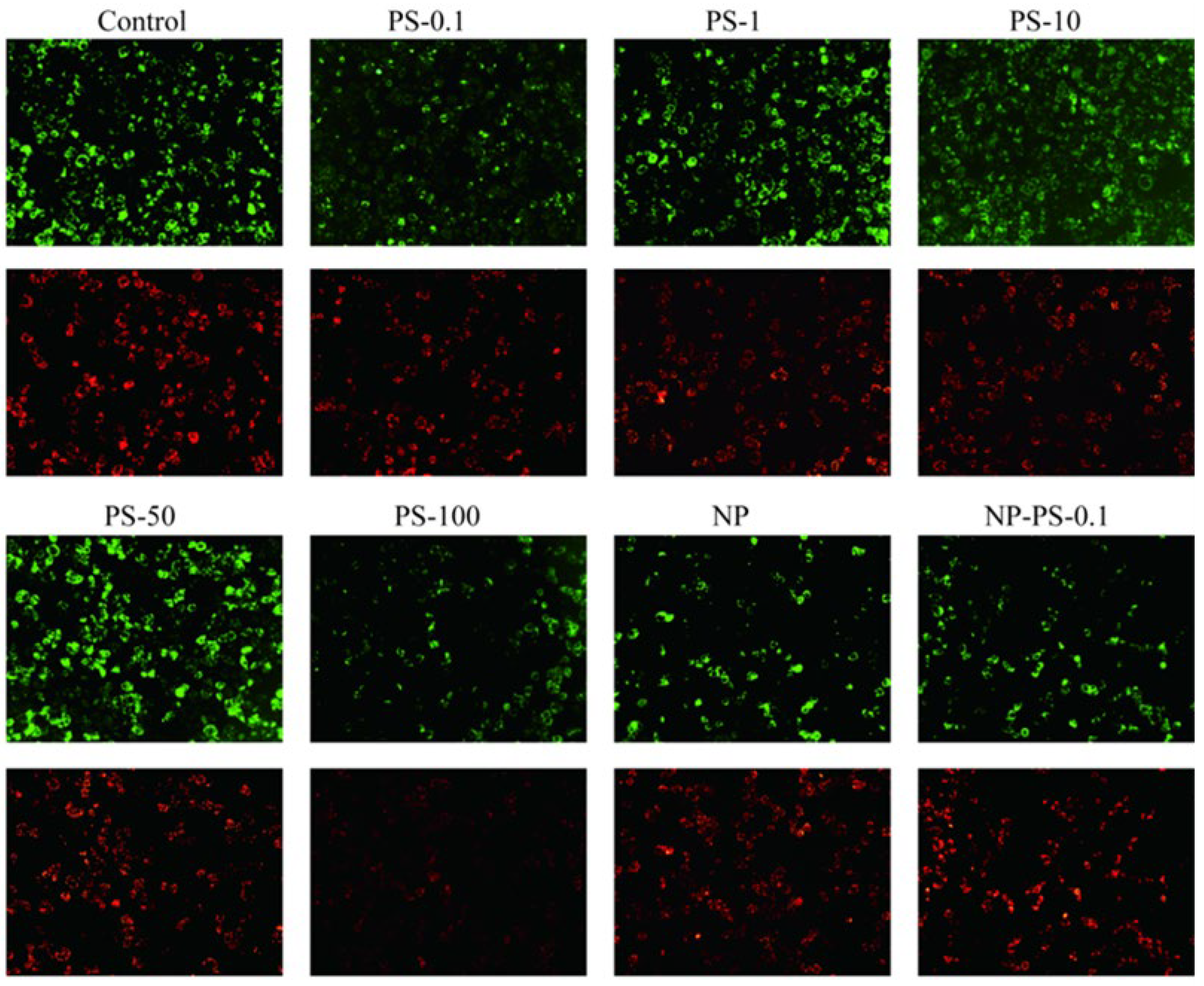
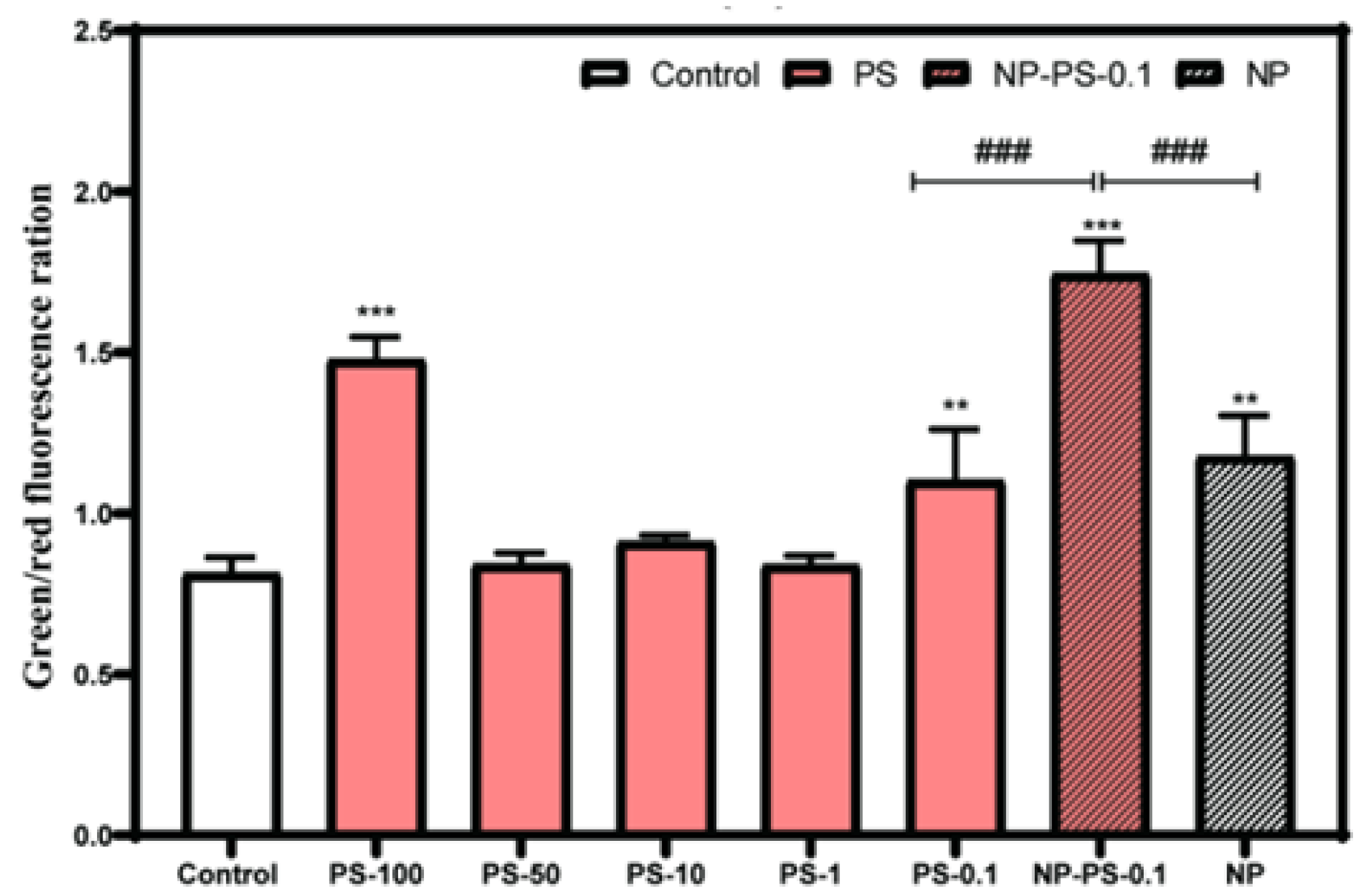
3.7.1. Mitochondrial Depolarization
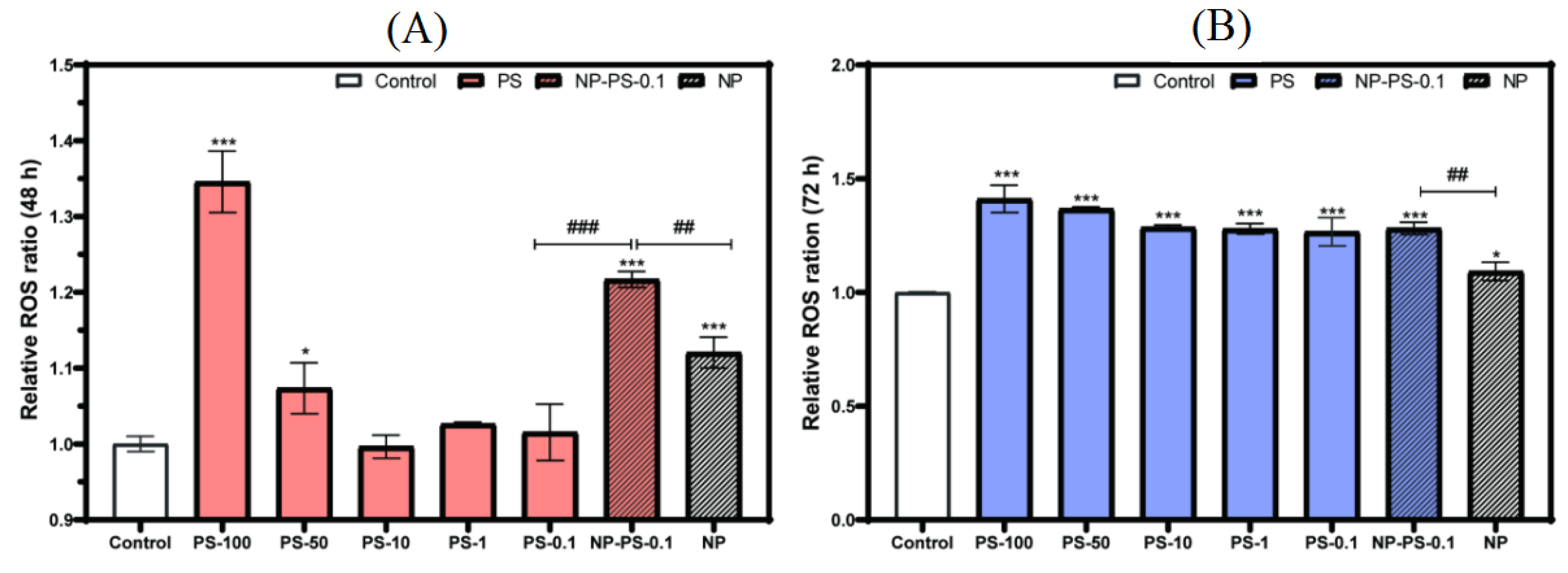
3.7.2. Reactive Oxygen Species Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Name | Equations | References |
|---|---|---|
| kinetics | ||
| Pseudo-first-order | [40] | |
| Pseudo-second-order | [41] | |
| Intra-particle diffusion | [42] | |
| Bangham | [43] | |
| isotherm | ||
| Langmuir | [44] | |
| Freundlich | [45] | |
| D-R | [46] | |
References
- Daniel, D.B.; Ashraf, P.M.; Thomas, S.N.; Thomson, K.T. Microplastics in the edible tissues of shellfishes sold for human consumption. Chemosphere 2021, 264, 128554. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Feng, Z.; Zhang, T.; Ma, C.; Shi, H. Microplastics in the commercial seaweed nori. J. Hazard. Mater. 2020, 388, 122060. [Google Scholar] [CrossRef] [PubMed]
- Nan, B.; Su, L.; Kellar, C.; Craig, N.J.; Keough, M.J.; Pettigrove, V. Identification of microplastics in surface water and Australian freshwater shrimp Paratya australiensis in Victoria, Australia. Environ. Pollut. 2020, 259, 113865. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Koppel, S.; Konigshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of various microplastics in human stool: A prospective case series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and accumulation of polystyrene microplastics in Zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.; Cedervall, T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef] [Green Version]
- Vieira, Y.; Lima, E.C.; Foletto, E.L.; Dotto, G.L. Microplastics physicochemical properties, specific adsorption modeling and their interaction with pharmaceuticals and other emerging contaminants. Sci. Total Environ. 2021, 753, 141981. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Qiao, R.; Bonilla, M.M.; Yang, X.; Ren, H.; Lemos, B. Evidence that microplastics aggravate the toxicity of organophosphorus flame retardants in mice (Mus musculus). J. Hazard. Mater. 2018, 357, 348–354. [Google Scholar] [CrossRef]
- Deng, Y.F.; Yan, Z.H.; Shen, R.Q.; Wang, M.; Huang, Y.C.; Ren, H.Q.; Zhang, Y.; Lemos, B. Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environ. Int. 2020, 143, 105916. [Google Scholar] [CrossRef]
- Soares, A.; Guieysse, B.; Jefferson, B.; Cartmell, E.; Lester, J.N. Nonylphenol in the environment: A critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ. Int. 2008, 34, 1033–1049. [Google Scholar] [CrossRef]
- Sun, D.; Chen, Q.; Zhu, B.; Zhao, H.; Duan, S. Multigenerational reproduction and developmental toxicity, and HPG axis gene expression study on environmentally-relevant concentrations of nonylphenol in zebrafish. Sci. Total Environ. 2021, 764, 144259. [Google Scholar] [CrossRef]
- Li, M.; You, M.; Li, S.; Qiu, Z.; Wang, Y. Effects of maternal exposure to nonylphenol on learning and memory in offspring involve inhibition of BDNF-PI3K/Akt signaling. Brain Res. Bull. 2019, 146, 270–278. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, M.; Xu, Y.; Ju, J.; Pan, M.; Pan, Z.; Li, X.; Sun, S. Nonylphenol exposure affects mouse oocyte quality by inducing spindle defects and mitochondria dysfunction. Environ. Pollut. 2020, 266, 114967. [Google Scholar] [CrossRef]
- Li, F.F.; Du, P.C.; Yang, W.Y.; Huang, D.F.; Nie, S.P.; Xie, M.Y. Polysaccharide from the seeds of Plantago asiatica L. alleviates nonylphenol induced intestinal barrier injury by regulating tight junctions in human Caco-2 cell line. Int. J. Biol. Macromol. 2020, 164, 2134–2140. [Google Scholar] [CrossRef]
- Uzun, P.; Farazande, S.; Guven, B. Mathematical modeling of microplastic abundance, distribution, and transport in water environments: A review. Chemosphere 2022, 288, 132517. [Google Scholar] [CrossRef]
- Bhandari, G.; Bagheri, A.R.; Bhatt, P.; Bilal, M. Occurrence, potential ecological risks, and degradation of endocrine disrupter, nonylphenol, from the aqueous environment. Chemosphere 2021, 275, 130013. [Google Scholar] [CrossRef]
- Stenholm, Å.; Holmström, S.; Hjärthag, S.; Lind, O. Development of a high-performance liquid chromatography-fluorescence detection method for analyzing nonylphenol/dinonylphenol-polyethoxylate-based phosphate esters. J. Chromatogr. 2009, 1216, 6974–6977. [Google Scholar] [CrossRef]
- Tsuda, T.; Suga, K.; Kaneda, E.; Ohsuga, M. Determination of 4-nonylphenol, nonylphenol monoethoxylate, nonylphenol diethoxylate and other alkylphenols in fish and shellfish by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. 2000, 746, 305–309. [Google Scholar] [CrossRef]
- Liu, P.; Wu, X.; Liu, H.; Wang, H.; Lu, K.; Gao, S. Desorption of pharmaceuticals from pristine and aged polystyrene microplastics under simulated gastrointestinal conditions. J. Hazard. Mater. 2020, 392, 122346. [Google Scholar] [CrossRef]
- Zuo, L.; Li, H.; Lin, L.; Sun, Y.; Diao, Z.; Liu, S.; Zhang, Z.; Xu, X. Sorption and desorption of phenanthrene on biodegradable poly(butylene adipate co-terephtalate) microplastics. Chemosphere 2019, 215, 25–32. [Google Scholar] [CrossRef]
- Ziino, G.; Nalbone, L.; Giarratana, F.; Romano, B.; Cincotta, F.; Panebianco, A. Microplastics in vacuum packages of frozen and glazed icefish (Neosalanx spp.): A freshwater fish intended for human consumption. Ital. J. Food Saf. 2021, 10, 9974. [Google Scholar] [CrossRef]
- Tang, S.; Lin, L.; Wang, X.; Feng, A.; Yu, A. Pb(II) uptake onto nylon microplastics: Interaction mechanism and adsorption performance. J. Hazard. Mater. 2020, 386, 121960. [Google Scholar] [CrossRef]
- Yu, F.; Yang, C.; Huang, G.; Zhou, T.; Zhao, Y.; Ma, J. Interfacial interaction between diverse microplastics and tetracycline by adsorption in an aqueous solution. Sci. Total Environ. 2020, 721, 137729. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; He, H.; Cheng, X.; Ma, T.; Hu, J.; Yang, S.; Li, S.; Zhang, L. Adsorption behavior and mechanism of 9-Nitroanthracene on typical microplastics in aqueous solutions. Chemosphere 2020, 245, 125628. [Google Scholar] [CrossRef]
- Sun, M.; Yang, Y.K.; Huang, M.L.; Fu, S.K.; Hao, Y.Y.; Hu, S.Y.; Lai, D.L.; Zhao, L. Adsorption behaviors and mechanisms of antibiotic norfloxacin on degradable and nondegradable microplastics. Sci. Total Environ. 2022, 807 Pt 3, 151042. [Google Scholar] [CrossRef]
- Calisto, V.; Jaria, G.; Silva, C.P.; Ferreira, C.I.A.; Otero, M.; Esteves, V.I. Single and multi-component adsorption of psychiatric pharmaceuticals onto alternative and commercial carbons. J. Env. Manag. 2017, 192, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Liu, X.; Wang, J. Ecotoxicological effects of microplastics and cadmium on the earthworm Eisenia foetida. J. Hazard. Mater. 2020, 392, 122273. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, K.; Ding, D.X.; Liu, J.T.; Lei, Z.; Chen, X.X.; Ye, G.Z.; Zhang, J.; Shen, H.Q.; Yan, C.Z.; et al. Size-dependent adverse effects of microplastics on intestinal microbiota and metabolic homeostasis in the marine medaka (Oryzias melastigma). Environ. Int. 2021, 151, 106452. [Google Scholar] [CrossRef]
- Liang, B.; Zhong, Y.; Huang, Y.J.; Lin, X.; Liu, J.; Lin, L.; Hu, M.J.; Jiang, J.Y.; Dai, M.Z.; Wang, B.; et al. Underestimated health risks: Polystyrene micro- and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef]
- Visalli, G.; Facciolà, A.; Pruiti Ciarello, M.; De Marco, G.; Maisano, M.; Di Pietro, A. Acute and Sub-Chronic Effects of Microplastics (3 and 10 microm) on the Human Intestinal Cells HT-29. Int. J. Environ. Res. Public Health 2021, 18, 15833. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.W.; Shull, G.M.; Fountain, J.H.; Guo, Z.; Musselman, L.P.; Fiumera, A.C.; Mahler, G.J. Titanium dioxide nanoparticle exposure alters metabolic homeostasis in a cell culture model of the intestinal epithelium and Drosophila melanogaster. Nanotoxicology 2018, 12, 390–406. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Blanco, C.; Juan-García, A.; Juan, C.; Font, G.; Ruiz, M. Alternariol induce toxicity via cell death and mitochondrial damage on Caco-2 cells. Food Chem. Toxicol. 2016, 88, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Lee, Y.H.; Hsu, Y.H.; Chiu, I.J.; Huang, C.C.Y.; Huang, C.C.; Chia, Z.C.; Lee, C.P.; Lin, Y.F.; Chiu, H.W. The kidney-related effects of polystyrene microplastics on human kidney proximal tubular epithelial cells HK-2 and male C57BL/6 mice. Environ. Health Perspect. 2021, 129, 57003. [Google Scholar] [CrossRef]
- Hesler, M.; Aengenheister, L.; Ellinger, B.; Drexel, R.; Straskraba, S.; Jost, C.; Wagner, S.; Meier, F.; von Briesen, H.; Büchel, C.; et al. Multi-endpoint toxicological assessment of polystyrene nano- and microparticles in different biological models in vitro. Toxicol. Vitr. 2019, 61, 104610. [Google Scholar] [CrossRef]
- Yu, Y.; Mo, W.Y.; Luukkonen, T. Adsorption behaviour and interaction of organic micropollutants with nano and microplastics—A review. Sci. Total Environ. 2021, 797, 149140. [Google Scholar] [CrossRef]
- Singh, N.; Bhagat, J.; Tiwari, E.; Khandelwal, N.; Darbha, G.K.; Shyama, S.K. Metal oxide nanoparticles and polycyclic aromatic hydrocarbons alter nanoplastic’s stability and toxicity to zebrafish. J. Hazard. Mater. 2021, 407, 124382. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Bai, J.L.; Ning, B.; Fan, L.X.; Sun, T.Q.; Fang, Y.J.; Wu, J.; Li, S.; Duan, C.H.; Zhang, Y.C.; et al. Effects of bisphenol A and nanoscale and microscale polystyrene plastic exposure on particle uptake and toxicity in human Caco-2 cells. Chemosphere 2020, 254, 126788. [Google Scholar] [CrossRef]
- Vagner, M.; Boudry, G.; Courcot, L.; Vincent, D.; Dehaut, A.; Duflos, G.; Huveta, A.; Tallec, K.; Zambonino-Infante, J.L. Experimental evidence that polystyrene nanoplastics cross the intestinal barrier of European seabass. Environ. Int. 2022, 166, 107340. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetensk. Handl. Band 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Ng, J.C.Y.; McKay, G. Kinetics of pollutant sorption by biosorbents. Separ. Purif. Method. 2000, 29, 189–232. [Google Scholar] [CrossRef]
- Morris, J.C.; Weber, W.J., Jr. Removal of biologically-resistant pollutants from waste waters by adsorption. Adv. Water Poll. Res. 1964, 269, 231–266. [Google Scholar]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Ueber die adsorption in loesungen. Z. Phys. Chem. 1996, 57, 385–470. [Google Scholar]
- Rey, F.; Calle, E.; Casado, J. Study of the effects of concentration and pH on the dissociation kinetics of Fe(II)-fulvic acid complexes. Int. J. Chem. Kinet. 1998, 30, 63–67. [Google Scholar] [CrossRef]
| Kinetic Model | Particle Size (μm) | |||||
|---|---|---|---|---|---|---|
| 0.1 | 1 | 10 | 50 | 100 | ||
| Pseudo-second-order model | ||||||
| k2 (g·mg−1·h−1) | 127.567 | 27.779 | 18.979 | 66.961 | 13.285 | |
| qe (mg·g−1) | 193.923 | 193.870 | 193.859 | 193.851 | 189.560 | |
| R2 | 0.9998 | 0.9998 | 0.9997 | 0.9982 | 0.9987 | |
| Intra-particle diffusion model | ||||||
| kip (mg·g−1 min−1) | k1p | 2.10013 | 0.15685 | 0.26952 | 1.30454 | 0.15907 |
| k2p | 0.20992 | 0.04903 | −0.11312 | 0.02179 | −0.55142 | |
| k3p | −0.01188 | −0.09727 | −0.01576 | −0.12874 | 0.19729 | |
| C (mg·g−1) | C1 | 191.949 | 192.81716 | 192.65587 | 192.38299 | 188.71922 |
| C2 | 193.385 | 193.36525 | 194.11567 | 193.68406 | 191.49437 | |
| C3 | 193.944 | 194.37526 | 193.43371 | 194.81593 | 181.1686 | |
| R2 | 0.9875 | 0.4645 | 0.8277 | 0.9451 | 0.2302 | |
| Bangham model | ||||||
| k | 6.35439 | 5.47685 | 5.40951 | 6.52911 | 7.89394 | |
| z | 0.11868 | 0.02838 | 0.03225 | 0.12528 | 0.3395 | |
| R2 | 0.9519 | 0.2615 | 0.5853 | 0.7581 | 0.4534 | |
| Isotherm Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Langmuir Model | |||||||||
| qmax (mg·g−1) | KL | R2 | RL | ||||||
| 1 | 4 | 10 | 20 | 30 | 40 | 50 | |||
| 1665.6118 | 3.9657 | 0.9880 | 0.2014 | 0.0593 | 0.0246 | 0.0125 | 0.0083 | 0.0067 | 0.0050 |
| D-R model | |||||||||
| qmax (mg·g−1) | KD-R (mol2·KJ−2) | R2 | E | ||||||
| 19.5600 | 1.52938 × 108 | 0.8062 | 5.717 × 10−5 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, F.; Zhao, Q.; Wang, L.; Ma, J.; Song, L.; Huang, D. Adsorption Behavior of Nonylphenol on Polystyrene Microplastics and Their Cytotoxicity in Human Caco-2 Cells. Water 2022, 14, 3288. https://doi.org/10.3390/w14203288
Ding F, Zhao Q, Wang L, Ma J, Song L, Huang D. Adsorption Behavior of Nonylphenol on Polystyrene Microplastics and Their Cytotoxicity in Human Caco-2 Cells. Water. 2022; 14(20):3288. https://doi.org/10.3390/w14203288
Chicago/Turabian StyleDing, Fangfang, Qianqian Zhao, Luchen Wang, Juan Ma, Lingmin Song, and Danfei Huang. 2022. "Adsorption Behavior of Nonylphenol on Polystyrene Microplastics and Their Cytotoxicity in Human Caco-2 Cells" Water 14, no. 20: 3288. https://doi.org/10.3390/w14203288
APA StyleDing, F., Zhao, Q., Wang, L., Ma, J., Song, L., & Huang, D. (2022). Adsorption Behavior of Nonylphenol on Polystyrene Microplastics and Their Cytotoxicity in Human Caco-2 Cells. Water, 14(20), 3288. https://doi.org/10.3390/w14203288










