Analysis of the Mechanism of Acid Mine Drainage Neutralization Using Fly Ash as an Alternative Material: A Case Study of the Extremely Acidic Lake Robule in Eastern Serbia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water Samples from the Lake Robule (AMD)
2.2. Fly ash Samples
2.3. Defining the Optimal Solid/Liquid Ratio for AMD Treatment
2.4. AMD Treatment with FA Samples
2.5. AMD Treatment with NaOH as a Conventional Neutralization Reagent
2.6. Acid Neutralization Capacity Test
2.7. Thermodynamic Modeling
3. Results and Discussion
3.1. Characterization of the Water Samples from Lake Robule
3.2. Characterization of the Fly Ashes Samples (EF and KOST)
3.2.1. Chemical Composition
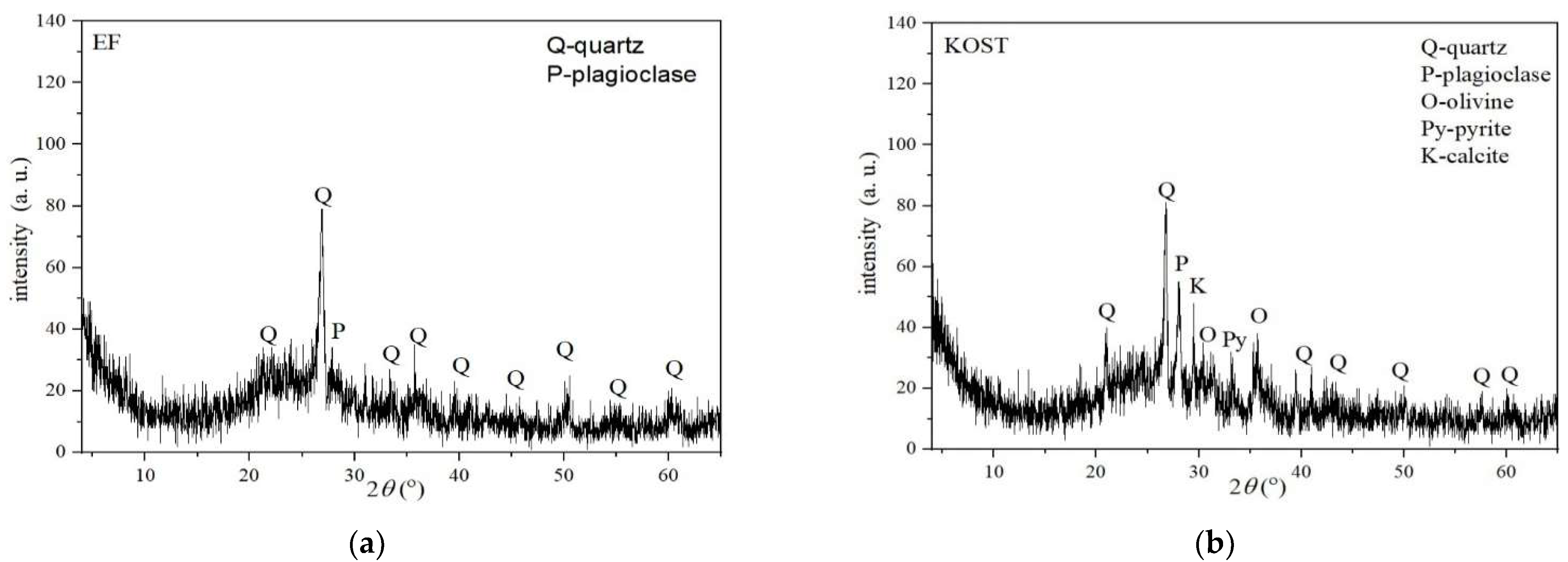
3.2.2. XRD Analyses of Fly Ashes
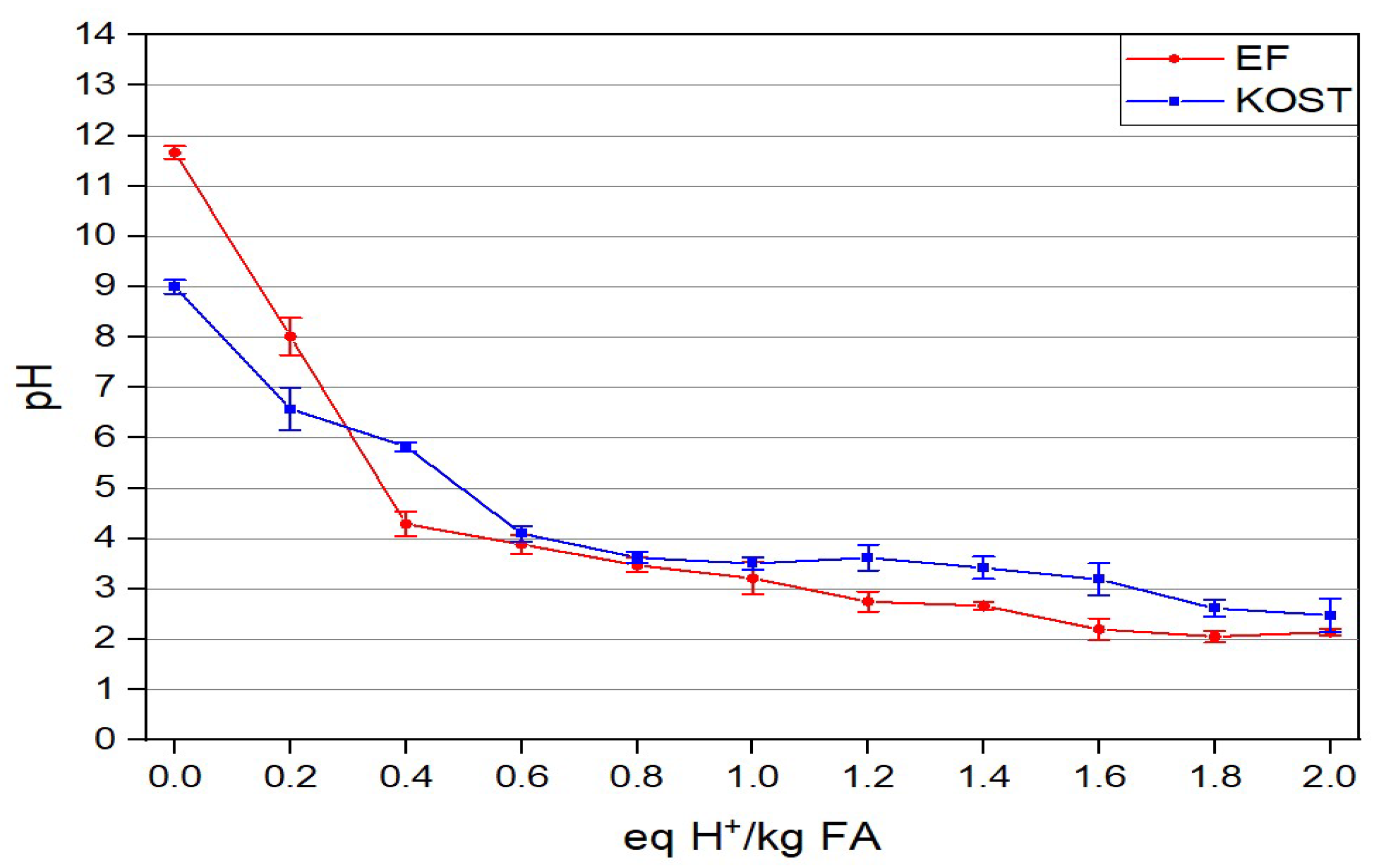
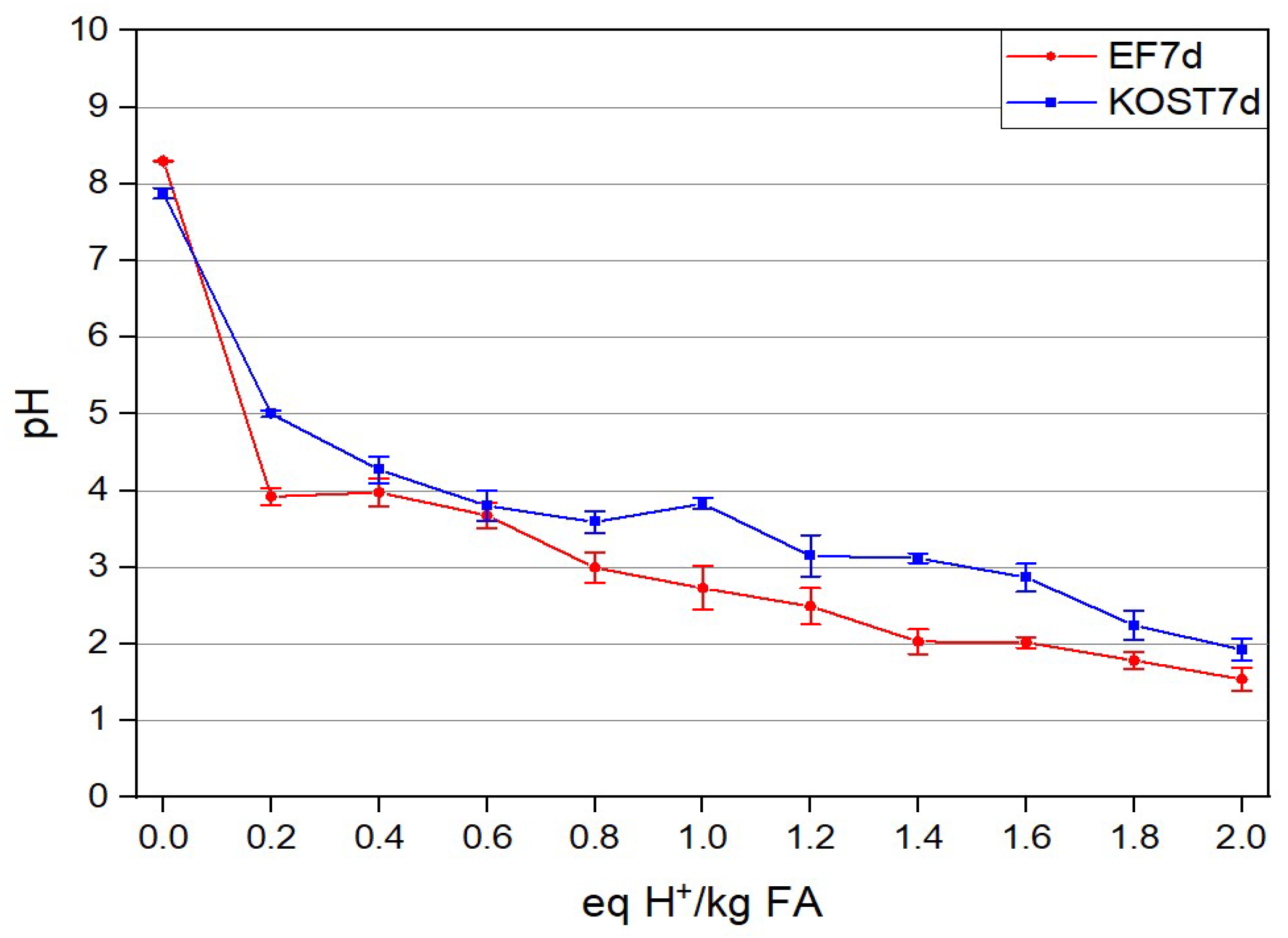
3.2.3. ANC of Fly Ashes
3.3. Results of AMD Treatment with Fly Ashes
3.3.1. Optimal S/L Ratio
3.3.2. Analysis of Solutions after the Treatment
3.3.3. Analysis of Solid Residues after the Treatment
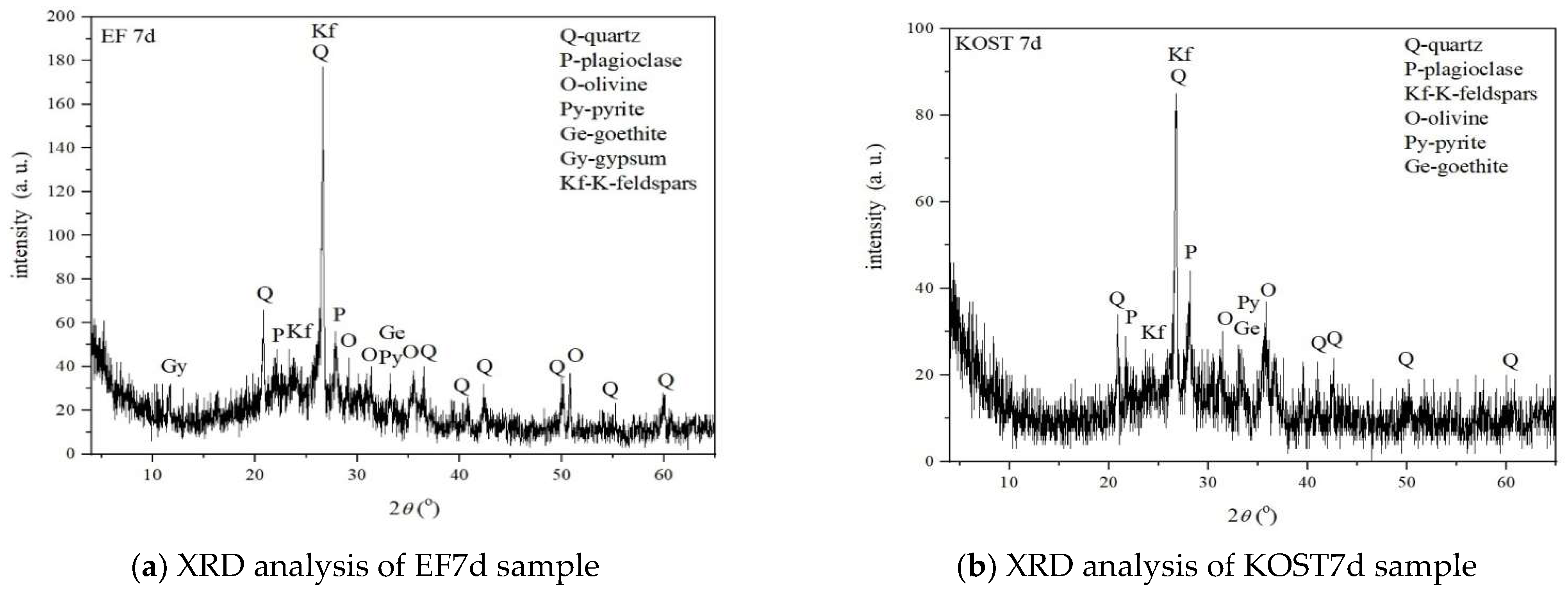
3.3.4. XRD Analyses of the Solid Residues
3.3.5. ANC of the Solid Residues
3.4. Mechanism of the Metals Removal from the AMD
3.4.1. Results of PHREEQC Modeling
3.4.2. Treatment with NaOH
3.4.3. Co-Precipitation of Metals Cations
Iron and Aluminum
Copper and Zinc
Nickel, Lead, and Cadmium
4. Conclusions
- Due to their alkaline nature, both samples of fly ash EF and KOST can neutralize acidic mine waters at optimal solid liquid rations with increasing pH values.
- The optimal solid liquid ratio for EF fly ash is 25%, while for KOST fly ash it is 20%.
- In laboratory conditions, after neutralization with either EF or KOST fly ash, more than 99% of Al, Fe, Cu, and Zn and over 89% of Pb have precipitated.
- The removal of Fe3+ and Al3+ ions and formation of insoluble (oxy)hydroxide compounds, that occurs in first 5 min of neutralization, at pH 4.38 for the EF sample and 5.11 for the KOST sample, creates favorable conditions for co-precipitation of other trace metals (Cu, Zn, Ni, Pb, and Cd) from AMD, which is further enhanced by cation adsorption on FA particles.
- The neutralizing efficiency was determined by comparative analysis between the EF and KOST fly ash. The more effective neutralizing agent between these two FA was found to be KOST fly ash, since it can elevate the pH to the alkali range within a day due to changes in mineral phases. These changes in the mineral phase occurred due to the aging of FA through the carbonization process of calcium oxide, originally present in FA, with carbon dioxide, leading to different neutralization capabilities. ANC test results confirmed the presence of calcite minerals in KOST sample formed due to the aging of FA and the presence of gypsum in the ANC test of both solid residue samples formed through the neutralization process, as XRD results have already shown.
- Effects of the changes in pH values on the leachability of metal ions, and the neutralization mechanisms were confirmed by solution chemistry modeling results (PHREEQC software). The modeling results showed the predominant effect of the formation of Goethite and Gibbsite on the precipitation mechanics during the neutralization treatment with both FA.
- The application of NaOH for neutralization indicated that FA samples have the ability to absorb metals on the surface of ash particles, since they obtained better efficiency results than this commercial neutralization material.
- Applying EF or KOST fly ash to AMD leads to effective neutralization, making the AMD safe for disposal according to Serbian environmental laws and regulations.
- Applying EF and KOST FA as alternative neutralization materials can lead to economic and environmental benefits
- Analysis of the mechanism of acid mine drainage neutralization using fly ash as an alternative material in a case study of the extremely acidic lake Robule in Eastern Serbia represents great potential for transferable knowledge from the laboratory to the pilot plant in order to increase the valorization of metals present in aqueous solutions.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kefeni, K.K.; Msagati, T.A.; Mamba, B. Acid mine drainage: Prevention, treatment options and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Chockalingam, E.; Subramanian, S. Studies on removal of metal ions and sulphate reduction using rice husk and Desulfotomaculum nigrificans with reference to remediation of acid mine drainage. Chemosphere 2006, 62, 699–708. [Google Scholar] [CrossRef]
- Radivojević, A. Cirkularna ekonomija-implementacija i primena tehnologije u njenoj funkciji. Ekon. Ideje Praksa 2018, 28, 33–46. [Google Scholar]
- Brar, K.K.; Magdouli, S.; Etteieb, S.; Zolfaghari, M.; Fathollahzadeh, H.; Calugaru, L.; Komtchou, S.-P.; Tanabene, R.; Brar, S.K. Integrated bioleaching-electrometallurgy for copper recovery—A critical review. J. Clean. Prod. 2021, 291, 125257. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ogata, F.; Saenjum, C.; Nakamura, T.; Kawasaki, N. Removal of Pb2+ from Aqueous Solutions Using K-Type Zeolite Synthesized from Coal Fly Ash. Water 2020, 12, 2375. [Google Scholar] [CrossRef]
- Buema, G.; Harja, M.; Lupu, N.; Chiriac, H.; Forminte, L.; Ciobanu, G.; Bucur, D.; Bucur, R.D. Adsorption Performance of Modified Fly Ash for Copper Ion Removal from Aqueous Solution. Water 2021, 13, 207. [Google Scholar] [CrossRef]
- Shirin, S.; Jamal, A.; Emmanouil, C.; Yadav, A.K. Assessment of Characteristics of Acid Mine Drainage Treated with Fly Ash. Appl. Sci. 2021, 11, 3910. [Google Scholar] [CrossRef]
- Kisić, D.M.; Miletić, S.R.; Radonjić, V.D.; Radanović, S.B.; Filipović, J.Z.; Gržetić, I.A. Prirodna radioaktivnost uglja i letećeg pepela u termoelektrani “Nikola Tesla B”. Hem. Ind. 2013, 67, 729–738. [Google Scholar] [CrossRef]
- Karličić, N. Svojstva Pepela iz Termoelektrana Značajna za Odlaganje i Primenu. Ph.D. Thesis, Univerzitet u Beogradu, Mašinski Fakultet, Belgrade, Serbia, 2018. [Google Scholar]
- Jovanović, N.; Rosić, A.; Kandić, L.; Živanović, B.; Logar, M.; Komljenović Petrašinović-Stojković, L.; Savić, M.; Živanćević, B. Rendgenska analiza sinterovanog portland cementnog klinkera na bazi elektrofilterskog pepela. Mater. Konstr. 2005, 48, 62–67. [Google Scholar]
- Bogdanović, G.; Trumić, M.; Stanković, V.; Antić, D.; Trumić, M.; Milanović, Z. Rudničke vode iz rudnika RTB Bor—Resurs za dobijanje bakra ili zagađivač životne sredine. Recycl. Sustain. Dev. 2013, 6, 41–50. [Google Scholar]
- Oreščanin, V.; Kollar, R. A combined CaO/electrochemical treatment of the acid mine drainage from the “Robule” Lake. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2012, 47, 1186–1191. [Google Scholar] [CrossRef]
- Pavlović, J.; Stopić, S.; Friedrich, B.; Kamberović, Ž. Selective removal of heavy metals from metal-bearing wastewater in a cascade line reactor. Environ. Sci. Pollut. Res. 2007, 14, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, Z.; Obradović, L.; Marković, R.; Jonović, R.; Avramović, L.; Bugarin, M.; Stevanović, J. Mine waste water management in the Bor municipality in order to protect the Bor River water. In Waste Water-Treatment Technologies and Recent Analytical Developments; Einschlag, F.S.G., Carlos, L., Eds.; InTech: Rijeka, Croatia, 2013; Chapter 2. [Google Scholar] [CrossRef] [Green Version]
- Petronijević, N.; Stanković, S.; Radovanović, D.; Sokić, M.; Marković, B.; Stopić, S.R.; Kamberović, Ž. Application of the Flotation Tailings as an Alternative Material for an Acid Mine Drainage Remediation: A Case Study of the Extremely Acidic Lake Robule (Serbia). Metals 2020, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Petronijević, N.; Alivojvodić, V.; Sokić, M.; Marković, B.; Stanković, S.; Radovanović, D. Sustainable mining towards accomplishing circular economy principles. J. Appl. Eng. Sci. 2020, 18, 493–499. [Google Scholar] [CrossRef]
- Utamia, U.B.L.; Susantob, H.; Cahyonoc, B. Neutralization Acid Mine Drainage (AMD) using NaOH at PT. Jorong Barutama Grestone, Tanah Laut, South Borneo. Anal. Chem. Indian J. 2020, 3, 17–21. [Google Scholar] [CrossRef]
- Skousen, J.G.; Hilton, T.; Faulkner, B. Overview of Acid Mine Drainage Treatment with Chemicals. In Acid Mine Drainage, Rock Drainage, and Acid Sulfate Soils: Causes, Assessment, Prediction, Prevention, and Remediation; Jacobs, J.A., Lehr, J.H., Testa, S.M., Eds.; Wiley: Hoboken, NJ, USA, 2014; Chapter 29. [Google Scholar] [CrossRef]
- Cravotta, C.A.; Means, B.P.; Arthur, W.; McKenzie, R.M.; Parkhurts, D.L. AMD Treat 5.0+ with PHREEQC Titration Module to Compute Caustic Chemical Quantity, Effluent Quality, and Sludge Volume. Mine Water Environ. 2015, 34, 136–152. [Google Scholar] [CrossRef]
- Kapil, N.; Bhattacharyya, K. A comparison of neutralization efficiency of chemicals with respect to acidic Kopili River water. Appl. Water Sci. 2017, 7, 2209–2214. [Google Scholar] [CrossRef]
- Skousen, J.G.; Sexstone, A.; Ziemkiewicz, P.F. Acid mine drainage control and treatment. Agronomy 2000, 41, 131–168. [Google Scholar] [CrossRef]
- Radovanović, D.; Kamberović, Ž.; Korać, M. A solidification/stabilization process for wastewater treatment sludge from a primary copper smelter. J. Serb. Chem. Soc. 2013, 78, 725–739. [Google Scholar] [CrossRef]
- Tomanec, R. Metode Ispitivanja Mineralnih Sirovina u Pripremi Mineralnih Sirovina; Špaldon, F., Salatić, D., Eds.; Rudarsko-geoloski fakultet, Univerzitet u Beogradu: Belgrade, Serbia, 2000. [Google Scholar]
- Radovanović, D.; Kamberović, Ž.; Korać, M.; Rogan, J. Solidified structure and leaching properties of metallurgical wastewater treatment sludge after solidification/stabilization process. J. Environ. Sci. Health Part A 2016, 51, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, J.A.; Cote, P.L. Summary of an investigation of test methods for solidified waste evaluation. Waste Manag. 1990, 10, 41–52. [Google Scholar] [CrossRef]
- Parkhurst, L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. In US Geological Survey Techniques and Methods; U.S. Geological Survey: Denver, CO, USA, 2013; Volume 6.A43, p. 497. [Google Scholar]
- Halim, C.E.; Short, S.A.; Scott, J.A.; Amal, R.; Low, G. Modelling the leaching of Pb, Cd, As, and Cr from cementitious waste using PHREEQC. J. Hazard. Mater. 2005, 125, 45–61. [Google Scholar] [CrossRef]
- Brännvall, E.; Andreas, L.; Sjöblom, R.; Diener, S.; Lagerkvist, A. Factors influencing chemical and mineralogical changes in RDF fly ashes during aging. J. Environ. Eng. 2014, 140, 04013014. [Google Scholar] [CrossRef]
- Qureshi, A.; Jia, Y.; Maurice, C.; Öhlander, B. Potential of fly ash for neutralisation of acid mine drainage. Environ. Sci. Pollut. Res. 2016, 23, 17083–17094. [Google Scholar] [CrossRef]
- Yan, J.; Bäverman, C.; Moreno, L.; Neretnieks, I. Neutralising processes of municipal solid waste incineration bottom ash in a flow-through system. Sci. Total Environ. 1999, 227, 1–11. [Google Scholar] [CrossRef]
- Yeheyis, M.B.; Shang, J.Q.; Yanful, E.K. Characterization and environmental evaluation of Atikokan coal fly ash for environmental applications. J. Environ. Eng. Sci. 2008, 7, 481–498. [Google Scholar] [CrossRef]
- Eary, L.E.; Williamson, M.A. Simulations of the neutralizing capacity of silicate rocks in acid mine drainage environments. J. Am. Soc. Min. Reclam. 2006, 2, 564–577. [Google Scholar] [CrossRef]
- Banwart, S.A.; Malmström, M.E. Hydrochemical modelling for preliminary assessment of minewater pollution. J. Geochem. Explor. 2001, 74, 73–97. [Google Scholar] [CrossRef]
- Linklater, C.M.; Sinclair, D.J.; Brown, P.L. Coupled chemistry and transport modelling of sulphidic waste rock dumps at the Aitik mine site, Sweden. Appl. Geochem. 2005, 20, 275–293. [Google Scholar] [CrossRef]
- Vadapalli, V.K.; Klink, M.J.; Etchebers, O.; Petrik, L.F.; Gitari, W.; White, R.A.; Iwuoha, E. Neutralization of acid mine drainage using fly ash, and strength development of the resulting solid residues. S. Afr. J. Sci. 2008, 104, 317–322. [Google Scholar]
- Yunusa, I.A.; Loganathan, P.; Nissanka, S.P.; Manoharan, V.; Burchett, M.D.; Skilbeck, C.G.; Eamus, D. Application of coal fly ash in agriculture: A strategic perspective. Crit. Rev. Environ. Sci. Technol. 2012, 42, 559–600. [Google Scholar] [CrossRef]
- Gitari, M.W.; Petrik, L.F.; Etchebers, O.; Key, D.L.; Iwuoha, E.; Okujeni, C. Treatment of acid mine drainage with fly ash: Removal of major contaminants and trace elements. J. Environ. Sci. Health Part A 2006, 41, 1729–1747. [Google Scholar] [CrossRef]
- Gitari, W.G.; Petrik, L.F.; Akinyemi, S.A. Treatment of Acid Mine Drainage with Coal Fly Ash: Exploring the Solution Chemistry and Product Water Quality. In Coal Fly Ash Beneficiation—Treatment of Acid Mine Drainage with Coal Fly Ash; Akinyemi, S.A., Gitari, M.W., Eds.; InTech: Rijeka, Croatia, 2018; Chapter 5. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Min, X.; Ke, Y.; Lin, Z.; Yang, Z.; Wang, S.; Wei, Y. Recent progress in understanding the mechanism of heavy metals retention by iron (oxyhydr) oxides. Sci. Total Environ. 2021, 752, 141930. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Pramanik, B.K.; Mainali, B.; Angove, M.J. Metal ion and contaminant sorption onto aluminium oxide-based materials: A review and future research. J. Environ. Chem. Eng. 2018, 6, 6853–6869. [Google Scholar] [CrossRef]
- Yan, Y.; Wan, B.; Mansor, M.; Wang, X.; Zhang, Q.; Kappler, A.; Feng, X. Co-sorption of metal ions and inorganic anions/organic ligands on environmental minerals: A review. Sci. Total Environ. 2022, 803, 149918. [Google Scholar] [CrossRef]
- Héquet, V.; Ricou, P.; Lecuyer, I.; Le Cloirec, P. Removal of Cu2+ and Zn2+ in aqueous solutions by sorption onto mixed fly ash. Fuel 2001, 80, 851–856. [Google Scholar] [CrossRef]
- Barrow, N.J.; Brümmer, G.W.; Fischer, L. Rate of desorption of eight heavy metals from goethite and its implications for understanding the pathways for penetration. Eur. J. Soil Sci. 2012, 63, 389–398. [Google Scholar] [CrossRef]
- Templeton, A.S.; Spormann, A.M.; Brown, G.E. Speciation of Pb (II) sorbed by Burkholderia cepacia/goethite composites. Environ. Sci. Technol. 2003, 37, 2166–2172. [Google Scholar] [CrossRef]
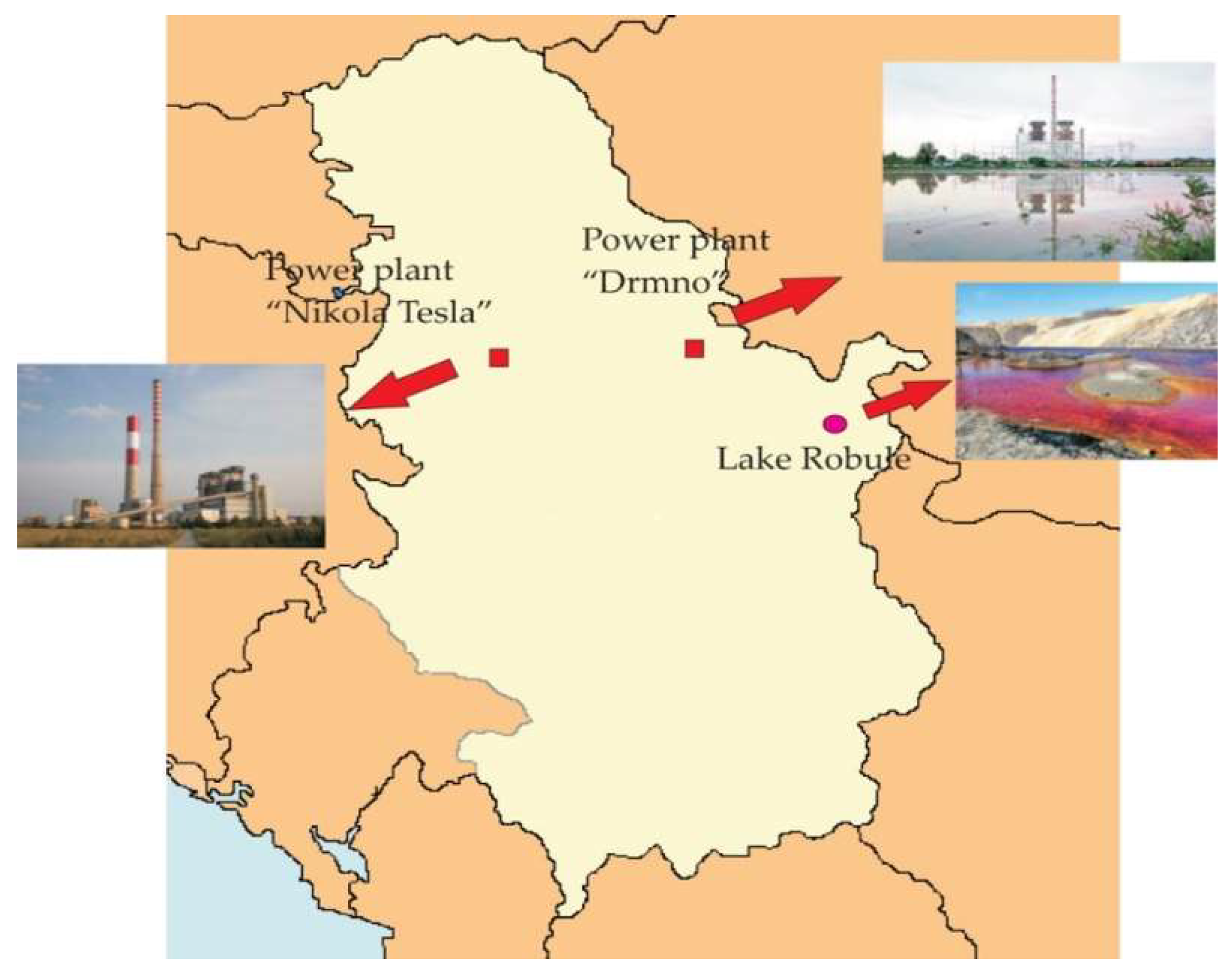
| Parameter | Results ± SD | Parameter | Results ± SD |
|---|---|---|---|
| Temperature °C | 7.0 ± 0.5 | pH | 2.46 ± 0.027 |
| Color and Odor | Yes/No | Eh | 615 ± 8.7 |
| (mg/L) | 0 | Zn (mg/L) | 17.5 ± 0.1 |
| (g/L) | 7.36 ± 0.703 | Pb (mg/L) | 0.19 ± 0.003 |
| (mg/L) | 278.9 ± 11.2 | Mn (mg/L) | 65.9 ± 1.77 |
| (mg/L) | 0.01 ± 0.004 | Al3+ (mg/L) | 1040 ± 37 |
| Fe (total) (mg/L) | 279 ± 11.2 | Mg (mg/L) | 1184 ± 59 |
| Cu (mg/L) | 65.9 ± 1.08 | Ca (mg/L) | 396 ± 7.5 |
| Ni (mg/L) | 0.61 ± 0.021 | Cd (mg/L) | 0.01 ± 0.004 |
| Major Components % ± SD | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | pH | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 | SO3 | MnO | |||||||||
| EF | 12 ± 0.15 | 53 ± 1.37 | 24 ± 0.76 | 8.13 ± 0.33 | 6.62 ± 0.308 | 1.53 ± 0.119 | 1.14 ± 0.067 | 0.39 ± 0.024 | 1.04 ± 0.015 | 0.91 ± 0.032 | 0.06 ± 0.003 | |||||||||
| KOST | 9.3 ± 0.17 | 45.3 ± 1.03 | 22.4 ± 0.46 | 8.99 ± 0.53 | 7.31 ± 0.459 | 1.75 ± 0.146 | 0.85 ± 0.04 | 0.33 ± 0.026 | 0.99 ± 0.046 | 1.24 ± 0.006 | 0.08 ± 0.003 | |||||||||
| Elements mg/kg ± SD | ||||||||||||||||||||
| Sample | Ba | F | Cl | Cu | V | Ni | Cr | As | Pb | |||||||||||
| EF | 79.8 ± 5.32 | 62.7 ± 2.54 | 32.1 ± 2.18 | 24.8 ± 0.15 | 23.9 ± 2.07 | 18.1 ± 0.44 | 15.7 ± 0.87 | 11.7 ± 0.99 | 9.46 ± 0.214 | |||||||||||
| KOST | 85.5 ± 0.25 | 7.3 ± 0.46 | 24.7 ± 1.52 | 34.6 ± 0.54 | 32.6 ± 1.09 | 16.5 ± 0.64 | 12.9 ± 1.04 | 24.2 ± 2.4 | 12 ± 0.351 | |||||||||||
| Elements mg/kg ± SD | ||||||||||||||||||||
| Sample | Zn | Mo | Se | Sb | Ag | Hg | Cd | LOI * | ||||||||||||
| EF | 6.32 ± 0.056 | 1.71 ± 0.054 | <2.3 | <1.2 | <1.0 | <0.05 | <1.2 | 4.4 ± 0.15 | ||||||||||||
| KOST | 10.45 ± 0.029 | 1.0 ± 0.085 | <2.3 | <1.2 | <1.0 | <0.05 | <1.2 | 11.2 ± 0.46 | ||||||||||||
| Element (mg/L) | Time (min) | P * (%) ± SD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 30 | 60 | 120 | 4320 | 10,080 | ||
| pH | 2.46 ± 0.027 | 4.38 ± 0.077 | 4.62 ± 0.085 | 4.87 ± 0.105 | 5.4 ± 0.075 | 6.02 ± 0.07 | 6.19 ± 0.056 | 7 ± 0.024 | 7.1 ± 0.061 | - |
| Cu | 65.9 ± 1.08 | 19.6 ± 0.06 | 18 ± 0.37 | 15.5 ± 0.31 | 9.5 ± 0.24 | 0.3 ± 0.01 | 0.2 ± 0.01 | 0.1 ± 0.01 | 0.3 ± 0.01 | 99.2 ± 1.05 |
| Cd | 0.01 ± 0.001 | 0.05 ± 0.004 | 0.05 ± 0.004 | 0.05 ± 0.004 | 0.05 ± 0.004 | 0.05 ± 0.004 | 0.02 ± 0.003 | <0.007 | <0.005 | 50 ± 7.825 |
| Ni | 0.61 ± 0.021 | 0.69 ± 0.008 | 0.75 ± 0.012 | 0.78 ± 0.008 | 0.8 ± 0.025 | 0.79 ± 0.034 | 0.74 ± 0.023 | 0.66 ± 0.008 | 0.14 ± 0.007 | 77.05 ± 2.386 |
| Pb | 0.19 ± 0.003 | 0.12 ± 0.003 | 0.16 ± 0.001 | 0.36 ± 0.007 | 0.44 ± 0.015 | 0.21 ± 0.007 | 0.03 ± 0.001 | <0.02 | <0.02 | 89.47 ± 1.552 |
| Zn | 17.5 ± 0.1 | 18.9 ± 0.19 | 19.6 ± 0.13 | 20.4 ± 0.35 | 22.5 ± 0.19 | 18.6 ± 0.17 | 7.6 ± 0.01 | 3 ± 0.02 | 0.2 ± 0.02 | 99 ± 0.86 |
| Fe | 279 ± 11.2 | 2.3 ± 0.1 | 1.2 ± 0.08 | 1.2 ± 0.06 | 1.1 ± 0.02 | 1.1 ± 0.03 | <0.1 | <0.1 | <0.1 | 99 ± 0.7 |
| Al | 1040 ± 37 | 8.5 ± 0.3 | 8.1 ± 0.1 | 7.4 ± 0.5 | 7.3 ± 0.2 | 5.2 ± 0.1 | <0.1 | <0.1 | <0.1 | 99.9 ± 0.6 |
| Mg | 1184 ± 59 | 1196 ± 47 | 1207 ± 67 | 1202 ± 70.9 | 1253 ± 78.3 | 1200 ± 50 | 1249 ± 43.9 | 1174 ± 81.7 | 1190 ± 13.5 | +0.51 ± 0.05 |
| Ca | 396 ± 7.5 | 433 ± 14.7 | 428 ± 6.9 | 428 ± 9.2 | 435 ± 9.1 | 437 ± 14.4 | 476 ± 4.2 | 459 ± 13.1 | 439 ± 11.7 | +10.86 ± 0.3 |
| Element (mg/L) | Time (min) | P * (%) ± SD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 30 | 60 | 120 | 4320 | 10,080 | ||
| pH | 2.46 ± 0.027 | 5.11 ± 0.104 | 5.65 ± 0.055 | 6.02 ± 0.078 | 6.43 ± 0.051 | 6.63 ± 0.129 | 7.18 ± 0.078 | 7.33 ± 0.155 | 7.42 ± 0.125 | - |
| Cu | 65.9 ± 1.08 | 11.6 ± 0.24 | 8.8 ± 0.1 | 0.3 ± 0.01 | 0.1 ± 0.01 | 0.3 ± 0.01 | 0.2 ± 0.01 | 0.1 ± 0.01 | 0.2 ± 0.01 | 99.4 ± 0.47 |
| Cd | 0.01 ± 0.001 | 0.052 ± 0.002 | 0.046 ± 0.002 | 0.039 ± 0.0055 | 0.026 ± 0.001 | 0.013 ± 0.0012 | 0.008 ± 0.0006 | <0.007 | <0.005 | 50 ± 1.763 |
| Ni | 0.61 ± 0.021 | 0.58 ± 0.025 | 0.57 ± 0.016 | 0.58 ± 0.023 | 0.58 ± 0.03 | 0.53 ± 0.026 | 0.43 ± 0.021 | 0.32 ± 0.01 | 0.17 ± 0.006 | 72.13 ± 2.158 |
| Pb | 0.19 ± 0.003 | 0.04 ± 0.002 | 0.05 ± 0.003 | 0.03 ± 0.003 | 0.03 ± 0.003 | 0.03 ± 0.003 | 0.03 ± 0.003 | 0.03 ± 0.003 | <0.02 | 89.47 ± 1.481 |
| Zn | 17.5 ± 0.1 | 19.7 ± 0.12 | 14.3 ± 0.2 | 7.5 ± 0.13 | 5.6 ± 0.02 | 4.9 ± 0.04 | 1.6 ± 0.01 | <0.02 | <0.02 | 99.8 ± 0.54 |
| Fe | 279 ± 11.2 | 1.4 ± 0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 99 ± 0.9 |
| Al | 1040 ± 37 | 1.3 ± 0.1 | 2.1 ± 0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 99.9 ± 0.1 |
| Mg | 1184 ± 59 | 1189 ± 65.9 | 1163 ± 65.9 | 1237 ± 43.6 | 1206 ± 25.4 | 1197 ± 45.2 | 1154 ± 35.7 | 1140 ± 14.2 | 1139 ± 68.7 | 3.8 ± 0.2 |
| Ca | 396 ± 7.5 | 439 ± 21.6 | 433 ± 25.9 | 458 ± 12.4 | 443 ± 8.2 | 451 ± 20.9 | 471 ± 14.1 | 417 ± 1.6 | 434 ± 9.1 | +9.6 ± 0.4 |
| Element Concentration (mg/kg) ± SD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Sb | Cu | V | Cd | Ni | Pb | Cr | Zn | S | Al |
| EF7d | 28.2 ± 0.35 | 210 ± 0.4 | 142 ± 2.5 | 2.86 ± 0.215 | 102 ± 3 | 23.2 ± 0.31 | 113 ± 3.7 | 120 ± 1.2 | 3881 ± 287.6 | 121441 ± 4661.4 |
| KOST7d | 20.9 ± 0.41 | 304 ± 11 | 196 ± 3.6 | 1.35 ± 0.083 | 87 ± 1.1 | 67.8 ± 0.31 | 80 ± 2.8 | 171 ± 2.6 | 4833 ± 31.7 | 107691 ± 2574 |
| Element Concentration (mg/kg) ± SD | ||||||||||
| Sample | Fe | B | Co | Mn | As | Ba | Mo | Se | LOI * | |
| EF7d | 45099 ± 2695.5 | <20 | 26.3 ± 0.82 | 391 ± 16 | 37.6 ± 0.33 | <100 | <0.5 | 25.4 ± 1.37 | 6.2 ± 0.1 | |
| KOST7d | 58343 ± 1870.7 | <20 | 32.4 ± 0.89 | 605 ± 7.4 | 68.5 ± 2.32 | <100 | 1.34 ± 0.047 | 37.5 ± 1.64 | 15.8 ± 0.12 | |
| Element | Mineral | t (Min) | 0 | 5 | 10 | 15 | 30 | 60 | 120 | 4320 | 10,080 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 2.46 | 4.38 | 4.62 | 4.87 | 5.4 | 6.02 | 6.19 | 7 | 7.1 | ||
| Formula | Saturation Index-SI | ||||||||||
| Al | Al(OH)3 (a) | Al(OH)3 | −5.80 | −2.00 | −3.13 | −0.69 | 0.53 | 1.26 | −0.36 | −0.71 | −0.8 |
| Gibbsite | Al(OH)3 | −3.11 | 0.69 | 0.44 | 2.00 | 3.22 | 3.95 | 2.33 | 1.98 | 1.89 | |
| Cd | Cd(OH)2 | Cd(OH)2 | −16.32 | −11.70 | −12.46 | −10.72 | −9.66 | −8.41 | −8.48 | −7.31 | −7.11 |
| Fe | Fe(OH)3 (a) | - | −9.37 | −5.58 | −6.38 | −4.39 | −2.84 | −0.98 | −1.52 | 0.84 | 1.09 |
| Goethite | FeOOH | −3.48 | 0.31 | 0.49 | 1.50 | 3.05 | 4.91 | 4.37 | 6.73 | 6.98 | |
| Pb | Pb(OH)2 | Pb(OH)2 | −9.81 | −6.08 | −6.72 | −4.62 | −3.48 | −2.56 | −3.07 | 1.65 | 1.46 |
| Zn | Zn(OH)2 (e) | Zn(OH)2 | −10.74 | −6.73 | −7.48 | −5.72 | −4.62 | −3.46 | −3.51 | −2.29 | −3.27 |
| Element | Mineral | t (Min) | 0 | 5 | 10 | 15 | 30 | 60 | 120 | 4320 | 10,080 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 2.46 | 5.11 | 5.65 | 6.02 | 6.43 | 6.63 | 7.18 | 7.33 | 7.42 | ||
| Formula | Saturation Index-SI | ||||||||||
| Al | Al(OH)3 (a) | Al(OH)3 | −5.80 | −0.96 | 0.42 | −0.45 | −0.45 | −0.43 | −0.88 | −1.02 | −1.11 |
| Gibbsite | Al(OH)3 | −3.11 | 1.73 | 3.11 | 2.24 | 2.24 | 2.26 | 1.81 | 1.67 | 1.58 | |
| Cd | Cd(OH)2 | Cd(OH)2 | −16.32 | −10.22 | −9.19 | −8.53 | −8.53 | −7.78 | −6.89 | −6.64 | −6.46 |
| Fe | Fe(OH)3 | Fe(OH)3 | −9.37 | −3.75 | −2.88 | −2.02 | −2.02 | −0.20 | 1.27 | 1.53 | 1.64 |
| Goethite | FeOOH | −3.48 | 2.14 | 3.04 | 3.87 | 3.87 | 5.69 | 7.16 | 7.42 | 7.53 | |
| Pb | Pb(OH)2 | Pb(OH)2 | −9.81 | −5.10 | −4.23 | −3.41 | −3.41 | −2.20 | −1.13 | 0.85 | 0.87 |
| Zn | Zn(OH)2 (e) | Zn(OH)2 | −10.74 | −5.25 | −4.31 | −3.86 | −3.86 | −2.82 | −2.20 | −3.80 | −3.63 |
| Element Concentration (mg/L) | |||||||
|---|---|---|---|---|---|---|---|
| Sample | Cu | Fe | Al | Cd | Ni | Zn | Pb |
| AMD | 65.9 ± 1.08 | 279 ± 11.2 | 1040 ± 37 | 0.01 ± 0.001 | 0.61 ± 0.021 | 17.5 ± 0.1 | 0.19 ± 0.003 |
| EF | 0.3 ± 0.01 | <0.1 | <0.1 | <0.007 | 0.14 ± 0.007 | 0.2 ± 0.02 | <0.02 |
| KOST | <0.4 | <0.1 | <0.1 | <0.007 | 0.17 ± 0.006 | <0.02 | <0.02 |
| NaOH | 0.5 ± 0.01 | 0.18 ± 0.007 | 1.4 ± 0.06 | 0.1 ± 0.013 | 0.13 ± 0.001 | 0.4 ± 0.01 | 0.67 ± 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petronijević, N.; Radovanović, D.; Štulović, M.; Sokić, M.; Jovanović, G.; Kamberović, Ž.; Stanković, S.; Stopic, S.; Onjia, A. Analysis of the Mechanism of Acid Mine Drainage Neutralization Using Fly Ash as an Alternative Material: A Case Study of the Extremely Acidic Lake Robule in Eastern Serbia. Water 2022, 14, 3244. https://doi.org/10.3390/w14203244
Petronijević N, Radovanović D, Štulović M, Sokić M, Jovanović G, Kamberović Ž, Stanković S, Stopic S, Onjia A. Analysis of the Mechanism of Acid Mine Drainage Neutralization Using Fly Ash as an Alternative Material: A Case Study of the Extremely Acidic Lake Robule in Eastern Serbia. Water. 2022; 14(20):3244. https://doi.org/10.3390/w14203244
Chicago/Turabian StylePetronijević, Nela, Dragana Radovanović, Marija Štulović, Miroslav Sokić, Gvozden Jovanović, Željko Kamberović, Srđan Stanković, Srecko Stopic, and Antonije Onjia. 2022. "Analysis of the Mechanism of Acid Mine Drainage Neutralization Using Fly Ash as an Alternative Material: A Case Study of the Extremely Acidic Lake Robule in Eastern Serbia" Water 14, no. 20: 3244. https://doi.org/10.3390/w14203244
APA StylePetronijević, N., Radovanović, D., Štulović, M., Sokić, M., Jovanović, G., Kamberović, Ž., Stanković, S., Stopic, S., & Onjia, A. (2022). Analysis of the Mechanism of Acid Mine Drainage Neutralization Using Fly Ash as an Alternative Material: A Case Study of the Extremely Acidic Lake Robule in Eastern Serbia. Water, 14(20), 3244. https://doi.org/10.3390/w14203244








