Analysis of Floating Macroalgae Distribution around Japan Using Global Change Observation Mission-Climate/Second-Generation Global Imager Data
Abstract
:1. Introduction
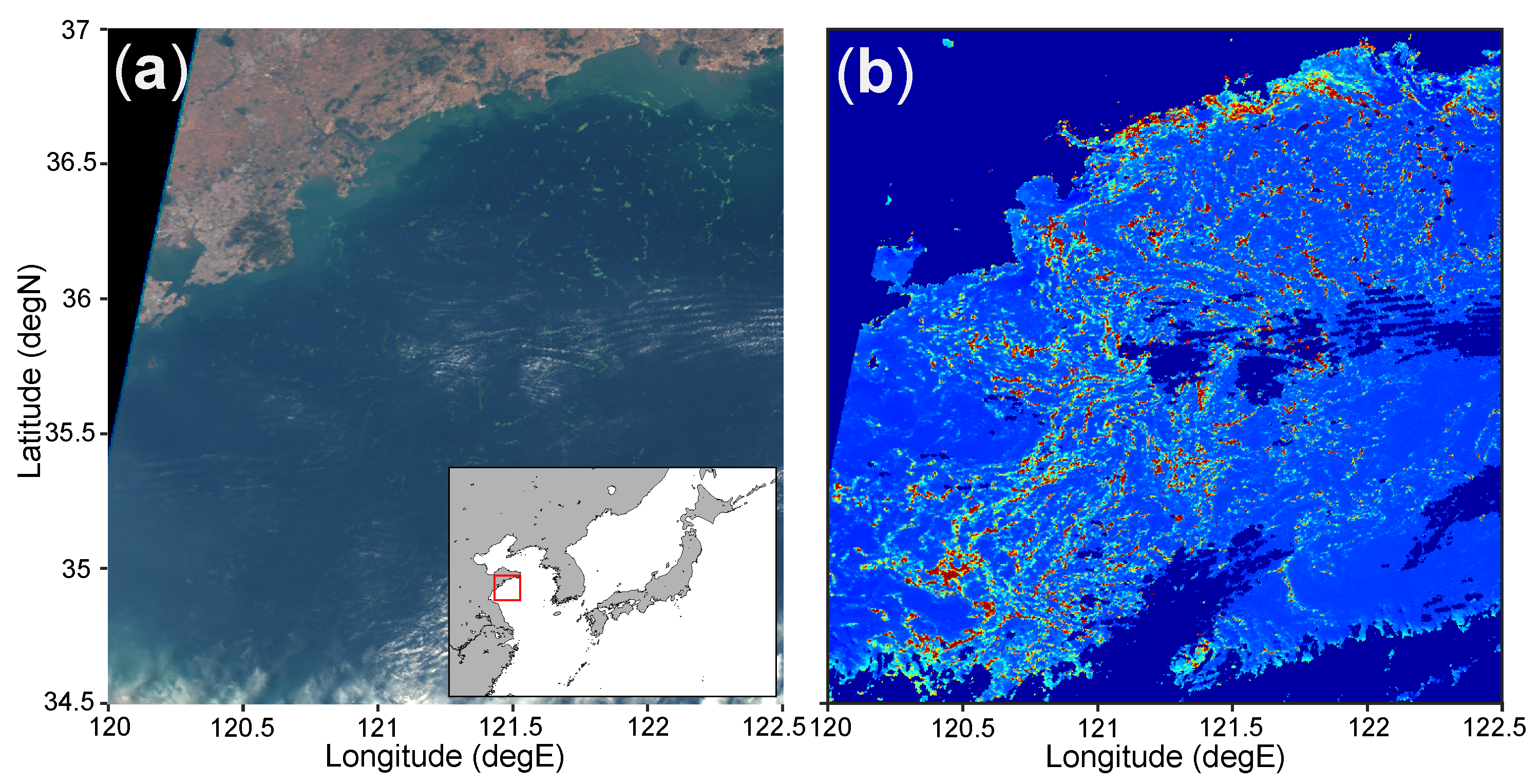
2. Materials and Methods
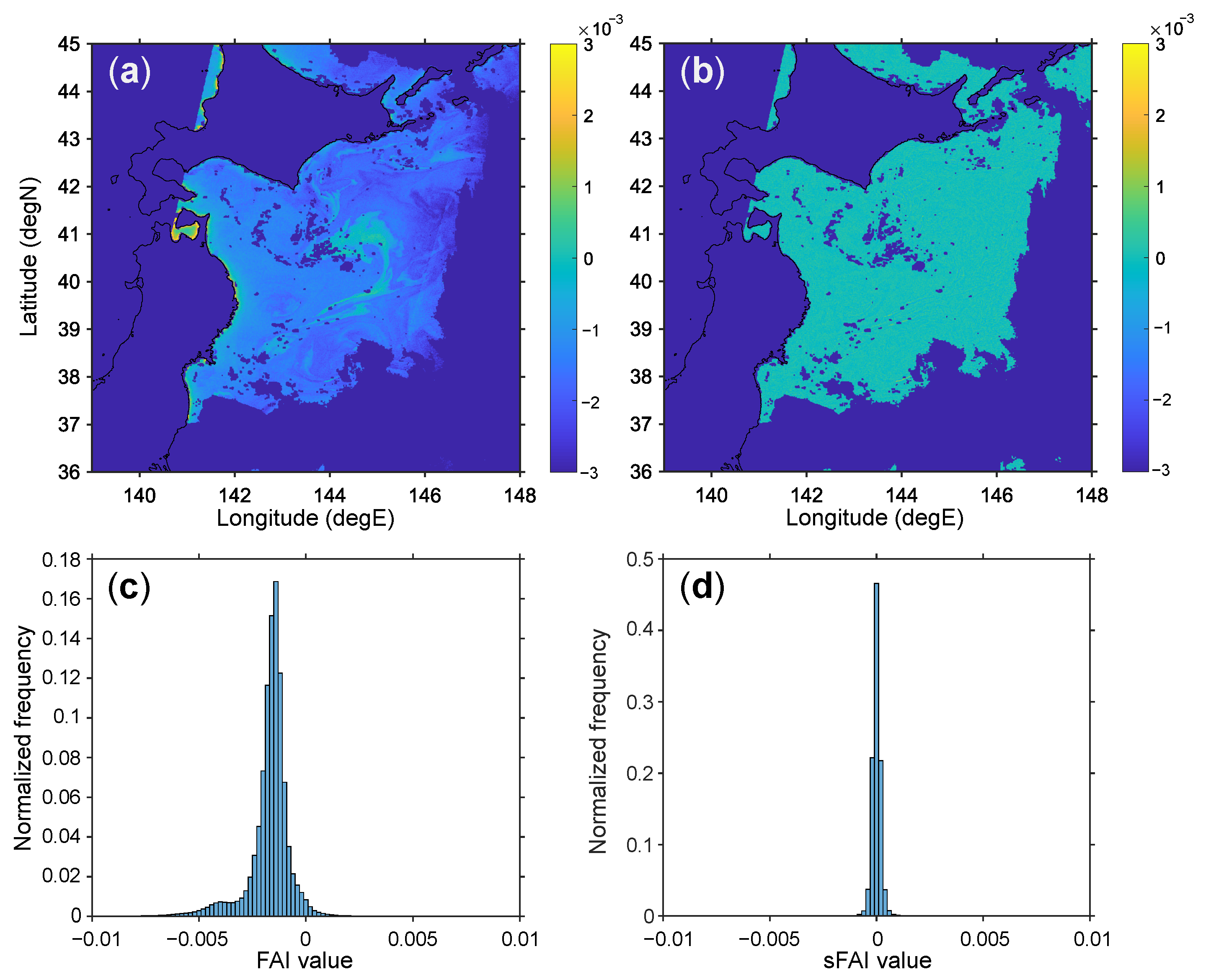
2.1. Floating Algae Index (FAI)
2.2. Scaling FAI
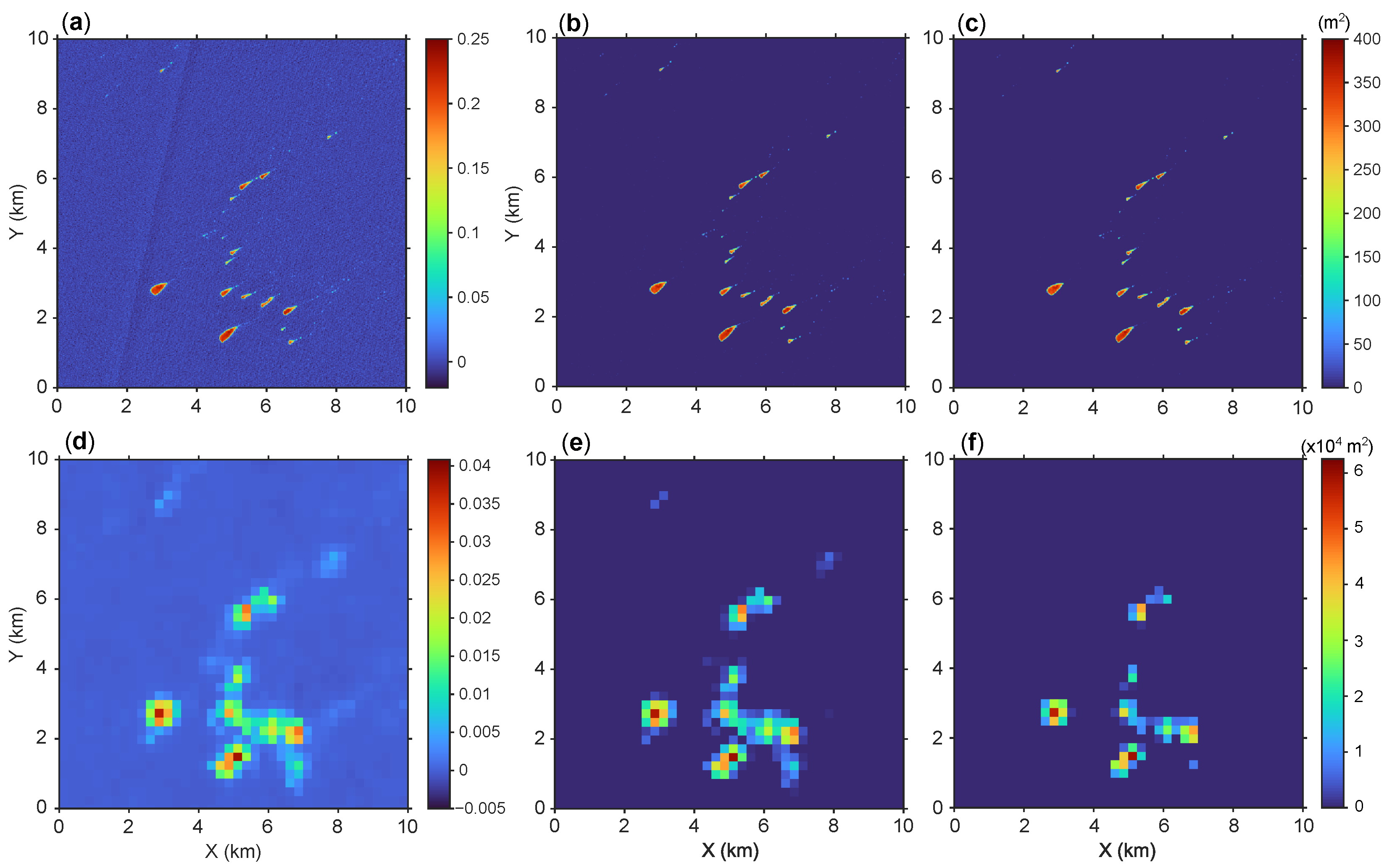
2.3. Algae Area Estimation
2.4. Algae Area Binning
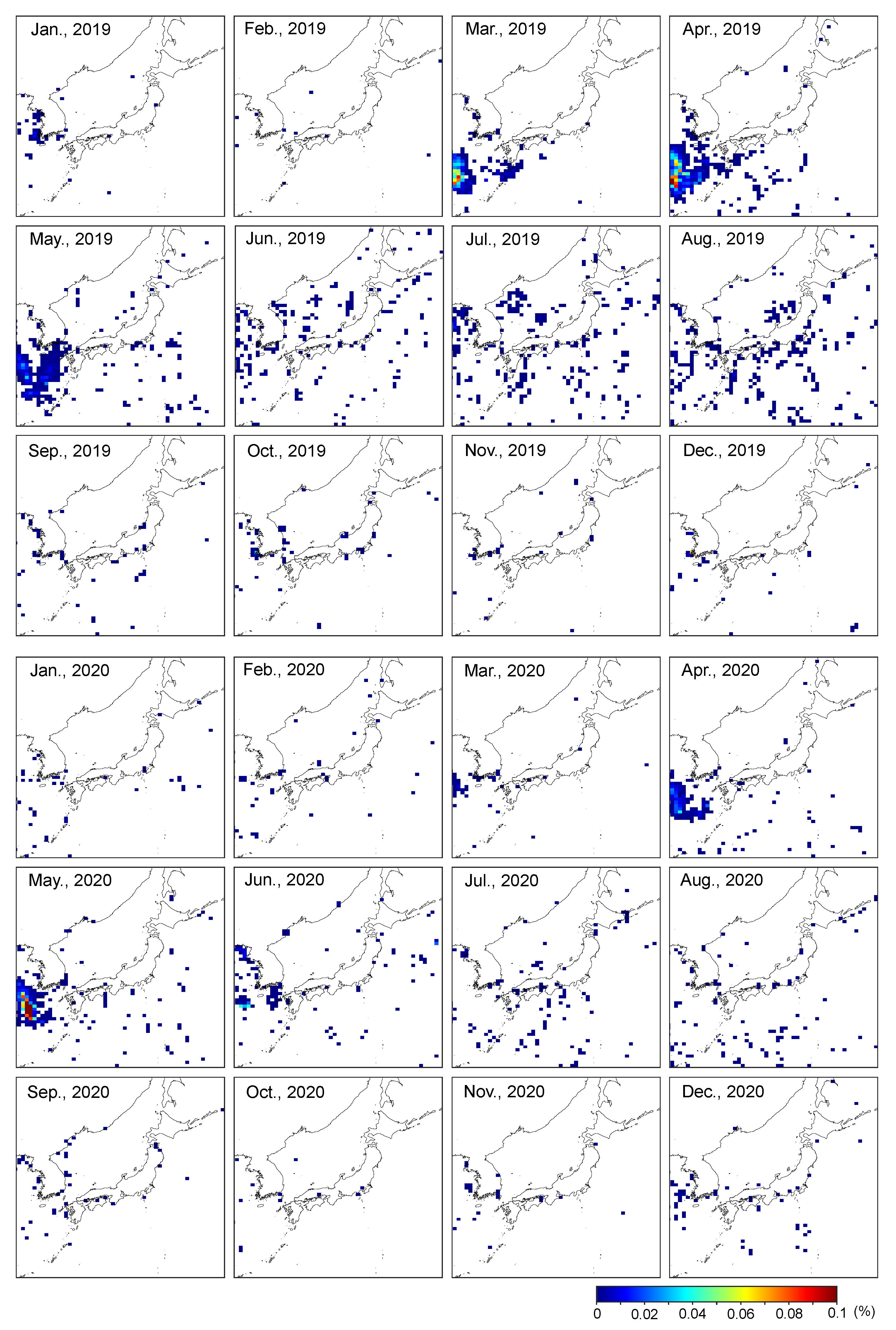
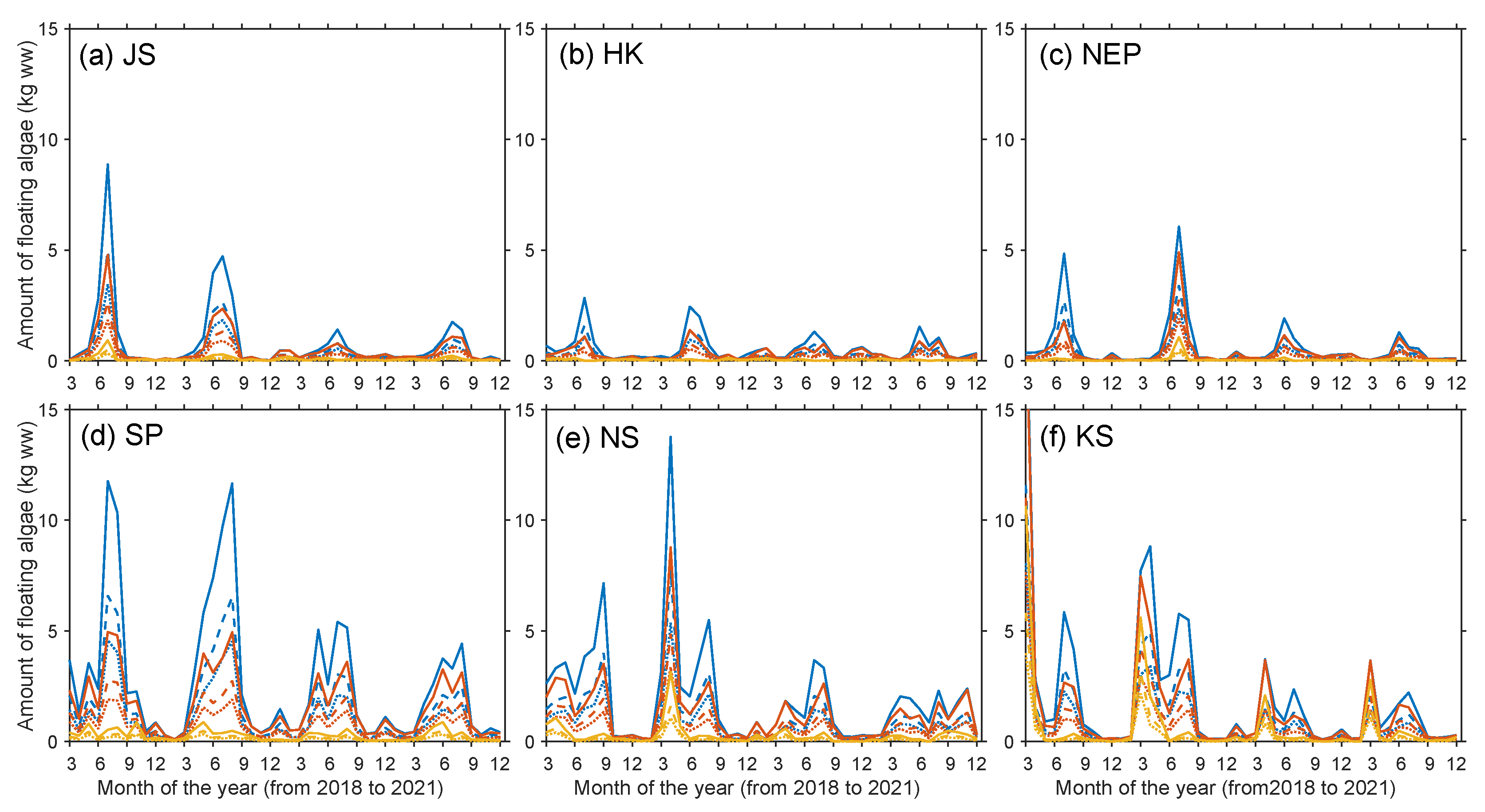
3. Results and Discussion
4. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GCOM-C | Global Change Observation Mission-Climate |
| SGLI | Second-Generation Global Imager |
| MSI | Multispectral Instrument (Multispectral Imager) |
| MODIS | Moderate Resolution Imaging Spectroradiometer |
| FAI | floating algae index |
| sFAI | scaled FAI |
| NDVI | normalized difference vegetation index |
| JAXA | Japan Aerospace Exploration Agency |
| JASMES | JAXA Satellite Monitoring for Environmental Studies |
| ATBD | algorithm theoretical basis document |
| R | Rayleigh corrected top-of-atmosphere reflectance |
| reflectance with correction shown in Equation (4) | |
| fractional coverage of macroalgae within an algae-containing pixel | |
| sFAI | lower threshold to determine if the pixel contains macroalgae or not. |
| If sFAI of a pixel is lower than sFAI, this pixel is 0% covered by macroalgae. | |
| sFAI | upper bound of sFAI. |
| If sFAI of a pixel is higher than sFAI, this pixel is 100% covered by macroalgae. | |
| f | monthly mean fractional macroalgae coverage. |
References
- Ryther, J.H. The Sargasso Sea. Sci. Am. 1956, 194, 98–108. [Google Scholar] [CrossRef]
- Sun, S.; Wang, F.; Li, C.; Qin, S.; Zhou, M.; Ding, L.; Pang, S.; Duan, D.; Wang, G.; Yin, B.; et al. Emerging challenges: Massive green algae blooms in the Yellow Sea. Nat. Preced. 2008. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, L.; Bao, X.; Zhao, L.D. Economic Cost of an Algae Bloom Cleanup in China’s 2008 Olympic Sailing Venue. Eos Trans. Am. Geophys. Union 2009, 90, 238–239. [Google Scholar] [CrossRef]
- Maurer, A.S.; De Neef, E.; Stapleton, S. Sargassum accumulation may spell trouble for nesting sea turtles. Front. Ecol. Environ. 2015, 13, 394–395. [Google Scholar] [CrossRef] [Green Version]
- Duarte, C.M.; Krause-Jensen, D. Export from Seagrass Meadows Contributes to Marine Carbon Sequestration. Front. Mar. Sci. 2017, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Gower, J.; Young, E.; King, S. Satellite images suggest a new Sargassum source region in 2011. Remote Sens. Lett. 2013, 4, 764–773. [Google Scholar] [CrossRef]
- Gower, J.; Hu, C.; Borstad, G.; King, S. Ocean Color Satellites Show Extensive Lines of Floating Sargassum in the Gulf of Mexico. IEEE Trans. Geosci. Remote Sens. 2006, 44, 3619–3625. [Google Scholar] [CrossRef]
- Hu, C.; He, M.X. Origin and Offshore Extent of Floating Algae in Olympic Sailing Area. Eos Trans. Am. Geophys. Union 2008, 89, 302–303. [Google Scholar] [CrossRef]
- Hu, C. A novel ocean color index to detect floating algae in the global oceans. Remote Sens. Environ. 2009, 113, 2118–2129. [Google Scholar] [CrossRef]
- Shi, W.; Wang, M. Green macroalgae blooms in the Yellow Sea during the spring and summer of 2008. J. Geophys. Res. Ocean. 2009, 114, C12010. [Google Scholar] [CrossRef]
- Hori, M.; Murakami, H.; Miyazaki, R.; Honda, Y.; Nasahara, K.; Kajiwara, K.; Nakajima, T.Y.; Irie, H.; Toratani, M.; Hirawake, T.; et al. GCOM-C Data Validation Plan for Land, Atmosphere, Ocean, and Cryosphere. Trans. Jpn. Soc. Aeronaut. Space Sci. 2018, 16, 218–223. [Google Scholar] [CrossRef] [Green Version]
- Murakami, H. ATBD of GCOM-C Floating Algae Index. 2019. Available online: https://suzaku.eorc.jaxa.jp/GCOM_C/data/files/ATBD_FAI_en.pdf (accessed on 10 September 2022).
- Yoshida, T. Studies on the distribution and drift of the floating sea weed [in Japanese with English Abstract]. Bull. Tohoku Reg. Fish. Res. Lab. 1963, 23, 141–186. [Google Scholar]
- Hirata, T.; Tanaka, J.; Iwami, T.; Ohmi, T.; Dazai, A.; Aoki, M.; Ueda, H.; Tsuchiya, Y.; Sato, T.; Yokohama, Y. Ecological studies on the community of drifting seaweeds in the south-eastern coastal waters of Izu Peninsula, central Japan. I: Seasonal changes of plants in species composition, appearance, number of species and size. Phycol. Res. 2001, 49, 215–229. [Google Scholar] [CrossRef]
- Yatsuya, K. Floating period of Sargassacean thalli estimated by the change in density. J. Appl. Phycol. 2007, 20, 797. [Google Scholar] [CrossRef]
- Komatsu, T.; Mizuno, S.; Natheer, A.; Kantachumpoo, A.; Tanaka, K.; Morimoto, A.; Hsiao, S.T.; Rothäusler, E.A.; Shishidou, H.; Aoki, M.; et al. Unusual distribution of floating seaweeds in the East China Sea in the early spring of 2012. J. Appl. Phycol. 2014, 26, 1169–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, S.; Ajisaka, T.; Lahbib, S.; Kokubu, Y.; Alabsi, M.N.; Komatsu, T. Spatial distributions of floating seaweeds in the East China Sea from late winter to early spring. J. Appl. Phycol. 2014, 26, 1159–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, L.; Hu, C.; Wang, M.; Shang, S.; Wilson, C. Floating Algae Blooms in the East China Sea. Geophys. Res. Lett. 2017, 44, 11,501–11,509. [Google Scholar] [CrossRef]
- Frouin, R.; Deschamps, P.Y.; Gross-Colzy, L.; Murakami, H.; Nakajima, T.Y. Retrieval of chlorophyll-a concentration via linear combination of ADEOS-II Global Imager data. J. Oceanogr. 2006, 62, 331–337. [Google Scholar] [CrossRef]
- Garcia, R.A.; Fearns, P.; Keesing, J.K.; Liu, D. Quantification of floating macroalgae blooms using the scaled algae index. J. Geophys. Res. Ocean. 2013, 118, 26–42. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Hu, C. Mapping and quantifying Sargassum distribution and coverage in the Central West Atlantic using MODIS observations. Remote Sens. Environ. 2016, 183, 350–367. [Google Scholar] [CrossRef]
- Komatsu, T.; Tatsukawa, K.; Filippi, J.B.; Sagawa, T.; Matsunaga, D.; Mikami, A.; Ishida, K.; Ajisaka, T.; Tanaka, K.; Aoki, M.; et al. Distribution of drifting seaweeds in eastern East China Sea. J. Mar. Syst. 2007, 67, 245–252. [Google Scholar] [CrossRef]
- Yamasaki, M.; Aono, M.; Ogawa, N.; Tanaka, K.; Imoto, Z.; Nakamura, Y. Drifting algae and fish: Implications of tropical Sargassum invasion due to ocean warming in western Japan. Estuar. Coast. Shelf Sci. 2014, 147, 32–41. [Google Scholar] [CrossRef]
- Nagano, A.; Yamashita, Y.; Hasegawa, T.; Ariyoshi, K.; Matsumoto, H.; Shinohara, M. Characteristics of an atypical large-meander path of the Kuroshio current south of Japan formed in September 2017. Mar. Geophys. Res. 2019, 40, 525–539. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Duarte, C.M. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Kokubu, Y.; Rothäusler, E.; Filippi, J.B.; Durieux, E.D.H.; Komatsu, T. Revealing the deposition of macrophytes transported offshore: Evidence of their long-distance dispersal and seasonal aggregation to the deep sea. Sci. Rep. 2019, 9, 4331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abo, K.; Sugimatsu, K.; Hori, M.; Yoshida, G.; Shimabukuro, H.; Yagi, H.; Nakayama, A.; Tarutani, K. Quantifying the Fate of Captured Carbon: From Seagrass Meadows to the Deep Sea. In Blue Carbon in Shallow Coastal Ecosystems: Carbon Dynamics, Policy, and Implementation; Kuwae, T., Hori, M., Eds.; Springer: Singapore, 2019; pp. 251–271. [Google Scholar] [CrossRef]
- Notoya, M. (Ed.) Moba-no-Kaisou-to-ZouseiGijutsu; Seizando: Tokyo, Japan, 2003. (In Japanese) [Google Scholar]
- Hirata, T.; Tanaka, J.; Iwami, T.; Ohmi, T.; Dazai, A.; Aoki, M.; Ueda, H.; Tsuchiya, Y.; Sato, T.; Yokohama, Y. Ecological studies on the community of drifting seaweeds in the south-eastern coastal waters of Izu Peninsula, central Japan. II: Seasonal changes in plants showing maximum stipe length in drifting seaweed communities. Phycol. Res. 2003, 51, 186–191. [Google Scholar] [CrossRef]
- Fujita, D. Current status and problems of isoyake in Japan. Bull. Fish. Res. Agency 2010, 32, 33–42. [Google Scholar]
- Terada, R.; Abe, M.; Abe, T.; Aoki, M.; Dazai, A.; Endo, H.; Kamiya, M.; Kawai, H.; Kurashima, A.; Motomura, T.; et al. Japan’s nationwide long-term monitoring survey of seaweed communities known as the “Monitoring Sites 1000”: Ten-year overview and future perspectives. Phycol. Res. 2021, 69, 12–30. [Google Scholar] [CrossRef]
- Hu, C.; Feng, L.; Hardy, R.F.; Hochberg, E.J. Spectral and spatial requirements of remote measurements of pelagic Sargassum macroalgae. Remote Sens. Environ. 2015, 167, 229–246. [Google Scholar] [CrossRef]
- Biermann, L.; Clewley, D.; Martinez-Vicente, V.; Topouzelis, K. Finding Plastic Patches in Coastal Waters using Optical Satellite Data. Sci. Rep. 2020, 10, 5364. [Google Scholar] [CrossRef]
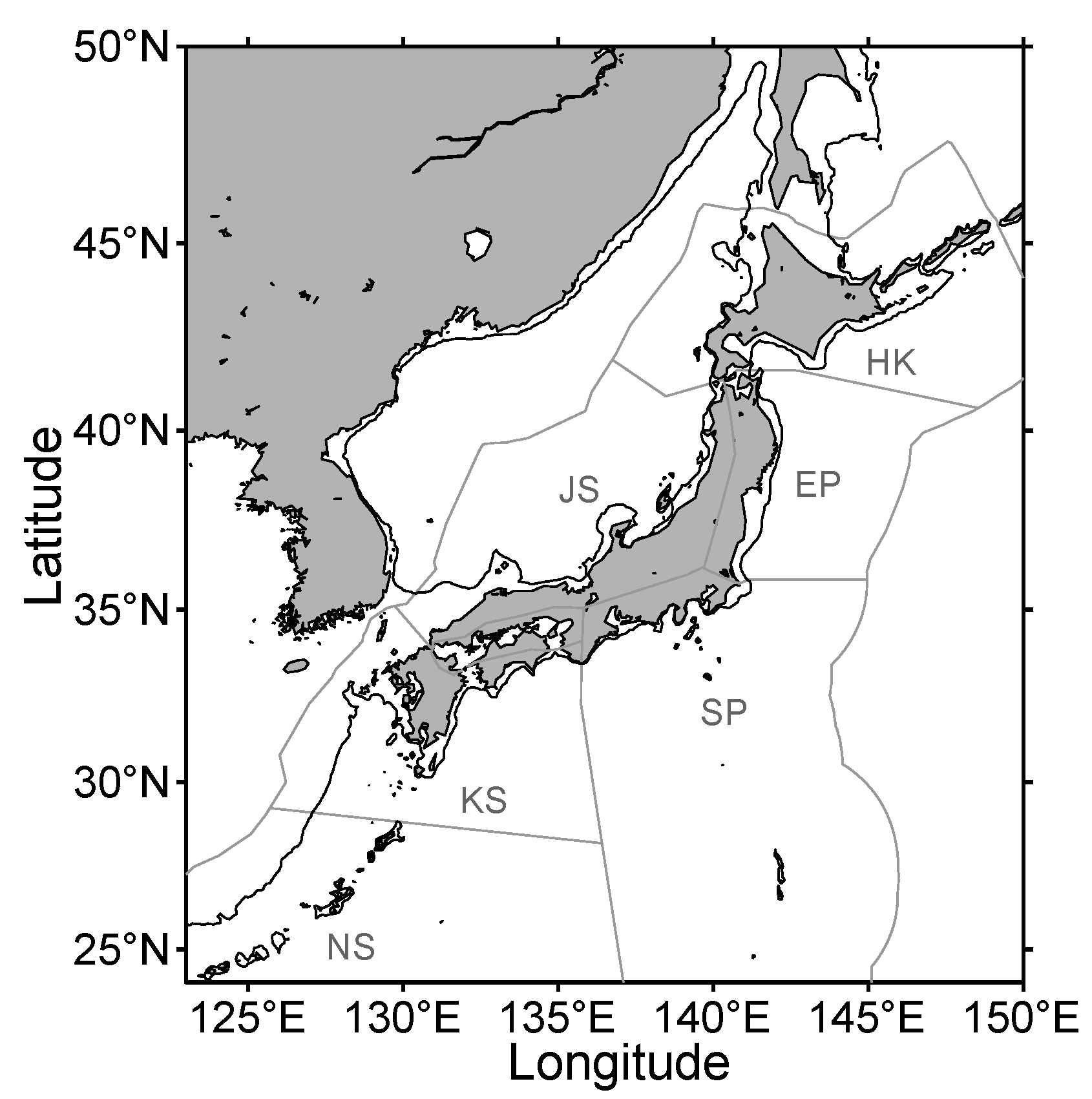

| JS | HK | NEP | SP | NS | KS | Sum | |
|---|---|---|---|---|---|---|---|
| 2018 | 640–14,350 | 250–6750 | 130–9310 | 1590–38,810 | 1340–27,690 | 4930–36,880 | 8880–133,790 |
| 2019 | 440–13,820 | 230–7030 | 580–11,250 | 1450–42,220 | 1950–32,730 | 3810–34,850 | 8460–141,900 |
| 2020 | 350–5640 | 240–6330 | 180–5800 | 950–25,470 | 860–14,820 | 1340–12,340 | 3910–70,380 |
| 2021 | 340–5820 | 200–5280 | 210–3720 | 1170–19,180 | 1000–16,050 | 1740–11,820 | 4620–61,870 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taniguchi, N.; Sakuno, Y.; Sun, H.; Song, S.; Shimabukuro, H.; Hori, M. Analysis of Floating Macroalgae Distribution around Japan Using Global Change Observation Mission-Climate/Second-Generation Global Imager Data. Water 2022, 14, 3236. https://doi.org/10.3390/w14203236
Taniguchi N, Sakuno Y, Sun H, Song S, Shimabukuro H, Hori M. Analysis of Floating Macroalgae Distribution around Japan Using Global Change Observation Mission-Climate/Second-Generation Global Imager Data. Water. 2022; 14(20):3236. https://doi.org/10.3390/w14203236
Chicago/Turabian StyleTaniguchi, Naokazu, Yuji Sakuno, Haoran Sun, Shilin Song, Hiromori Shimabukuro, and Masakazu Hori. 2022. "Analysis of Floating Macroalgae Distribution around Japan Using Global Change Observation Mission-Climate/Second-Generation Global Imager Data" Water 14, no. 20: 3236. https://doi.org/10.3390/w14203236
APA StyleTaniguchi, N., Sakuno, Y., Sun, H., Song, S., Shimabukuro, H., & Hori, M. (2022). Analysis of Floating Macroalgae Distribution around Japan Using Global Change Observation Mission-Climate/Second-Generation Global Imager Data. Water, 14(20), 3236. https://doi.org/10.3390/w14203236








