Toxicological Effects of Mercuric Chloride Exposure on Scenedesmus quadricauda
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture of Algal Cells
2.2. Preparation of HgCl2 Solution and Exposure Treatment of Algal Cells
2.3. Algal Cell Growth Measurements and Sub-Microscopic Structure Observation
2.4. Determination of Antioxidant Enzyme Activity
2.5. Determination of Membrane Permeability
2.6. Determination of Photosynthesis
Chlorophyll b (mg/mL) = 24.96 × OD (649 nm) − 7.32 × OD (665 nm)
3. Results
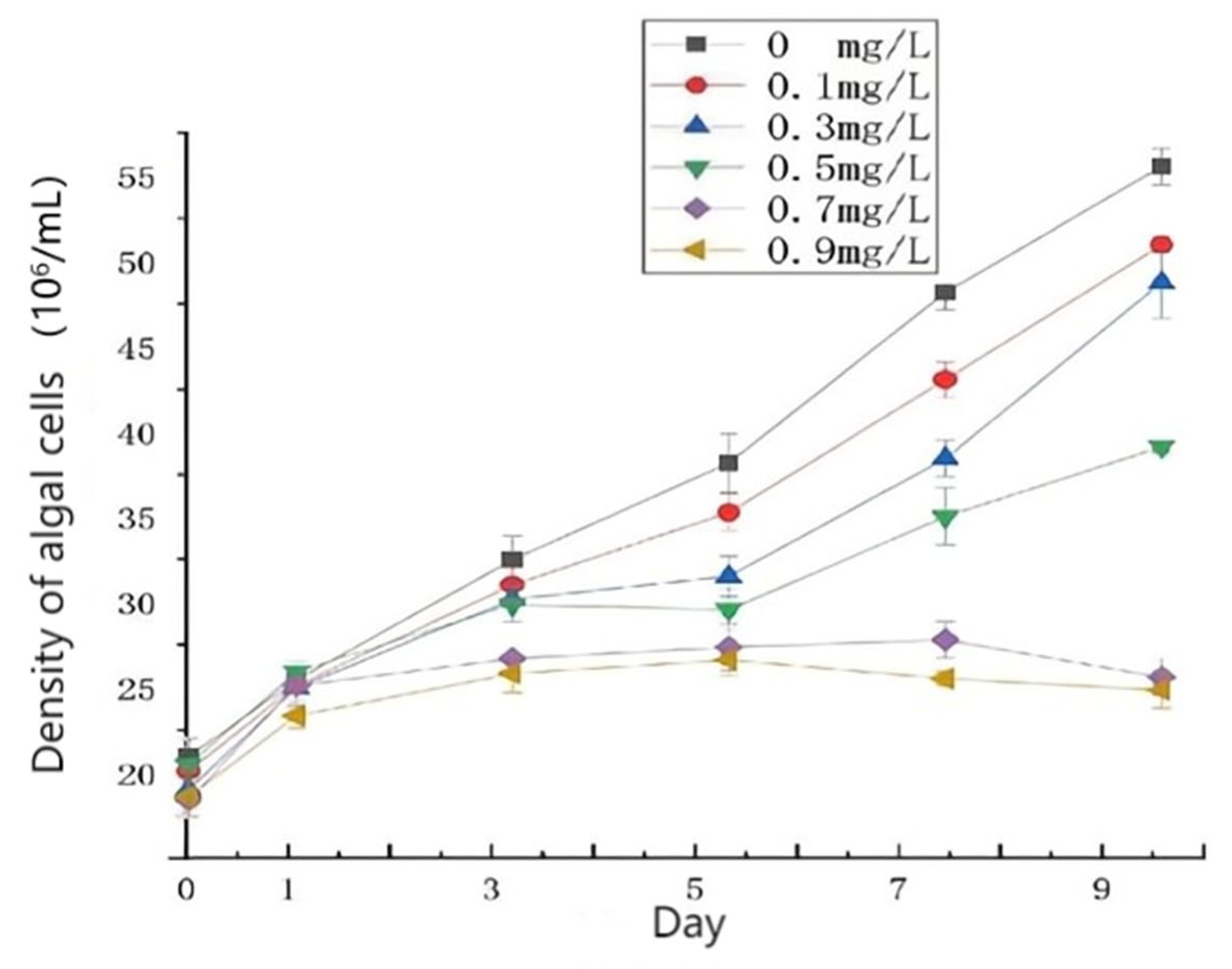
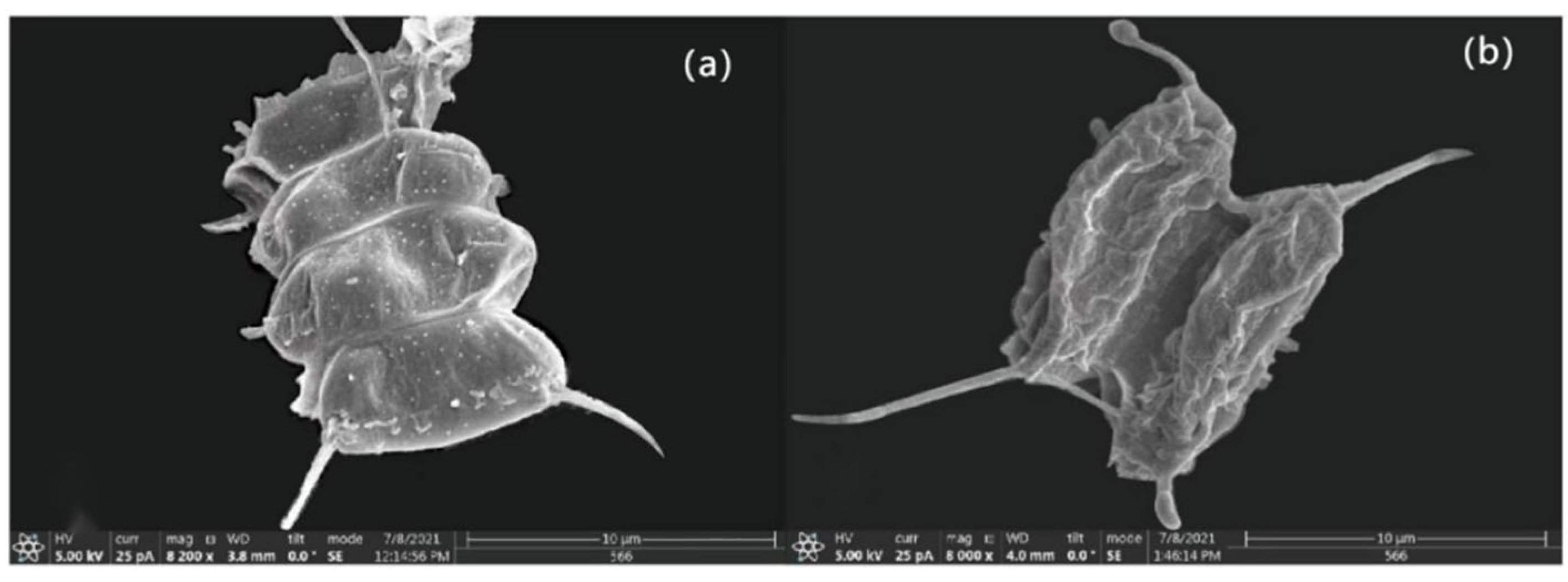
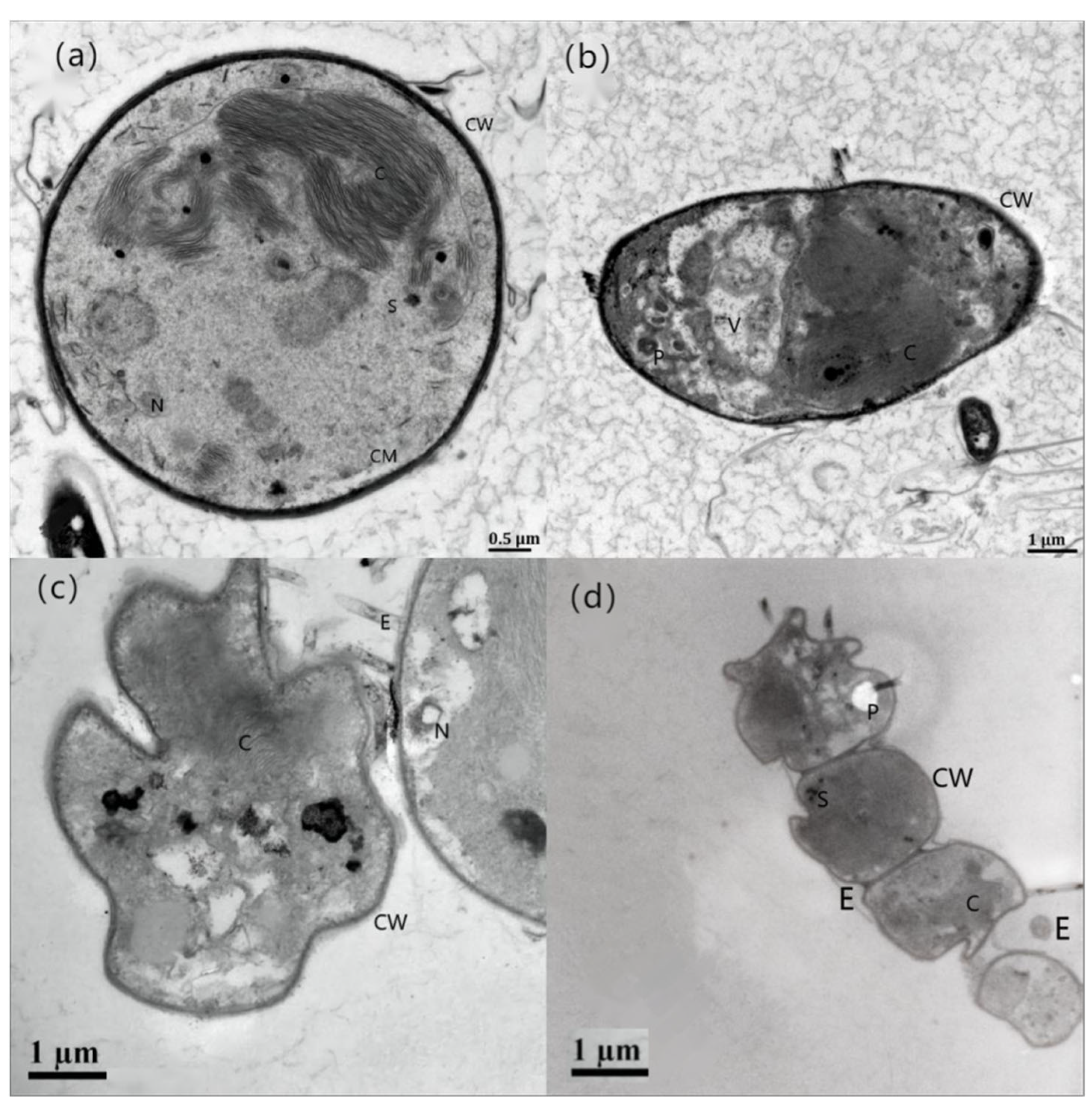
3.1. Effects of Different Concentrations of HgCl2 on the Growth and Cell Morphology of Scenedesmus quadricauda
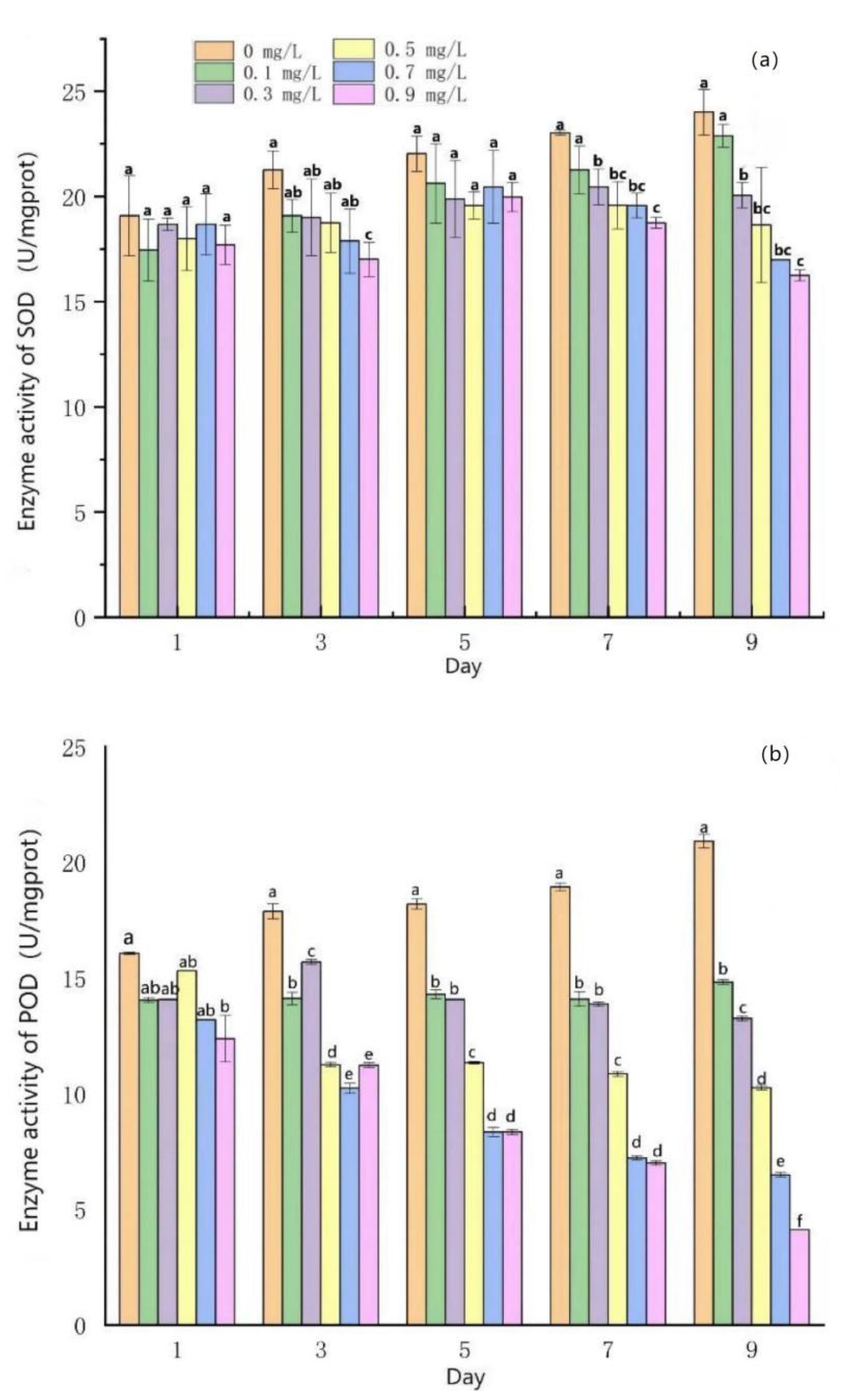
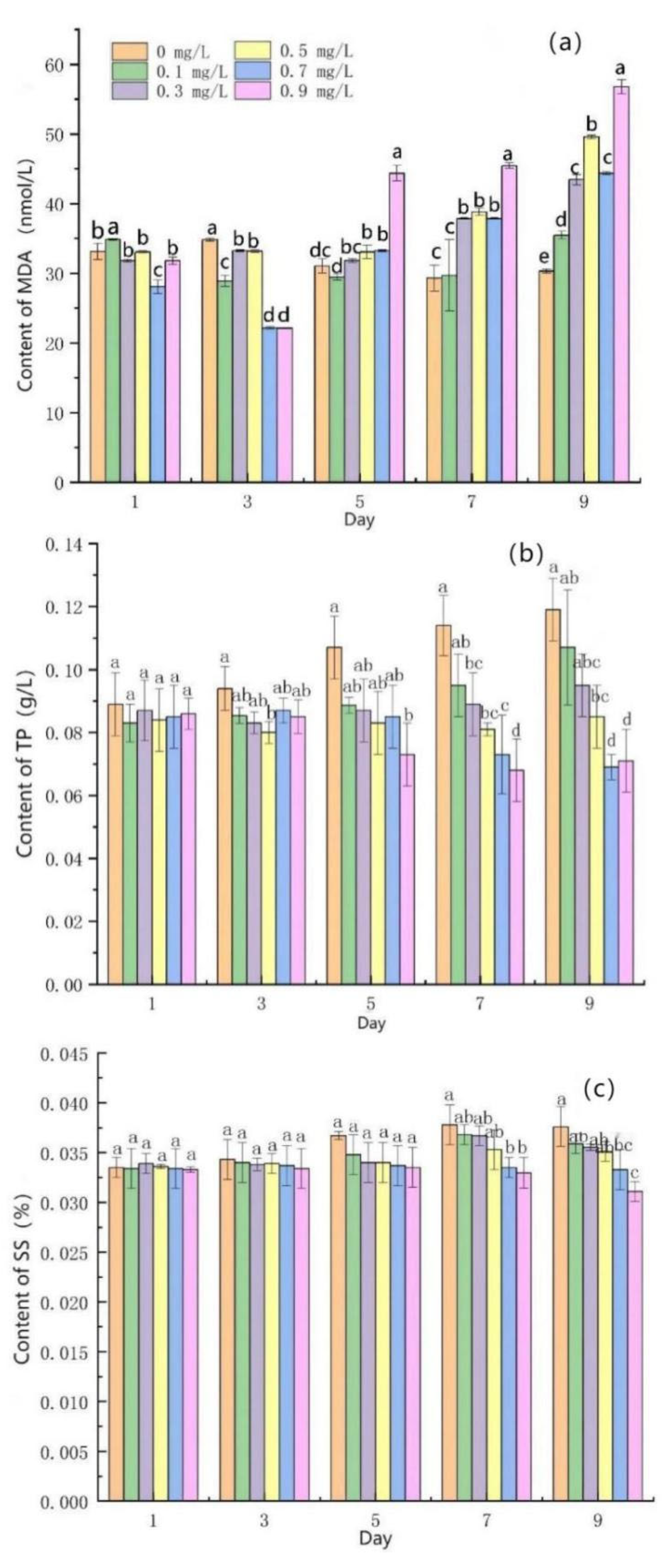
3.2. Effects of Mercuric Chloride Concentration on the Antioxidant System of Scenedesmus quadricauda
3.3. Effects of Mercuric Chloride Concentration on the Membrane Permeability of Scenedesmus quadricauda
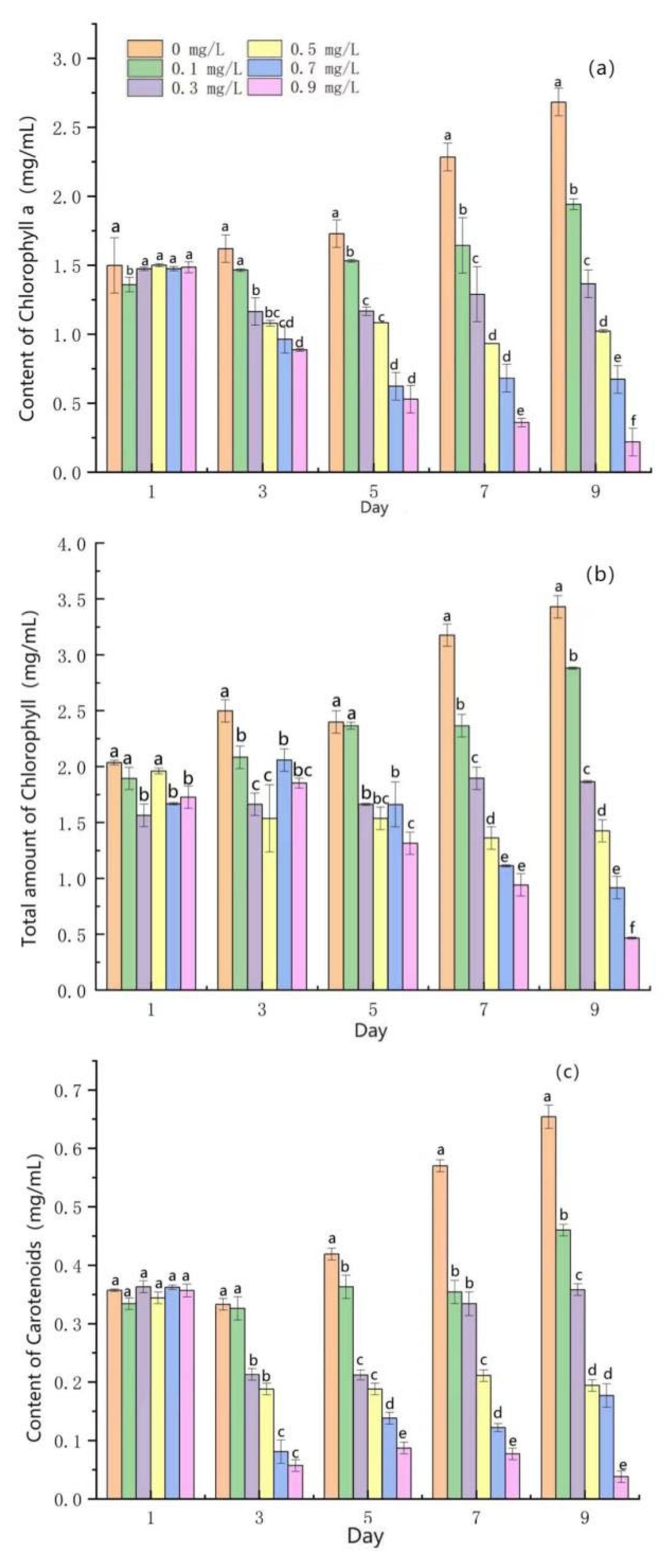
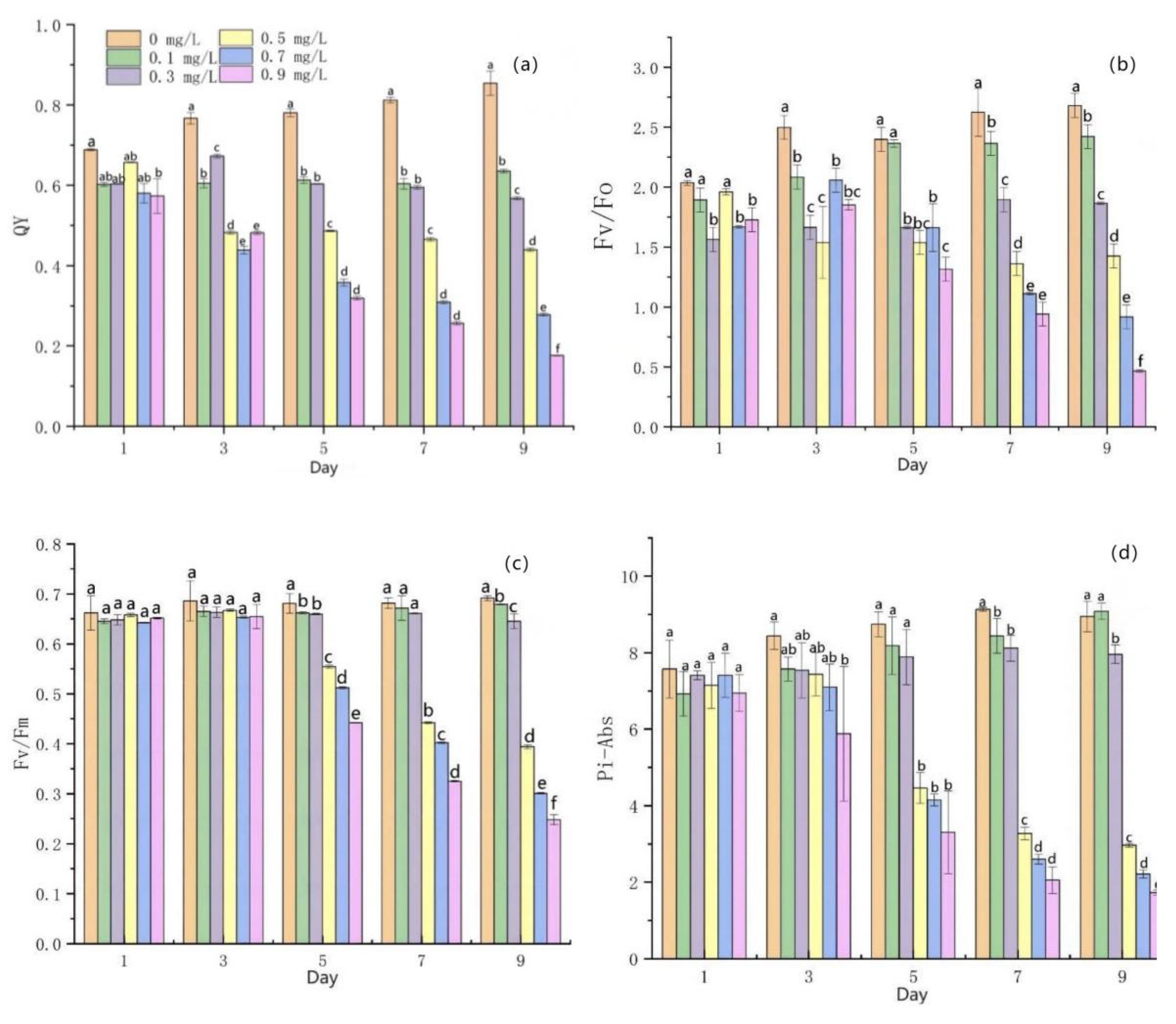
3.4. Effects of Mercuric Chloride Concentration on the Photosynthesis of Scenedesmus quadricauda
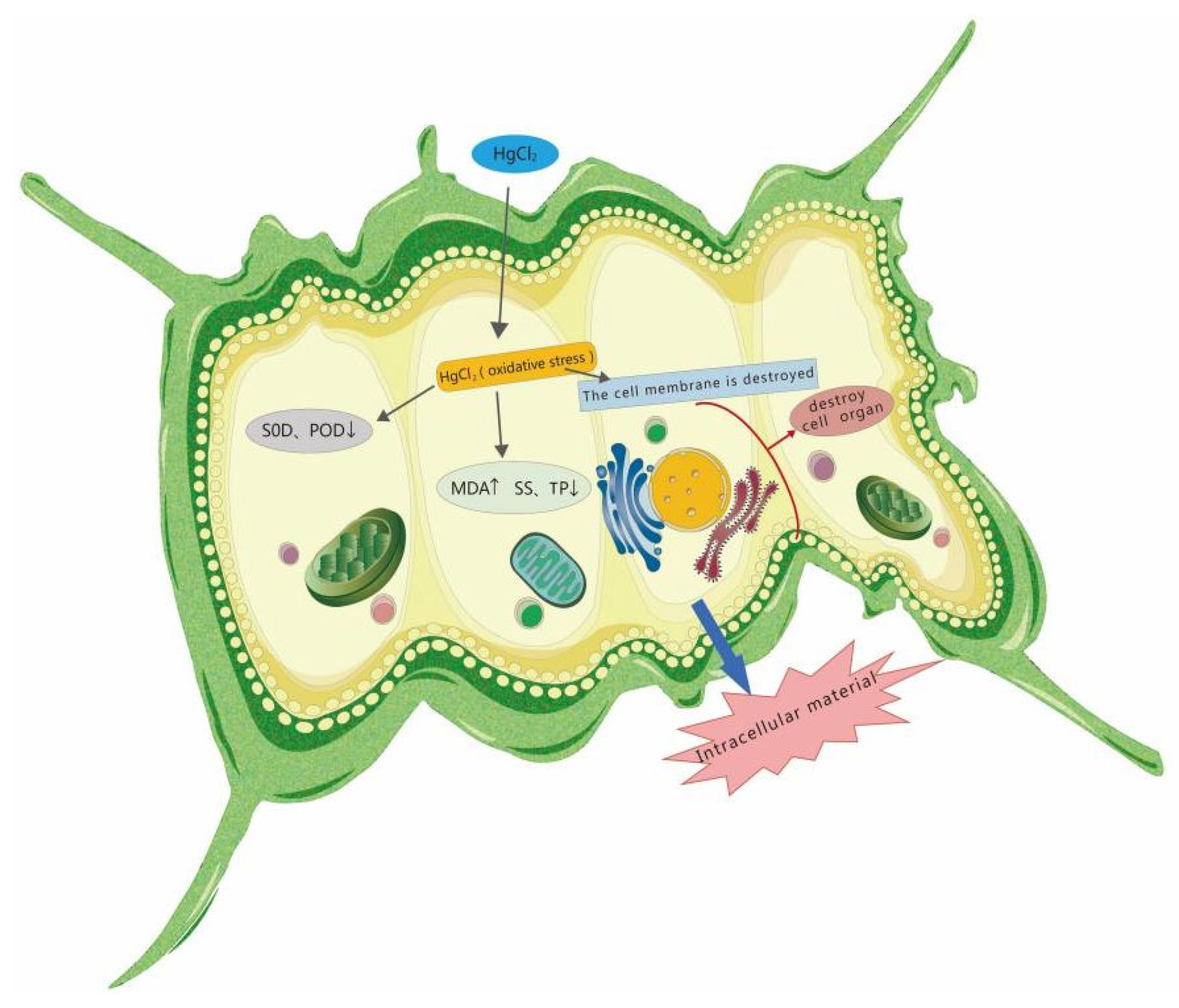
4. Discussion
5. Conclusions
- The proliferation of algal cells was inhibited, the growth cycle was shortened, and the process of transition to decay was accelerated. Under high HgCl2 concentrations (≥0.7 mg/L), the cell density of the algae was seriously affected. Through observation, it was found that the original green color of the algal culture faded and turned yellow-brown and white, and the algae aggregated in flocs and bottom sediments. The ultrastructures of the algal cells were damaged greatly, and the algal cells became irregular and obviously deformed, with obvious plasma–wall separation. The nuclei and protein nuclei became seriously faded, almost becoming transparent, the vacuole volume decreased, the photosynthetic lamellae structures of the chloroplasts became indistinct, and the original ordered arrangement was lost.
- Under low HgCl2 concentrations (≤0.3 mg/L), the activities of SOD and POD in S. quadricauda cells increased to some extent, but the degree of the increase was small compared to the control group. The activities of SOD and POD in algal cells decreased when the concentrations of HgCl2 were greater than 0.5 mg/L.
- Under medium and high HgCl2 concentrations (≥0.5 mg/L), the content of malondialdehyde (MDA) in algal cells obviously increased, while the content of total protein (TP) and soluble sugar (SS) obviously decreased.
- Under medium and high HgCl2 concentrations (≥0.5 mg/L), the rate of photosynthetic pigment production in algal cells decreased and, correspondingly, the content of photosynthetic pigment decreased. Furthermore, the PS II reaction center was damaged beyond the scope of self-repair, the yield of PS II photons decreased, the photoelectric transmission and energy-conversion efficiency decreased sharply, the photochemical reaction ability decreased, and photosynthesis weakened sharply.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drbal, K.; Vèber, K.; Zahradnìk, J. Toxicity and accumulation of copper and cadmium in the alga Scenedesmus obliquus LH. Bull. Environ. Contam. Toxicol. 1985, 34, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wong, M.H. Environmental mercury contamination in China: Sources and impacts. Environ. Int. 2007, 33, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Fitzgerald, W.F.; Moral, M.M. The biogeochemical cycling of element mercury: Anthropogenic influences. Geochim. Cosmochim. Acta 1994, 58, 3191–3198. [Google Scholar] [CrossRef]
- Lindqvist, O.; Rodhe, H. Atmosphric mercury—A review. Tellus 1985, 37B, 135–159. [Google Scholar]
- Navas-Acien, A.; Silbergeld, E.K.; Pastor-Barriuso, R.; Guallar, E. Arsenic exposure and prevalence of type 2 diabetes in US adults. Int. J. Mod. Phys. B 2008, 300, 814–822. [Google Scholar] [CrossRef] [Green Version]
- Joshi, S. Mercury hazards and how the community responses in developing country. Occup. Environ. Med. 2019, 76 (Suppl. 1), 81. [Google Scholar] [CrossRef]
- Du, X.; Zhu, Y.G.; Liu, W.J.; Zhao, X.S. Uptake of mercury (Hg) by seedlings of rice (Oryza sativa L.) grown in solution culture and interactions with arsenate uptake. Environ. Exp. Bot. 2005, 54, 1–7. [Google Scholar] [CrossRef]
- Ahmad, S.; Mahmood, R. Mercury chloride toxicity in human erythrocytes: Enhanced generation of ROS and RNS, hemoglobin oxidation, impaired antioxidant power, and inhibition of plasma membrane redox system. Environ. Sci. Pollut. Res. 2019, 26, 5645–5657. [Google Scholar] [CrossRef]
- Čelechovská, O.; Malota, L.; Zima, S. Entry of heavy metals into food chains: A 20-year comparison study in Northern Moravia (Czech Republic). Acta Vet. Brno 2008, 77, 645–652. [Google Scholar] [CrossRef]
- Liu, L.H.; Zhang, Y.; Yun, Z.J.; He, B.; Jiang, G.B. Estimation of bioaccessibility and potential human health risk of mercury in Chinese patent medicines. J. Environ. Sci. 2016, 39, 37–44. [Google Scholar] [CrossRef]
- Chan, H.M.; Egeland, G.M. Fish consumption, mercury exposure, and heart diseases. Nutr. Rev. 2004, 62, 68–72. [Google Scholar]
- Nabi, S. Toxic Effects of Mercury; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Ekere, N.R.; Ukoha, P.O.; Udeogu, U.V.; Ihedioha, J.N.; Agbazue, V.E. Human exposures and potential health hazard assessment of Hg and Pb in some major imported frozen fish species in Nigeria. Hum. Ecol. Risk Assess. Int. J. 2015, 22, 393–400. [Google Scholar] [CrossRef]
- Ricketts, P.; Fletcher, H.; Voutchkov, M. Factors associated with mercury levels in human placenta and the relationship to neonatal anthropometry in Jamaica and Trinidad & Tobago. Reprod. Toxicol. 2017, 71, 78–83. [Google Scholar]
- Capolino, E.; Tredici, M.; Pepi, M.; Baldi, F. Tolerance to mercury chloride in Scenedesmus strains. BioMetals 1997, 10, 85–94. [Google Scholar] [CrossRef]
- Bezzubova, E.M.; Drits, A.V.; Mosharov, S.A. Effect of mercury chloride on the chlorophyl a and pheophytin content in marine microalgae: Measuring the flow of autotrophic phytoplankton using sediment traps data. Oceanology 2018, 58, 479–486. [Google Scholar] [CrossRef]
- Han, H.Y. Effect of mercury on growth, development and photosynthesis of Scenedesmus obliquus. Acta Sci. Circumstantiae 1984, 4, 157–164. [Google Scholar]
- Li, Y.; Zhu, L.; Liu, S. Individual and joint stress of lead and mercury on growthglutathione and glutathione-related enzymes of Scenedesmus quadricauda. Enviromental Sci. 2009, 30, 248–253. [Google Scholar]
- Zhou, X.P.; Xia, L.; Ge, H.M.; Zhang, D.L.; Hu, C.X. Feasibility of biodiesel production by microalgal Chlorella sp. (FACHB-1748) under outdoor conditions. Bioresour. Technol. 2013, 138, 131–135. [Google Scholar] [CrossRef]
- Ates, M.; Cimen, I.C.C.; Unal, I.; Kutlu, B.; Tastan, B.E.; Danabas, D.; Aksu, O.; Arslan, Z. Assessment of impact of α-Fe2O3 and γ-Fe2O3 nanoparticles on phytoplankton species Selenastrum capricornutum and Nannochloropsis oculata. Environ. Toxicol. 2019, 35, 385–394. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgal beats bioethanol. Trends Biotechnol. 2008, 26, 126–131. [Google Scholar] [CrossRef]
- Lin, L.; Wang, Y.; Wang, F.X.; He, J.Y.; Zhang, H.L. Determination of polysaccharides content of Gentiana farreri from different producing areas based on anthrone-sulfuric acid method. China J. Chin. Mater. Med. 2014, 39, 2774–2776. [Google Scholar]
- Mera, R.; Torres, E.; Abalde, J. Effect of sodium sulfate on the freshwater microalga Chlamydomonas moewusii: Implications for the optimization of algal culture media. J. Phycol. 2016, 52, 75–88. [Google Scholar] [CrossRef]
- Markou, G.; Muylaert, K. Effect of light intensity on the degree of ammonia toxicity on PSII activity of Arthrospira platensis and Chlorella vulgaris. Bioresour. Technol. 2016, 216, 453–461. [Google Scholar] [CrossRef]
- Wang, C.L.; Zheng, Y.F.; Li, R.K.; Yin, Q.R.; Song, C.F. Removal of cefradine by Chlorella sp. L166 and Scenedesmus quadricauda: Toxicity investigation, degradation mechanism and metabolic pathways. Process Saf. Environ. Prot. 2022, 160, 632–640. [Google Scholar] [CrossRef]
- Lamai, C.; Kruatrachue, M.; Pokethitiyook, P.; Upatham, E.S.; Soonthornsarathool, V. Toxicity and accumulation of lead and cadmium in the filamentous green alga Cladophora fracta (O.F. Muller ex Vahl) Kutzing: A laboratory study. ScienceAsia 2005, 31, 121–127. [Google Scholar] [CrossRef]
- Elstner, E.F. Oxygen activation and oxygen toxicity. Annu. Rev. Plant Physiol. 1982, 33, 73–96. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Kaduková, J.; Bačkor, M. Physiology of Matricaria chamomilla exposed to nickel excess. Ecotoxicol. Environ. Saf. 2009, 72, 603–609. [Google Scholar] [CrossRef]
- Yang, Y.J.; Zhao, X.L.; Zhang, J.F. Effects of heavy metal Hg2+ on growth and antioxidant enzymes of Achnanthes kryophila. Environ. Prot. Technol. 2018, 4, 1–4. [Google Scholar]
- Song, Y.Z.; Kong, F.F.; Xue, Y.; Qin, B.Q. Responses of chlorophyll and MDA of Vallisneria natans to nitrogen and phosphorus availability and epiphytic algae. J. Freshw. Ecol. 2015, 30, 85–97. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the cytotoxicity of semicon- ductor quantum dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Stratton, G.W. Effect of the solvent acetone on membrane integrity in the green alga Chlorella pyrenoidosa. Bull. Environ. Contam. Toxicol. 1989, 42, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.P.; Liu, W.J.; Luo, Y.M.; Wu, H.; Diao, P.P.; Duan, S.S. Toxicity of Cd2+ and Pb2+ to the growth and physiology of marina microalga Karenia mikimotoi. Ecol. Sci. 2019, 38, 211–217. [Google Scholar]
- Wang, H.; Zhu, R.; Zhang, J.; Ni, L.Y.; Shen, H.; Xie, P. A novel and convenient method for early warning of algal cell density by chlorophyll fluorescence parameters and its application in a highland lake. Front. Plant Sci. 2018, 9, 869. [Google Scholar] [CrossRef] [Green Version]
- Rizza, F.; Pagani, D.; Stanca, A.M. Use of chlorophyll fluorescence to evaluate the cold acclimation and freezing tolerance of winter and spring oats. Plant Breed 2001, 120, 389–396. [Google Scholar] [CrossRef]
- Zhang, B.W.; Zhao, J.T.; Wu, E.W.; Li, Y.Y.; Li, N.; Qiao, X.W.; Wu, G.; Gao, Y.X. Water pollution characteristics of heavy metals in Fuyang River system and their toxicity to Vibrio qinghaiensis sp. Q67 and Scenedesmus obliquus. Asian J. Ecotoxicol. 2018, 13, 179–189. [Google Scholar]
- Pang, M.X.; Huang, Z.L.; Liu, X.L.; Tang, Y.J.; Li, X.D.; Jin, G. Effects of heavy metal cadmium on Caulerpa lentillifera based on transcriptome analysis. Chin. J. Appl. Ecol. 2021, 32, 4447–4456. [Google Scholar]
- He, Z.Y.; Wei, J.B.; Cheng, B.; Chen, L.F.; Cao, M.X.; Yin, Y.G.; Liang, Y.; Cai, Y. Bioremediation of mercury pollution in land surface environment: Review and prospects. Earth Environ. 2022, 50, 415–425. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, Y.; Liu, X.; Nan, F.; Liu, Q.; Lv, J.; Feng, J.; Xie, S. Toxicological Effects of Mercuric Chloride Exposure on Scenedesmus quadricauda. Water 2022, 14, 3228. https://doi.org/10.3390/w14203228
Ge Y, Liu X, Nan F, Liu Q, Lv J, Feng J, Xie S. Toxicological Effects of Mercuric Chloride Exposure on Scenedesmus quadricauda. Water. 2022; 14(20):3228. https://doi.org/10.3390/w14203228
Chicago/Turabian StyleGe, Yuheng, Xudong Liu, Fangru Nan, Qi Liu, Junping Lv, Jia Feng, and Shulian Xie. 2022. "Toxicological Effects of Mercuric Chloride Exposure on Scenedesmus quadricauda" Water 14, no. 20: 3228. https://doi.org/10.3390/w14203228




