Arsenic Accumulation and Biotransformation Affected by Nutrients (N and P) in Common Blooming-Forming Microcystis wesenbergii (Komárek) Komárek ex Komárek (Cyanobacteria)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Cell Counting and Specific Growth Rate
2.3. Reagents and Standards
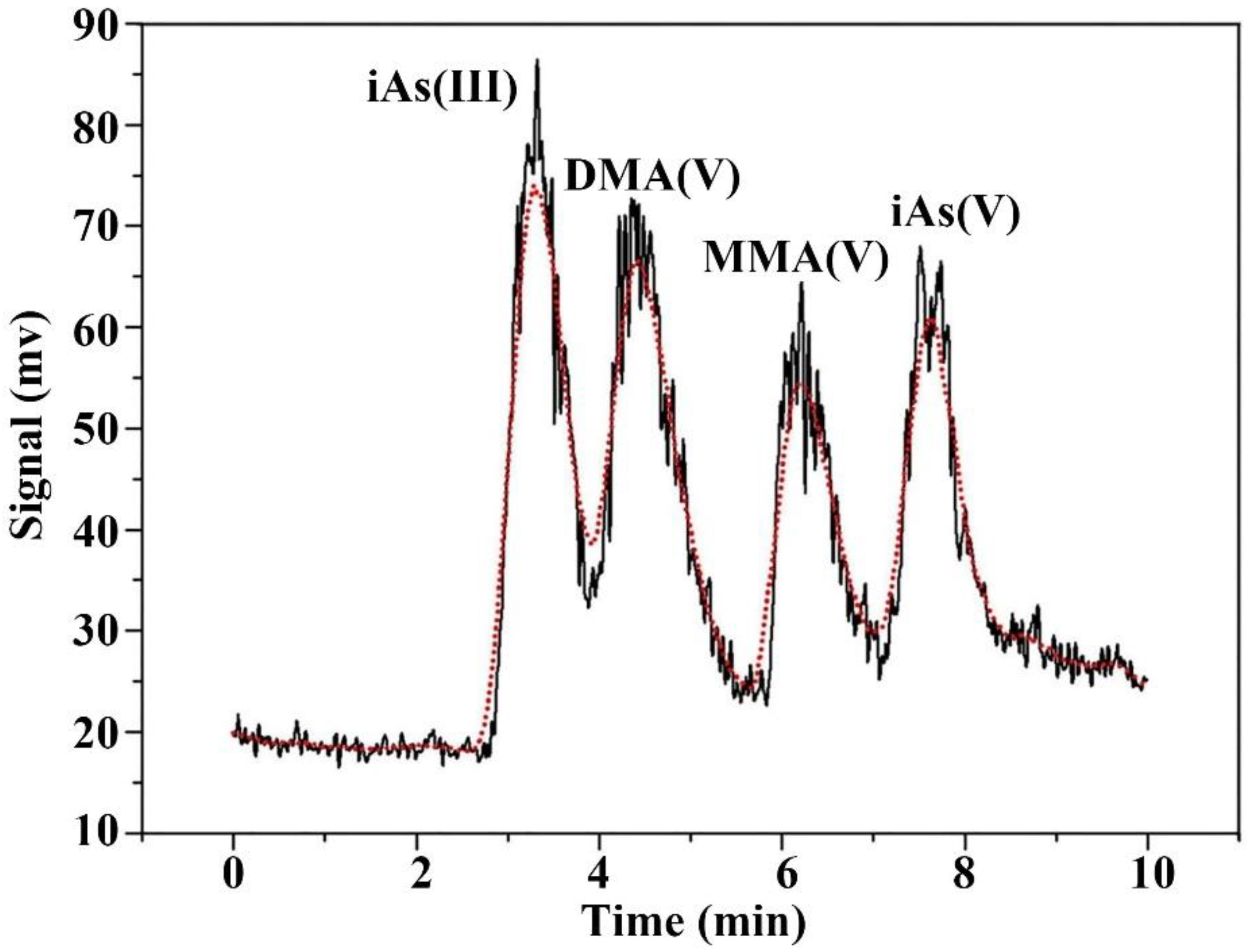
2.4. Arsenic Speciation and Concentration
2.5. Statistical Analysis
3. Results
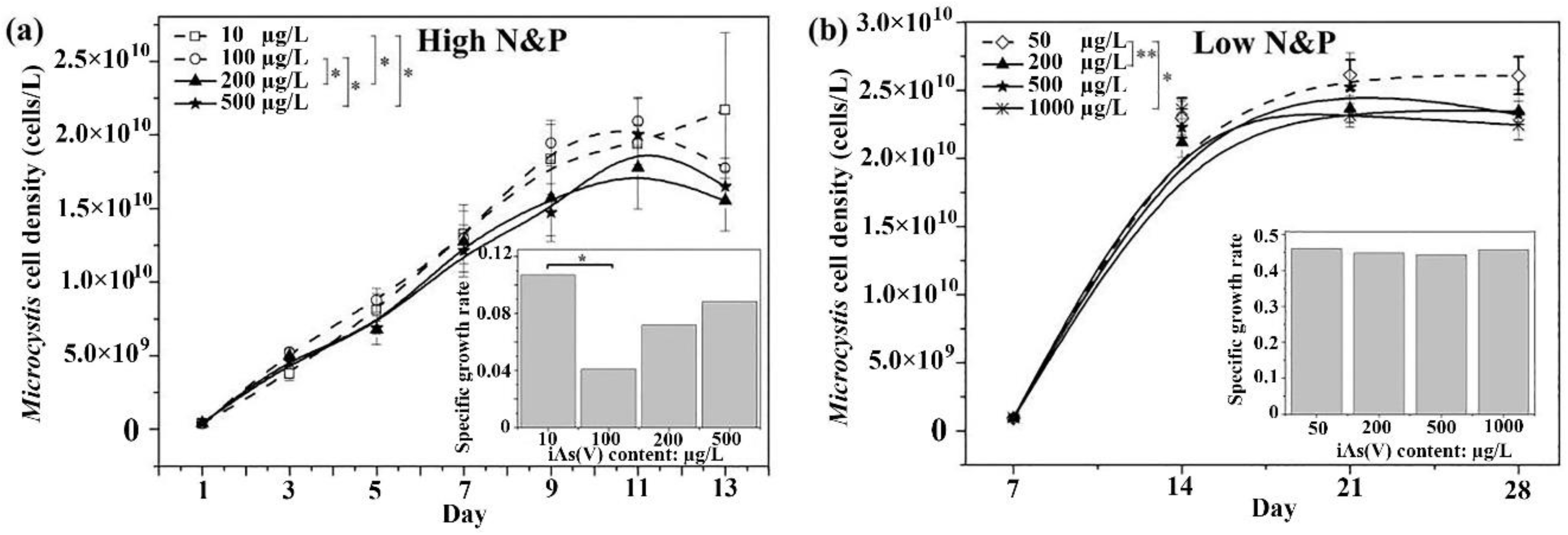
3.1. Effect of Arsenate and Nutrients on Growth
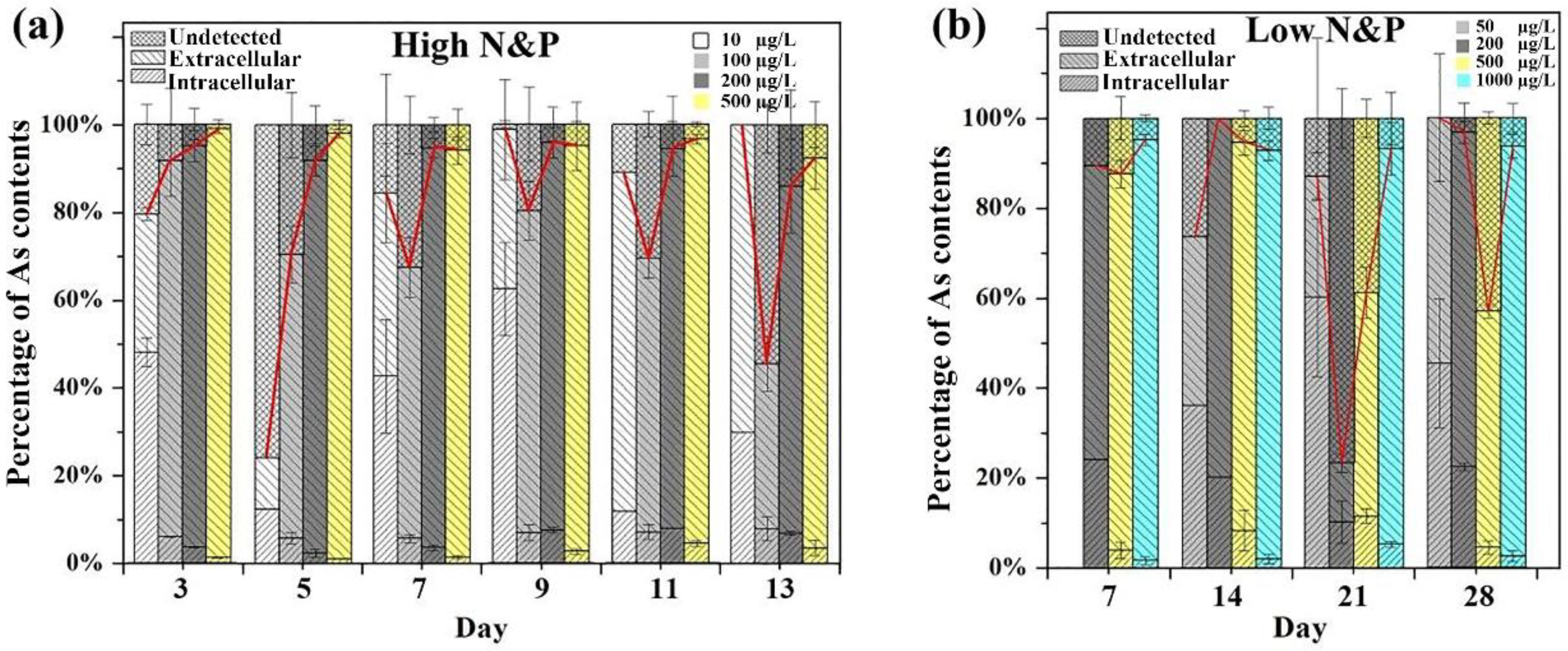
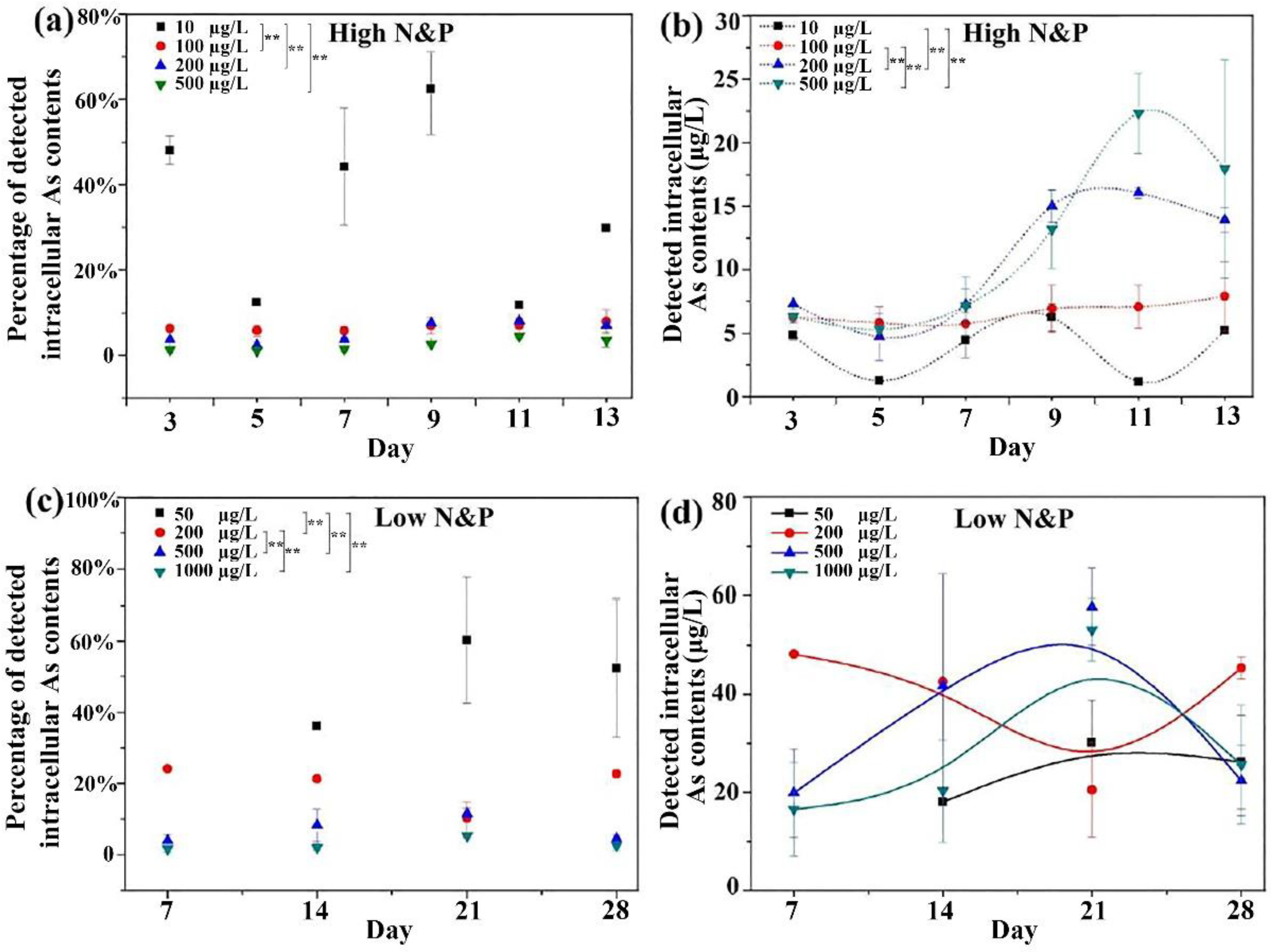
3.2. Intracellular and Extracellular Arsenic Concentrations.
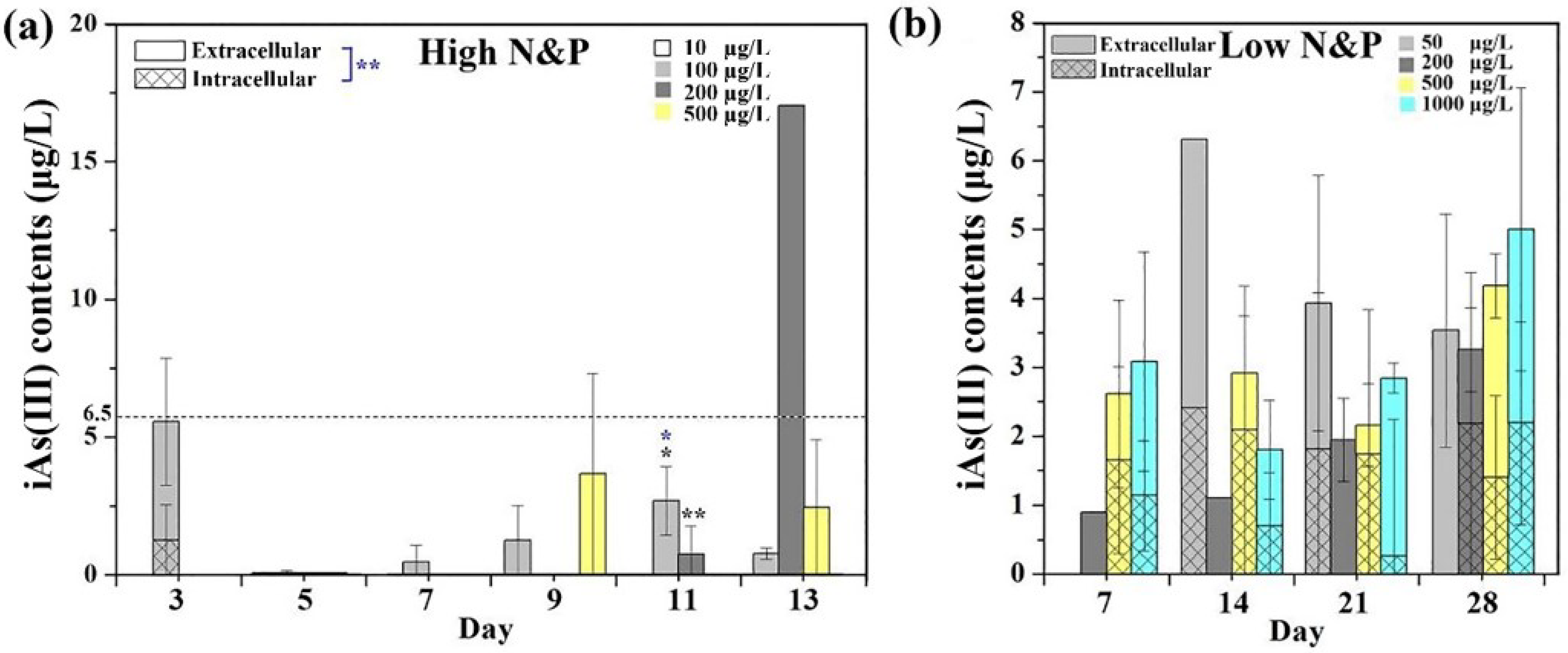
3.3. Intracellular and Extracellular Arsenic Species and Concentrations
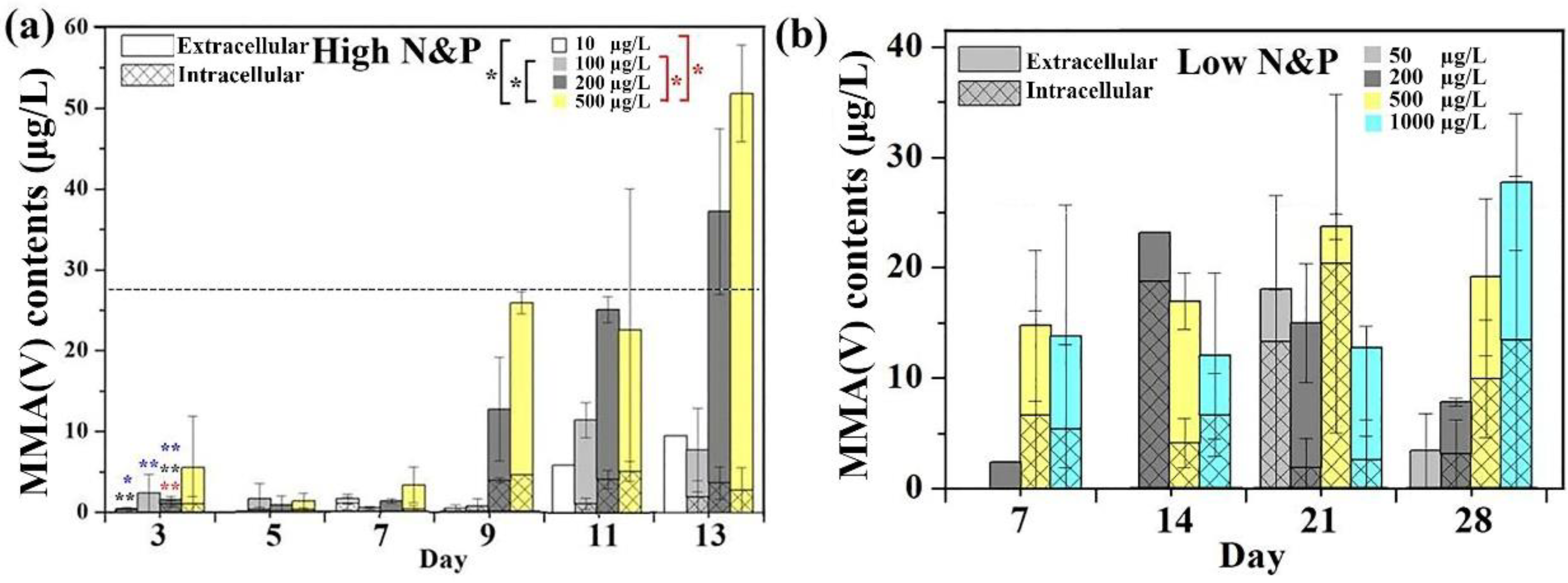
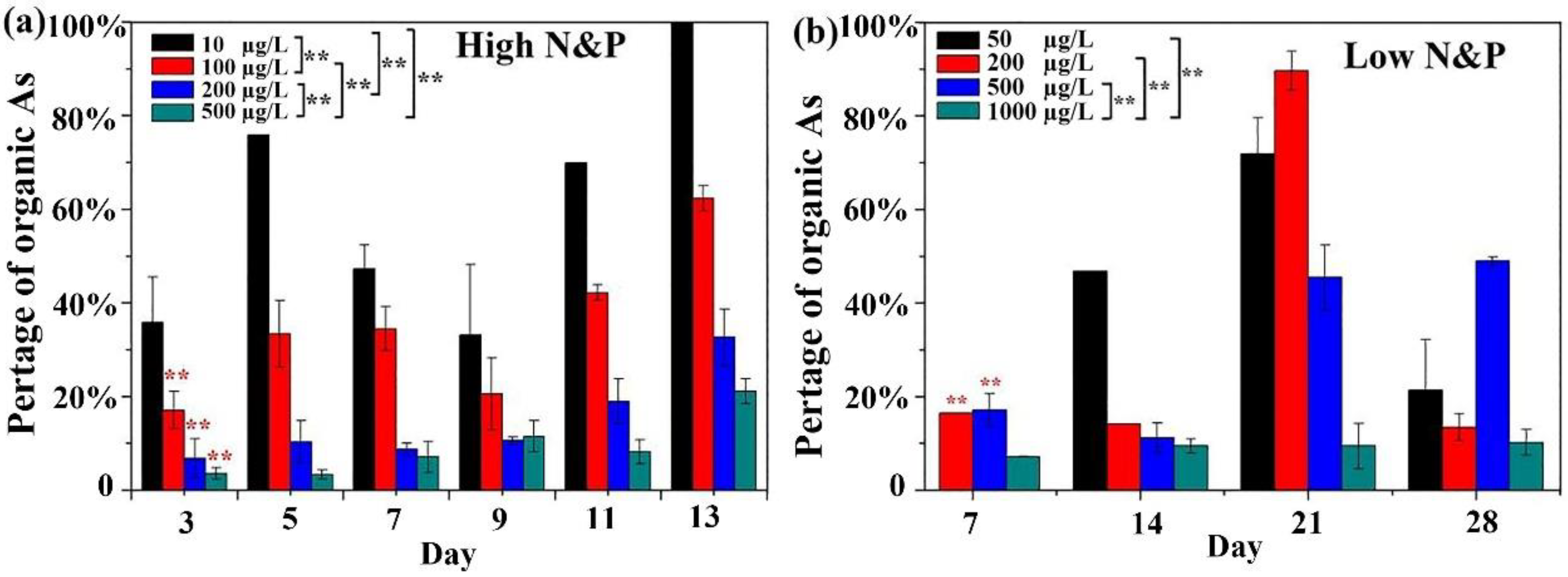
3.4. Arsenic Methylation
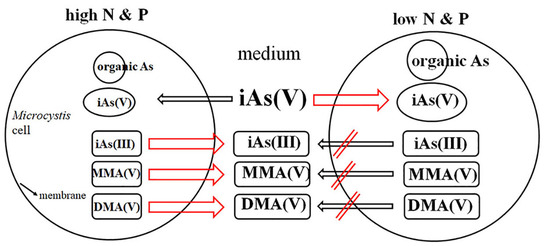
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Francesconi, K.A. Current perspectives in arsenic environmental and biological research. Environ. Chem. 2005, 2, 141–145. [Google Scholar] [CrossRef]
- Guo, Y.; Xue, X.; Yan, Y.; Zhu, Y.; Yang, G.; Ye, J. Arsenic methylation by an arsenite S-adenosylmethionine methyltransferase from Spirulina platensis. J. Environ. Sci. 2016, 49, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.M.; Yan, Y.; Xiong, C.; Raber, G.; Francesconi, K.; Pan, T.; Ye, J.; Zhu, Y.G. Arsenic biotransformation by a cyanobacterium Nostoc sp. PCC 7120. Environ. Pollut. 2017, 228, 111–117. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sohn, M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ. Intern. 2009, 35, 743–759. [Google Scholar] [CrossRef]
- Raber, G.; Raml, R.; Goessler, W.; Francesconi, K.A. Quantitative speciation of arsenic compounds when using organic solvent gradients in HPLC-ICPMS. J. Anal. Atom. Spectrom. 2010, 25, 570–576. [Google Scholar] [CrossRef]
- Yoshinaga, M.; Cai, Y.; Rosen, B.P. Demethylation of methylarsonic acid by a microbial community. Environ. Microbiol. 2011, 13, 1205–1215. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Xue, X.M.; Guo, Y.Q.; Zhu, Y.G.; Ye, J. Co-expression of Cyanobacterial genes for arsenic methylation and demethylation in Escherichia coli offers insights into arsenic resistance. Front. Microbiol. 2017, 8, 60. [Google Scholar] [CrossRef]
- Wang, P.P.; Sun, G.X.; Zhu, Y.G. Identification and characterization of arsenite methyltransferase from an archaeon, Methanosarcina acetivorans C2A. Environ. Sci. Technol. 2014, 48, 12706–12713. [Google Scholar] [CrossRef]
- Huber, V.; Wagner, C.; Gerten, D.; Adrian, R. To bloom or not to bloom: Contrasting responses of cyanobacteria to recent heat waves explained by critical thresholds of abiotic drivers. Oecologia 2012, 169, 245–256. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Yin, X.; Chen, J.; Qin, J.; Sun, G.; Rosen, B.; Zhu, Y. Biotransformation and volatilization of arsenic by three photosynthetic cyanobacteria. Plant Physiol. 2011, 156, 1631–1638. [Google Scholar] [CrossRef]
- Pandey, S.; Rai, R.; Rai, L.C. Proteomics combines morphological, physiological and biochemical attributes to unravel the survival strategy of Anabaena sp. PCC7120 under arsenic stress. J. Proteom. 2012, 75, 921–937. [Google Scholar] [CrossRef]
- Yan, Y.; Ye, J.; Xue, X.M.; Zhu, Y.G. Arsenic demethylation by a C·As lyase in cyanobacterium Nostoc sp. PCC 7120. Environ. Sci. Technol. 2015, 49, 14350–14358. [Google Scholar] [CrossRef]
- Chen, J.; Rosen, B.P. Organoarsenical biotransformations by Shewanella putrefaciens. Environ. Sci. Technol. 2016, 50, 7956–7963. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Bhattacharjee, H.; Rosen, B.P. ArsH is an organoarsenical oxidase that confers resistance to trivalent forms of the herbicide monosodium methylarsenate and the poultry growth promoter roxarsone. Mol. Microbiol. 2015, 96, 1042–1052. [Google Scholar] [CrossRef] [Green Version]
- Guo, P.; Gong, Y.; Wang, C.; Liu, X.; Liu, J. Arsenic speciation and effect of arsenate inhibition in a Microcystis aeruginosa culture medium under different phosphate regimes. Environ. Toxicol. Chem. 2011, 30, 1754–1759. [Google Scholar] [CrossRef]
- Markley, C.T.; Herbert, B.E. Modeling phosphate influence on arsenate reduction kinetics by a freshwater cyanobacterium. Environ. Model. Assess. 2010, 15, 361–368. [Google Scholar] [CrossRef]
- Zhang, S.; Rensing, C.; Zhu, Y.G. Cyanobacteria-mediated arsenic redox dynamics is regulated by phosphate in aquatic environments. Environ. Sci. Technol. 2013, 48, 994–1000. [Google Scholar] [CrossRef]
- López-Maury, L.; Florencio, F.J.; Reyes, J.C. Arsenic sensing and resistance system in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 2003, 185, 5363–5371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyashita, S.I.; Fujiwara, S.; Tsuzuki, M.; Kaise, T. Cyanobacteria produce arsenosugars. Environ. Chem. 2012, 9, 474–484. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.M.; Raber, G.; Foster, S.; Chen, S.C.; Francesconi, K.A.; Zhu, Y.G. Biosynthesis of arsenolipids by the cyanobacterium Synechocystis sp. PCC 6803. Environ. Chem. 2014, 11, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171. [Google Scholar] [CrossRef]
- García-Salgado, S.; Quijano, M.A.; Bonilla, M.M. Arsenic speciation in edible alga samples by microwave-assisted extraction and high performance liquid chromatography coupled to atomic fluorescence spectrometry. Anal. Chim. Acta 2012, 714, 38–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Ariza, J.L.; Sánchez-Rodas, D.; Giráldez, I.; Morales, E. A comparison between ICP-MS and AFS detection for arsenic speciation in environmental samples. Talanta 2000, 51, 257–268. [Google Scholar] [CrossRef]
- Roldán, N.; Salinas-Parra, N.; Gonzalez, A.A.; Cifuentes-Araneda, F.; Arias, H.; Bravo, M.; Quiroz, W. Implementation of an analytical method for the determination of inorganic arsenic species in occupationally exposed human urine samples and its toxic effects on epithelial cells of renal collecting tubule. J. Chil. Chem. Soc. 2016, 61, 3214–3218. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Shi, X.; Liu, K.J. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol. Cell. Biochem. 2004, 255, 67–78. [Google Scholar] [CrossRef]
- Requejo, R.; Tena, M. Proteome analysis of maize roots reveals that oxidative stress is a main contributing factor to plant arsenic toxicity. Phytochemistry 2005, 66, 1519–1528. [Google Scholar] [CrossRef]
- Requejo, R.; Tena, M. Maize response to acute arsenic toxicity as revealed by proteome analysis of plant shoots. Proteomics 2006, 6, 156–162. [Google Scholar] [CrossRef]
- Xu, P.; Yang, G.J.; Zhou, J.; Qin, B.Q.; Zhang, G.S.; Zou, H.; Hu, X.Q. Effect of nitrogen and phosphorus concentration on colony growth of Microcystis flos-aquae in Lake Taihu. J. Lake Sci. 2014, 26, 213. [Google Scholar]
- Chen, W.; Zhang, Q.; Dai, S. Effects of nitrate on intracellular nitrite and growth of Microcystis aeruginosa. J. Appl. Phycol. 2009, 21, 701–706. [Google Scholar] [CrossRef]
- Sivasankar, S.; Oaks, A. Nitrate assimilation in higher plants: The effect of metabolites and light. Plant Physiol. Biochem. 1996, 34, 609–620. [Google Scholar]
- Spiller, H.; Boger, P. Photosynthetic nitrite reduction by dithioerythritol and the effect of nitrite on electron transport in isolated chloroplasts. Photochem. Photobiol. 1977, 26, 397–402. [Google Scholar] [CrossRef]
- Almeida, J.S.; Júlio, S.M.; Reis, M.A.M.; Carrondo, M.J.T. Nitrite inhibition of denitrification by Pseudomonas fluorescens. Biotechnol. Bioeng. 1995, 46, 194–201. [Google Scholar] [CrossRef]
- McCormick, P.V.; O’Dell, M.B.; Shuford, R.B.E., III; Backus, J.G.; Kennedy, W.C. Periphyton responses to experimental phosphorus enrichment in a subtropical wetland. Aquat. Bot. 2001, 71, 119–139. [Google Scholar] [CrossRef]
- Yi, W.L.; Jin, X.C.; Chu, Z.S.; Hu, X.Z.; Ma, Z.Y.; Wang, G.D.; Zhang, S.Y. Effect of different P mass concentrations on growth and P-in-cell of Microcystis aeruginosa. Res. Environ. Sci. 2004, 17, 58–61. [Google Scholar]
- Murray, L.A.; Raab, A.; Marr, I.L.; Feldmann, J. Biotransformation of arsenate to arsenosugars by Chlorella vulgaris. Appl. Organomet. Chem. 2003, 17, 669–674. [Google Scholar] [CrossRef]
- Levy, J.L.; Stauber, J.L.; Adams, M.S.; Maher, W.A.; Kirby, J.K.; Jolley, D.F. Toxicity, biotransformation, and mode of action of arsenic in two freshwater microalgae (Chlorella sp. and Monoraphidium arcuatum). Environ. Toxicol. Chem. 2005, 24, 2630–2639. [Google Scholar] [CrossRef]
- Che, F.; Du, M.; Yan, C. Arsenate biotransformation by Microcystis aeruginosa under different nitrogen and phosphorus levels. J. Environ. Sci. 2018, 66, 41–49. [Google Scholar] [CrossRef]
- Slaughter, D.C.; Macur, R.E.; Inskeep, W.P. Inhibition of microbial arsenate reduction by phosphate. Microbiol. Res. 2012, 167, 151–156. [Google Scholar] [CrossRef]
- Wang, N.X.; Li, Y.; Deng, X.H.; Miao, A.J.; Ji, R.; Yang, L.Y. Toxicity and bioaccumulation kinetics of arsenate in two freshwater green algae under different phosphate regimes. Water Res. 2013, 47, 2497–2506. [Google Scholar] [CrossRef]
- Hellweger, F.L.; Lall, U. Modeling the effect of algal dynamics on arsenic speciation in Lake Biwa. Environ. Sci. Technol. 2004, 38, 6716–6723. [Google Scholar] [CrossRef] [PubMed]
- Hellweger, F.L. Greedy algae reduce arsenate. Limnol. Oceanogr. 2003, 48, 2275–2288. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.S.; Heinemann, J.; Bothner, B.; Rensing, C.; Mcdermott, T.R. Integrated co-regulation of bacterial arsenic and phosphorus metabolisms. Environ. Microbiol. 2012, 14, 3097–3109. [Google Scholar] [CrossRef]
- De Figueiredo, D.R.; Azeiteiro, U.M.; Esteves, S.M.; Gonçalves, F.J.; Pereira, M.J. Microcystin-producing blooms—A serious global public health issue. Ecotoxicol. Environ. Saf. 2004, 59, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Ao, H.Y.; Liu, B.B.; Wen, S.; Wang, Z.; Hu, D.J.; Zhang, X.Z.; Song, L.R.; Liu, J.T. Effects of inorganic arsenic on growth and microcystin production of a Microcystis strain isolated from an algal bloom in Dianchi Lake, China. Chin. Sci. Bull. 2011, 56, 2337–2342. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Chou, H.N.; Tu, C.D.; Liu, X.; Liu, J.; Song, L. Effects of arsenate on the growth and microcystin production of Microcystis aeruginosa isolated from Taiwan as influenced by extracellular phosphate. J. Appl. Phycol. 2009, 21, 225–231. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Z.; Li, X.; Huo, S. Arsenic Accumulation and Biotransformation Affected by Nutrients (N and P) in Common Blooming-Forming Microcystis wesenbergii (Komárek) Komárek ex Komárek (Cyanobacteria). Water 2022, 14, 245. https://doi.org/10.3390/w14020245
Xiao Z, Li X, Huo S. Arsenic Accumulation and Biotransformation Affected by Nutrients (N and P) in Common Blooming-Forming Microcystis wesenbergii (Komárek) Komárek ex Komárek (Cyanobacteria). Water. 2022; 14(2):245. https://doi.org/10.3390/w14020245
Chicago/Turabian StyleXiao, Zhe, Xiaochuang Li, and Shouliang Huo. 2022. "Arsenic Accumulation and Biotransformation Affected by Nutrients (N and P) in Common Blooming-Forming Microcystis wesenbergii (Komárek) Komárek ex Komárek (Cyanobacteria)" Water 14, no. 2: 245. https://doi.org/10.3390/w14020245
APA StyleXiao, Z., Li, X., & Huo, S. (2022). Arsenic Accumulation and Biotransformation Affected by Nutrients (N and P) in Common Blooming-Forming Microcystis wesenbergii (Komárek) Komárek ex Komárek (Cyanobacteria). Water, 14(2), 245. https://doi.org/10.3390/w14020245