Abstract
Illegal gold mining is on the rise in the tropical Andes. The Santiago-Cayapas watershed is located in the north of the Pacific basin of Ecuador, in the Chocó biogeographical region. It is recognized for its high biodiversity, as 62 fish species have been described in the area, and because it contains two of the largest protected areas in the Pacific coast of Ecuador: the mangroves of the Cayapas and Mataje Rivers and the Cotacachi-Cayapas Ecological Reserve. Open-pit gold mining has been described in the area since 2006 and most mining fronts operate illegally and lack any environmental control. Heavy-metal concentrations and fish communities were studied in streams that drain active and abandoned mines, in larger rivers located downstream of the mined areas and in control sites without mining activities. Open-pit mining causes a reduction of dissolved oxygen concentrations and an increase of water temperature, turbidity, and concentrations of Al, Cr, Co, Cu, Fe, Mn, and V. Fish abundance decreased in streams that drain active mines, however, metrics of taxonomic diversity remain unchanged among the study sites. The response of fish communities to open-pit gold mining was complex and driven by the pollution tolerance of each species, the presence of specific adaptions to turbid waters, and changes in the fishing pressure as locals avoid fishing activities in mined areas. Finally, streams that drain abandoned mines showed chemical characteristics, metal concentrations, and fish communities that were similar to control sites, but maintained higher water temperatures than control sites.
1. Introduction
Mining causes large impacts on the quality of water and sediment in aquatic ecosystems located downstream of mined areas [1,2,3]. Moreover, mine wastewater discharges have significant effects on aquatic communities, causing structural, functional, and distribution changes in macroinvertebrates, fish, and trophic chains [4,5,6]. Such impacts may persist decades after the closure of mining facilities [3,7].
Impacts of artisanal gold mining in the tropics are well known and include changes to surface drainage, soil and water contamination with mining wastes, increased water turbidity, reductions in water quality and water for human consumption, losses of natural habitats, biodiversity, and vulnerable species, degradation of fishing grounds, and severe decline of human living conditions and health [5,8,9,10,11,12,13,14,15,16,17]. Many studies have also alerted of mercury in water, sediment and fish from water-bodies impacted by auriferous mining in Ecuador [18], Colombia [19,20,21], Venezuela [22], Peru [23], and Bolivia [24,25]
In the south of Ecuador, small gold mines have existed since the XVth century [26]. In these mining areas, impoverishment of stream benthic communities and high concentrations of toxic metals in water, in sediments, in fish consumed by the local populations, and in the blood and urine of miners and locals with clear negative effects on their moor skills have been documented [14,27,28,29,30,31,32]. This knowledge has resulted in proposals to improve mine management and the living conditions of the affected populations [18,26,33]. However, there is less information about the characteristics and the impacts of gold mining in the Santiago-Cayapas watershed and in other areas of Ecuador [34].
The Santiago-Cayapas watershed is located within the Chocó biogeographical region, one of the world biodiversity hotspots that contains an estimate of 6300 species of plants, 830 species of birds, 235 species of mammals, 350 species of amphibians, and 210 species of reptiles [35]. In this watershed, Jiménez-Prado et al. (2015) identified 62 species of freshwater fish, of which 5 are endemic. Gold panning has been a traditional activity of the Afro and Chachi communities in the Santiago-Cayapas watershed, but open-pit gold mining has been documented since 2006 [36]. Most of the mining fronts operate without permits, lack any environmental control, and require the processing of large amounts of sediments for profitability because of the low ore grade. Therefore, open-pit gold mining may lead to large impacts in the rivers and streams of the Santiago-Cayapas watershed, as the amount of waste and its management are determinant of the impacts of mining activities on aquatic ecosystems [37,38]. The aim of this work is to study water quality and fish communities of rivers and streams in the Santiago-Cayapas watershed. Our central hypothesis is that gold mining has triggered changes in fish communities that are related to the presence and proximity of mining activities. It is likely that fish that scavenge on hard substrates or dwell near the streambed, such as Loricaridae, Gobiidae, Eleotridae, Cichlidae, and Heptapteridae, are likely to be more affected by the presence of mining sediments.
2. Materials and Methods
2.1. Study Area
The Santiago-Cayapas watershed, located in the north of the province of Esmeraldas, has an area of 6321 km2 and discharges into the Pacific Ocean at the southern-most end of the Manglares Cayapas-Mataje Ecological Reserve, the second largest mangrove reserve in Ecuador. The Santiago-Cayapas watershed is the third largest hydrological basin in Ecuador, situated in a seismically active zone [39,40,41,42,43]. The annual rainfall averages 3300 mm, which allows for high water levels throughout the year [44], but discharge is seasonal with higher flows between January and April. The upper part of the watershed belongs to the Cotacachi-Cayapas Ecological Reserve, the largest protected area in Ecuador with 243,638 ha that contains the largest forest patch remaining in the Ecuadorian Chocó (Figure 1).
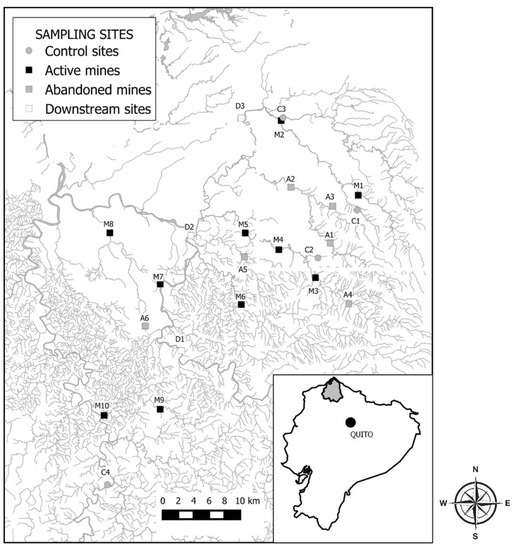
Figure 1.
Location of the Santiago-Cayapas watershed within Ecuador and study sites (C, control sites; M, active mines; A, abandoned mines; D, downstream sites).
2.2. Mining Activities in the Area
After removing the vegetation and about six to eight meters of the topsoil layer, minerals and metals are dug from square shaped pits of 20–30 m width. The ore, a layer of auriferous grey clay located at six to eight meters depth, is processed in Z-shaped industrial sieves under a high-pressure water flow to wash sediments out and concentrate the gold particles. Because operating gold mines consume about 36 m3 of water per hour, they are always located near rivers, streams, and other water bodies [36]. When the exploitation of a pit finishes, it is abandoned and fills with rain and groundwater and the mining front advances another 20–30 m to start the process over. In 2011, 4889 abandoned ponds were counted in an area of 5709 ha by aerial imagery [36] and that number has likely doubled since then.
2.3. Study Sites
Twenty-five sites were selected in the Bogotá, Tululbí, Palabí, Cachaví, Wimbi, Wimbicito, Santiago, and Cayapas rivers and in small tributaries, including the San Antonio, Durango, Comba, Zapallito, María, and Las Antonias streams (Table 1, Figure 1). Sampling sites were grouped into four categories as a function of the presence of mining activities: (1) control sites included streams with no mining activities upstream of the sampling site; (2) active mines included sampling sites located downstream of operating mines; (3) abandoned mines included sites located downstream of mined areas that stopped activity more than 6 months ago; (4) downstream sites located at least 10 km downstream of any mining activity and receive water from tributaries with and without mining influence.

Table 1.
UTM Coordinates of Location (WGS84, 17N) and characteristics of sampling sites.
2.4. Field and Laboratory Work
Study sites were visited 14 times between May 2011 and January 2014 with an irregular schedule to measure water temperature (°C), dissolved oxygen concentration (mg O2L), with a oximeter Mettler Toledo SG-6 Seven Go ProTM Greifensee, Switzerland; electrical conductivity (µScm), pH, and turbidity (NTU) with the multiparameters Hanna HI9829 Woonsocket, RI, 02895, USA and Wagtech Potalab, Palintest House, Kingsway Team Valley Gateshead Tyne Wear NE11 ONS United Kindgdom.
In October and December 2013, one-liter water samples were collected, refrigerated in coolers with ice packs, and submitted to an accredited laboratory to measure total concentrations of 20 metals and metalloids by mass spectrometry with inductively coupled plasma according to the EPA 6020A reference method within 48 h of collection. Additionally, fish were sampled by active fishing methods with the help of local fishermen that had the ability to use the fishing gear in a variety of habitats. Two fishermen used 1.25 cm mesh-size cast-nets and two others used a 1 cm mesh-size sweep-net (1.5 × 4 m). At each site, we collected 12 casts with the cast-net and six sweeps with the sweep-net on river turns, banks with submerged vegetation, woody debris accumulations, and shadowed areas. To capture Chaetostoma marginatum scold in rocky reaches with fast flowing waters, a cast-net was swept by two fishermen for two minutes along the current.
Captured specimens were refrigerated and identified to species in the laboratory according to Jiménez et al. (2015) [45] and their conservation status was taken from the International Union for Nature Conservation (www.iucnredlist.org (accessed on 10 March 2015)) and Aguirre et al. (2021) [46]. Species richness, absolute and relative species abundances, alpha diversity with the Shannon H index, and the proportion of specimens with deformities were calculated for each sample.
2.5. Statistical Analyses
Physical and chemical variables and metal concentrations were fitted to a generalized linear model with the glm function using the location factor (control sites, active mines, abandoned mines, and downstream sites) as the independent variable, the Gaussian error distribution, and the identity link function. One-way ANOVA tables were built with the ANOVA function of the car package [47]. Multiple comparisons by the Tukey test were performed with the emmeans and contrast functions of the emmeans package [48]. To search for patterns among the study sites, a principal component analysis (PCA) was performed with the prcomp function and relevant factors for each axis were selected with the broken-stick method [49]. Prior to these analyses, turbidity and heavy metal concentrations were log-transformed.
Fish, abundances, species richness, and alpha diversity were analyzed with a glm model like the physical and chemical variables. For the fish richness and abundance models, the Poisson error distribution and the identity link function were used. For the alfa diversity model, the Gaussian error distribution and the identity link function were used. Patterns in the distribution of fish species were analyzed with a non-metric multidimensional analysis (NMDS) with the metaNMDS function of the vegan package [50]. Total fish catch of the two samplings was calculated and converted to relative abundances for the NMDS analysis. Only species that were present at more than two study sites were included in the NMDS analysis. Gradients of species’ relative abundances and environmental variables including study site characteristics (Table 1), physicochemical variables, and heavy metal concentrations were added to the NMDS plots with the envfit function. Physicochemical variables and heavy metal concentrations of the two samplings were averaged and log-transformed prior to the NMDS analysis. Watershed area was also log-transformed prior to the NMDS analysis. All statistical analyses were performed in R [51].
3. Results
3.1. Water Quality
There were large differences in the water physical and chemical characteristics among the study sites (Figure 2, Table A1 and Table A2). Water temperature in the streams that drain active and abandoned mines was significantly higher than in the control and downstream sites with an increase of about 1.5 °C (F3,193 = 13.3, p 0.001). Moreover, streams that drain active mines showed significantly lower oxygen concentrations than the other study sites (F3,172 = 12.6, p 0.001). Differences in conductivity were only significant between the streams that drain active mines and the control sites (F3,149 = 3.03, p 0.05). In this case, mean conductivity at the streams that drain active mines almost doubled the value observed at the control sites. There were significant differences in pH among the studied sites (F3,177 = 4.90, p 0.01), but the differences were negligible and mean pH varied between 6.9 and 7.3 among the sites. Turbidity showed the largest significant differences among the study sites (F3,190 = 4.90, p 0.01). Mean turbidity in the streams that drain active mines was seven-fold higher than in the control sites and the streams that drain abandoned mines, while it was intermediate in the downstream sites.
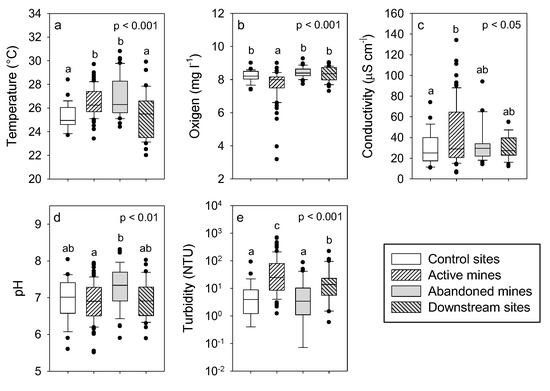
Figure 2.
Boxplots of physical and chemical variables at the study sites (a) = Temperature, (b) = Dissolved oxygen, (c) = Electrical conductivity, (d) = pH and (e) = Turbidity. Results of one-factor ANOVAs (location) and multiple comparisons with Tukey test are also shown. There are no significant differences among locations with the same letter. Mean values and statistical analyses are detailed in Table A1 and Table A2.
Heavy metal concentrations showed two distinct patterns among the study sites (Figure 3, Table A1 and Table A2). The first group showed no significant differences among the study sites and included As, Cd, Se, Sr, and Zn (As: F3,39 = 0.16, p 0.05; Cd: F3,39 = 1.89, p 0.05; Mg: F3,39 = 2.70, p 0.05; Se: F3,39 = 0.25, p 0.05; Sr: F3,39 = 0.75, p 0.05; Zn: F3,39 = 0.16, p 0.05). Additionally, a few metals including Hg, Ag, Tl, and U with concentrations below 0.2 µg L−1 in more than 95% of the samples that were not included in Figure 3 also showed the same pattern (Hg: F3,39 = 0.16, p 0.05; Ag: F3,39 = 0.16, p 0.05; Tl: F3,39 = 0.16, p 0.05; U: F3,39 = 0.16, p 0.05). The second group of metals showed significant differences among the study sites and included Al, Ba, Cr, Co, Cu, Fe, Mn, Ni, Pb, and V (Al: F3,39 = 16.9, p 0.001; Ba: F3,39 = 7.04, p 0.001; Cr: F3,39 = 15.1, p 0.001; Co: F3,39 = 11.9, p 0.001; Cu: F3,39 = 8.22, p 0.001; Fe: F3,39 = 12.1, p 0.001; Mn: F3,39 = 8.03, p 0.001; Ni: F3,39 = 4.47, p 0.01; Pb: F3,39 = 3.98, p 0.05; V: F3,39 = 8.03, p 0.001). Concentrations of these metals in the streams that drain active mines were higher than in the control sites or in the streams that drain abandoned mines.
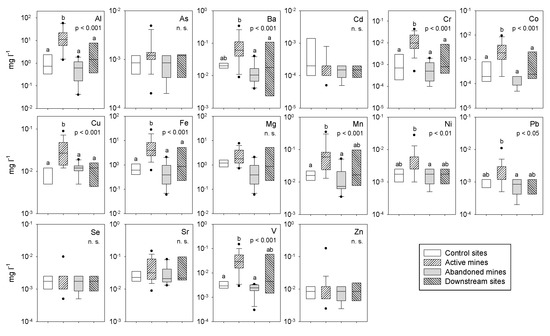
Figure 3.
Boxplots of heavy metal concentrations at the study sites. Results of one-factor ANOVAs (location) and multiple comparisons with Tukey are also shown. There are no significant differences among locations with the same letter. Mean values and statistical analyses are detailed in Table A1 and Table A2.
The two first axes of the PCA analysis explained 60% of the variance in the data (Table 2). The first axis explained 45% of the variance and was positively correlated with turbidity and the concentrations of Al, Co, Cr, St, Fe, Mn, V, and Zn. The second axis explained 15% of the variance and was positively correlated with pH and turbidity and the concentrations of Mg, and negatively correlated with the concentrations of Cd, Se, and Zn. Most samples from streams that drain active mines were located on the right side of the first axis with positive scores, which indicates higher turbidity and concentrations of Al, Co, Cr, St, Fe, Mn, V, and Zn (Figure 4). Samples were segregated depending on the sampling date along the second axis. Most samples collected in December were located on the upper side of the second axis, which indicates higher pH and turbidity and higher concentrations of Mg, but a dilution of Cd, Se, and Zn concentrations. The first axis of the PCA is showing the impact of mining on water chemistry, while the second axis is showing the effect of rain and higher water discharges at the study sites. Under rainy conditions, the higher dispersion of the samples collected in December along the first axis suggest a stronger impact of active mines on water chemistry.

Table 2.
Results of the PCA on the physical and chemical variables and heavy metal concentrations. The variance explained by the axes and loadings of variables that were selected by the broken-stick method are listed.
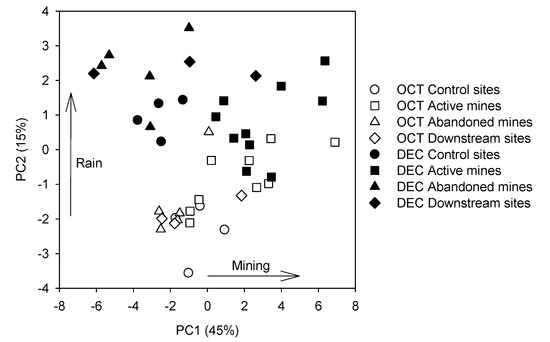
Figure 4.
Results of the PCA on the physical and chemical variables and heavy metal concentrations at the study sites (OCT, October samples; DEC, December samples, CS, control sites; AcM, active mines; AbM, abandoned mines; DS, downstream sites).
3.2. Fish Communities
A total of 33 fish species from 14 families were captured in this study (Table A3). The most abundant families were Characidae, Loricaridae, Cichlidae, and Eliotridae which represented 88% of the 1135 individuals captured. The Characidae predominated in all study sites and represented 57% of the total catch. All species collected were native to Ecuador. Among them, 5 endemic species were captured: Bryconamericus simus [52] and Andinoacara blombergi [53] that are endemic to the Santiago-Cayapas and Mira rivers; Sturisoma frenatum [54] that is endemic to the Santiago-Cayapas, Guayas, and Esmeralda rivers; Sternopygus arenatus [55] that is endemic to lowland rivers in the central and northern coast of Ecuador; and Sycidium rosembergi [56] that is endemic to the Santiago-Cayapas and Esmeraldas rivers. Of the collected species, 4 appear in the IUNC red list: Pseudochalceus longianalis [57] as vulnerable, S. rosembergi and Brycon posadae [58] as near threatened, and S. frenatum as critically endangered, but the remaining 6 species have not been evaluated or lack sufficient data for evaluation.
There were significant differences in fish abundance among the study sites (F3,38 = 3.22, p 0.05). Fish abundance in streams that drain active mines and in the downstream sites was lower than in the streams that drain abandoned mines and the control sites (Figure 5a). Furthermore, fish abundance in the streams that drain abandoned mines was lower than in the control sites. There were lower abundances of endemic species in streams that drain active mines and in the downstream sites (Figure 5b), but the differences were not significant (F3,38 = 1.61, p 0.05). The abundance of species cataloged as critically endangered, near threatened, and vulnerable by the IUCN in streams that drain active mines was significantly lower than in control sites and streams that drain abandoned mines (F2,34 = 15.1, p 0.001). Mean species richness varied between 5 and 6 and mean alpha diversity varied between 1.2 and 1.4 among the studied sites (Figure 5a,c, Table A4 and Table A5) and these variables showed no significant differences (richness: F3,38 = 0.43, p 0.05; alpha diversity: F3,38 = 0.51, p 0.05).
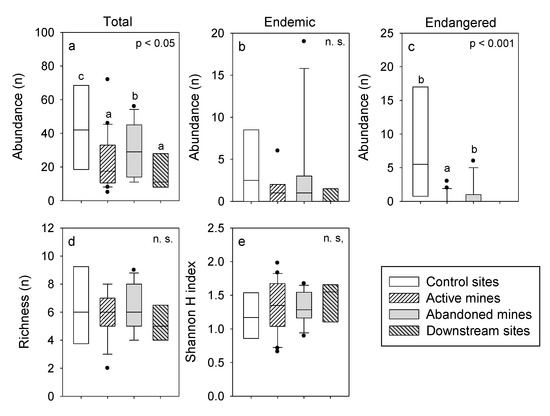
Figure 5.
Boxplots of fish richness (d), abundance (a) = total fishes, (b) = endemic fishes and (c) = endangered fishes, and alpha diversity measured as the Shannon H index (e) at the study sites. Results of one-factor ANOVAs (location) and multiple comparisons with Tukey are also shown. There are no significant differences among locations with the same letter. Mean values and statistical analyses are detailed in Table A4 and Table A5. ns means not statical differences.
The most abundant and widely distributed species was Bryconamericus dahli [59], which represented almost 40% of the total catch. This species appeared in 21 of the 23 study sites and showed no preferences about the presence or absence of mining activities (Table 3). Seven or 3% of the specimens of B. dahli from streams that drain active mines showed large body deformities, but no deformities were observed in the specimens from other study sites. The second most abundant species was Chaestostoma marginatum [60], which accounted for 13% of the total catch, but it was absent from the control sites. The last species that was common was Roeboides occidentalis [61], which represented 10% of the total catch and showed no preferences for the presence or absence of mining activities. Other species showed relative abundances below 10%. Among them, A. blombergi and P. longianalis were absent from the streams that drain active mines; S. arenatus, Rineloricaria jubata [62], and Astyanax ruberrinus [63] were absent from the control sites; and S. frenatum was absent from streams that drain abandoned mines.

Table 3.
Relative abundances of fish species collected at the study sites. Only species that appeared in three or more sites are represented ⬤ 20%; •, 10–20%; ○, 5–10%; +, 5%).
Many species including P. longianalis, S. arenatus, Brycon dentex [64], S. rosembergi, A. banana, S. frenatum, and Hoplias malabaricus [65] were absent from the downstream sites. Other species that were present on all the types of study sites were Hemieleotris latifasciata [66], Pimelodella elongata [64], Gobiomorus maculatus [67], and Pseudocurimata lineopunctata [68]. Finally, a group of 12 species (B simus, Astyanax festae [69], Pseudopoecilia fria [70], Eleotris picta [71], Pseudophallus starksii [72], Lebiasina astrigata [73], B. posadae, Chaestostoma fischeri [74], Hemiancistrus sp. [75], Pimelodella sp. [76], Strongylura fluviatilis [73] and Andinoacara rivulatus [64]) that represented less than 5% of the total catch were too scarce to infer any site preference. These species were not listed in Table 3 and were not included in the NMDS analysis.
Despite some overlapping, a segregation of the study sites was observed along the first axis of the NMDS analysis (Figure 6a). Control sites were located on the left side of the first NMDS axis, while streams that drain active mines were located on the right side. Also, several antagonistic species were observed along the first NMDS axis. P. longianalis (ps1) was associated with control sites, while S. arenatus (st1) and P. elongatus (pi) were associated with streams that drain active mines. Segregation of study sites along the second NMDS axis was not related to mining activities. The second NMDS axis seems to be related to habitat differences among the study sites as C. marginatum (ch) and B. dentex (br2), with preference for shallow and fast waters, were associated with positive values along the second NMDS axis, and P. lineopunctatus (ps2) and G. maculatus (go), with preference for deep and slow waters were associated with negative values. The third axis of the NMDS segregated the control site in the Palabí river from the other study sites (Figure 6c). This site showed a distinct fish community of low richness (4 species) that contained 67% of the captured individuals of A. blombergi.
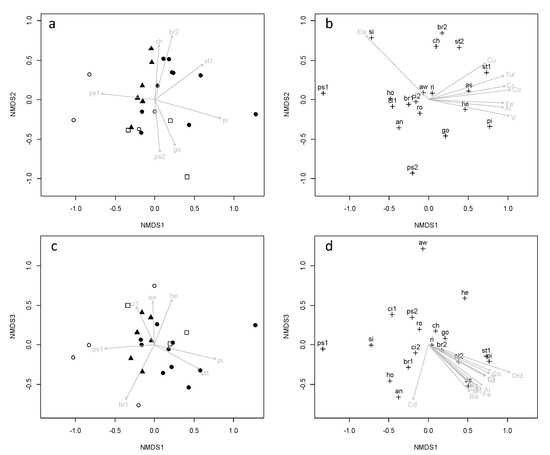
Figure 6.
Results of NMDS of relative abundances of fish species at the study sites (stress = 0.14; • control sites; ▪, active mines; Δ, abandoned mines; ◊, downstream sites, empty figures correspond to october and black figures correspond to december). Species included in the analysis were present at more than two study sites: br1, Briconamericus dalhi; ch, Chaestostoma marginatum; ro, Roeboides occidentalis; he, Hemielotris latifasciata; ps1, Pseudochalceus longianalis; ci1, Cichlasoma festae; pi, Pimelodella elongatus; go, Gobiomorus maculatus; ci2, Cichlasoma ornatum; st1, Sternopygus arenatus; an, Andinoacara blombergi; ps2, Pseudocurimata lineopunctatus; br2, Brycon dentex; si, Sicydium rosembergi; ri, Rinelocaria jubata; aw, Awaous banana; as, Astianax ruberrinus; st2, Sturisoma frenatum; ho, Hoplias malabaricus. Gradients of environmental variables also shown: Ord, channel order; Ele, elevation; Tur, turbidity.
Turbidity and heavy metal concentrations were the main environmental variables related to the gradients of fish abundances along the first NMDS axis (Figure 6b). Further, higher relative abundances of S. rosembergi were related to higher elevation of the study sites. There were no environmental variables related to the second NMDS axis, probably because our study contained no metrics related to in-stream habitat. Finally, the control site in the Palabí river is characterized by a higher concentration of Cd than other study sites (Figure 6d).
4. Discussion
Although mining activities in northern Esmeraldas are usually referred as artisanal, they cannot be considered artisanal because of the size of the labor force and the volume of processed ore. According to Hammond et al. (2013) [77], they fall between the medium- to large-scale categories and lack appropriate environmental management systems to contain and treat the effluents they produce [44,78]. Bulldozers and retro excavators move large volumes of mine waste and create physical barriers that modify water flow, displacing the natural channel from its original position. Open pits fill with water and miners pump this water or water from nearby rivers for washing the gold ore. Overtopping or collapse of the pit walls and leakages in the pipes that feed the pumps cause the discharge of large volumes of water and fine sediment into rivers and streams.
Most studies about the impacts of gold mining have focused on the detrimental effects of Hg on aquatic organisms, gold miners, and related human populations [16,79], which have led to global regulations on the use of Hg [80]. In the Santiago-Cayapas watershed, gold is found in the form of flakes that are recovered gravimetrically and there is no evidence of Hg or other amalgamating compounds. Nor is there evidence that the removable filters of the Z-shaped sieves are being transported elsewhere for treatment with Hg. As amalgamating compounds are not used in the study area and Hg concentrations were below the detection level in all the samples, this is an opportunity to observe environmental impacts caused by other changes in water quality, such as the excess of fine sediments and of heavy metals.
Effluents from open-pit gold mining activities in the Santiago-Cayapas watershed have reduced the water quality of the receiving rivers and streams. Streams receiving the effluents of active mines showed significantly higher concentrations of Al, Cr, Co, Cu, Fe, Mn, and V than control sites. A few metals, including Ba, Ni, and Pb, also showed higher concentrations in streams that drain active mines, but the differences with control sites were not significant. Streams that receive the effluents of active mines in the Santiago-Cayapas watershed have lower As and Cu concentrations, similar Cd concentrations, and higher Pb concentrations than in mining areas in the south of Ecuador [28,29,31]. However, concentrations of heavy metals in a mining area of the Amazonas watershed [81] were higher than in impacted streams in the Santiago-Cayapas watershed, except for Al and Fe. Heavy metals released from mining areas pose a potential risk for human populations and the environment [32]. According to the Ecuadorian legislation [82], water from sites that received effluents from active mines surpassed the reference values of Al, Cr, Cu, Fe, Mn, Ni, Pb, and Se for the preservation of aquatic life (Table 4). However, there were also samples from the control sites that surpassed some reference values, so other activities such as agriculture and deforestation are also potential sources of heavy metals in the study area.

Table 4.
Percentage of samples that comply with reference values for the support of aquatic life according to the Ecuadorian environmental law. Only parameters with established reference values are listed.
The presence of individuals with body deformities is a clear indicator of deleterious effects of mine effluents on aquatic life as there is ample evidence of histopathological damage and deformities in fish exposed to heavy metals [83,84,85,86,87]. However, an evidence approach is required to demonstrate this situation, since there are other pressures in the environment.
Compared to control sites, we also observed higher temperature, conductivity, and turbidity, lower oxygen concentrations, and the formation of thick sediment layers on the stream-bed in streams that drain active mines. Abundance of fish declined in streams that receive effluents from active mines, which indicates that fish avoid the conditions created by mine effluents. Excess of fine sediment is a condition that many fish species avoid seeking refuge in unimpacted reaches, because it reduces food availability and can cause abrasion and clogging of the gill epithelium reducing gas exchange [88,89,90,91]. The presence of individuals of endemic and endangered species also decreased in streams that receive effluents from active mines. Therefore, open-pit gold mining is a big threat for endemic species of reduced distribution already known to be at risk, such as S. frenatum (critically endangered) and S. rosembergi (near threatened), and for others whose conservation status has not been evaluated, such as B. simus. Among the species of ample distribution, it would be advisable to take actions to avoid the local extinction of P. longianalis (vulnerable) and B. posadae (near threatened) in the Santiago-Cayapas watershed. Mining is considered a major threat to aquatic ecosystems and biodiversity across South America [77,92]. In Ecuador, mining has also been identified as a major threat to freshwater fish in the eastern and western slopes of the Andes, which is exacerbated when mining fronts are illegal and operate without environmental controls [46]. Otherwise, there were no significant differences in species richness or alpha diversity among the study sites. Species richness and other measures of taxonomic diversity have been used to monitor the impacts of mining on fish assemblages with mixed results [5,93,94,95], but are considered as poor indicators of these impacts [96]. In the Santiago-Cayapas watershed, some species disappear in the streams that receive effluents from active mines, but they are substituted by other species that are pollution tolerant and take advantage of the newly created conditions, so broad metrics of taxonomic composition remain unchanged among the study sites.
Analysis of the impacts of mining on fish communities is based on current knowledge about the distribution and the ecology of freshwater fish in western Ecuador [45]. The most abundant species, B. dahli (Characidae), is omnivorous and tolerant to contamination and was present in almost all the study sites. C. marginatum (Loricariidae) scavenges on biofilms and was only found in streams that drain active and abandoned mines. This species is attached to rocks and boulders in areas of strong current where sedimentation is limited, so it may tolerate the effluents from active mines. R. occidentalis (Characidae) is omnivorous and sometimes found associated to floating garbage and was present in controls and streams that receive effluents from active mines. P. longianalis (Characidae) and A. blombergi (Cichlidae) were absent from streams that receive effluents from active mines. P. longianalis is found in coastal rivers of Ecuador and Colombia associated to gravel and bedrock bottoms in small- to medium-sized channels with clear water. A. blombergi is endemic to the Santiago-Cayapas and Esmeraldas watersheds, but there is not much information about its biology and ecology. Our study suggests that both species can be considered as indicators of good water quality in the study area. On the contrary, S. arenatus (Sternopygidae) and P. elongatus (Heptapteriadae) were mainly found in streams that receive the effluents of active mines. Both species are predators that are active at night and use electrical sensors (S. arenatus) and antennae (P. elongatus) to detect their prey. A few other species showed no preference for control or impacted streams, but responded to some other environmental variables. S. rosembergii appeared mainly in streams located at an elevation of 100 m or higher, so this threatened species may have found refuge on higher grounds of the Santiago-Cayapas watershed outside the mined areas. A group of species including C. festae, G. maculatus, and P. lineapunctatus were more abundant in downstream sites, which indicates a preference for larger streams.
In opposition to our initial hypothesis that prioritized habitat preferences to explain the impact of mining on fish, the response of fish communities to environmental changes caused by open-pit gold mining was complex and driven by the pollution tolerance of each species, the presence of specific adaptions to turbid waters, and changes in the fishing pressure as locals avoid or reduce fishing activities in mined areas. It has been observed that functional composition of fish assemblages is more stable than taxonomic composition in streams impacted by mines [96,97]. On the contrary, functional adaptations play a major role to explain the impact of mining on the distribution of fish in the Santiago-Cayapas watershed. Night predators that forage using tactile and olfactory senses are favored in the turbid waters of streams that drain active mines [88]. Prevalence of night predators has also been observed in streams impacted by gold mining in Suriname [13], so it could be a typical response of fish assemblages to increasing turbidity of the Chocó and other regions. Furthermore, fish species of ample distribution in the watershed and known to be pollution tolerant are found in streams that drain active mines. The distribution of the population near rivers in the Cayapas-Santiago watershed and their dependence on fishing for survival also influences the response of fish communities to mining. Population is relatively high in the study area and miners operate near population centers, sometimes surrounding them and even isolating some houses from others. Locals avoid fishing in streams that drain active mines, either because they are suspicious about the pollution caused by mine effluents or because they recognize the lower fish abundances. Therefore, streams that drain active and abandoned mines become a refuge for C. marginatum, a species that is highly appreciated and actively fished elsewhere.
In contrast with some studies that found impacts that persist for decades after the termination of mining activities [3,7], we observed that streams that drain abandoned mines have chemical characteristics, metal concentrations, and fish communities that are similar to control sites but maintained higher water temperatures than control sites. Higher temperatures in streams of mined areas are caused by the removal of the riparian vegetation on the banks during the excavation of the pits, as riparian vegetation regulates stream temperature through shading [98,99]. Legacy impacts from mining activities in the Cayapas-Santiago watershed were non-existent or were difficult to detect within the scope and time limitations of this study.
The use of active fishing methods may lead to some uncertainty about the observed differences in fish communities among the study sites, because fishing gear is more likely to be undetected by fish in turbid waters. However, the lower fish catches obtained in streams that receive mine effluents suggest that the collaboration of expert local fishermen in the study may have overcome any potential bias caused by the methodology.
Finally, some limitations of our study should be highlighted. First, some study sites could not be visited continuously because of unfordable rivers or to ensure the safety of the monitoring team. The lack of easy access to the study sites, which is further complicated during rainy periods, and the violence in the study area limit the opportunities to establish a monitoring program and some impacts may have been undetected. Despite this scenario it is necessary to expand studies towards other effects associated with water quality, such as microbiological analysis and the bioaccumulation of metal both in fish and in inhabitants who consume water from the rivers north of Esmeraldas; however, the analysis capacity in Ecuador to these purposes is limited.
5. Conclusions
Illegal open-pit gold mining in the north of Esmeraldas reduces water quality, restricts fish habitats, and modifies the fish assemblages in rivers and streams of the Santiago-Cayapas watershed. Mining causes a reduction of dissolved oxygen concentrations and an increase of water temperature, turbidity, and concentrations of some heavy metals. These changes are also observed in larger rivers located downstream of the mining areas.
The presence of body deformities in fish is probably related to exposure to heavy metals from mine effluents, requiring an evidence approach with specific studies to confirm this situation as there are other sources of pollution in the area such as the abuse of agrochemicals. Two species, P. longianalis (Characidae) and A. blombergi (Cichlidae), can be considered as bioindicators of good water quality in the study area. Nocturnal predator species that do not rely on visual prey detection increase their presence and activity in mined areas.
In general, most fish species avoid the conditions caused by mine effluents, so open-pit gold mining reduces habitat availability and poses a risk to those species already threatened or endemic to the Santiago-Cayapas watershed. Otherwise, most rivers and streams seem to recover after mining activities cease, although the difficulty of establishing a monitoring program in the study area may mean that some effects caused by open-pit gold mining have been undetected in this study.
Author Contributions
Conceptualization, E.R.M. and P.J.P.; methodology, E.R.M. and J.M.O.; software, P.J.P.; validation, E.R.M., J.M.O. and P.J.P.; formal analysis, E.R.M. and J.M.O.; investigation, P.J.P. and J.M.O.; resources, E.R.M. and J.M.O.; data curation, E.R.M.; writing—original draft preparation, T.T. and E.R.M.; writing—review and editing, T.T.; visualization, T.T.; supervision, E.R.M.; project administration, J.M.O.; funding acquisition, E.R.M. and P.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been financed by the Programa de Reparación Ambiental y Social (PRAS) del Ministerio del Ambiente Agua y Transicion Ecologica del Ecuador (MAATE) contracts 064 (2011) and 048 (2013), the Apostolic Vicariate of Esmeraldas donations, and the Pontificia Universidad Católica del Ecuador Sede Esmeraldas internal funds (PUCESE).
Data Availability Statement
Not applicable.
Acknowledgments
We thank the Pontificia Universidad Católica del Ecuador Sede Esmeraldas (PUCESE) and the Universidad de las Fuerzas Armadas ESPE for their technical support.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Mean, standard deviation and range of variation of physical and chemical variables and heavy metal concentrations at the study sites.
Table A1.
Mean, standard deviation and range of variation of physical and chemical variables and heavy metal concentrations at the study sites.
| Control Sites | Active Mines | Abandoned Mines | Downstream Sites | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max | |
| Temperature (°C) | 25.2 ± 1.1 | 23.7–28.4 | 26.5 ± 1.2 | 23.4–29.7 | 26.9 ± 1.7 | 24.4–30.8 | 25.3 ± 1.9 | 22–0–29.9 |
| Oxygen (mg L−1) | 8.2 ± 0.4 | 7.4–9.0 | 7.7 ± 0.9 | 3.2–9.0 | 8.4 ± 0.3 | 7.7–9.3 | 8.3 ± 0.4 | 7.3–9.0 |
| Conductivity (µS cm−1) | 29.5 ± 15.7 | 10.7–74.0 | 42.5 ± 29.5 | 6.0–134.0 | 33.9 ± 19.7 | 14.0–94.0 | 30.1 ± 11.5 | 12.0–55.0 |
| pH | 7.0 ± 0.6 | 5.6–8.0 | 6.9 ± 0.5 | 5.5–7.9 | 7.3 ± 0.6 | 5.9–8.3 | 7.0 ± 0.5 | 5.9–8.0 |
| Turbidity (NTU) | 9 ± 18 | 0–92 | 75 ± 130 | 0–690 | 11 ± 18 | 0–87 | 30 ± 45 | 0–222 |
| Aluminum (mg L−1) | 1.2 ± 1.2 | 0.2–3.3 | 15.8 ± 16.6 | 1.4–58.0 | 0.7 ± 0.6 | 0.0–1.9 | 3.4 ± 4.1 | 0.1–10.0 |
| Arsenic (µg L−1) | 0.9 ± 0.4 | 0.5–1.2 | 1.5 ± 1.2 | 0.2–4.9 | 0.8 ± 0.4 | 0.2–1.2 | 1.0 ± 0.5 | 0.2–1.4 |
| Barium (µg L−1) | 20 ± 7 | 8–34 | 90 ± 85 | 9–340 | 14 ± 11 | 4–40 | 63 ± 113 | 1–290 |
| Cadmium (µg L−1) | 0.9 ± 1.6 | 0.1–4.6 | 0.3 ± 0.2 | 0.1–0.8 | 0.1 ± 0.1 | 0.1–0.2 | 0.1 ± 0.1 | 0.1–0.2 |
| Chrome (µg L−1) | 2.0 ± 2.7 | 0.2–8.0 | 32.2 ± 21.9 | 12.0–89.0 | 0.8 ± 0.7 | 0.1–2.0 | 3.4 ± 4.5 | 0.1–10.0 |
| Cobalt (µg L−1) | 0.4 ± 0.5 | 0.1–1.4 | 2.7 ± 2.6 | 0.2–9.5 | 0.2 ± 0.1 | 0.1–0.2 | 1.0 ± 1.5 | 0.1–4.0 |
| Copper (µg L−1) | 9.1 ± 4.9 | 0.5–14.0 | 32.2 ± 21.9 | 12.0–89.0 | 11.7 ± 4.2 | 5.0–19.0 | 11.8 ± 8.5 | 2.5–27.0 |
| Iron (mg L−1) | 0.9 ± 0.7 | 0.3–2.5 | 6.7 ± 6.8 | 0.6–28.0 | 0.6 ± 0.7 | 0.1–2.1 | 2.4 ± 3.3 | 0.0–8.4 |
| Magnesium (mg L−1) | 1.2 ± 0.5 | 0.7–2.1 | 2.6 ± 2.0 | 0.7–7.7 | 1.4 ± 0.9 | 0.6–3.4 | 1.8 ± 1.0 | 1.1–3.8 |
| Manganese (µg L−1) | 20 ± 14 | 8–52 | 88 ± 97 | 13–350 | 15 ± 17 | 4–52 | 51 ± 72 | 6–190 |
| Mercury (µg L−1) | 0.14 ± 0.05 | 0.10–0.20 | 0.14 ± 0.06 | 0.05–0.20 | 0.14 ± 0.07 | 0.05–0.20 | 0.14 ± 0.07 | 0.05–0.20 |
| Nickel (µg L−1) | 1.8 ± 0.8 | 1.0–2.5 | 5.3 ± 6.4 | 1.0–28.0 | 1.7 ± 0.9 | 0.5–2.5 | 1.7 ± 0.9 | 0.5–2.0 |
| Silver (µg L−1) | 0.55 ± 0.84 | 0.10–2.50 | 0.14 ± 0.06 | 0.05–0.20 | 0.14 ± 0.07 | 0.05–0.20 | 0.14 ± 0.07 | 0.05–0.20 |
| Lead (µg L−1) | 1.4 ± 1.1 | 0.5–4.0 | 2.3 ± 2.5 | 0.5–11.0 | 0.8 ± 0.4 | 0.2–1.2 | 0.9 ± 0.4 | 0.2–1.2 |
| Selenium (µg L−1) | 1.8 ± 0.8 | 1.0–2.5 | 2.2 ± 2.1 | 0.5–10.0 | 1.7 ± 0.9 | 0.5–2.5 | 1.7 ± 0.9 | 0.5–2.5 |
| Strontium (µg L−1) | 28 ± 13 | 16–55 | 49 ± 42 | 9–150 | 31 ± 23 | 13–83 | 67 ± 105 | 19–280 |
| Thallium (µg L−1) | 0.15 ± 0.05 | 0.10–0.20 | 0.14 ± 0.06 | 0.05–0.20 | 0.14 ± 0.07 | 0.05–0.20 | 0.14 ± 0.07 | 0.05–0.20 |
| Uranium (µg L−1) | 0.15 ± 0.05 | 0.10–0.20 | 0.22 ± 0.17 | 0.05–0.70 | 0.14 ± 0.07 | 0.05–0.20 | 0.14 ± 0.07 | 0.05–0.20 |
| Vanadium (µg L−1) | 3.8 ± 2.4 | 2.1–9.3 | 40.5 ± 38.8 | 2.9–150.0 | 2.4 ± 1.0 | 0.3–3.4 | 30.4 ± 54.9 | 1.1–140.0 |
| Zinc (µg L−1) | 20 ± 35 | 5–106 | 19 ± 39 | 3–180 | 8 ± 4 | 3–12 | 10 ± 9 | 3–26 |

Table A2.
Results of one-way ANOVA (location) and multiple comparisons with Tukey’s test of physical and chemical variables and heavy metal concentrations at the study sites (CS, control sites; AcM, active mines; AbM, abandoned mines; DS, downstream sites; n. s., not significant). There are no significant differences between locations with the same superscript.
Table A2.
Results of one-way ANOVA (location) and multiple comparisons with Tukey’s test of physical and chemical variables and heavy metal concentrations at the study sites (CS, control sites; AcM, active mines; AbM, abandoned mines; DS, downstream sites; n. s., not significant). There are no significant differences between locations with the same superscript.
| Metal | F Value | p | Multiple Comparisons |
|---|---|---|---|
| Temperature | F3,193 = 13.3 | p 0.001 | CS a DS a AcM b AbM b |
| Oxygen | F3,172 = 12.6 | p 0.001 | AcM a CS b DS b AbM b |
| Conductivity | F3,149 = 3.03 | p 0.05 | CS a DS ab AbM ab AcM b |
| pH | F3,177 = 4.90 | p 0.01 | AcM a DS ab CS ab AbM b |
| Turbidity | F3,190 = 19.9 | p 0.001 | CS a AbM a DS b AcM c |
| Aluminum | F3,39 = 16.9 | p 0.001 | AbM a CS a DS a AcM b |
| Arsenic | F3,39 = 0.16 | n. s. | --- |
| Barium | F3,39 = 7.04 | p 0.001 | AbM a DS a CS ab AcM b |
| Cadmium | F3,39 = 1.89 | n. s. | --- |
| Chrome | F3,39 = 15.1 | p 0.001 | AbM a CS a DS a AcM b |
| Cobalt | F3,39 = 11.9 | p 0.001 | AbM a CS a DS a AcM b |
| Copper | F3,39 = 8.22 | p 0.001 | CS a DS a AbM a AcM b |
| Iron | F3,39 = 12.1 | p 0.001 | AbM a CS a DS a AcM b |
| Magnesium | F3,39 = 2.70 | n. s. | --- |
| Manganese | F3,39 = 8.03 | p 0.001 | AbM a CS a DS ab AcM b |
| Mercury | F3,39 = 0.08 | n. s. | --- |
| Nickel | F3,39 = 4.47 | p 0.01 | AbM a CS ab DS ab AcM b |
| Silver | F3,39 = 2.35 | n. s. | --- |
| Lead | F3,39 = 3.98 | p 0.05 | AbM a CS ab DS ab AcM b |
| Selenium | F3,39 = 0.25 | n. s. | --- |
| Strontium | F3,39 = 0.75 | n. s. | --- |
| Thallium | F3,39 = 0.15 | n. s. | --- |
| Uranium | F3,39 = 1.31 | n. s. | --- |
| Vanadium | F3,39 = 8.03 | p 0.001 | AbS a CS a DS ab AcM b |
| Zinc | F3,39 = 0.50 | n. s. | --- |

Table A3.
Taxonomical classification of the collected fish specimens (Det, detritivore; Pre, predator; Ins, insectivorous; Omn, omnivorous; Herb, herbivorous; LC, least concern; NE, not evaluated; DD, data deficient; VU, vulnerable; NT, near threatened; CR, critically endangered).
Table A3.
Taxonomical classification of the collected fish specimens (Det, detritivore; Pre, predator; Ins, insectivorous; Omn, omnivorous; Herb, herbivorous; LC, least concern; NE, not evaluated; DD, data deficient; VU, vulnerable; NT, near threatened; CR, critically endangered).
| Family | Species | Type | Feeding | IUNC Status |
|---|---|---|---|---|
| Curimatidae | Pseudocurimata lineopunctata [68] | Native | Det | LC |
| Erythrinidae | Hoplias malabaricus [65] | Native | Pre | LC |
| Lebiasinidae | Lebiasina astrigata [73] | Native | Ins | LC |
| Characidae | Astyanax festae [69] | Native | Omn | NE |
| Astyanax ruberrinus [63] | Native | Omn | NE | |
| Bryconamericus dahli [59] | Native | Omn | LC | |
| Bryconamericus simus [69] | Endemic | --- | DD | |
| Pseudochalceus longianalis [57] | Native | --- | VU | |
| Roeboides occidentalis [61] | Native | Omn | LC | |
| Bryconidae | Brycon dentex [64] | Native | Omn | LC |
| Brycon posadae [58] | Native | --- | NT | |
| Heptapteridae | Pimelodella longate [64] | Native | Omn | LC |
| Pimelodella sp [76] | --- | --- | --- | |
| Loricariidae | Chaestostoma fischeri [74] | Native | Herb | LC |
| Chaestostoma marginatum [60] | Native | Herb | LC | |
| Hemiancistrus sp [75] | --- | --- | --- | |
| Rineloricaria jubata [54] | Native | Herb | LC | |
| Sturisoma frenatum [54] | Endemic | Herb | CR | |
| Sternopygidae | Sternopygus arenatus [55] | Endemic | Pre | NE |
| Poeciliidae | Pseudopoecilia fria [70] | Native | Ins | LC |
| Belonidae | Strongylura fluviatilis [73] | Native | Pre | NE |
| Syngnathidae | Pseudophallus starksii [72] | Native | --- | LC |
| Cichlidae | Andinoacara blombergi [53] | Endemic | Pre | LC |
| Andinoacara rivulatus [64] | Native | Omn | NE | |
| Cichlasoma ornatus [100] | Native | Pre | LC | |
| Cichlasoma festae [56] | Native | Omn | NE | |
| Andinoacara rivulatus [64] | Native | Ins | NE | |
| Eleotridae | Gobiomorus maculatus [67] | Native | Pre | LC |
| Eleotris picta [71] | Native | Pre | LC | |
| Hemieleotris latifasciata [66] | Native | Ins | LC | |
| Gobiidae | Awaous banana [101] | Native | Herb | LC |
| Sycidium rosembergi [56] | Endemic | --- | NT |

Table A4.
Mean, standard deviation, and range of variation of fish richness, abundance, and alpha diversity at the study sites.
Table A4.
Mean, standard deviation, and range of variation of fish richness, abundance, and alpha diversity at the study sites.
| Control Sites | Active Mines | Abandoned Mines | Downstream Sites | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max | |
| Abundance (count) | 45 ± 28 | 17–88 | 22 ± 16 | 5–72 | 31 ± 16 | 11–56 | 17 ± 11 | 7–31 |
| Endemic (count) | 4 ± 4 | 0–10 | 1 ± 2 | 0–6 | 3 ± 6 | 0–19 | 1 ± 1 | 0–2 |
| Endangered (count) | 8 ± 9 | 0–23 | 0 ± 1 | 0–3 | 1 ± 2 | 0–6 | --- | --- |
| Richness (count) | 6 ± 3 | 3–10 | 6 ± 2 | 2–8 | 6 ± 2 | 4–9 | 5 ± 1 | 4–7 |
| Alpha diversity | 1.2 ± 0.4 | 0.4–1.7 | 1.3 ± 0.4 | 0.7–2.0 | 1.3 ± 0.2 | 0.9–1.7 | 1.4 ± 0.3 | 0.9–1.7 |

Table A5.
Results of one-way ANOVA (location) and multiple comparisons with Tukey’s test of fish richness, abundance, and alpha diversity (CS, control sites; AcM, active mines; AbM, abandoned mines; DS, downstream sites; n. s., not significant). There are no significant differences between locations with the same superscript.
Table A5.
Results of one-way ANOVA (location) and multiple comparisons with Tukey’s test of fish richness, abundance, and alpha diversity (CS, control sites; AcM, active mines; AbM, abandoned mines; DS, downstream sites; n. s., not significant). There are no significant differences between locations with the same superscript.
| Variable | F Value | p | Multiple Comparisons |
|---|---|---|---|
| Abundance | F3,38 = 3.22 | p 0.05 | DS a AcM a AbM b CS c |
| Endemic | F3,38 = 1.61 | n. s. | --- |
| Endangered | F2,34 = 15.1 | p 0.001 | AcM a AbM b CS b |
| Richness | F3,38 = 0.43 | n. s. | --- |
| Alpha diversity | F3,38 = 0.51 | n. s. | --- |
References
- Liu, W.; Coveney, R.; Chen, J. Environmental quality assessment on a river system polluted by mining activities. Appl. Geochem. 2003, 18, 749–764. [Google Scholar] [CrossRef]
- Thorslund, J.; Jarsjö, J.; Chalov, S.R.; Belozerova, E.V. Gold mining impact on riverine heavy metal transport in a sparsely monitored region: The upper Lake Baikal Basin case. J. Environ. Monit. 2012, 14, 2780–2792. [Google Scholar] [CrossRef]
- Resongles, E.; Casiot, C.; Freydier, R.; Dezileau, L.; Viers, J.; Elbaz-Poulichet, F. Persisting impact of historical mining activity to metal (Pb, Zn, Cd, Tl, Hg) and metalloid (As, Sb) enrichment in sediments of the Gardon River, Southern France. Sci. Total Environ. 2014, 481, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, M. Sondeo ecolygico rapido de las comunidades de peces tropicales en un área de explotación minera en Costa Rica. Rev. De Biol. Trop. 2007, 56, 1971–1990. [Google Scholar]
- Yule, C.M.; Boyero, L.; Marchant, R. Effects of sediment pollution on food webs in a tropical river (Borneo, Indonesia). Mar. Freshw. Res. 2010, 61, 204–213. [Google Scholar] [CrossRef]
- Wantzen, K.; Mol, J. Soil Erosion from Agriculture and Mining: A Threat to Tropical Stream Ecosystems. Agriculture 2013, 3, 660–683. [Google Scholar]
- Coulthard, T.J.; Macklin, M.G. Modeling long-term contamination in river systems from historical metal mining. Geology 2003, 31, 451. [Google Scholar] [CrossRef]
- Salomons, W. Environmental impact of metals derived from mining activities: Processes, predictions, prevention. J. Geochem. Explor. 1995, 52, 5–23. [Google Scholar] [CrossRef]
- Hilson, G. The environmental impact of small-scale gold mining in Ghana: Identifying problems and possible solutions. Geogr. J. 2002, 168, 57–72. [Google Scholar] [CrossRef]
- Ogola, J.S.; Mitullah, W.V.; Omulo, M.A. Impact of gold mining on the environment and human health: A case study in the Migori gold belt, Kenya. Environ. Geochem. Health 2002, 24, 141–157. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Marins, R.V. Anthropogenic mercury emissions to the atmosphere in Brazil: The impact of gold mining. J. Geochem. Explor. 1997, 58, 223–229. [Google Scholar] [CrossRef]
- Smolders, A.; Lock, R.; Van der Velde, G.; Medina Hoyos, R.; Roelofs, J. Effects of mining activities on heavy metal concentrations in water, sediment, and macroinvertebrates in different reaches of the Pilcomayo River, South America. Arch. Environ. Contam. Toxicol. 2003, 44, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Mol, J.H.; Ouboter, P.E. Downstream effects of erosion from small-scale gold mining on the instream habitat and fish community of a small neotropical rainforest stream. Conserv. Biol. 2004, 18, 201–214. [Google Scholar] [CrossRef]
- Betancourt, O.; Narváez, A.; Roulet, M. Small-scale Gold Mining in the Puyango River Basin, Southern Ecuador: A Study of Environmental Impacts andHuman Exposures. EcoHealth 2005, 2, 323–332. [Google Scholar] [CrossRef]
- Schueler, V.; Kuemmerle, T.; Schröder, H. Impacts of surface gold mining on land use systems in Western Ghana. Ambio 2011, 40, 528–539. [Google Scholar] [CrossRef]
- Peplow, D.; Augustine, S. Community-Led Assessment of Risk from Exposure to Mercury by Native Amerindian Wayana in Southeast Suriname. J. Environ. Public Health 2012, 2012, 674596. [Google Scholar] [CrossRef]
- Klubi, E.; Abril, J.M.; Mantero, J.; García-Tenorio, R.; Nyarko, E. Environmental radioactivity and trace metals in surficial sediments from estuarine systems in Ghana (Equatorial Africa), impacted by artisanal gold-mining. J. Environ. Radioact. 2020, 218, 106260. [Google Scholar] [CrossRef]
- Mestanza-Ramón, C.; Paz-Mena, S.; Lopez-Paredes, C.; Jimenez-Gutierrez, M.; Herrera Morales, G.; Dório, G.; Straface, S.S. History, Current Situation and Challengues of Gold Mining in Ecuador’s Litoral Region. Land 2021, 10, 1220. [Google Scholar] [CrossRef]
- Olivero, J.; Johnson, B. El Lado Gris de la Mineria del oro: La Contaminación con Mercurio en el Norte de Colombia; Universidad de Cartagena: Cartagena, Colombia, 2002. [Google Scholar]
- Olivero-Verbel, J.; Johnson-Restrepo, B.; Mendoza-Marin, C.; Paz-Martinez, R.; Olivero-Verbel, R. Mercury in the aquatic environment of the village of Caimito at the Mojana region, north of Colombia. Water Air Soil Pollut. 2004, 159, 409–420. [Google Scholar] [CrossRef]
- Vargas Licona, S.P.; Marrugo Negrete, J.L. Mercurio, metilmercurio y otros metales pesados en peces de Colombia: Riesgo por ingesta. Acta Biolygica Colomb. 2019, 24, 232–242. [Google Scholar] [CrossRef]
- Trujillo, F.; Lasso, C.; Diaz-Granados, M.; Farina, O.; Pérez, L.; Barbarino, A.; González, M.; Usma, J. Evaluación de la contaminación por mercurio en peces de interés comercial y de la concentración de organoclorados y organofosforados en el agua y sedimentos de la Orinoquia. In Biodiversidad de la cuenca del Orinoco; Lasso, C.A., Usma, J.S., Trujillo, F., Rial, A., Eds.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, WWF Colombia, Fundación Omacha, Fundación La Salle e Instituto de Estudios de la Orinoquia (Universidad Nacional de Colombia): Bogotá, Colombia, 2010; pp. 340–355. [Google Scholar]
- Swenson, J.J.; Carter, C.E.; Domec, J.-C.; Delgado, C.I. Gold Mining in the Peruvian Amazon: Global Prices, Deforestation, and Mercury Imports. PLoS ONE 2011, 6, e18875. [Google Scholar] [CrossRef] [PubMed]
- Poully, M.; Pérez, T.; Ovando, A.; Guzmán, F.; Paco, P.; Duprey, J.; Chincheros, J.; Carranzas, B.; Barbieri, F.; Gardon, J. Diagnystico de la Contaminaciyn por el Mercurio en la Cuenca Itánez; IRD-WWF Report; IRD: La Paz, Bolivia, 2008. [Google Scholar]
- Ramos, R.; Verde, A.; García, E.M. Heavy metals in Venezuelan marine sediments: Concentrations, degree of contamination, and distribution. Cienc. Mar. 2021, 47, 185–199. [Google Scholar] [CrossRef]
- Miserendino, R.A.; Bergquist, B.A.; Adler, S.E.; Guimarães, J.R.D.; Lees, P.S.; Niquen, W.; Velasquez-Lypez, P.C.; Veiga, M.M. Challenges to measuring, monitoring, and addressing the cumulative impacts of artisanal and small-scale gold mining in Ecuador. Resour. Policy 2013, 38, 713–722. [Google Scholar] [CrossRef]
- Appleton, J.D. A study of the extent of mercury and related chemical pollution along the Naboc River, Monkayo, Davao del Norte; Hijo River, Apokon ore processing site; and their neighbouring areas (rice fields and banana plantations)—A report for UNIDO Project DP/PHI/98/00511–02. Br. Geol. Surv. 2000, 108, 34. [Google Scholar]
- Tarras-Wahlberg, N.H.; Flachier, A.; Fredriksson, G.; Lane, S.; Lundberg, B.; Sangfors, O. Environmental impact of small-scale and artisanal gold mining in southern Ecuador. AMBIO J. Hum. Environ. 2000, 29, 484–491. [Google Scholar] [CrossRef]
- Tarras-Wahlberg, N.; Flachier, A.; Lane, S.; Sangfors, O. Environmental impacts and metal exposure of aquatic ecosystems in rivers contaminated by small scale gold mining: The Puyango River basin, southern Ecuador. Sci. Total Environ. 2001, 278, 239–261. [Google Scholar] [CrossRef]
- Harari, R.; Harari, F.; Gerhardsson, L.; Lundh, T.; Skerfving, S.; Strömberg, U.; Broberg, K. Exposure and toxic effects of elemental mercury in gold-mining activities in Ecuador. Toxicol. Lett. 2012, 213, 75–82. [Google Scholar] [CrossRef]
- Carling, G.T.; Diaz, X.; Ponce, M.; Perez, L.; Nasimba, L.; Pazmino, E.; Rudd, A.; Merugu, S.; Fernandez, D.P.; Gale, B.K.; et al. Particulate and dissolved trace element concentrations in three Southern Ecuador rivers impacted by artisanal gold mining. Water Air Soil Pollut. 2013, 224, 1415. [Google Scholar] [CrossRef]
- Salgado-Almeida, B.; Falquez-Torres, D.A.; Romero-Crespo, P.L.; Valverde-Armas, P.E.; Guzmán-Martínez, F.; Jiménez-Oyola, S. Risk Assessment of Mining Environmental Liabilities for Their Categorization and Prioritization in Gold-Mining Areas of Ecuador. Sustainability 2022, 14, 6089. [Google Scholar] [CrossRef]
- Warnaars, X.S. Why be poor when we can be rich? Constructing responsible mining in El Pangui, Ecuador. Resour. Policy 2012, 37, 223–232. [Google Scholar] [CrossRef]
- Galarza, E.; Cabrera, M.; Espinosa, R.; Espitia, E.; Moulatlet, G.M.; Capparelli, M.V. Assessing the Quality of Amazon Aquatic Ecosystems with Multiple Lines of Evidence: The Case of the Northeast Andean Foothills of Ecuador. Bull. Environ. Contam. Toxicol. 2021, 107, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Critical Ecosystems Partnerships Funds (CEPF). Ecosystems Profiles. Tumbes Choco Madgalena. I’Agence Francaise de Developpement, Conservation International, European Union, Global Environmental Facility, The Government of Japan and World Bank. 2022. Available online: https://www.cepf.net/our-work/biodiversity-hotspots/tumbes-choco-magdalena (accessed on 10 August 2022).
- Programa de Reparacion Ambiental y Social (PRAS); Ministerio del Ambiente del Ecuador y Centro de Investigacion y Desarrollo; Pontificia Universidad Catolica del Ecuador Sede Esmeraldas (CID PUCESE). Informe de Valoración de Pasivos Socio Ambientales Vinculados a la Actividad Minera Aurífera Ilegal en el Norte de Esmeraldas. 2011, p. 69. Available online: https://www.researchgate.net/publication/280052412_Economic_valuation_of_the_environmental_assestments_of_gold_mining_activity_in_the_north_of_Ecuador (accessed on 10 August 2022).
- Ashton, P.J.; Love, D.; Mahachi, H.; Dirks, P. An overview of the impact of mining and mineral processing operations on water resources and water quality in the Zambezi, Limpopo and Olifants Catchments in Southern Africa. Contract Report to the Mining, Minerals and Sustainable Development (Southern Africa) Project, by CSIR-Environmentek, Pretoria and Geology Department, University of Zimbabwe-Harare. Report No. ENV-PC. 2001, Volume 42, pp. 1–362. Available online: https://pubs.iied.org/sites/default/files/pdfs/migrate/G00599.pdf? (accessed on 15 March 2022).
- Hammond, D.S.; Gond, V.; De Thoisy, B.; Forget, P.-M.; DeDijn, B.P. Causes and consequences of a tropical forest gold rush in the Guiana Shield, South America. AMBIO J. Hum. Environ. 2007, 36, 661–670. [Google Scholar] [CrossRef]
- Toulkeridis, T.; Chunga, K.; Rentería, W.; Rodriguez, F.; Mato, F.; Nikolaou, S.; Cruz D’Howitt, M.; Besenzon, D.; Ruiz, H.; Parra, H.; et al. The 7.8 Mw Earthquake and Tsunami of the 16th April 2016 in Ecuador—Seismic evaluation, geological field survey and economic implications. Sci. Tsunami Hazards 2017, 36, 197–242. [Google Scholar]
- Toulkeridis, T.; Parra, H.; Mato, F.; Cruz D’Howitt, M.; Sandoval, W.; Padilla Almeida, O.; Rentería, W.; Rodríguez Espinosa, F.; Salazar Martinez, R.; Cueva Girón, J. Contrasting results of potential tsunami hazards in Muisne, central coast of Ecuador. Sci. Tsunami Hazards 2017, 36, 13–40. [Google Scholar]
- Rodríguez Espinosa, F.; Toulkeridis, T.; Salazar Martinez, R.; Cueva Girón, J.; Taipe Quispe, A.; Bernaza Quiñonez, L.; Padilla Almeida, O.; Mato, F.; Cruz D’Howitt, M.; Parra, H. Economic evaluation of recovering a natural protection with concurrent relocation of the threatened public of tsunami hazards in central coastal Ecuador. Sci. Tsunami Hazards 2017, 36, 293–306. [Google Scholar]
- Chunga, K.; Toulkeridis, T.; Vera-Grunauer, X.; Gutierrez, M.; Cahuana, N.; Alvarez, A. A review of earthquakes and tsunami records and characterization of capable faults on the northwestern coast of Ecuador. Sci. Tsunami Hazards 2017, 36, 100–127. [Google Scholar]
- Toulkeridis, T.; Porras, L.; Tierra, A.; Toulkeridis-Estrella, K.; Cisneros, D.; Luna, M.; Carrión, J.L.; Herrera, M.; Murillo, A.; Perez-Salinas, J.C. Two independent real-time precursors of the 7.8 Mw earthquake in Ecuador based on radioactive and geodetic processes—Powerful tools for an early warning system. J. Geodyn. 2019, 126, 12–22. [Google Scholar] [CrossRef]
- Jiménez-Prado, P.; Rebolledo-Monsalve, Y.E. La cuenca del río Santiago-Cayapas, Provincia de Esmeraldas, Noroccidente del Ecuador: Importancia en las Comunidades Locales y Relación con las Actividades Industriales. In Capítulo 8.9 XII. Cuencas Pericontinentales de Colombia, Ecuador, Perú y Venezuela. Tipología, Biodiversidad, Servicios eco Sistémicos y Sostenibilidad de los ríos, Quebradas y Arroyos Costeros; Carlos, A., Lasso, Blanco-Libreros, J.P., Sánchez-Duarte, P., Eds.; Serie Editorial Recursos Hidrobiológicos y Pesqueros Continentales de Colombia; Instituto de Investigación de los Recursos Biológicos Alexander von Humboldt (IAvH): Bogotá, DC, Colombia, 2015; Available online: http://repository.humboldt.org.co/handle/20.500.11761/9279 (accessed on 22 April 2018).
- Jiménez Prado, P.; Aguirre, W.; Laaz, E.; Navarrete, R.; Nugra, F.; Rebolledo, E.; Zárate, E.; Torres, A.; Valdivieso, J. Guía de Peces para Aguas Continentales en la Vertiente Occidental del Ecuado; Pontificia Universidad Católica del Ecuador: Esmeraldas, Ecuador, 2015. [Google Scholar]
- Aguirre, W.E.; Alvarez-Mieles, G.; Anaguano-Yancha, F.; Morón, R.B.; Cucalyn, R.V.; Escobar-Camacho, D.; Jácome-Negrete, I.; Jiménez-Prado, P.; Laaz, E.; Miranda-Troya, K.; et al. Conservation threats and prospects for the freshwater fishes of Ecuador: A hotspot of Neotropical fish diversity. J. Fish Biol. 2021, 99, 1158–1189. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S.; Adler, D.; Bates, D.; Baud-Bovy, G.; Ellison, S.; Firth, D.; Friendly, M.; Gorjanc, G.; Graves, S.; et al. Package ‘car’. Vienna R Found. Stat. Comput. 2012, 16. [Google Scholar]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means. R package Version 1.4.8.; University of Iowa: Iowa City, IA, USA, 2020. [Google Scholar]
- Peres-Neto, P.R.; Jackson, D.A.; Somers, K.M. Giving meaningful interpretation to ordination axes: Assessing loading significance in principal component analysis. Ecology 2003, 84, 2347–2363. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H.; et al. Package ‘Vegan’. Community Ecology Package, Version 2; Rstudio: Integrated Development Environment for R: Boston, MA, USA, 2013; pp. 1–295. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 10 August 2022).
- Boulenger, G.A. Report on the collection of fishes made by Mr. J.E.S. Moore in lake Tanganyika during his expedition 1895–1896, with ab appendix by J.E.S. Moore. Trans. Zool. Soc. Lond. 1989, 15, 1–30. [Google Scholar]
- Wijkmark, N.; Kullander, S.O.; Barriga Salazar, R.E. Andinoacara blombergi, a new species from the río Esmeraldas basin in Ecuador and a review of A. rivulatus (Teleostei: Cichlidae). Ichthyol. Explor. Freshw. 2012, 23, 117. [Google Scholar]
- Boulenger, G.A. XLVII.—Descriptions of new fishes and reptiles discovered by Dr. F. Silvestri in South America. J. Nat. Hist. 1902, 9, 284–288. [Google Scholar] [CrossRef]
- Eydoux, J.F.T.; Souleyet, F.A. Poissons. In Voyage Autour du Monde Exécuté Pendant les Années 1836 et 1837 sur la Corvette La Bonite, Commandée par M. Vaillant. Zoologie, Paris; Arthus Bertrand: Paris, France, 1850; Volume 1, pp. 155–216. [Google Scholar]
- Boulenger, G.A. XII.—Description of a new genus of Gobioid fishes from the Andes of Ecuador. J. Nat. Hist. 1899, 4, 125–126. [Google Scholar] [CrossRef]
- Géry, J. Poissons Characoïdes des Guyanes: I. Généralités. II. Famille des Serrasalmidae; EJ Brill: Leiden, The Netherlands, 1972. [Google Scholar]
- Fowler, H.W. Colombian Zoological Survey. Part I: The Fresh-Water Fishes Obtained in 1945. Proc. Acad. Nat. Sci. Phila. 1945, 97, 93–135. [Google Scholar]
- Román-Valencia, C. Tres nuevas especies de Bryconamericus (Ostariophysi: Characidae) de Colombia y diagnóstico del género. Rev. Biol. Trop. 2000, 48, 449–464. [Google Scholar] [CrossRef]
- Regan, C.T. A Monograph of the Fishes of the Family Loricariidæ. Trans. Zool. Soc. Lond. 1904, 17, 191–350. [Google Scholar] [CrossRef]
- Meek, S.E.; Hildebrand, S.F. The Fishes of the Fresh Waters of Panama; Field Museum of Natural History: London, UK, 1916; Volume 10. [Google Scholar]
- Boulenger, G.A. LIX.—Descriptions of new fishes from the collection made by Mr. E. Degen in Abyssinia. J. Nat. Hist. 1902, 10, 421–439. [Google Scholar] [CrossRef][Green Version]
- Eigenmann, C.H.; Kettleborough, C.; Holmes, C.D.V.; Jackson, D.E.; McDonald, E.D.; Mathers, F.C.; Williams, K.P.; Adams, L.C.; Esarey, L.; Lockhart, O.C.; et al. Some Results from an Ichthyological Reconnaissance of Colombia, South America, Part II; Indiana University: Bloomington, IN, USA, 1913. [Google Scholar]
- Günther, A.C.L.G. Catalogue of the Fishes in the British Museum: Acanthopterygian Fishes: Squamipinnes, Cirrhitidœ, Triglidœ, Trachinidœ, Sciœnidœ, Polynemidœ, Sphyrœnidœ, Trichiuridœ, Scombridœ, Carangidœ, Xiphiidœ; The Trustees: London, UK, 1860; Volume 2. [Google Scholar]
- Bloch, M.E. Der Malabarische Hecht; Naturgeschichte des Auslandische Fische: Berlin, Germany, 1794; pp. 149–150. [Google Scholar]
- Meek, S.E.; Hildebrand, S.F. Descriptions of New Fishes from Panama; Field Museum of Natural History: Berlin, Germany, 1912. [Google Scholar]
- Günther, A.C.L.G. Sexual Differences Found in Bones of Some Recent and Fossil Species of Frogs and Fishes; Annals and Magazine of Natural History: London, UK, 1859. [Google Scholar]
- Boulenger, G.A. XLI.—Descriptions of new freshwater fishes discovered by Dr. WJ Ansorge in Portuguese Guinea. Ann. Mag. Nat. Hist. 1911, 7, 373–376. [Google Scholar] [CrossRef]
- Boulenger, G.A. LVIII.—Descriptions of two new Siluroid fishes from Brazil. J. Nat. Hist. 1898, 2, 477–478. [Google Scholar] [CrossRef]
- Eigenmann, C.H.; Henn, A.W. On New Species of Fishes from Colombia, Ecuador, and Brazil; Indiana University: Bloomington, IN, USA, 1914. [Google Scholar]
- Kner, R. Eine Uebersicht der Ichthyologischen Ausbeute des Herrn Professors Doktor Moritz Wagner in Central-Amerika; Sitzungsberichte der königl. bayer.; Akademie der Wissenschaften: Munich, Germany, 1863. [Google Scholar]
- Jordan, D.S. The Fishes of Sinaloa; Leland Stanford, Jr. University: Palo Alto, CA, USA, 1895; Volume 1. [Google Scholar]
- Regan, C.T. LXXIX. Description of a new fish of the genus Chætostomus from Venezuela. J. Nat. Hist. 1903, 11, 601–602. [Google Scholar] [CrossRef]
- Steindachner, F. Beiträge zur Kenntniss der Flussfische Südamerika’s; Gerold Kaiserlich-Königlichen Hof-und Staatsdruckerei: Vienna, Austria, 1879; Volume 1. [Google Scholar]
- Bleeker, P. Atlas Ichthyologique; Frederic Muller: Amsterdam, The Netherlands, 1862. [Google Scholar]
- Eigenmann, C.H.; Eigenmann, R.S. A Revision of the South American Nematognathi or Catfishes (No. 1); California Academy of Sciences: San Francisco, CA, USA, 1890. [Google Scholar]
- Hammond, D.S.; Rosales, J.; Ouboter, P.E. Gestión del impacto de la explotación minera a cielo abierto sobre el agua dulce en América Latina. Banco Interam. Desarro. 2013, 40. [Google Scholar]
- Programa de Reparacion Ambiental y Social (PRAS), Ministerio del Ambiente del Ecuador y Centro de Investigacion y Desarrollo, Pontificia Universidad Catolica del Ecuador Sede Esmeraldas (CID PUCESE), 2011b. Producto 2: Indicadores ecológicos, económicos y sociales vinculados a la mineria de oro en el norte de Esmeraldas. Esmeraldas, 9 de septiembre del 2011. 23.
- Toulkeridis, T.; Tamayo, E.; Simón-Baile, D.; Merizalde-Mora, M.J.; Reyes–Yunga, D.F.; Viera-Torres, M.; Heredia, M. Climate Change according to Ecuadorian academics–Perceptions versus facts. Granja. Rev. Cienc. Vida 2020, 31, 21–46. [Google Scholar] [CrossRef]
- Konkel, L. A Safer Gold Rush? Curbing Mercury Pollution in Artisanal and Small-Scale Gold Mining. Environ. Health Perspect. 2019, 127, 112001. [Google Scholar] [CrossRef]
- Capparelli, M.V.; Moulatlet, G.M.; de Souza Abessa, D.M.; Lucas-Solis, O.; Rosero, B.; Galarza, E.; Tuba, D.; Carpintero, N.; Ochoa-Herrera, V.; Cipriani-Avila, I. An integrative approach to identify the impacts of multiple metal contamination sources on the Eastern Andean foothills of the Ecuadorian Amazonia. Sci. Total Environ. 2020, 709, 136088. [Google Scholar] [CrossRef]
- República de Ecuador, 2015. Acuerdo A97. Ministerio del Ambiente de la República del Ecuador. Registro Oficial Año III-N°387- Quito, Miercoles 4 de Noviembre de. 2015. Available online: https://www.gob.ec/regulaciones/acuerdo-ministerial-097-anexos-normativa-reforma-libro-vi-texto-unificado-legislacion-secundaria-ministerio-ambiente (accessed on 10 August 2022).
- Figueiredo-Fernandes, A.; Ferreira-Cardoso, J.V.; Garcia-Santos, S.; Monteiro, S.M.; Carrola, J.; Matos, P.; Fontainhas-Fernandes, A. Histopathological changes in liver and gill epithelium of Nile tilapia, Oreochromis niloticus, exposed to waterborne copper. Pesqui. Vet. Bras. 2007, 27, 103–109. [Google Scholar] [CrossRef]
- Coban, M.; Eroglu, M.; Canpolat, O.; Calta, M.; Sen, D. Effect of chromium on scale morphology in scaly carp (Cyprinus carpio L.). J. Anim. Plant Sci. 2013, 23, 1455–1459. [Google Scholar]
- Badr, A.M.; Mahana, N.A.; Eissa, A. Assessment of heavy metal levels in water and their toxicity in some tissues of Nile Tilapia (Oreochromis niloticus) in River Nile basin at Greater Cairo, Egypt. Glob. Vet. 2014, 13, 432–443. [Google Scholar]
- Dane, H.; Sisman, T. A morpho-histopathological study in the digestive tract of three fish species influenced with heavy metal pollution. Chemosphere 2020, 242, 125212. [Google Scholar] [CrossRef]
- Sfakianblakis, D.; Renieri, E.; Kentouri, M.; Tsatsakis, A. Effect of heavy metals on fish larvae deformities: A review. Environ. Res. 2015, 137, 246–255. [Google Scholar] [CrossRef]
- Kemp, P.; Sear, D.; Collins, A.; Naden, P.; Jones, I. The impacts of fine sediment on riverine fish. Hydrol. Process. 2011, 25, 1800–1821. [Google Scholar] [CrossRef]
- Cavanagh, J.; Harding, J. Effects of Suspended Sediment on Freshwater Fish; Landcare Research: South Beach, New Zealand, 2014; p. 29. [Google Scholar]
- Ramezani, J.; Rennebeck, L.; Closs, G.P.; Matthaei, C.D. Effects of fine sediment addition and removal on stream invertebrates and fish: A reach-scale experiment. Freshw. Biol. 2014, 59, 2584–2604. [Google Scholar] [CrossRef]
- Affandi, F.A.; Ishak, M.Y. Impacts of suspended sediment and metal pollution from mining activities on riverine fish population: A review. Environ. Sci. Pollut. Res. 2019, 26, 16939–16951. [Google Scholar] [CrossRef] [PubMed]
- Torremorell, A.; Hegoburu, C.; Brandimarte, A.L.; Rodrigues, E.H.C.; Pompko, M.; da Silva, S.C.; Moschini-Carlos, V.; Caputo, L.; Fierro, P.; Mojica, J.I.; et al. Current and future threats for ecological quality management of South American freshwater ecosystems. Inland Waters 2021, 11, 125–140. [Google Scholar] [CrossRef]
- Storey, A.W.; Yarrao, M.; Tenakanai, C.; Figa, B.; Lynas, J. Chapter 12 Use of Changes in Fish Assemblages in the Fly River System, Papua New Guinea, to Assess Effects of the Ok Tedi Copper Mine. Dev. Earth Environ. Sci. 2008, 9, 427–462. [Google Scholar]
- Allard, L.; Popée, M.; Vigouroux, R.; Brosse, S. Effect of reduced impact logging and small-scale mining disturbances on Neotropical stream fish assemblages. Aquat. Sci. 2015, 78, 315–325. [Google Scholar] [CrossRef]
- Silva, N.C.D.S.; Soares, B.E.; Teresa, F.B.; Caramaschi, E.P.; Albrecht, M.P. Fish functional diversity is less impacted by mining than fish taxonomic richness in an Amazonian stream system. Aquat. Ecol. 2022, 56, 815–827. [Google Scholar] [CrossRef]
- Brosse, S.; Grenouillet, G.; Gevrey, M.; Khazraie, K.; Tudesque, L. Small-scale gold mining erodes fish assemblage structure in small neotropical streams. Biodivers. Conserv. 2011, 20, 1013–1026. [Google Scholar] [CrossRef]
- Dos Dantos, J.A.; Barbosa Silva, C.; Soares de Santana, H.; Cano-Barbacil, C.; Agostinho, A.A.; Normando, T.F.; Cabeza, J.R.; Roland, F.; Garcia-Berthou, E. Assessing the short-term response of fish assemblages to damming of a Amazonias river. J. Environ. Manag. 2022, 307, 114571. [Google Scholar] [CrossRef]
- Roth, T.R.; Westhoff, M.C.; Huwald, H.; Huff, J.A.; Rubin, J.F.; Barrenetxea, G.; Vetterli, M.; Parriaux, A.; Selker, J.S.; Parlange, M.B. Stream temperature response to three riparian vegetation scenarios by use of a distributed temperature validated model. Environ. Sci. Technol. 2010, 44, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Bowler, D.E.; Mant, R.; Orr, H.; Hannah, D.M.; Pullin, A.S. What are the effects of wooded riparian zones on stream temperature? Environ. Evid. 2012, 1, 3. [Google Scholar] [CrossRef]
- Regan, C.T. XLII. A Revision of the Fishes of the South-American Cichlid Genera Acara, Nannacara, Acaropsis, and Astronotus. J. Nat. Hist. 1905, 15, 329–347. [Google Scholar] [CrossRef]
- Valenciennes, A. Des Chironectes (Chironectes, Cuv., Antennarius, Comm.). Hist. Nat. Poisson. Levrault Paris Strasburg 1837, 11, 389–437. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).