Stimulating Nitrate Removal with Significant Conversion to Nitrogen Gas Using Biochar-Based Nanoscale Zerovalent Iron Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
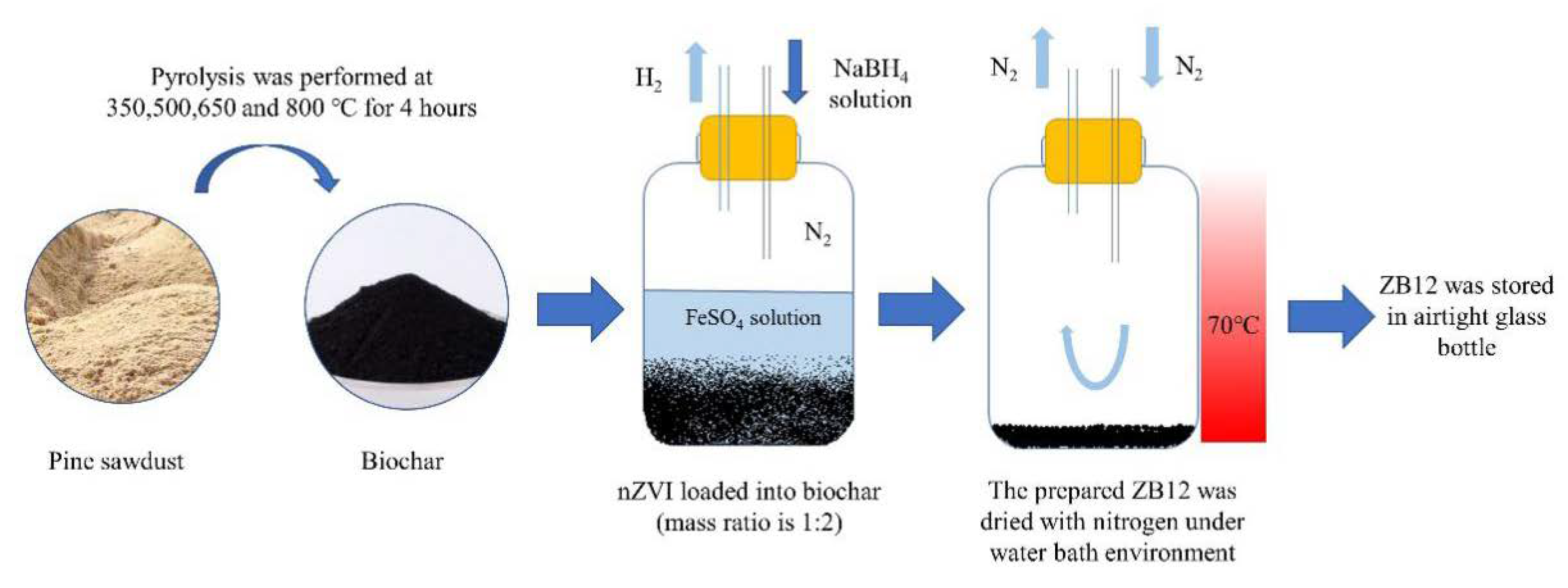
2.2. Synthesis of ZB12
2.3. Characterization
2.4. Experiments for Chemical Reduction of Nitrate by ZB12
2.5. Analysis Method
2.5.1. Calculation Method
2.5.2. Kinetic Studies
3. Results
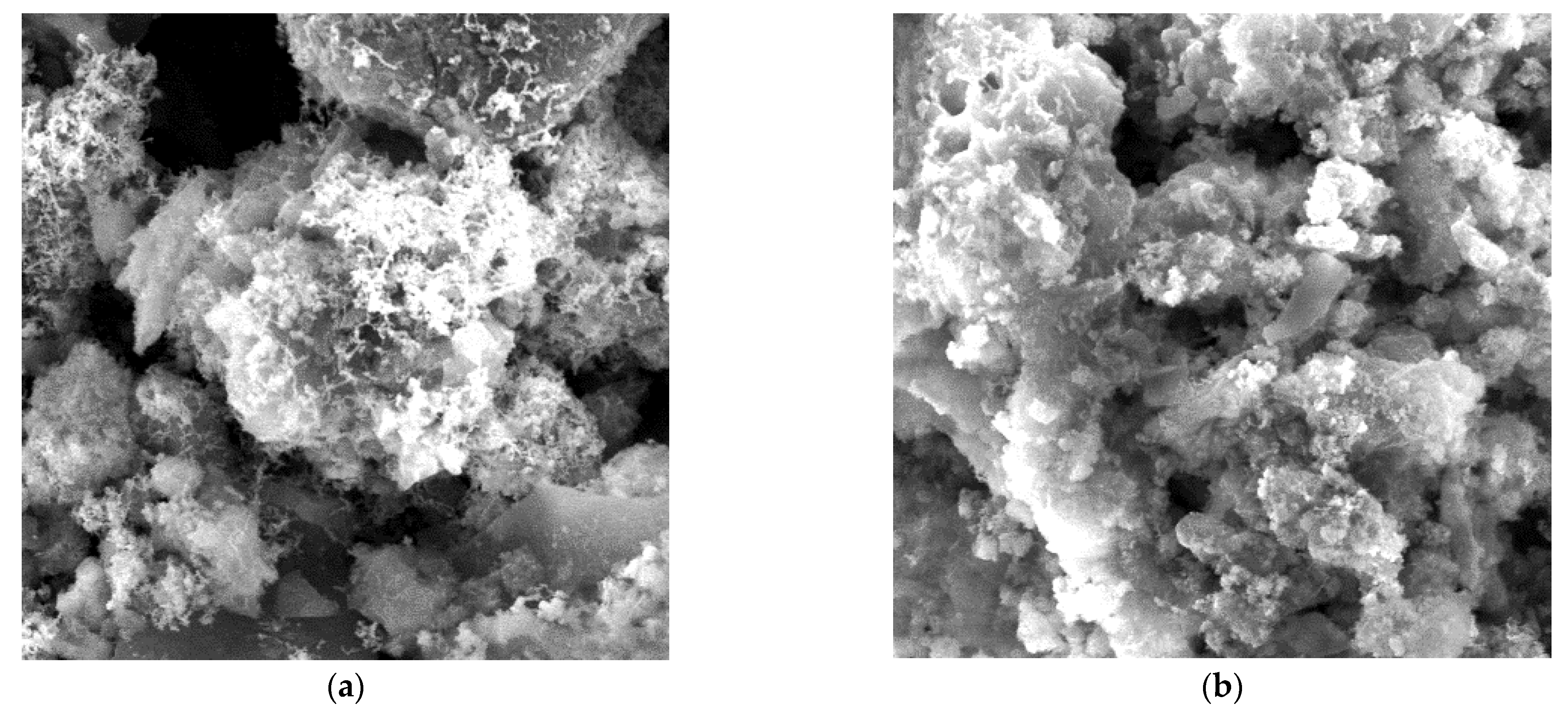
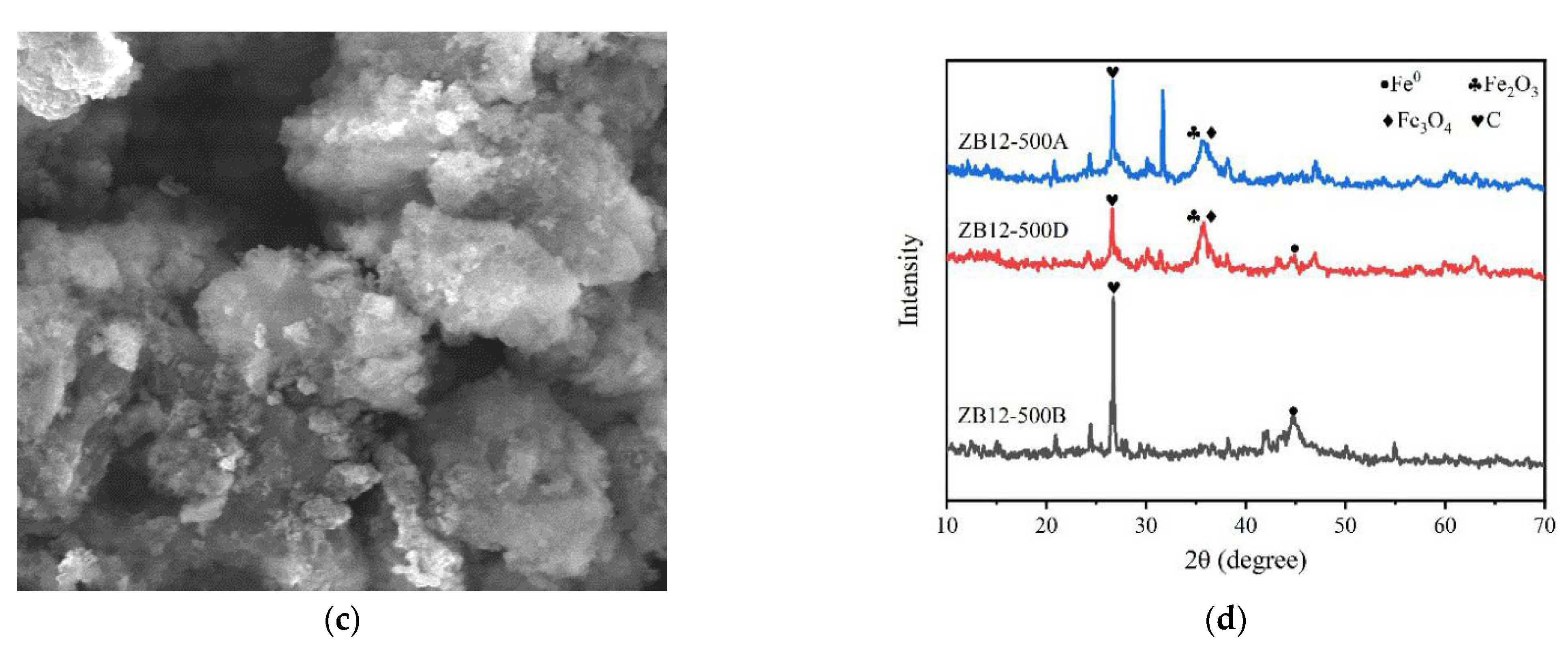
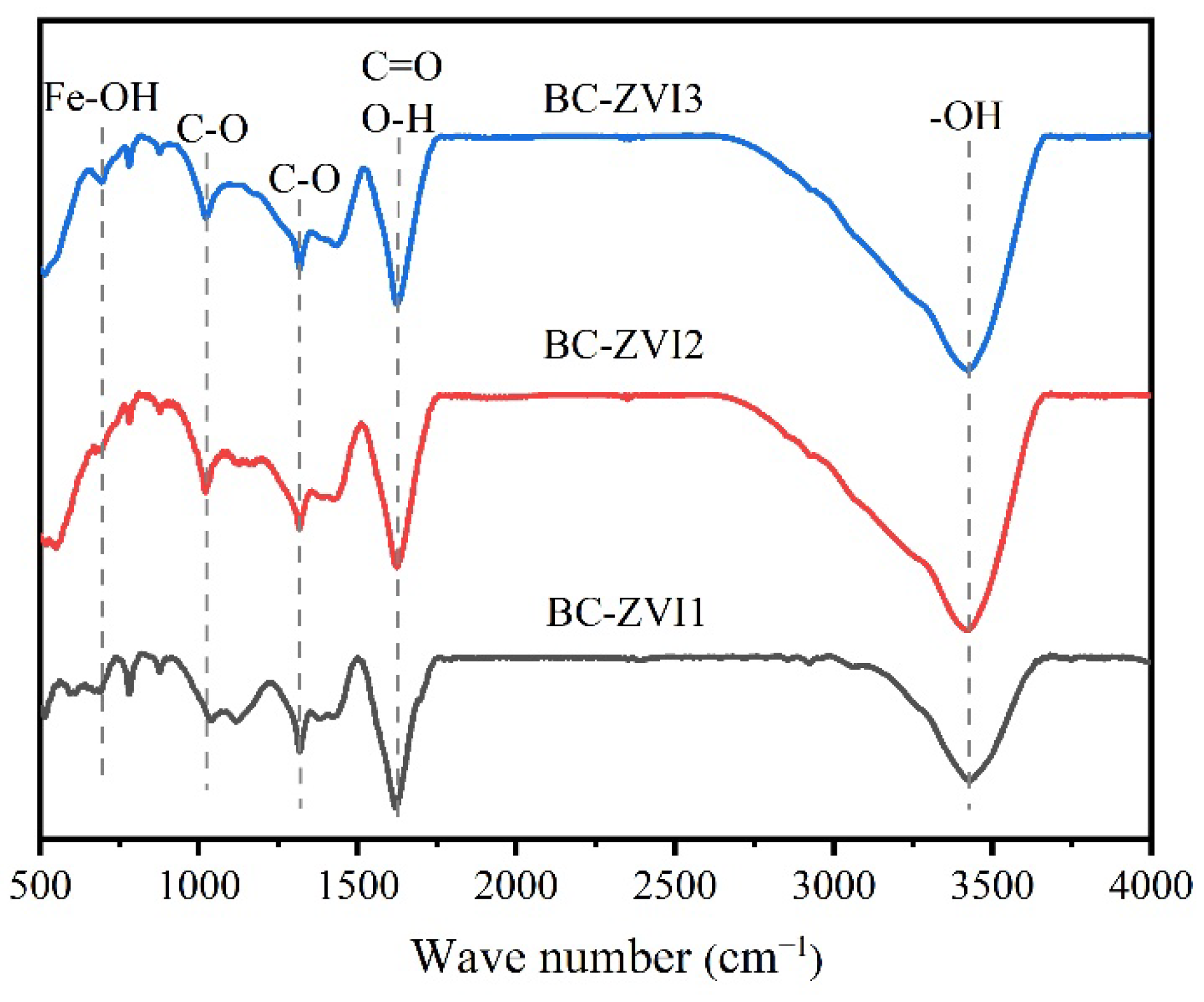
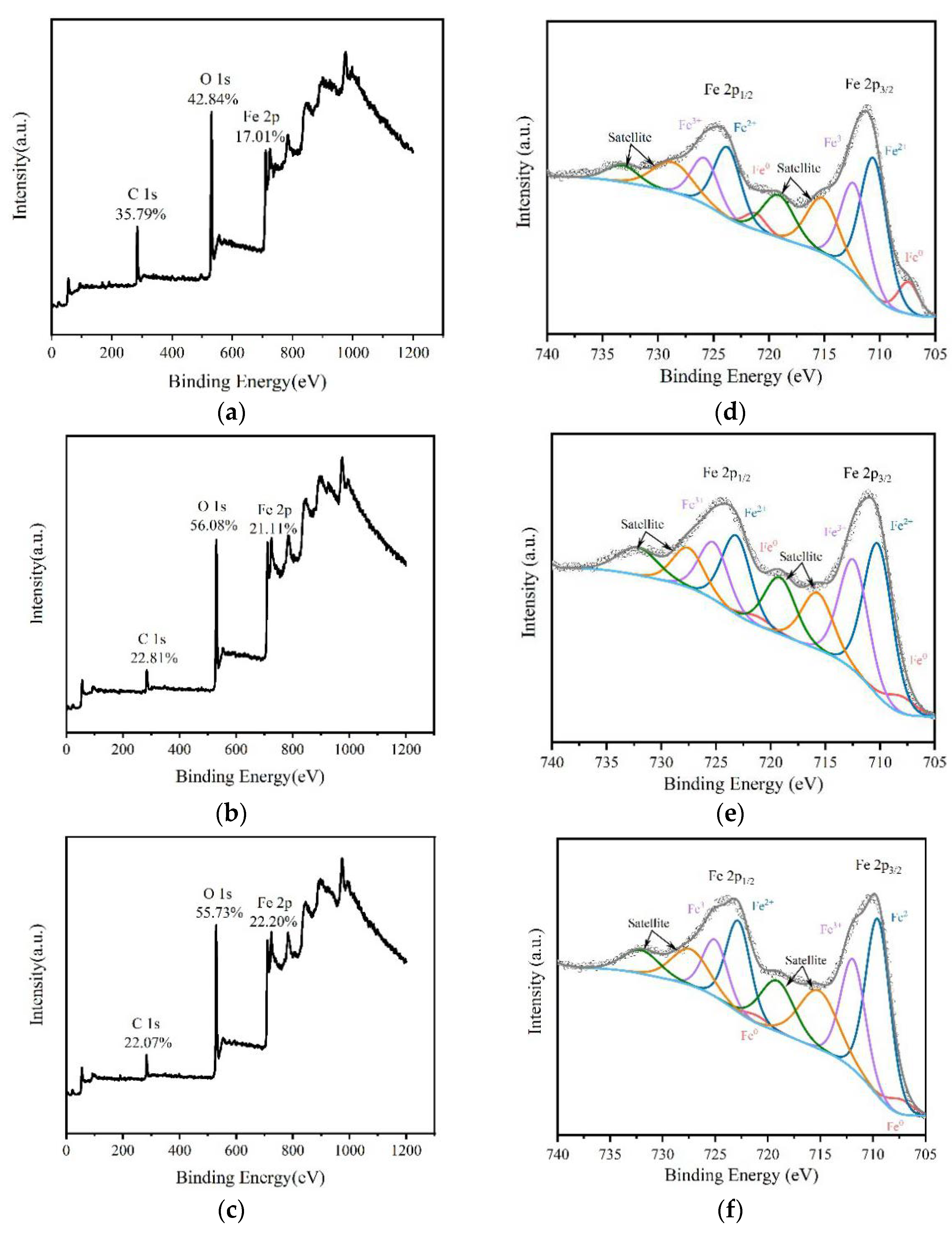
3.1. Characterization of ZB12-500
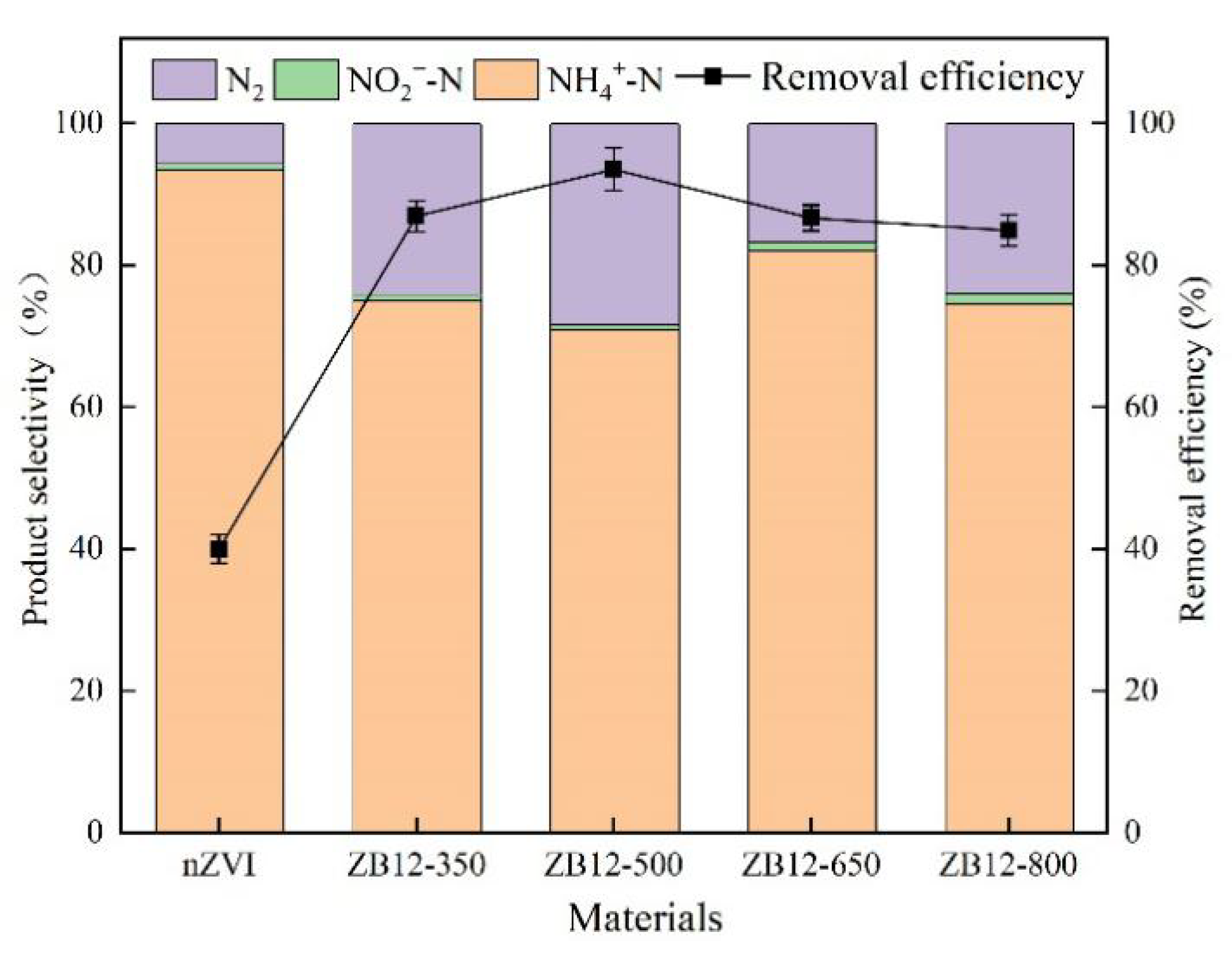
3.2. Effect of the Pyrolysis Temperature of ZB12-500 on Biochar
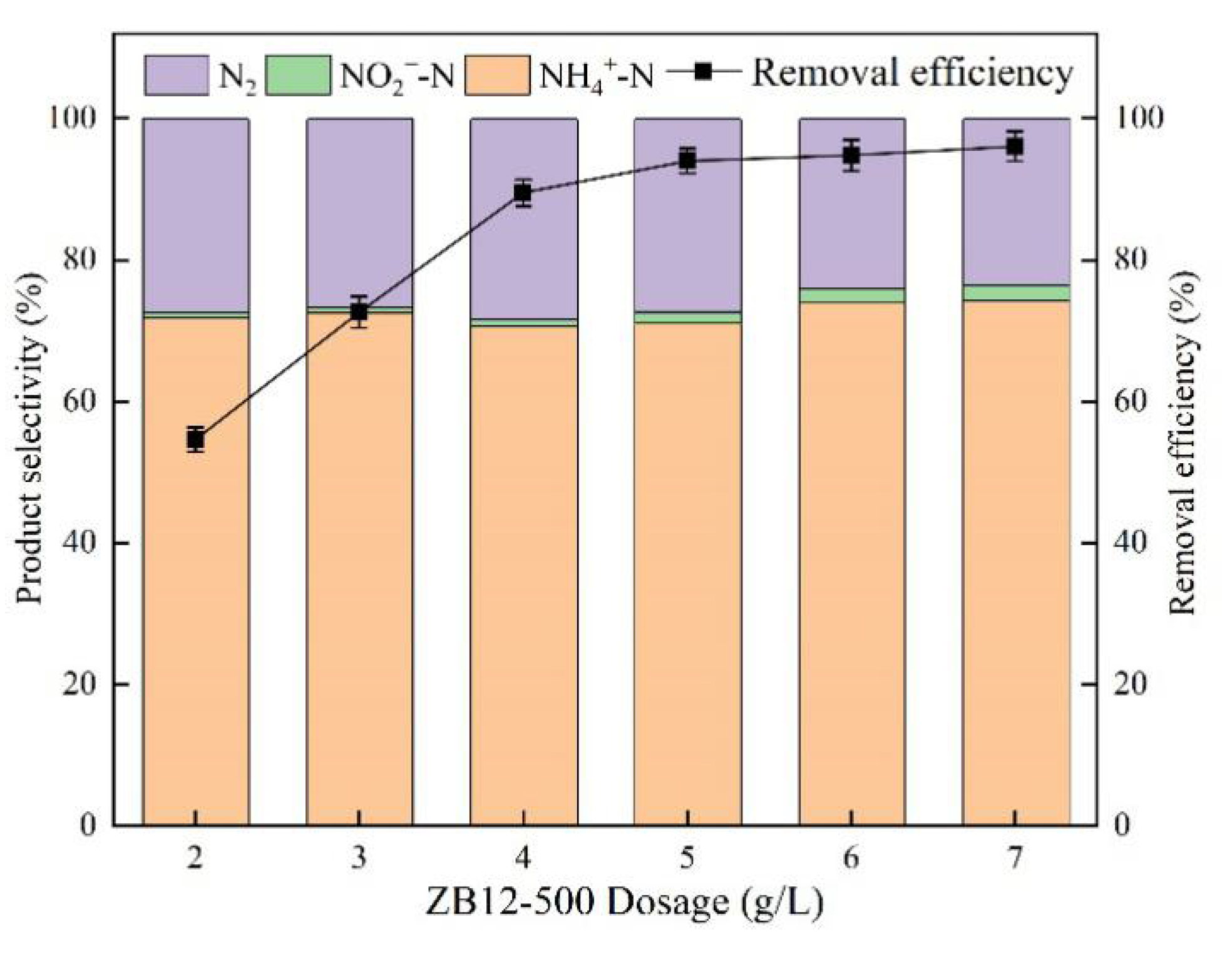
3.3. Effect of the Dosage of ZB12-500
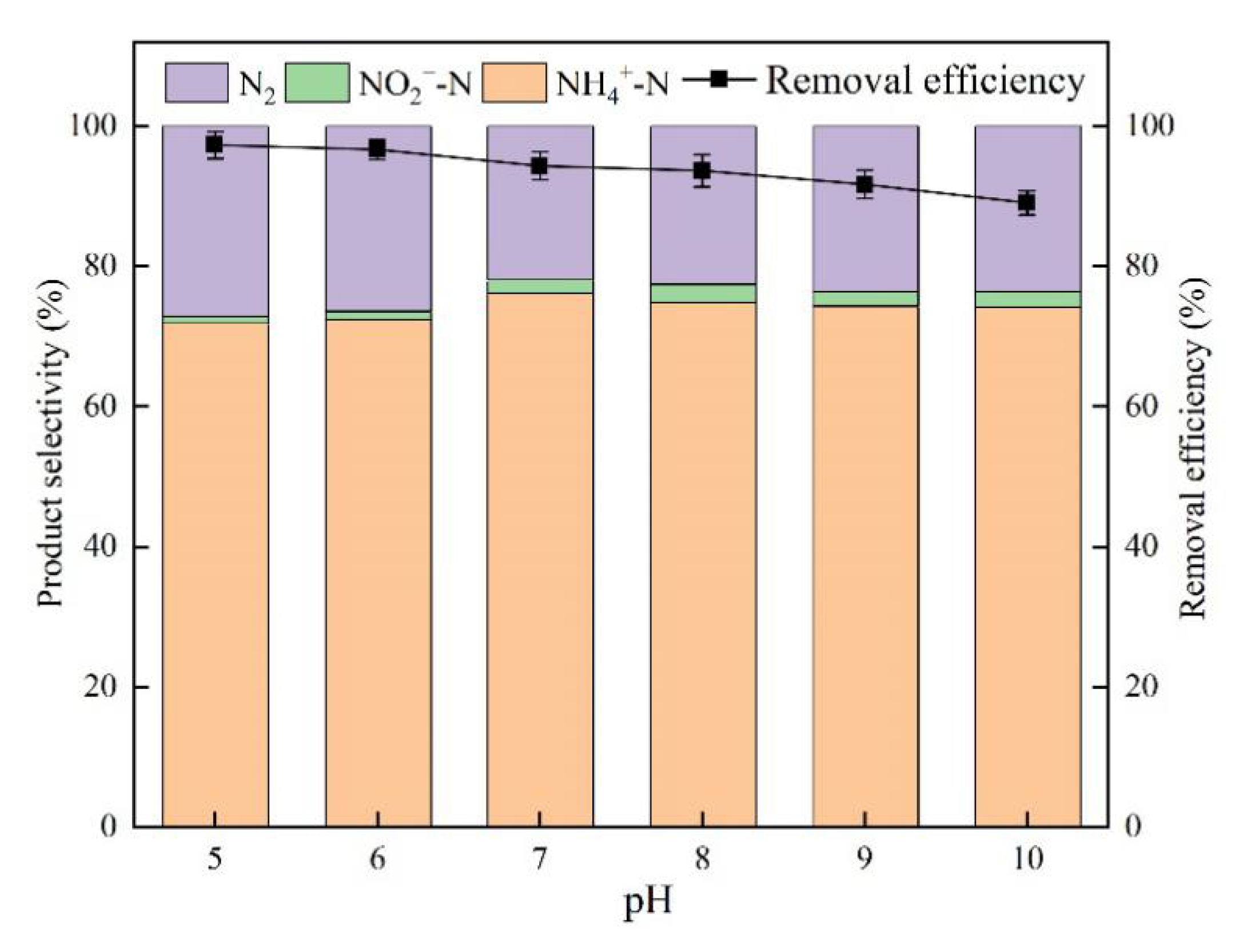
3.4. Effect of pH
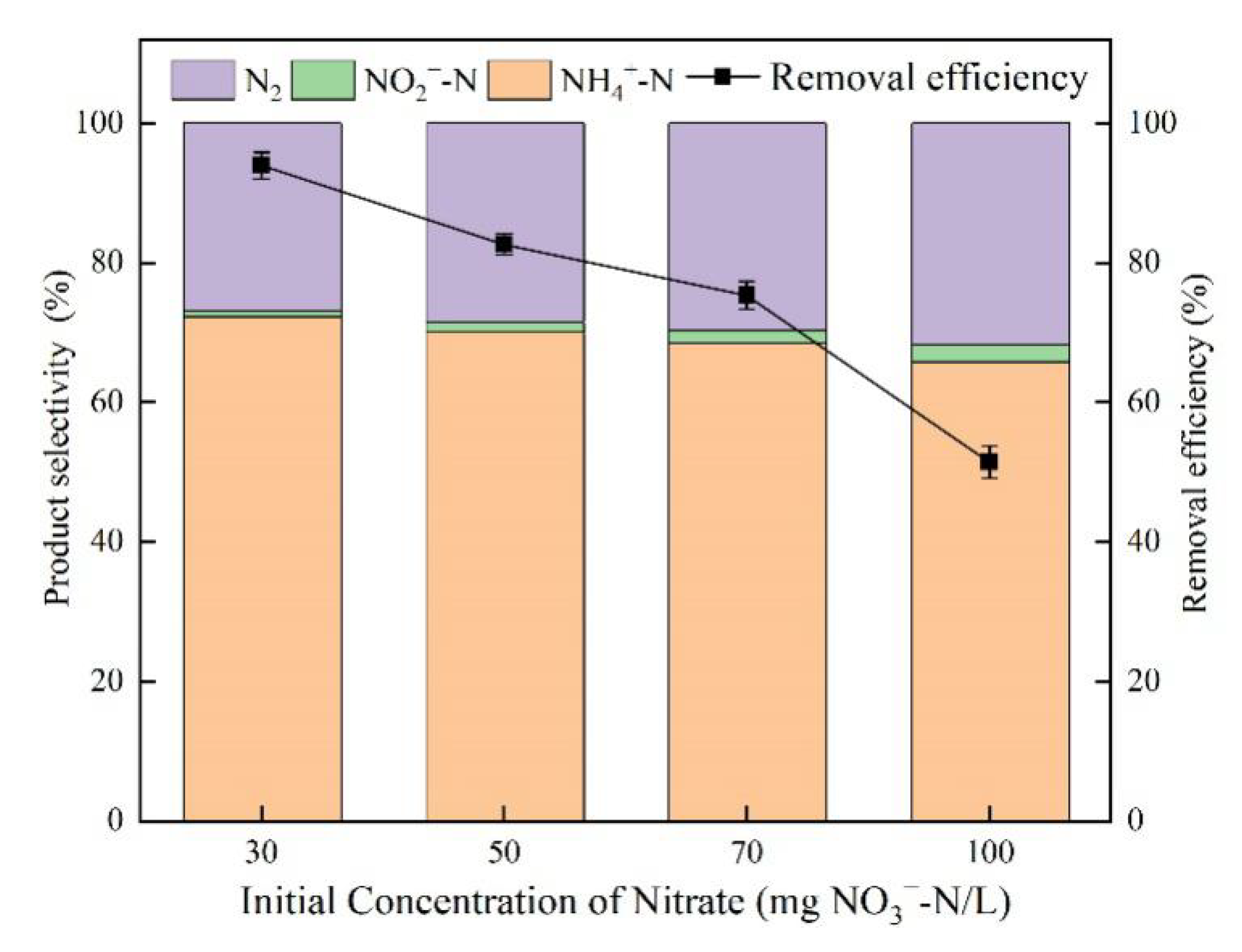
3.5. Effect of Initial Nitrate Concentration
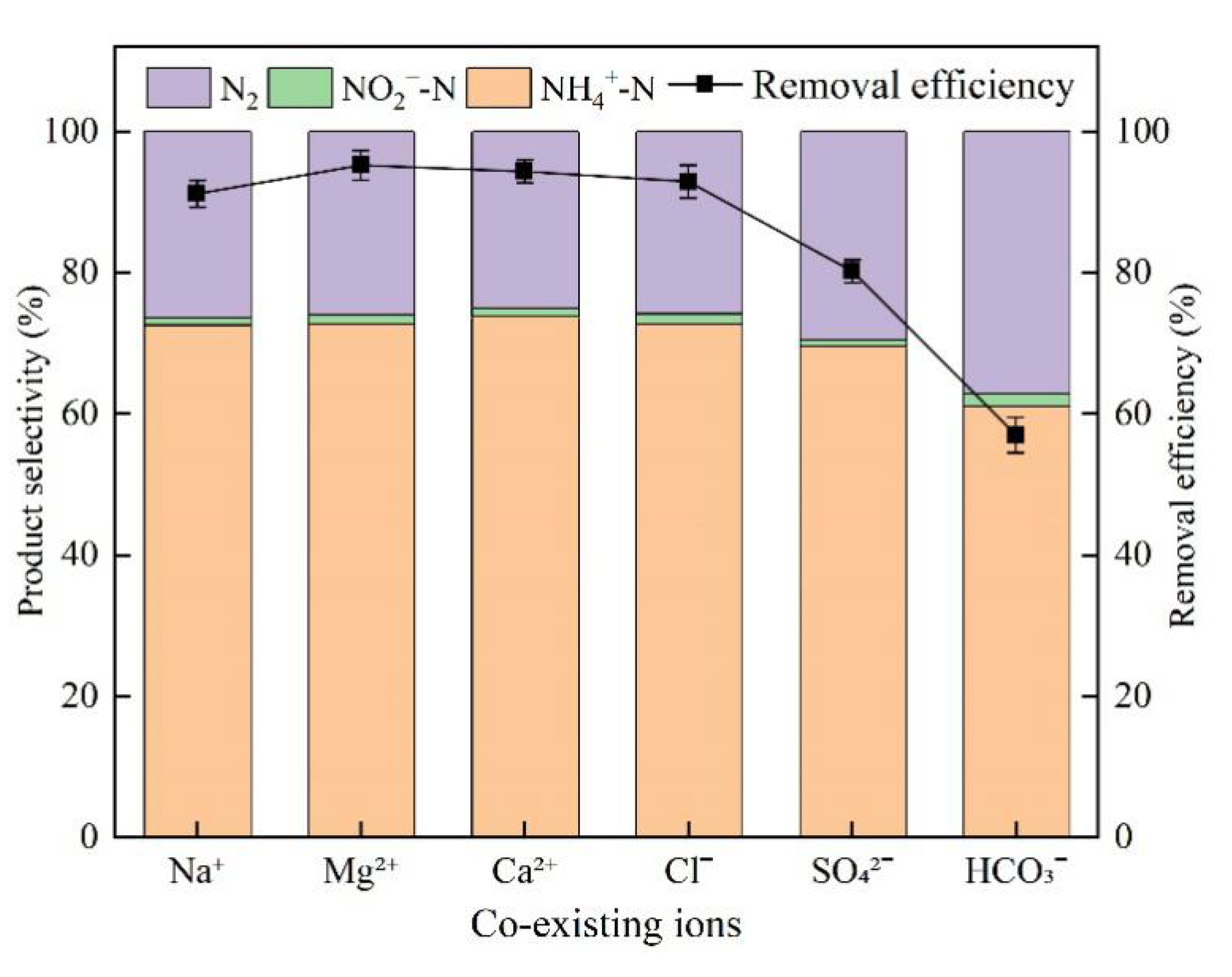
3.6. Effect of Co-Existing Ions
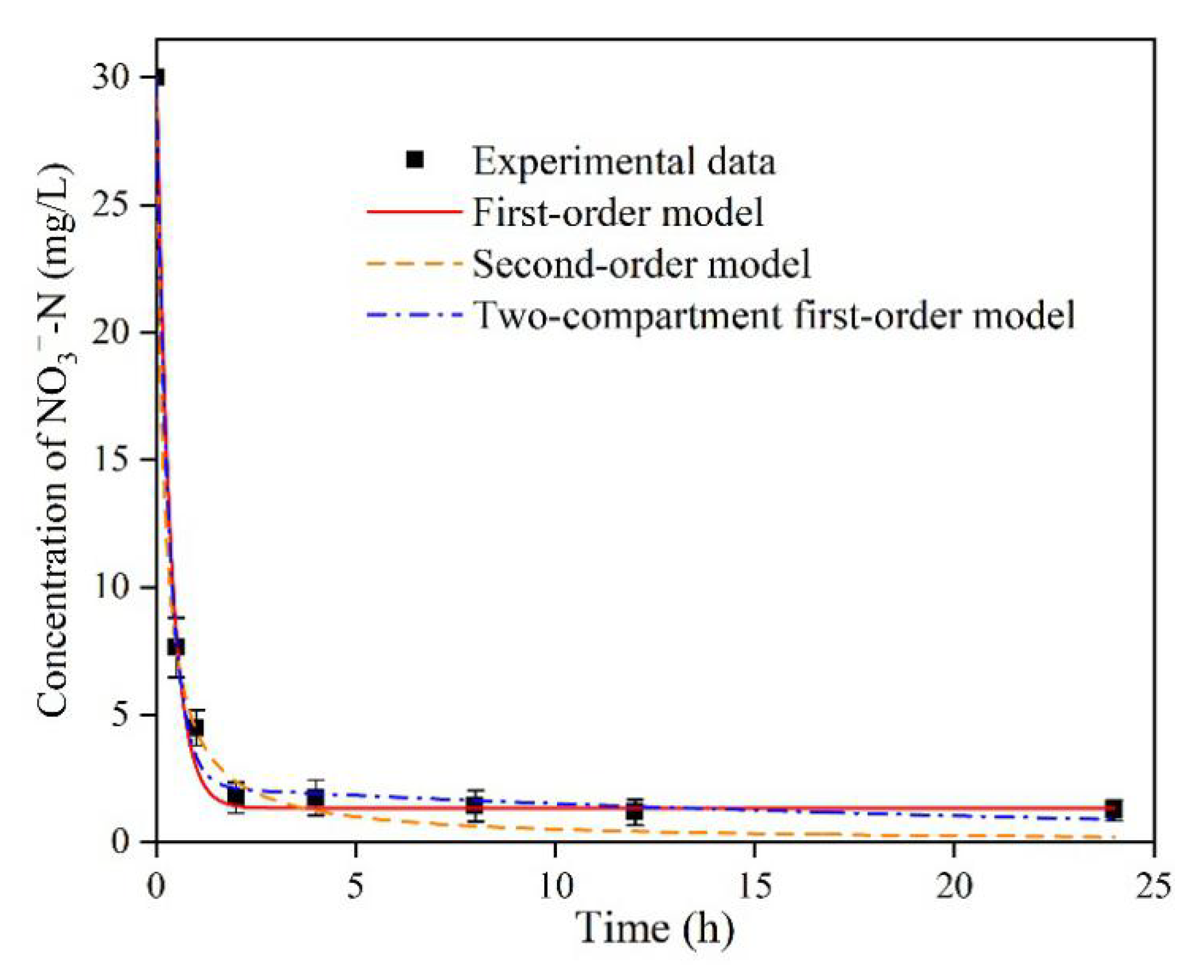
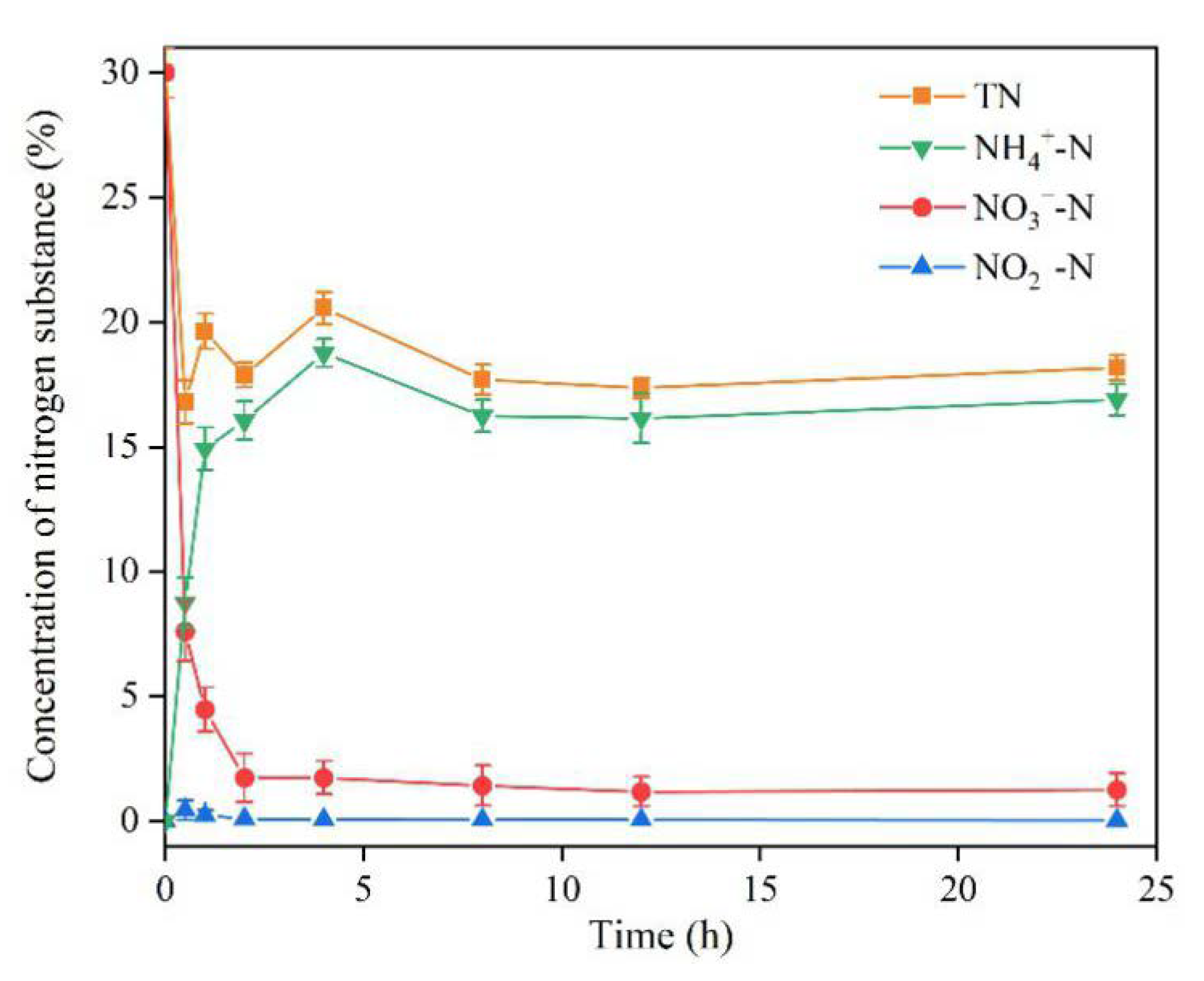
3.7. Kinetics
4. Discussion
4.1. Effect of the Pyrolysis Temperature of ZB12-500 on Biochar
4.2. Effect of the Dosage of ZB12-500
4.3. Effect of pH
4.4. Effect of Initial Nitrate Concentration
4.5. Effect of Co-Existing Ions
4.6. Kinetics
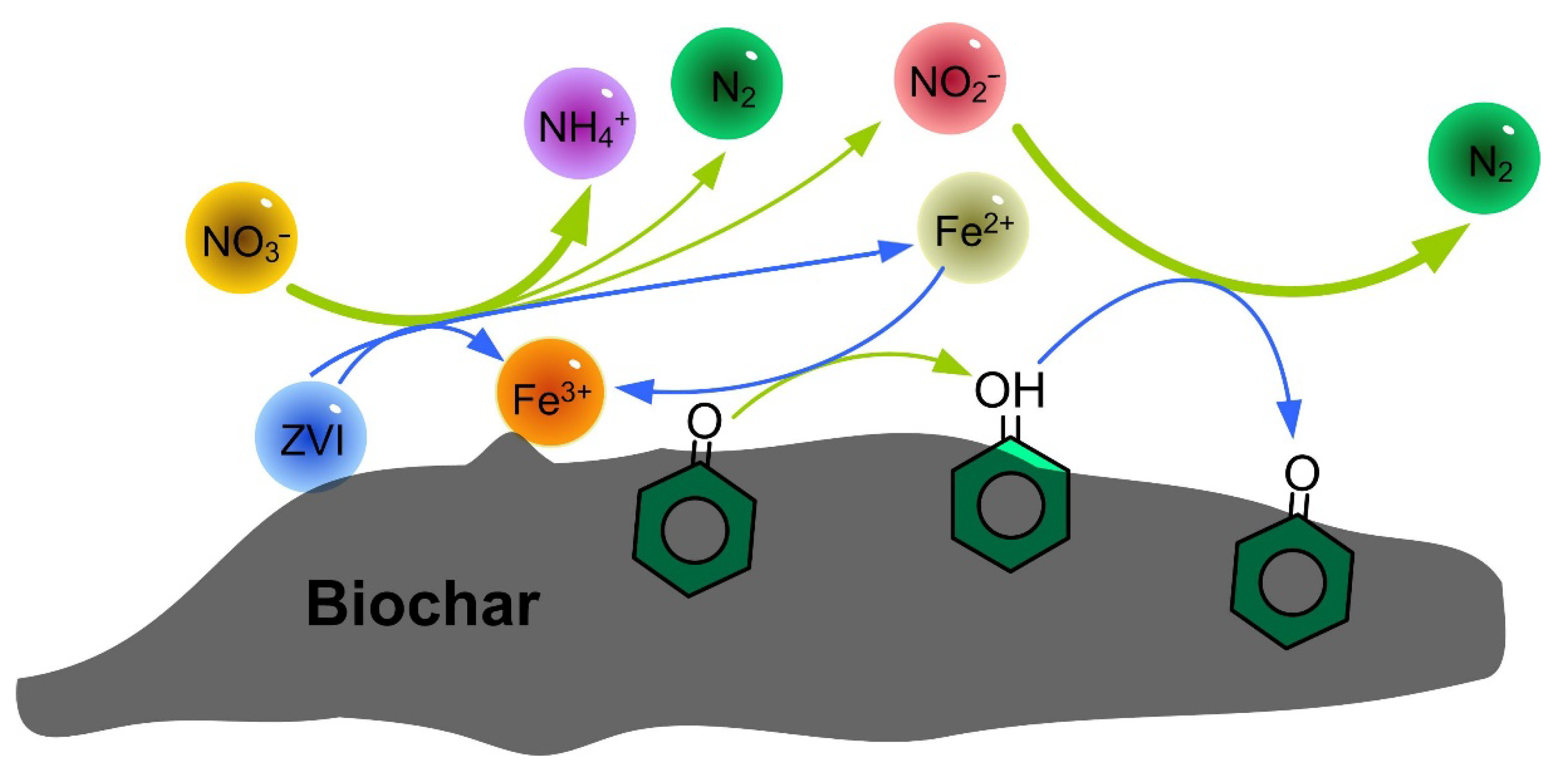
4.7. Nitrate Reduction Mechanism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhai, Y.Z.; Lei, Y.; Wu, J.; Teng, Y.; Wang, J.; Zhao, X.; Pan, X. Does the groundwater nitrate pollution in China pose a risk to human health? A critical review of published data. Environ. Sci. Pollut. Res. 2017, 24, 3640–3653. [Google Scholar] [CrossRef] [PubMed]
- Allaire, M.; Wu, H.W.; Lall, U. National trends in drinking water quality violations. Proc. Natl. Acad. Sci. USA 2018, 115, 2078–2083. [Google Scholar] [CrossRef] [PubMed]
- Han, D.M.; Currell, M.J.; Cao, G.L. Deep challenges for China’s war on water pollution. Environ. Pollut. 2016, 218, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Zghibi, A.; Tarhouni, J.; Zouhri, L. Assessment of seawater intrusion and nitrate contamination on the groundwater quality in the Korba coastal plain of Cap-Bon (North-east of Tunisia). J. Afr. Earth Sci. 2013, 87, 1–12. [Google Scholar] [CrossRef]
- Kuang, P.J.; Natsui, K.; Einaga, Y. Comparison of performance between boron-doped diamond and copper electrodes for selective nitrogen gas formation by the electrochemical reduction of nitrate. Chemosphere 2018, 210, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.J.; Li, G.; Lei, Z.C.; Zhu, T.H.; Xue, Y.Z.; Wei, C.H.; Feng, C.H. Highly active and durable carbon electrocatalyst for nitrate reduction reaction. Water Res. 2019, 161, 126–135. [Google Scholar] [CrossRef]
- Fajardo, A.S.; Westerhoff, P.; Sanchez-Sanchez, C.M.; Garcia-Segura, S. Earth-abundant elements a sustainable solution for electrocatalytic reduction of nitrate. Appl. Catal. B-Environ. 2021, 281, 119465. [Google Scholar] [CrossRef]
- Martinez, J.; Ortiz, A.; Ortiz, I. State-of-the-art and perspectives of the catalytic and electrocatalytic reduction of aqueous nitrates. Appl. Catal. B-Environ. 2017, 207, 42–59. [Google Scholar] [CrossRef]
- Chen, J.; Wu, H.; Qian, H.; Gao, Y.Y. Assessing Nitrate and Fluoride Contaminants in Drinking Water and Their Health Risk of Rural Residents Living in a Semiarid Region of Northwest China. Expo. Health 2017, 9, 183–195. [Google Scholar] [CrossRef]
- Vilardi, G.; Di Palma, L. Kinetic Study of Nitrate Removal from Aqueous Solutions Using Copper-Coated Iron Nanoparticles. Bull. Environ. Contam. Toxicol. 2017, 98, 359–365. [Google Scholar] [CrossRef]
- Peng, L.; Liu, Y.W.; Gao, S.H.; Chen, X.M.; Xin, P.; Dai, X.H.; Ni, B.J. Evaluation on the Nanoscale Zero Valent Iron Based Microbial Denitrification for Nitrate Removal from Groundwater. Sci. Rep. 2015, 5, 12331. [Google Scholar] [CrossRef] [PubMed]
- Cecconet, D.; Devecseri, M.; Callegari, A.; Capodaglio, A.G. Effects of process operating conditions on the autotrophic denitrification of nitrate-contaminated groundwater using bioelectrochemical systems. Sci. Total Environ. 2018, 613, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, C.; Belgiorno, V.; Meric, S. Overview of in-situ applicable nitrate removal processes. Desalination 2007, 204, 46–62. [Google Scholar] [CrossRef]
- Guan, X.H.; Sun, Y.K.; Qin, H.J.; Li, J.X.; Lo, I.M.C.; He, D.; Dong, H.R. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef] [PubMed]
- Sparis, D.; Mystrioti, C.; Xenidis, A.; Papassiopi, N. Reduction of nitrate by copper-coated ZVI nanoparticles. Desalination Water Treat. 2013, 51, 2926–2933. [Google Scholar] [CrossRef]
- Lubphoo, Y.; Chyan, J.M.; Grisdanurak, N.; Liao, C.H. Nitrogen gas selectivity enhancement on nitrate denitrification using nanoscale zero-valent iron supported palladium/copper catalysts. J. Taiwan Inst. Chem. Eng. 2015, 57, 143–153. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Zhou, H.G.; Chen, C.; Wei, S.Q.; Zhang, W.M. The Enhancement of Nitrate Reduction by Supported Pd-Fe Nanoscale Particle. Sci. Adv. Mater. 2015, 7, 1734–1740. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, J.H.; Li, J.; Lai, B. Strengthening the reactivity of Fe-0/(Fe/Cu) by premagnetization: Implications for nitrate reduction rate and selectivity. Chem. Eng. J. 2017, 330, 813–822. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.L. Reduction of nitrate by zero valent iron (ZVI)-based materials: A review. Sci. Total Environ. 2019, 671, 388–403. [Google Scholar] [CrossRef]
- Zeng, Y.B.; Walker, H.; Zhu, Q.Z. Reduction of nitrate by NaY zeolite supported Fe, Cu/Fe and Mn/Fe nanoparticles. J. Hazard. Mater. 2017, 324, 605–616. [Google Scholar] [CrossRef]
- Lee, C.S.; Gong, J.; Huong, C.V.; Oh, D.S.; Chang, Y.S. Macroporous alginate substrate-bound growth of Fe-0 nanoparticles with high redox activities for nitrate removal from aqueous solutions. Chem. Eng. J. 2016, 298, 206–213. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Zhang, S.J.; Pan, B.C.; Wang, W.F.; Wang, X.S.; Lv, L.; Zhang, W.M.; Zhang, Q.X. A fabrication strategy for nanosized zero valent iron (nZVI)-polymeric anion exchanger composites with tunable structure for nitrate reduction. J. Hazard. Mater. 2012, 233, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jia, Y.; Wu, X.; Wang, H. Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J. Hazard. Mater. 2009, 172, 1591–1596. [Google Scholar] [CrossRef]
- Li, P.J.; Lin, K.R.; Fang, Z.Q.; Wang, K.M. Enhanced nitrate removal by novel bimetallic Fe/Ni nanoparticles supported on biochar. J. Clean. Prod. 2017, 151, 21–33. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Chen, J.J.; Zhang, M.; Inyang, M.; Li, Y.C.; Alva, A.; Yang, L.Y. Engineered carbon (biochar) prepared by direct pyrolysis of Mg-accumulated tomato tissues: Characterization and phosphate removal potential. Bioresour. Technol. 2013, 138, 8–13. [Google Scholar] [CrossRef]
- Namasivayam, C.; Sangeetha, D. Removal and recovery of nitrate from water by ZnCl2 activated carbon from coconut coir pith, an agricultural solid waste. Indian J. Chem. Technol. 2005, 12, 513–521. [Google Scholar]
- Shirvanimoghaddam, K.; Czech, B.; Tyszczuk-Rotko, K.; Kończak, M.; Fakhrhoseini, S.M.; Yadav, R.; Naebe, M. Sustainable synthesis of rose flower-like magnetic biochar from tea waste for environmental applications. J. Adv. Res. 2021, 34, 13–27. [Google Scholar] [CrossRef]
- Wei, A.L.; Ma, J.; Chen, J.J.; Zhang, Y.; Song, J.X.; Yu, X.Y. Enhanced nitrate removal and high selectivity towards dinitrogen for groundwater remediation using biochar-supported nano zero-valent iron. Chem. Eng. J. 2018, 353, 595–605. [Google Scholar] [CrossRef]
- Oh, S.Y.; Seo, Y.D.; Kim, B.; Kim, I.Y.; Cha, D.K. Microbial reduction of nitrate in the presence of zero-valent iron and biochar. Bioresour. Technol. 2016, 200, 891–896. [Google Scholar] [CrossRef]
- Gao, J.; Yang, L.Z.; Liu, Y.Y.; Shao, F.L.; Liao, Q.J.H.; Shang, J.G. Scavenging of Cr(VI) from aqueous solutions by sulfide-modified nanoscale zero-valent iron supported by biochar. J. Taiwan Inst. Chem. Eng. 2018, 91, 449–456. [Google Scholar] [CrossRef]
- Yang, G.C.C.; Lee, H.L. Chemical reduction of nitrate by nanosized iron: Kinetics and pathways. Water Res. 2005, 39, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Zhang, T.C. Effects of low pH on nitrate reduction by iron powder. Water Res. 2004, 38, 2631–2642. [Google Scholar] [CrossRef] [PubMed]
- Alowitz, M.J.; Scherer, M.M. Kinetics of nitrate, nitrite, and Cr(VI) reduction by iron metal. Environ. Sci. Technol. 2002, 36, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Zhang, T.C. Kinetics of nitrate reduction by iron at near neutral pH. J. Environ. Eng. 2002, 128, 604–611. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Mitch, W.A.; Xu, W.Q. Activity and Reactivity of Pyrogenic Carbonaceous Matter toward Organic Compounds. Environ. Sci. Technol. 2017, 51, 8893–8908. [Google Scholar] [CrossRef]
- Nayyar, D.; Shaikh, M.A.N.; Nawaz, T. Remediation of Emerging Contaminants by Naturally Derived Adsorbents. In New Trends in Emerging Environmental Contaminants; Singh, S.P., Agarwal, A.K., Gupta, T., Maliyekkal, S.M., Eds.; Springer: Singapore, 2022; pp. 225–260. [Google Scholar]
- Jiang, S.F.; Ling, L.L.; Chen, W.J.; Liu, W.J.; Li, D.C.; Jiang, H. High efficient removal of bisphenol A in a peroxymonosulfate/iron functionalized biochar system: Mechanistic elucidation and quantification of the contributors. Chem. Eng. J. 2019, 359, 572–583. [Google Scholar] [CrossRef]
- Oh, S.Y.; Seo, Y.D.; Ryu, K.S. Reductive removal of 2,4-dinitrotoluene and 2,4-dichlorophenol with zero-valent iron-included biochar. Bioresour. Technol. 2016, 216, 1014–1021. [Google Scholar] [CrossRef]
- Fakhrhoseini, S.M.; Czech, B.; Shirvanimoghaddam, K.; Naebe, M. Ultrafast microwave assisted development of magnetic carbon microtube from cotton waste for wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125449. [Google Scholar] [CrossRef]
- Yan, J.C.; Han, L.; Gao, W.G.; Xue, S.; Chen, M.F. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour. Technol. 2015, 175, 269–274. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.Y.; Xuan, W.D.; Li, S.P.; Wei, A.L. Efficient Nitrate Adsorption from Groundwater by Biochar-Supported Al-Substituted Goethite. Sustainability 2022, 14, 7824. [Google Scholar] [CrossRef]
- Qiu, J. China to Spend Billions Cleaning Up Groundwater. Science 2011, 334, 745. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Bae, S.; Lee, W. Nitrate reduction by maghemite supported Cu-Pd bimetallic catalyst. Appl. Catal. B-Env. 2012, 127, 148–158. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Kamiya, Y.; Okuhara, T. Selective hydrogenation of nitrate to nitrite in water over Cu-Pd bimetallic clusters supported on active carbon. J. Mol. Catal. A-Chem. 2006, 250, 80–86. [Google Scholar] [CrossRef]
- Jung, S.; Bae, S.; Lee, W. Development of Pd-Cu/hematite catalyst for selective nitrate reduction. Environ. Sci. Technol. 2014, 48, 9651–9658. [Google Scholar] [CrossRef] [PubMed]
- Shuai, D.; Choe, J.K.; Shapley, J.R.; Werth, C.J. Enhanced activity and selectivity of carbon nanofiber supported Pd catalysts for nitrite reduction. Environ. Sci. Technol. 2012, 46, 2847–2855. [Google Scholar] [CrossRef]
- Choe, S.; Chang, Y.Y.; Hwang, K.Y.; Khim, J. Kinetics of reductive denitrification by nanoscale zero-valent iron. Chemosphere 2000, 41, 1307–1311. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Torrey, J.D.; Amaro, R.L.; Shaw, J.M. Kinetics of Zero Valent Iron Nanoparticle Oxidation in Oxygenated Water. Environ. Sci. Technol. 2012, 46, 12913–12920. [Google Scholar] [CrossRef]
- Liu, C.-M.; Diao, Z.-H.; Huo, W.-Y.; Kong, L.-J.; Du, J.-J. Simultaneous removal of Cu2+ and bisphenol A by a novel biochar-supported zero valent iron from aqueous solution: Synthesis, reactivity and mechanism. Environ. Pollut. 2018, 239, 698–705. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, B.; Gong, X.; Yang, Z.; Liu, Y. Selective reduction of nitrate to nitrogen gas by novel Cu2O-Cu0@Fe0 composite combined with HCOOH under UV radiation. Chem. Eng. J. 2019, 359, 1195–1204. [Google Scholar] [CrossRef]
- Wang, T.; Jin, X.Y.; Chen, Z.L.; Megharaj, M.; Naidu, R. Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater. Sci. Total. Environ. 2014, 466–467, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Ritter, K.; Odziemkowski, M.S.; Gillham, R.W. An in situ study of the role of surface films on granular iron in the permeable iron wall technology. J. Contam. Hydrol. 2002, 55, 87–111. [Google Scholar] [CrossRef]
- Kohn, T.; Livi, K.J.T.; Roberts, A.L.; Vikesland, P.J. Longevity of granular iron in groundwater treatment processes: Corrosion product development. Environ. Sci. Technol. 2005, 39, 2867–2879. [Google Scholar] [CrossRef]
- Odziemkowski, M.S.; Simpraga, R.P. Distribution of oxides on iron materials used for emediation of organic groundwater contaminants-Implications for hydrogen evolution reactions. Can. J. Chem. 2004, 82, 1495–1506. [Google Scholar] [CrossRef]
- Sajjadi, B.; Broome, J.W.; Chen, W.Y.; Mattern, D.L.; Egiebor, N.O.; Hammer, N.; Smith, C.L. Urea functionalization of ultrasound-treated biochar: A feasible strategy for enhancing heavy metal adsorption capacity. Ultrason. Sonochem. 2019, 51, 20–30. [Google Scholar] [CrossRef]
- Oh, W.D.; Lisak, G.; Webster, R.D.; Liang, Y.N.; Veksha, A.; Giannis, A.; Moo, J.G.S.; Lim, J.W.; Lim, T.T. Insights into the thermolytic transformation of lignocellulosic biomass waste to redox-active carbocatalyst: Durability of surface active sites. Appl. Catal. B-Environ. 2018, 233, 120–129. [Google Scholar] [CrossRef]
- Zhao, G.X.; Li, J.X.; Ren, X.M.; Chen, C.L.; Wang, X.K. Few-Layered Graphene Oxide Nanosheets As Superior Sorbents for Heavy Metal Ion Pollution Management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.L.; Zhang, R.; Wang, X.K. Nanoscale Zero-Valent Iron Particles Supported on Reduced Graphene Oxides by Using a Plasma Technique and Their Application for Removal of Heavy-Metal Ions. Chem. Asian J. 2015, 10, 1410–1417. [Google Scholar] [CrossRef]
- Qian, L.B.; Shang, X.; Zhang, B.; Zhang, W.Y.; Su, A.Q.; Chen, Y.; Ouyang, D.; Han, L.; Yan, J.C.; Chen, M.F. Enhanced removal of Cr(VI) by silicon rich biochar-supported nanoscale zero-valent iron. Chemosphere 2019, 215, 739–745. [Google Scholar] [CrossRef]
- Zuo, X.J.; Liu, Z.G.; Chen, M.D. Effect of H2O2 concentrations on copper removal using the modified hydrothermal biochar. Bioresour. Technol. 2016, 207, 262–267. [Google Scholar] [CrossRef]
- Xing, M.; Wang, J.L. Nanoscaled zero valent iron/graphene composite as an efficient adsorbent for Co(II) removal from aqueous solution. J. Colloid Interface Sci. 2016, 474, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Dong, X.L.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.S.; Ma, L.N.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Lua, A.C.; Yang, T.; Guo, J. Effects of pyrolysis conditions on the properties of activated carbons prepared from pistachio-nut shells. J. Anal. Appl. Pyrolysis 2004, 72, 279–287. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Oh, S.Y.; Seo, Y.D. Factors Affecting Sorption of Nitro Explosives to Biochar: Pyrolysis Temperature, Surface Treatment, Competition, and Dissolved Metals. J. Environ. Qual. 2015, 44, 833–840. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Salam, M.A.; Fageeh, O.; Al-Thabaiti, S.A.; Obaid, A.Y. Removal of nitrate ions from aqueous solution using zero-valent iron nanoparticles supported on high surface area nanographenes. J. Mol. Liq. 2015, 212, 708–715. [Google Scholar] [CrossRef]
- Zhang, J.H.; Hao, Z.W.; Zhang, Z.; Yang, Y.P.; Xu, X.H. Kinetics of nitrate reductive denitrification by nanoscale zero-valent iron. Process. Saf. Env. 2010, 88, 439–445. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Su, C.M.; Ford, R.G.; Paul, C.J. Chromium-removal processes during groundwater remediation by a zerovalent iron permeable reactive barrier. Environ. Sci. Technol. 2005, 39, 4599–4605. [Google Scholar] [CrossRef]
- Bock, E.; Smith, N.; Rogers, M.; Coleman, B.; Reiter, M.; Benham, B.; Easton, Z.M. Enhanced Nitrate and Phosphate Removal in a Denitrifying Bioreactor with Biochar. J. Environ. Qual. 2015, 44, 605–613. [Google Scholar] [CrossRef]
- Huang, L.H.; Liu, G.F.; Dong, G.H.; Wu, X.Y.; Wang, C.; Liu, Y.Y. Reaction mechanism of zero-valent iron coupling with microbe to degrade tetracycline in permeable reactive barrier (PRB). Chem. Eng. J. 2017, 316, 525–533. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Chen, X.J.; Yang, Z.M.; Liu, Y.; Zhou, Z.Y.; Ren, Z.Q. Investigating the influences of electrode material property on degradation behavior of organic wastewaters by iron-carbon micro-electrolysis. Chem. Eng. J. 2018, 338, 46–54. [Google Scholar] [CrossRef]
- Chen, S.S.; Hsu, H.D.; Li, C.W. A new method to produce nanoscale iron for nitrate removal. J. Nanoparticle Res. 2004, 6, 639–647. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Mikami, I.; Sakamoto, Y.; Yoshinaga, Y.; Okuhara, T. Kinetic and adsorption studies on the hydrogenation of nitrate and nitrite in water using Pd-Cu on active carbon support. Appl. Catal. B-Environ. 2003, 44, 79–86. [Google Scholar] [CrossRef]
- Mehdinejadiani, B.; Amininasab, S.M.; Manhooei, L. Enhanced adsorption of nitrate from water by modified wheat straw: Equilibrium, kinetic and thermodynamic studies. Water Sci. Technol. 2019, 79, 302–313. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.J.; Liu, F.; Liang, X.L.; Feng, X.H.; Tan, W.F.; Zheng, L.R.; Yin, H. Effects of Al3+ doping on the structure and properties of goethite and its adsorption behavior towards phosphate. J. Env. Sci 2016, 45, 18–27. [Google Scholar] [CrossRef]
- Suzuki, T.; Moribe, M.; Oyama, Y.; Niinae, M. Mechanism of nitrate reduction by zero-valent iron: Equilibrium and kinetics studies. Chem. Eng. J. 2012, 183, 271–277. [Google Scholar] [CrossRef]
- Chen, Z.M.; Xiao, X.; Chen, B.L.; Zhu, L.Z. Quantification of Chemical States, Dissociation Constants and Contents of Oxygen-containing Groups on the Surface of Biochars Produced at Different Temperatures. Environ. Sci. Technol. 2015, 49, 309–317. [Google Scholar] [CrossRef]
- Sun, T.R.; Levin, B.D.A.; Schmidt, M.P.; Guzman, J.J.L.; Enders, A.; Martinez, C.E.; Muller, D.A.; Angenent, L.T.; Lehmann, J. Simultaneous Quantification of Electron Transfer by Carbon Matrices and Functional Groups in Pyrogenic Carbon. Environ. Sci. Technol. 2018, 52, 8538–8547. [Google Scholar] [CrossRef]
- Kluepfel, L.; Keiluweit, M.; Kleber, M.; Sander, M. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2014, 48, 5601–5611. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Ataie-Ashtiani, B.; Kholghi, M. Nitrate reduction by nano-Fe/Cu particles in packed column. Desalination 2011, 276, 214–221. [Google Scholar] [CrossRef]
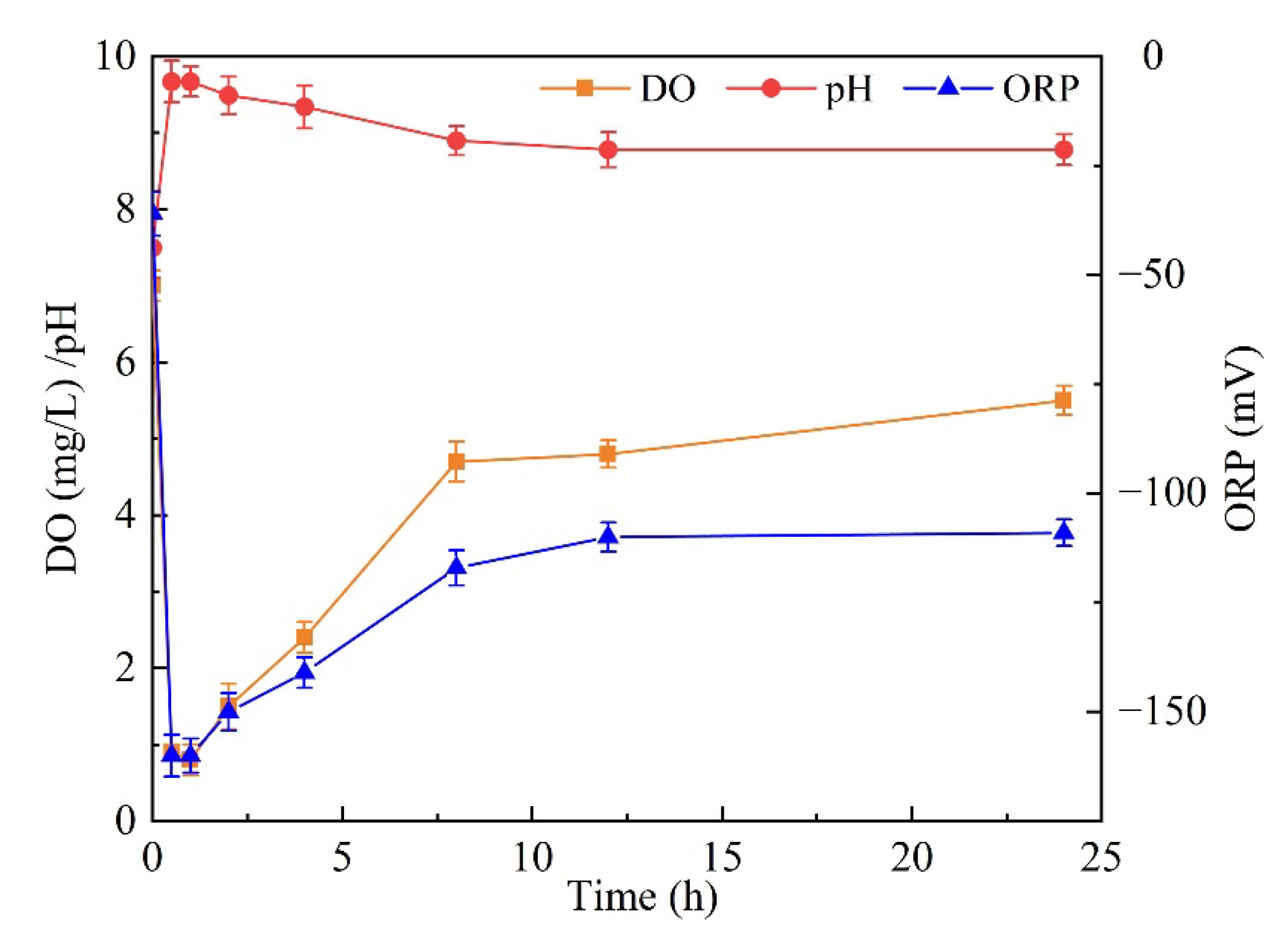
| Water Type | Monitoring Index | Maximum Value | Minimum Value | Average Value | Experimental Value |
|---|---|---|---|---|---|
| Ground water | K+ | 9.18 | 0.87 | 2.90 | 0 |
| Na+ | 953.74 | 12.98 | 286.12 | 250 | |
| Mg2+ | 274.62 | 14.56 | 61.93 | 60 | |
| Ca2+ | 140.98 | 13.05 | 43.66 | 40 | |
| Cl− | 358.32 | 15.07 | 68.87 | 60 | |
| SO42− | 604.65 | 12.26 | 42.30 | 40 | |
| HCO3− | 388.64 | 28.81 | 78.05 | 70 | |
| NO3− | 141.49 | 0.81 | 32.96 | 30 |
| Instruments | Models |
|---|---|
| Scanning electron microscope | Vega-3XMU, Tescan, Czech |
| Surface area BET analyzer | Micromeritics ASAP 2020, Norcross, USA |
| X-ray diffractometer | TTR-III, Rigaku, Japan |
| Fourier transform infrared spectroscopy | Equinox 55, Bruker Banner Lane, Germany |
| X-ray photoelectron spectroscopy | Escalab 250, Thermo Fisher Scientifific, USA |
| DO meter | HQ30d, Hach, USA |
| pH meter | MP220, Mettler Toledo, Switzerland |
| spectrophotometer | UV-1802, BeifenRuili, China |
| GC-TCD | GC-8A, Shimadzu, Japan |
| ZB12-500 Samples | SSA/(m2/g) |
|---|---|
| before reaction | 10.69 |
| during the reaction | 63.47 |
| after reaction | 83.32 |
| Fe Valence States | ZB12-500B | ZB12-500D | ZB12-500A |
|---|---|---|---|
| Fe0 | 9.87% | 6.03% | 4.87% |
| Fe2+ | 54.39% | 54.36% | 58.59% |
| Fe3+ | 35.74% | 39.61% | 36.54% |
| Sample | Material | First-Order | Second-Order | Two-Compartment First-Order | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1/(1/h) | R2 | k2/(1/h) | R2 | ff | kf/(1/h) | fs | ks/(1/h) | R2 | ||
| ZB12-500 | NO3−-N | 2.905 | 0.993 | 0.166 | 0.996 | 0.925 | 3.093 | 0.075 | 0.038 | 0.997 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Han, X.; Li, S.; Xuan, W.; Wei, A. Stimulating Nitrate Removal with Significant Conversion to Nitrogen Gas Using Biochar-Based Nanoscale Zerovalent Iron Composites. Water 2022, 14, 2877. https://doi.org/10.3390/w14182877
Liu S, Han X, Li S, Xuan W, Wei A. Stimulating Nitrate Removal with Significant Conversion to Nitrogen Gas Using Biochar-Based Nanoscale Zerovalent Iron Composites. Water. 2022; 14(18):2877. https://doi.org/10.3390/w14182877
Chicago/Turabian StyleLiu, Siyuan, Xiao Han, Shaopeng Li, Wendi Xuan, and Anlei Wei. 2022. "Stimulating Nitrate Removal with Significant Conversion to Nitrogen Gas Using Biochar-Based Nanoscale Zerovalent Iron Composites" Water 14, no. 18: 2877. https://doi.org/10.3390/w14182877






