Accumulation of Vanadium by Nanoscale Zero-Valent Iron Supported by Activated Carbon under Simulation Water Conditions: A Batch Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Instrumentation
2.2. Synthesis and Characterization of NZVI/AC
2.3. Batch Adsorption Experiments
2.4. Desorption of Adsorbed V(V)
2.5. Models
2.6. Analytical Methods
3. Results
3.1. The Iron Decorating on AC/Biochar
3.2. Dosage of Adsorbent
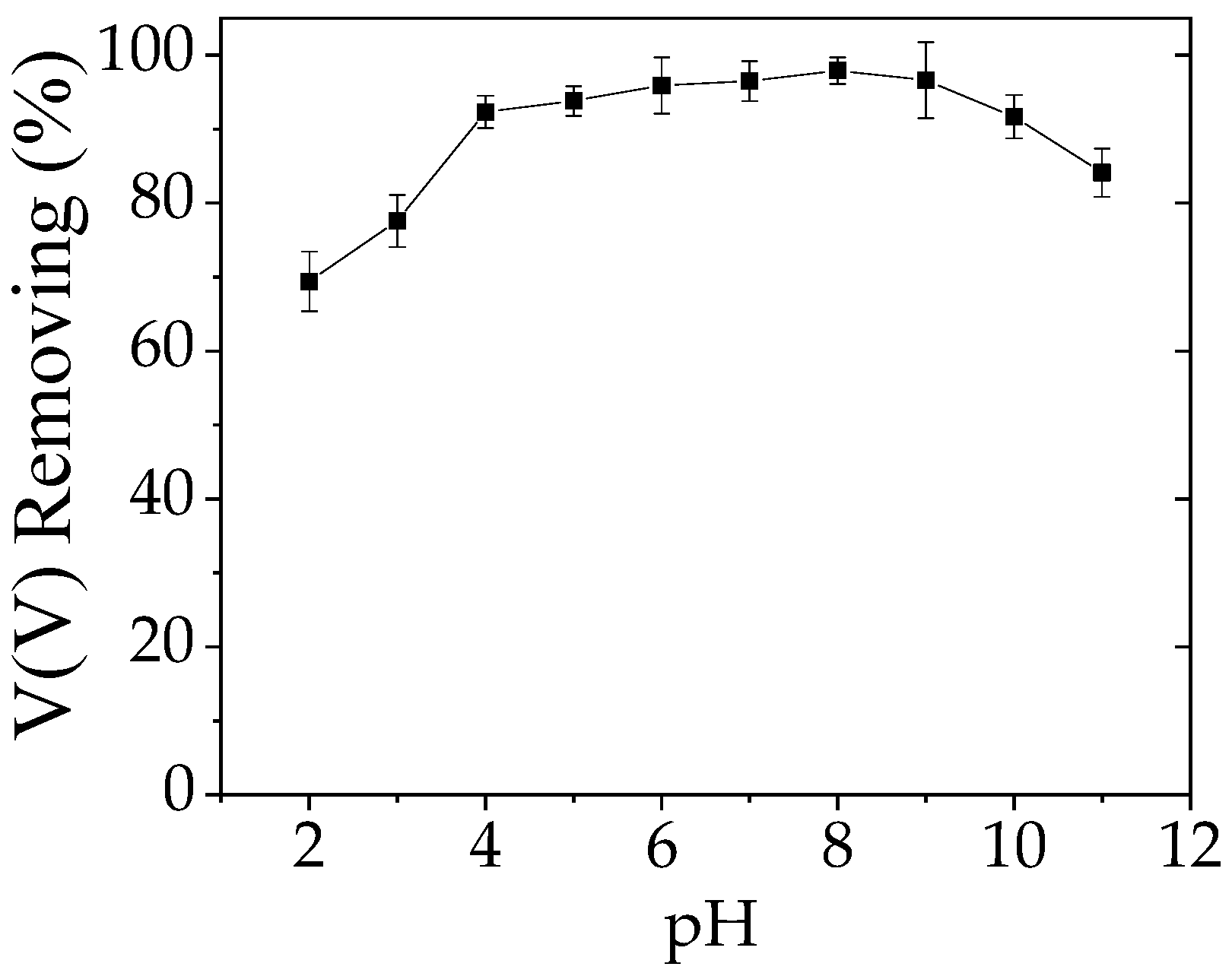
3.3. Solution pH
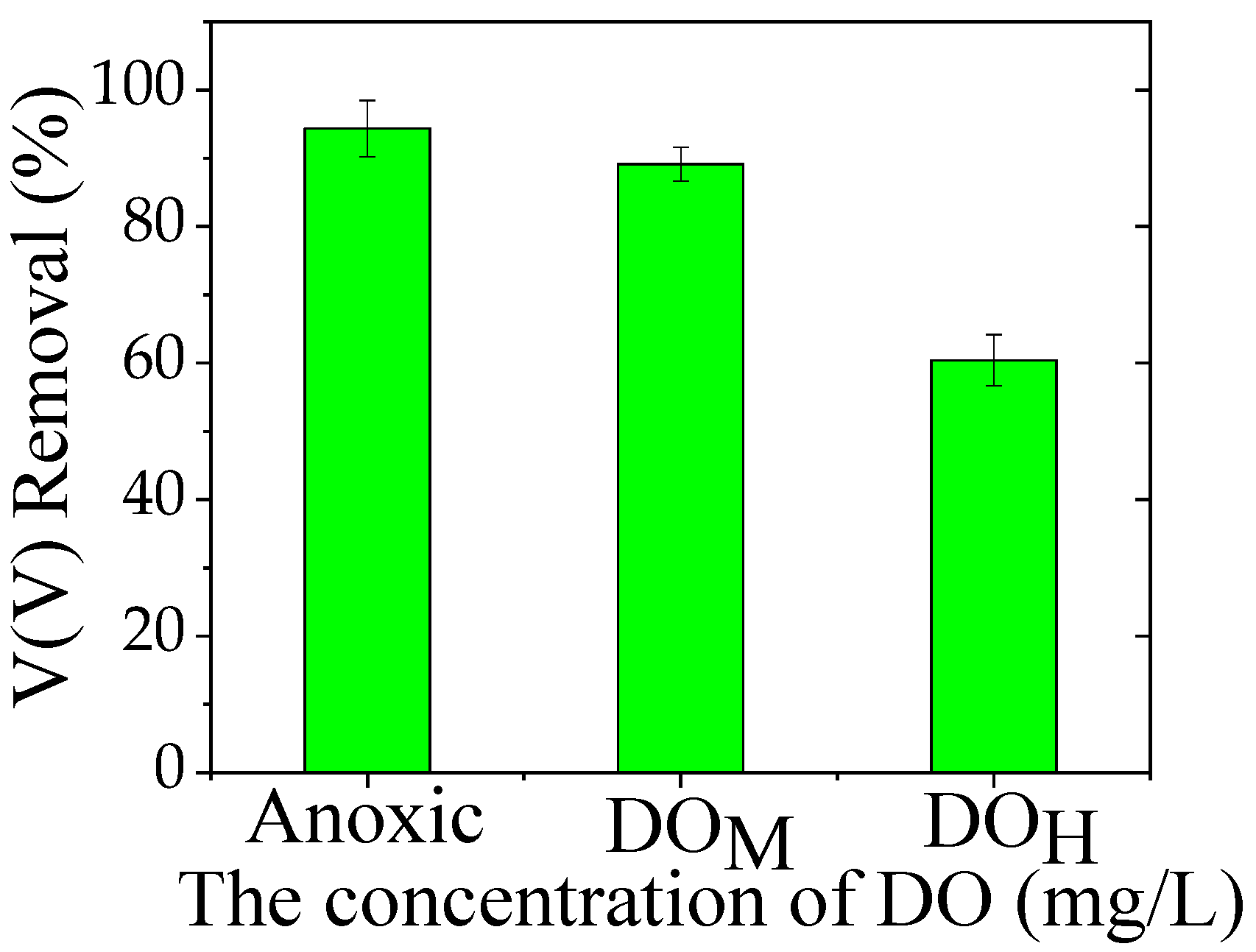
3.4. Impact of DO Concentration
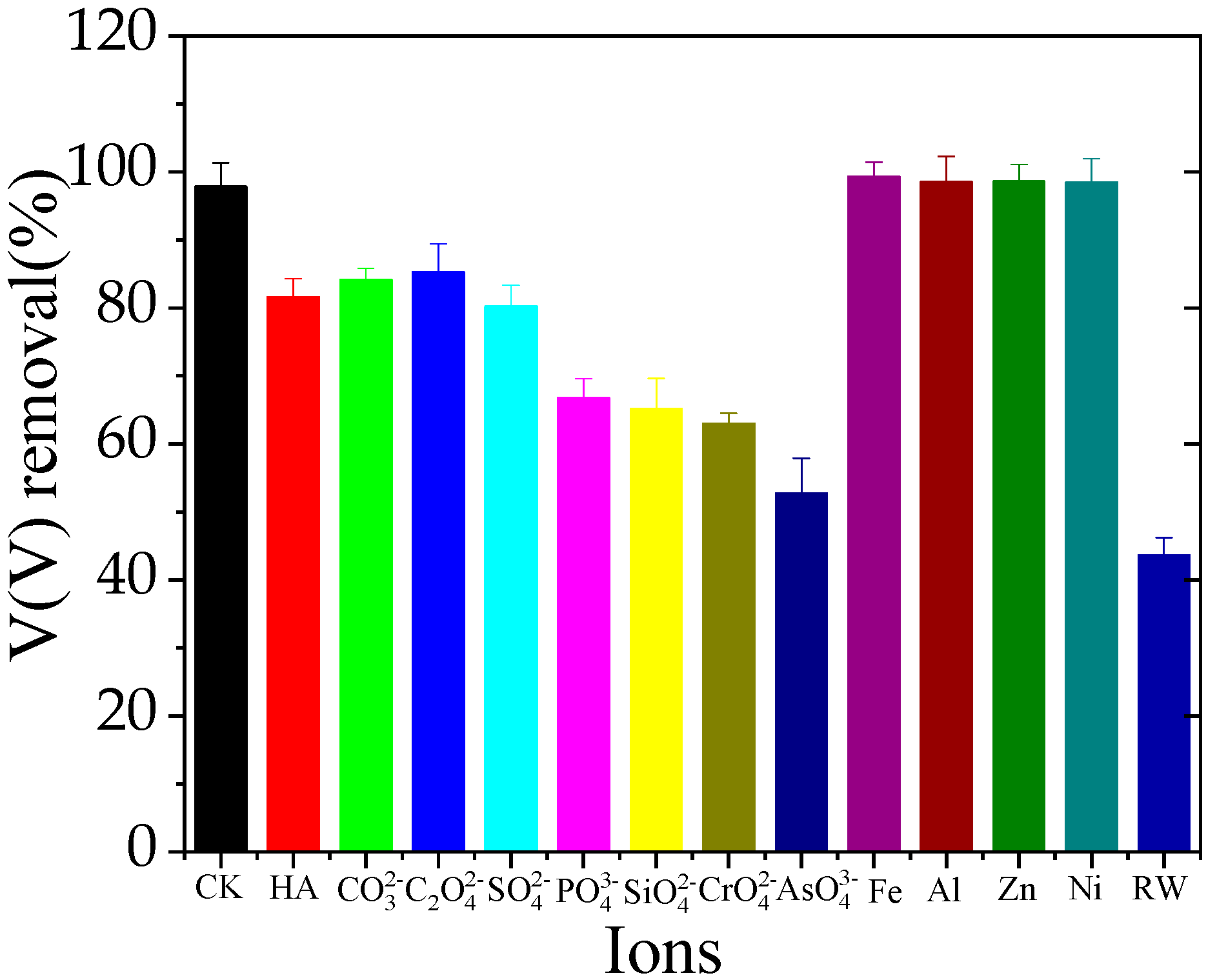
3.5. Effect of Coexisting Ions
3.5.1. Inorganic Ions
3.5.2. Organic Ions
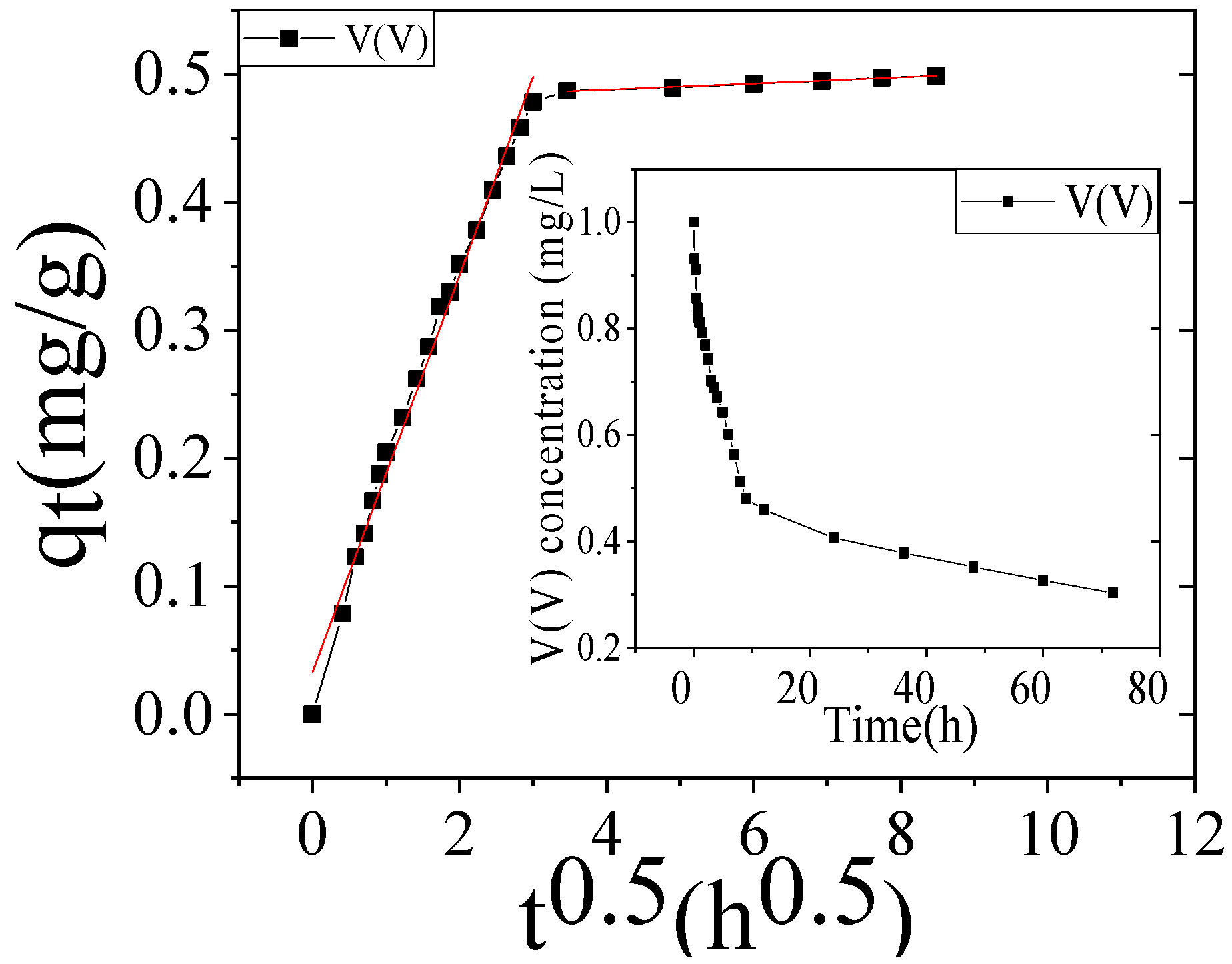
3.6. Adsorption Kinetics
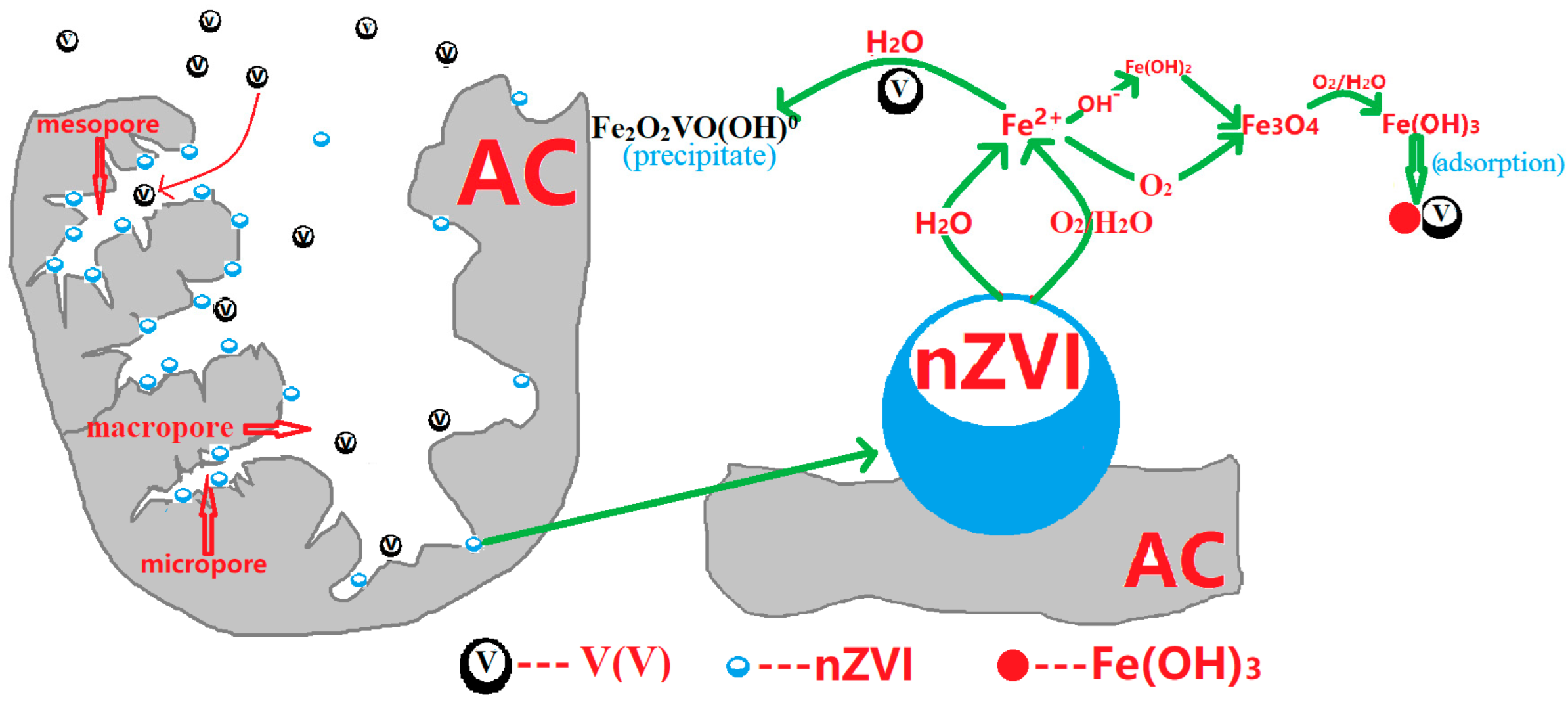
3.7. Removal Mechanisms by Adsorption
3.8. Regeneration of NZVI/AC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.-C.; Kurniawan; Kim, E.-Y.; Chung, K.W.; Kim, R.; Jeon, H.-S. A review on the metallurgical recycling of vanadium from slags: Towards a sustainable vanadium production. J. Mater. Res. Technol. 2021, 12, 343–364. [Google Scholar] [CrossRef]
- Imtiaz, M.; Rizwan, M.S.; Xiong, S.; Li, H.; Ashraf, M.; Shahzad, S.M.; Shahzad, M.; Tu, S. Vanadium, recent advancements and research prospects: A review. Environ. Int. 2015, 80, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Chen, N.; Qin, J.; Yang, Y.; Feng, C.; Li, M.; Gao, Y. Biochar stabilized nano zero-valent iron and its removal performance and mechanism of pentavalent vanadium(V(V)). Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124882. [Google Scholar] [CrossRef]
- Chen, G.; Liu, H. Understanding the Reduction Kinetics of Aqueous Vanadium(V) and Transformation Products Using Rotating Ring-Disk Electrodes. Environ. Sci. Technol. 2017, 51, 11643–11651. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, B.; Zou, S.; Liu, Q.; Yang, M. Highly selective adsorption of vanadium (V) by nano-hydrous zirconium oxide-modified anion exchange resin. J. Hazard. Mater. 2019, 384, 121386. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, W.; Zhan, S.; Qiu, J.; Wang, X.; Wu, Z.; Li, H.; Qiu, Z.; Peng, H. Adsorption and mechanistic study for humic acid removal by magnetic biochar derived from forestry wastes functionalized with Mg/Al-LDH. Sep. Purif. Technol. 2021, 276, 119296. [Google Scholar] [CrossRef]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.-V.N.; Manan, F.A. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2020, 278, 123805. [Google Scholar] [CrossRef]
- Hao, L.; Liu, Y.; Chen, N.; Hao, X.; Zhang, B.; Feng, C. Microbial removal of vanadium (V) from groundwater by sawdust used as a sole carbon source. Sci. Total Environ. 2020, 751, 142161. [Google Scholar] [CrossRef]
- He, C.; Zhang, B.; Lu, J.; Qiu, R. A newly discovered function of nitrate reductase in chemoautotrophic vanadate transformation by natural mackinawite in aquifer. Water Res. 2020, 189, 116664. [Google Scholar] [CrossRef]
- Mangini, L.F.K.; Valt, R.B.G.; Ponte, M.J.J.D.S.; Ponte, H.D.A. Vanadium removal from spent catalyst used in the manufacture of sulfuric acid by electrical potential application. Sep. Purif. Technol. 2020, 246, 116854. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, J.; Zhang, Y.; Liu, T.; Hu, P.; Liu, H.; Luo, D. Separation and recovery of vanadium and aluminum from oxalic acid leachate of shale by solvent extraction with Aliquat 336. Sep. Purif. Technol. 2020, 249, 116867. [Google Scholar] [CrossRef]
- Roccaro, P.; Vagliasindi, F.G.A. Coprecipitation of vanadium with iron(III) in drinking water: A pilot-scale study. Desalin Water Treat. 2014, 55, 799–809. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, B.; Chen, D.; Guo, Z.; Peng, Z. Simultaneous Reduction of Vanadium (V) and Chromium (VI) in Wastewater by Nanosized ZnWO4 Photocatalysis. J. Nanosci. Nanotechnol. 2016, 16, 2847–2852. [Google Scholar] [CrossRef] [PubMed]
- Rivas, B.L.; Espinosa, C.; Sánchez, J. Application of the liquid-phase polymer-based retention technique to the sorption of molybdenum(VI) and vanadium(V). Polym. Bull. 2018, 76, 539–552. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Czech, B.; Tyszczuk-Rotko, K.; Kończak, M.; Fakhrhoseini, S.M.; Yadav, R.; Naebe, M. Sustainable synthesis of rose flower-like magnetic biochar from tea waste for environmental applications. J. Adv. Res. 2021, 34, 13–27. [Google Scholar] [CrossRef]
- Shin, J.; Lee, Y.-G.; Kwak, J.; Kim, S.; Lee, S.-H.; Park, Y.; Chon, K. Adsorption of radioactive strontium by pristine and magnetic biochars derived from spent coffee grounds. J. Environ. Chem. Eng. 2021, 9, 105119. [Google Scholar] [CrossRef]
- Yayayürük, A.E.; Yayayürük, O. Adsorptive performance of nanosized zero-valent iron for V(V) removal from aqueous solutions. J. Chem. Technol. Biotechnol. 2017, 92, 1891–1898. [Google Scholar] [CrossRef]
- Saitoh, H.; Sato, T.; Tanikami, M.; Ikeda, K.; Machida, A.; Watanuki, T.; Taguchi, T.; Yamamoto, S.; Yamaki, T.; Takagi, S.; et al. Hydrogen storage by earth-abundant metals, synthesis and characterization of Al3FeH3.9. Mater. Des. 2021, 208, 109953. [Google Scholar] [CrossRef]
- Cui, B.; Chen, Z.; Wang, F.; Zhang, Z.; Dai, Y.; Guo, D.; Liang, W.; Liu, Y. Facile Synthesis of Magnetic Biochar Derived from Burley Tobacco Stems towards Enhanced Cr(VI) Removal: Performance and Mechanism. Nanomaterials 2022, 12, 678. [Google Scholar] [CrossRef]
- Zuliani, A.; Kikhtyanin, O.; Cova, C.M.; Rodriguez-Padron, D.; Kubička, D.; Luque, R. Boosting the Ni-Catalyzed Hydrodeoxygenation (HDO) of Anisole Using Scrap Catalytic Converters. Adv. Sustain. Syst. 2022, 6, 2100394. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Y.; Wu, X.; Wang, H. Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J. Hazard. Mater. 2009, 172, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zhang, Y.; Zeng, X.; Kuang, H.; Huang, S. Enhancement of ball-miling on pyrite/zero-valent iron for arsenic removal in water: A mechanistic study. Chemosphere 2020, 249, 126130. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jin, J.; Li, J.; Zou, Z.; Lei, R.; Sun, J.; Xia, J. Enhanced Phosphorus Recovery as Vivianite from Anaerobically Digested Sewage Sludge with Magnetic Biochar Addition. Sustainability 2022, 14, 8690. [Google Scholar] [CrossRef]
- Keränen, A.; Leiviskä, T.; Salakka, A.; Tanskanen, J. Removal of nickel and vanadium from ammoniacal industrial wastewater by ion exchange and adsorption on activated carbon. Desalination Water Treat. 2013, 53, 2645–2654. [Google Scholar] [CrossRef]
- Ardakani, S.S.; Zandipak, R.; Fili, Z.; Ghoochian, M.; Sahraei, R.; Farmany, A. Removal of V(V) ions from aqueous solutions using oxidized multi-walled carbon nanotubes. J. Water Supply Res. Technol. 2015, 64, 425–433. [Google Scholar] [CrossRef]
- Omidinasab, M.; Rahbar, N.; Ahmadi, M.; Kakavandi, B.; Ghanbari, F.; Kyzas, G.Z.; Martinez, S.S.; Jaafarzadeh, N. Removal of vanadium and palladium ions by adsorption onto magnetic chitosan nanoparticles. Environ. Sci. Pollut. Res. 2018, 25, 34262–34276. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Chen, J.; Tang, Y.; Lv, Y.; Chen, T.; Wang, H. Enhanced removal of vanadium(V) from groundwater by layered double hydroxide-supported nanoscale zerovalent iron. J. Hazard. Mater. 2020, 392, 122392. [Google Scholar] [CrossRef]
- Zhang, R.; Leivisk¨a, T.; Tanskanen, J. Utilization of ferric groundwater treatment residuals for inorganic-organic hybrid biosorbent preparation and its use for vanadium removal. Chem. Eng. J. 2019, 361, 680–689. [Google Scholar] [CrossRef]
- Zhang, R.; Leivisk¨a, T. Surface modification of pine bark with quaternary ammonium groups and its use for vanadium removal. Chem. Eng. J. 2020, 385, 123967. [Google Scholar] [CrossRef]
- Zhu, H.; Xiao, X.; Guo, Z.; Han, X.; Liang, Y.; Zhang, Y.; Zhou, C. Adsorption of vanadium (V) on natural kaolinite and montmorillonite: Characteristics and mechanism. Appl. Clay Sci. 2018, 161, 310–316. [Google Scholar] [CrossRef]
- Bello, A.; Leivisk¨a, T.; Zhang, R. Synthesis of zerovalent iron from water treatment residue as a conjugate with kaolin and its application for vanadium removal. J. Hazard. Mater. 2019, 374, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Li, Q.; Xiao, L. Extraction of vanadium from the leach solution of stone coal using ion exchange resin. Hydrometallurgy 2009, 97, 194–197. [Google Scholar] [CrossRef]
- Fan, P.; Sun, Y.; Qiao, J.; Lo, I.M.; Guan, X. Influence of weak magnetic field and tartrate on the oxidation and sequestration of Sb(III) by zerovalent iron: Batch and semi-continuous flow study. J. Hazard. Mater. 2018, 343, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, J.; Huang, T.; Guan, X. The influences of iron characteristics, operating conditions and solution chemistry on contaminants removal by zero-valent iron: A review. Water Res. 2016, 100, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Komnitsas, K.; Bartzas, G.; Fytas, K.; Paspaliaris, I. Long-term efficiency and kinetic evaluation of ZVI barriers during clean-up of copper containing solutions. Miner. Eng. 2007, 20, 1200–1209. [Google Scholar] [CrossRef]
- Zhu, H.; Xiao, X.; Guo, Z.; Peng, C.; Wang, X.; Yang, A. Characteristics and behaviour of vanadium(V) adsorption on goethite and birnessite. Environ. Earth Sci. 2020, 79, 240. [Google Scholar] [CrossRef]
- Ghanim, B.; Murnane, J.; O’Donoghue, L.; Courtney, R.; Pembroke, J.T.; O’Dwyer, T.F. Removal of vanadium from aqueous solution using a red mud modified saw dust biochar. J. Water Process Eng. 2019, 33, 101076. [Google Scholar] [CrossRef]
- Salehi, S.; Mandegarzad, S.; Anbia, M. Preparation and characterization of metal organic framework-derived nanoporous carbons for highly efficient removal of vanadium from aqueous solution. J. Alloys Compd. 2019, 812, 152051. [Google Scholar] [CrossRef]
- Namasivayam, C.; Sangeetha, D. Removal and recovery of vanadium(V) by adsorption onto ZnCl2 activated carbon: Kinetics and isotherms. Adsorption 2006, 12, 103–117. [Google Scholar] [CrossRef]
- Thamilarasi, M.J.V.; Anilkumar, P.; Theivarasu, C.; Sureshkumar, M.V. Removal of vanadium from wastewater using surface-modified lignocellulosic material. Environ. Sci. Pollut. Res. 2018, 25, 26182–26191. [Google Scholar] [CrossRef]
- Hu, Q.; Paudyal, H.; Zhao, J.; Huo, F.; Inoue, K.; Liu, H. Adsorptive recovery of vanadium(V) from chromium(VI)-containing effluent by Zr(IV)-loaded orange juice residue. Chem. Eng. J. 2014, 248, 79–88. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Xia, W.; Zhang, W. Preparation and characterization of chitosan-zirconium(IV) composite for adsorption of vanadium(V). Int. J. Biol. Macromol. 2013, 64, 155–161. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L. Insight into the adsorption mechanisms of vanadium(V) on a high-efficiency biosorbent (Ti-doped chitosan bead). Int. J. Biol. Macromol. 2015, 79, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Anbia, M. Performance comparison of chitosan–clinoptilolite nanocomposites as adsorbents for vanadium in aqueous media. Cellulose 2019, 26, 5321–5345. [Google Scholar] [CrossRef]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The Chemistry and Biochemistry of Vanadium and the Biological Activities Exerted by Vanadium Compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef]
- Islam, A.; Morton, D.W.; Johnson, B.B.; Angove, M.J. Adsorption of humic and fulvic acids onto a range of adsorbents in aqueous systems, and their effect on the adsorption of other species: A review. Sep. Purif. Technol. 2020, 247, 116949. [Google Scholar] [CrossRef]
- Zhu, H.; Shi, M.; Zhang, X.; Liu, B.; Yao, D. Adsorption Kinetics of Arsenic (V) on Nanoscale Zero-Valent Iron Supported by Activated Carbon. Nanomaterials 2020, 10, 1791. [Google Scholar] [CrossRef]
- Hameed, B.H.; Ahmad, A.; Aziz, N. Isotherms, kinetics and thermodynamics of acid dye adsorption on activated palm ash. Chem. Eng. J. 2007, 133, 195–203. [Google Scholar] [CrossRef]
- Ong, S.A.; Seng, C.E.; Lim, P. Kinetics of adsorption of Cu (II) and Cd (II) from aqueous solution on rice husk and modified rice husk. Electron. J. Environ. Agric. Food Chem. 2007, 6, 1764–1774. [Google Scholar]
- Yang, C.; Zhang, J.; Zhu, X.; Liu, Y.; Chen, Y.; Wang, C. Deep and efficient removal of vanadium from molybdate solution using magnetic γ-Fe2O3 nanoparticles. Appl. Surf. Sci. 2020, 529, 147060. [Google Scholar] [CrossRef]
- Peacock, C.L.; Sherman, D.M. Vanadium(V) adsorption onto goethite (α-FeOOH) at pH 1.5 to 12: A surface complexation model based on ab initio molecular geometries and EXAFS spectroscopy. Geochim. Cosmochim. Acta. 2004, 68, 1723–1733. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Jalajamony, S.; Divya, L. Efficiency of Amine-Modified Poly(glycidyl methacrylate)-Grafted Cellulose in the Removal and Recovery of Vanadium(V) from Aqueous Solutions. Ind. Eng. Chem. Res. 2009, 48, 2118–2124. [Google Scholar] [CrossRef]
- Manohar, D.M.; Noeline, B.F.; Anirudhan, T.S. Removal of Vanadium(IV) from Aqueous Solutions by Adsorption Process with Aluminum-Pillared Bentonite. Ind. Eng. Chem. Res. 2005, 44, 6676–6684. [Google Scholar] [CrossRef]
- Benjamin, M.M.; Sletten, R.S.; Bailey, R.P. Sorption and filtration of metals using iron-oxide-coated sand. Water Res. 1996, 30, 2609–2620. [Google Scholar] [CrossRef]

| Thickness | Diameter | Shape | Total Pore Volume | Fe Content | BET Surface Area |
|---|---|---|---|---|---|
| ~20 nm | <100 nm | flakes | 0.45 cm3/g | ~8.2% | 821.7 m2/g |
| Carbon from Raw Material | Shape | Total Pore Volume cm3/g | Fe Content | BET Surface Area m2/g | Form of Fe | Average Pore Diameter | Target Removal of Contaminants |
|---|---|---|---|---|---|---|---|
| Coal [This study] | flakes | 0.45 | ~8.2% | 821.7 | FexOy | 20 nm | Vanadium |
| Forestry wastes [6] | strip-like, brush hollow/hierarchical structure | 0.588 | - | 116.095 | CoFe2O4@BC-LDH | <10 | HA |
| Tea waste [15] | rose flower like pattern | 0.201 | - | 111.215 | Fe3O4 | 1.5–10 | |
| Pristine [16] | relatively conspicuous pore structures | 0.186 | 0.9% | 431.7 | Fe3O4 | - | Sr2+ |
| Burley Tobacco Stems [19] | - | 0.008 | - | 4.33 | Fe3O4/Fe2O3 | - | Cr(VI) |
| Anaerobically Digested Sewage Sludge [23] | spherical or irregular nodular | 0.1494 | 8.359% | 44.75 | Fe3O4/FeO | 13.358 | P |
| Parameter | Weber–Morris Diffusion | |||
|---|---|---|---|---|
| 1st Step | 2nd Step | |||
| C0 (0.50 mg/L) | kid1 | R2 | kid2 | R2 |
| Simulation water | 0.1550 | 0.9902 | 0.00239 | 0.9937 |
| Cycle | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Vanadium removal (%) | 95.2 | 97.4 | 93.1 | 96.7 | 95.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Fu, S.; Zhu, H.; Song, H.; Yang, Z.; Zhang, X.; Bie, J.; Lu, J.; Shi, M.; Liu, B. Accumulation of Vanadium by Nanoscale Zero-Valent Iron Supported by Activated Carbon under Simulation Water Conditions: A Batch Study. Water 2022, 14, 2867. https://doi.org/10.3390/w14182867
Huang Q, Fu S, Zhu H, Song H, Yang Z, Zhang X, Bie J, Lu J, Shi M, Liu B. Accumulation of Vanadium by Nanoscale Zero-Valent Iron Supported by Activated Carbon under Simulation Water Conditions: A Batch Study. Water. 2022; 14(18):2867. https://doi.org/10.3390/w14182867
Chicago/Turabian StyleHuang, Qiang, Shuai Fu, Huijie Zhu, Huaihui Song, Zhe Yang, Xiuji Zhang, Junhong Bie, Jianhong Lu, Mingyan Shi, and Bo Liu. 2022. "Accumulation of Vanadium by Nanoscale Zero-Valent Iron Supported by Activated Carbon under Simulation Water Conditions: A Batch Study" Water 14, no. 18: 2867. https://doi.org/10.3390/w14182867





