Insights into the Kinetics, Theoretical Model and Mechanism of Free Radical Synergistic Degradation of Micropollutants in UV/Peroxydisulfate Process
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Experimental Procedures
2.3. Analytical Methods
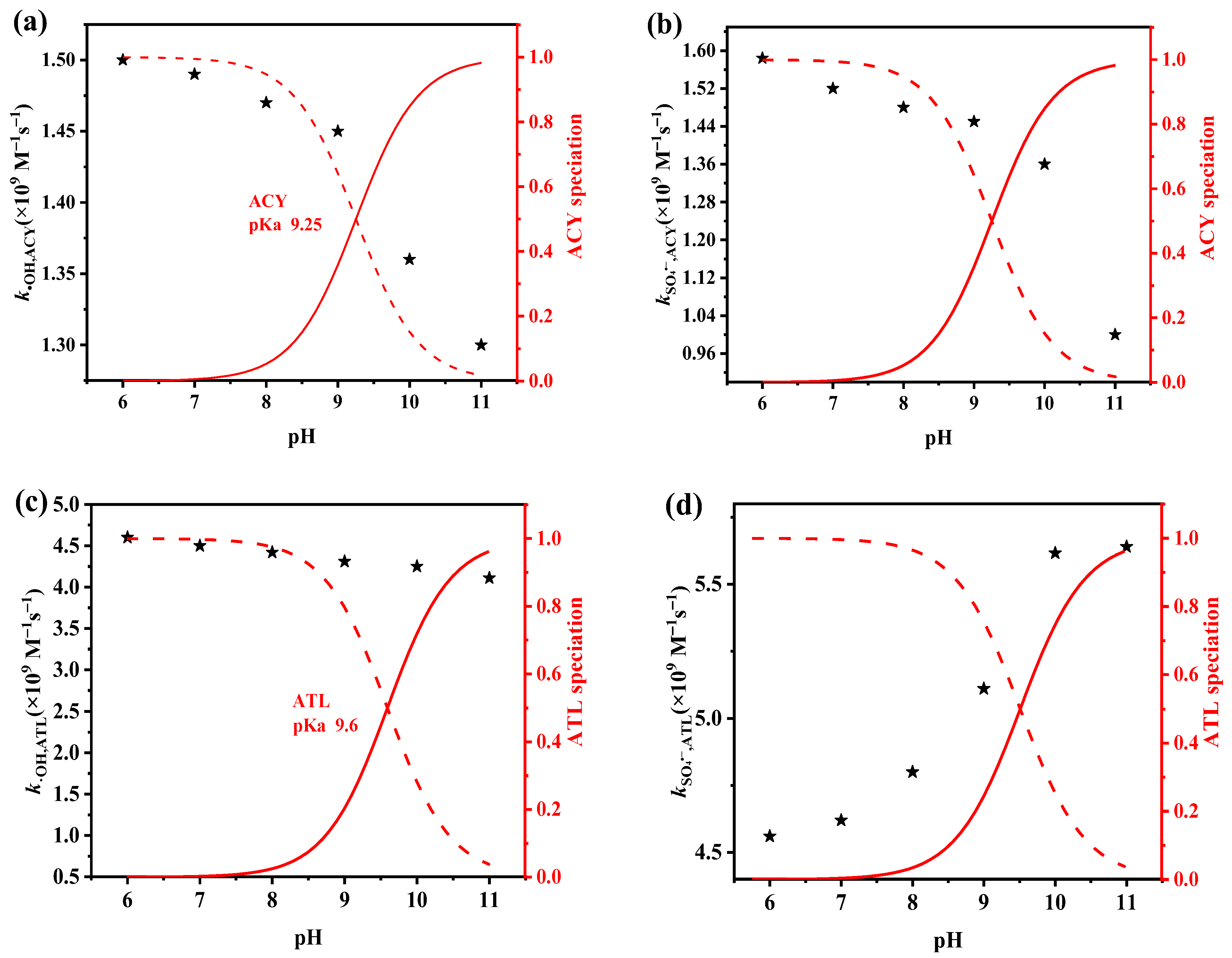
2.4. Determination of the Second-Order Reaction Rate Constants
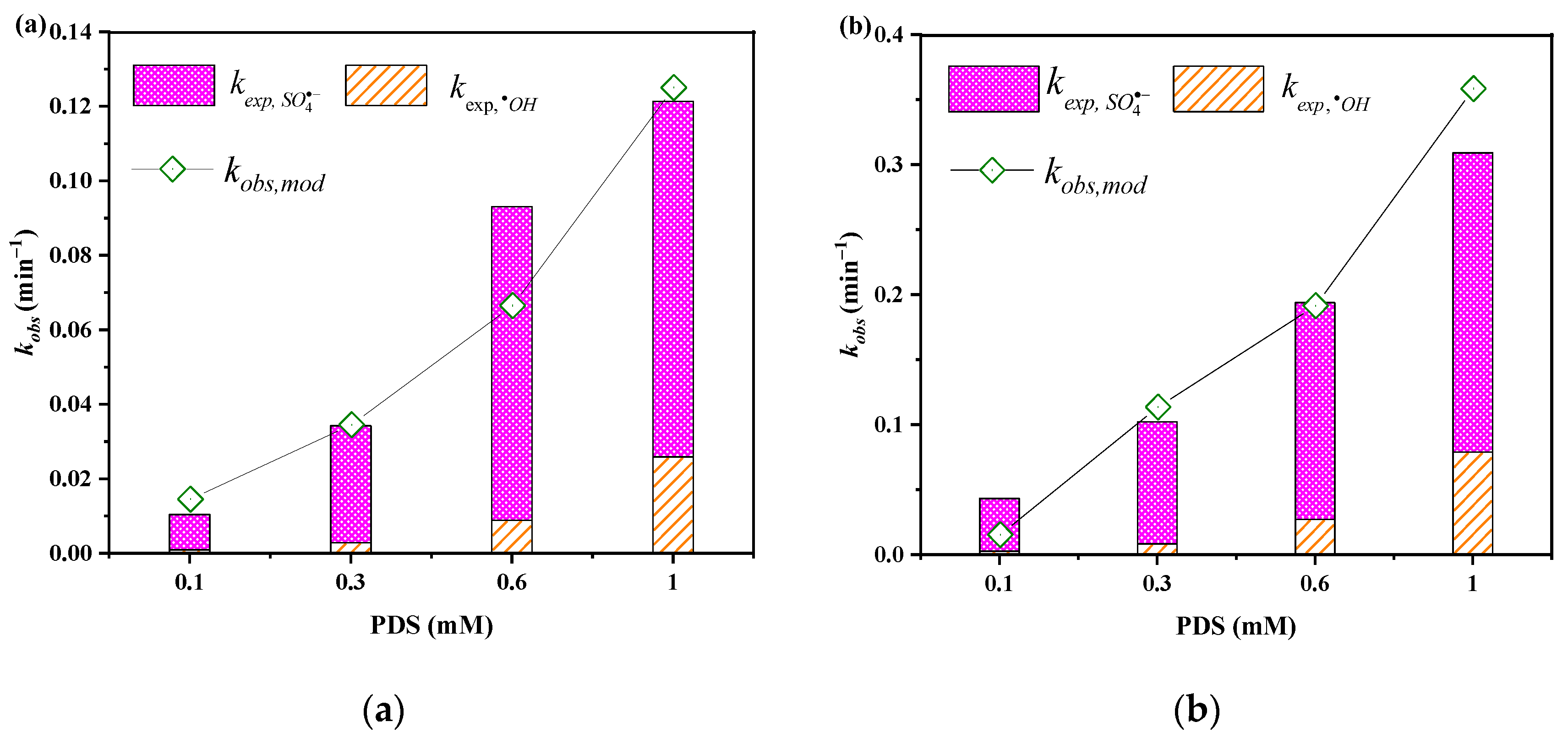
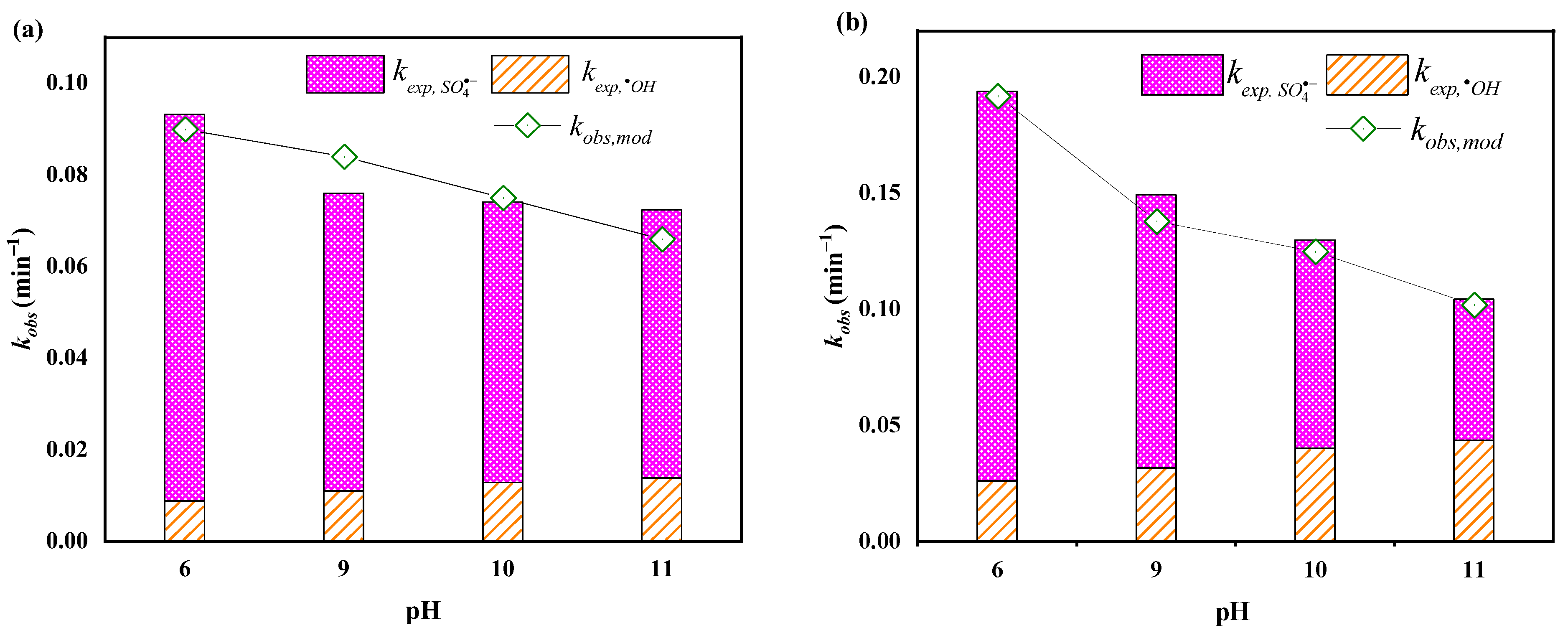
2.5. Determination of the Relative Contributions of •OH and SO4•−
2.6. Kinetic Model
2.7. Quantum Chemistry Calculation
3. Results and Discussion
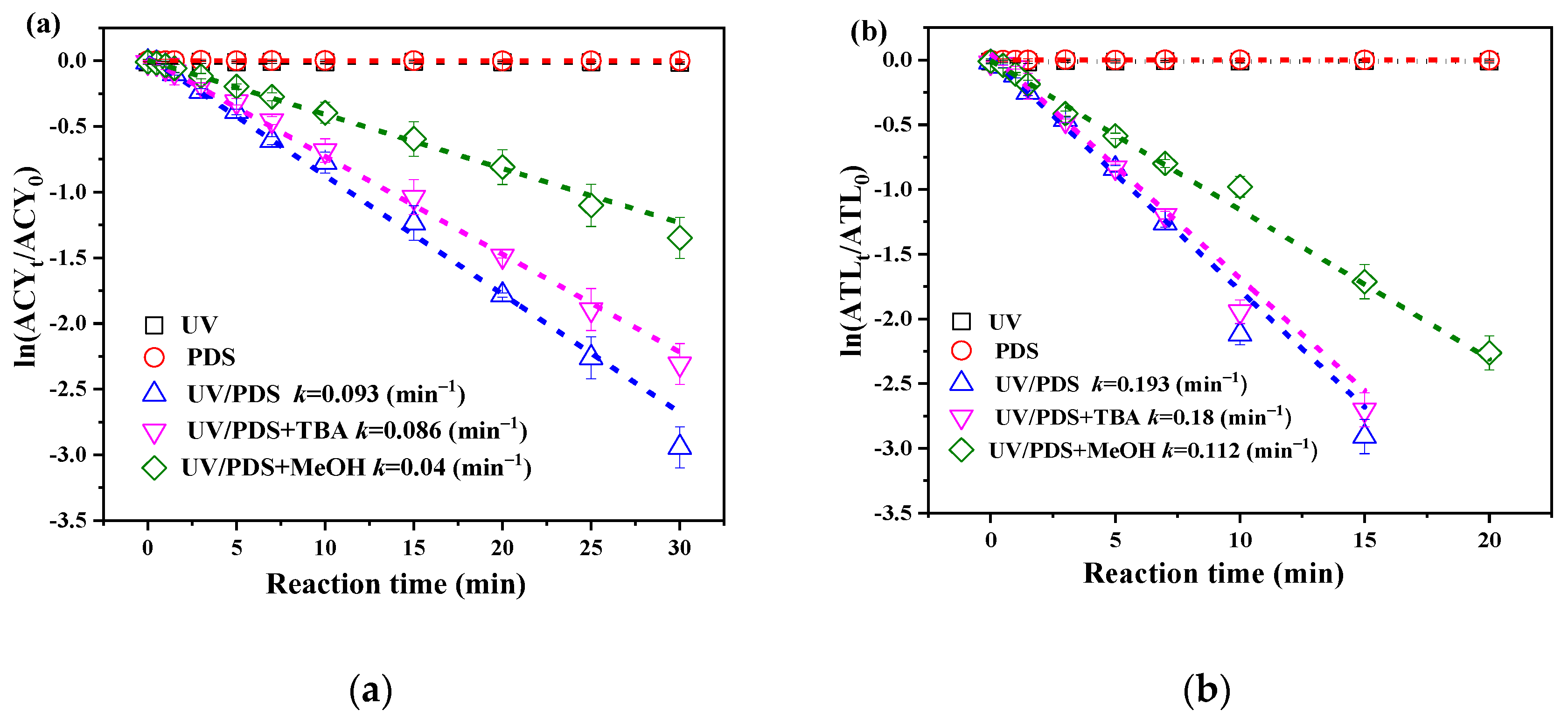
3.1. Degradation Efficiencies of ACY and ATL in Different Processes
3.2. Effects of PDS Dosage
3.3. Effects of Solution pH
3.4. Effects of Operation Parameters in UV/PDS Process
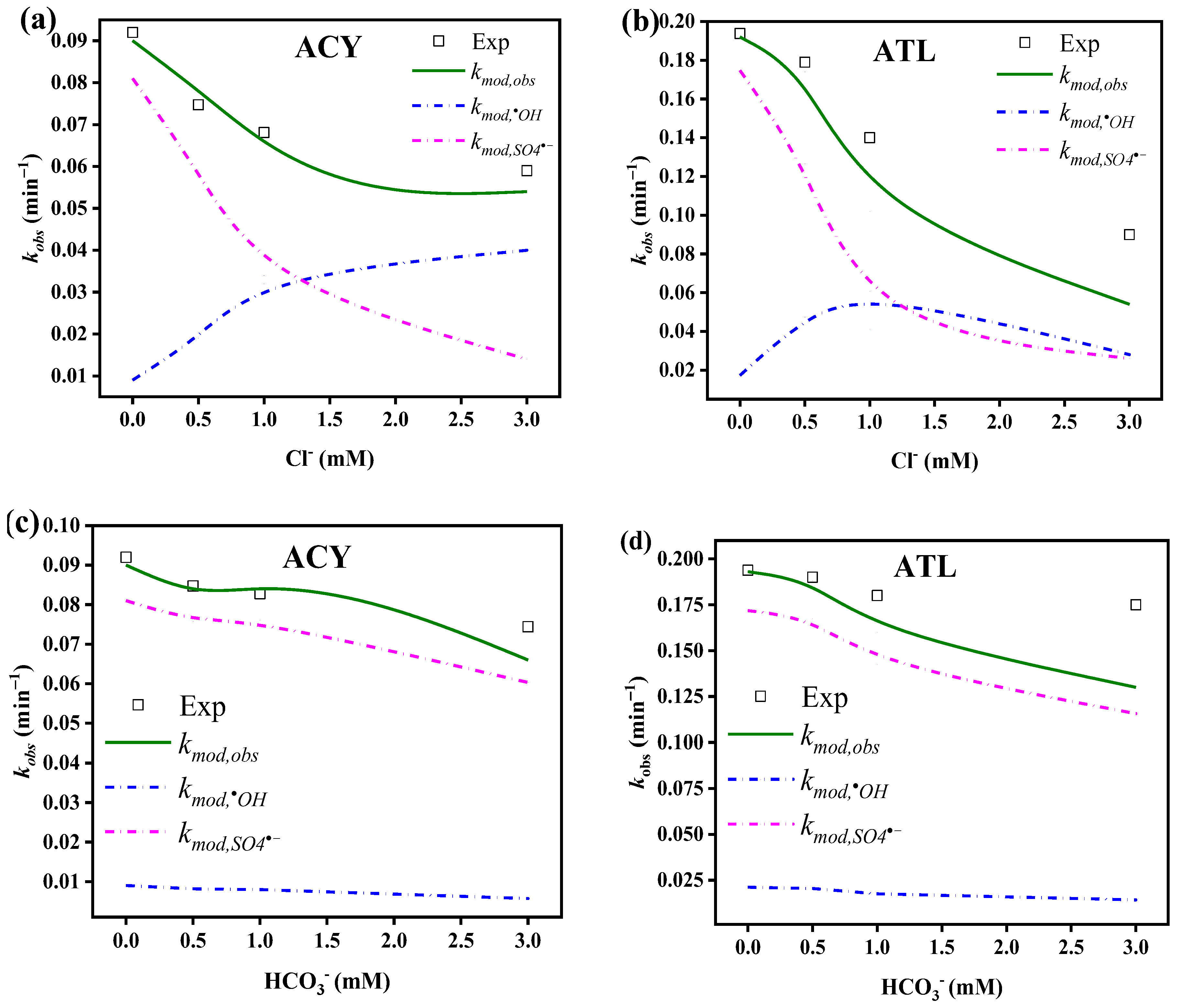
3.4.1. Chloride
3.4.2. Bicarbonate
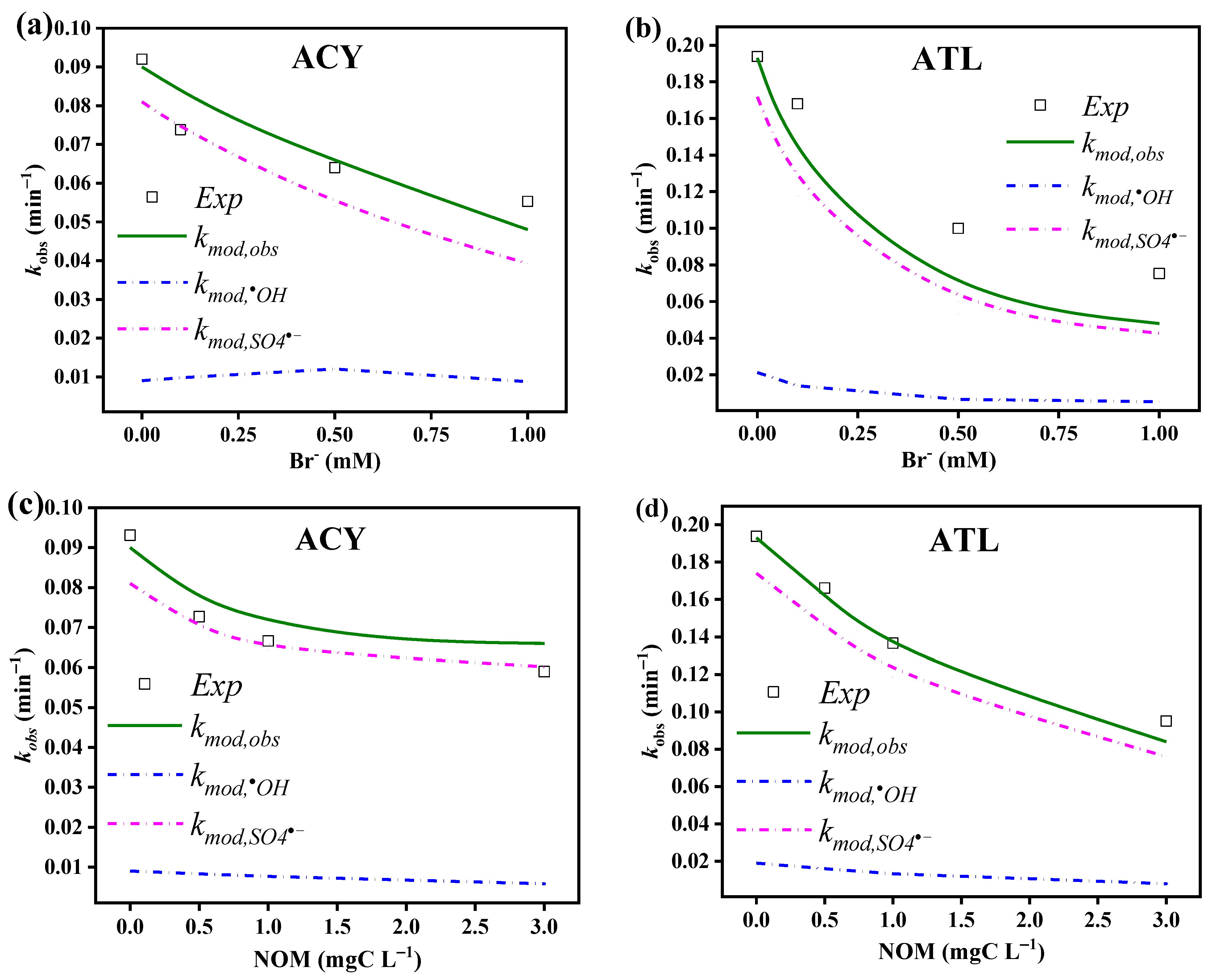
3.4.3. Bromide
3.4.4. NOM
3.4.5. Sulfate and Nitrate
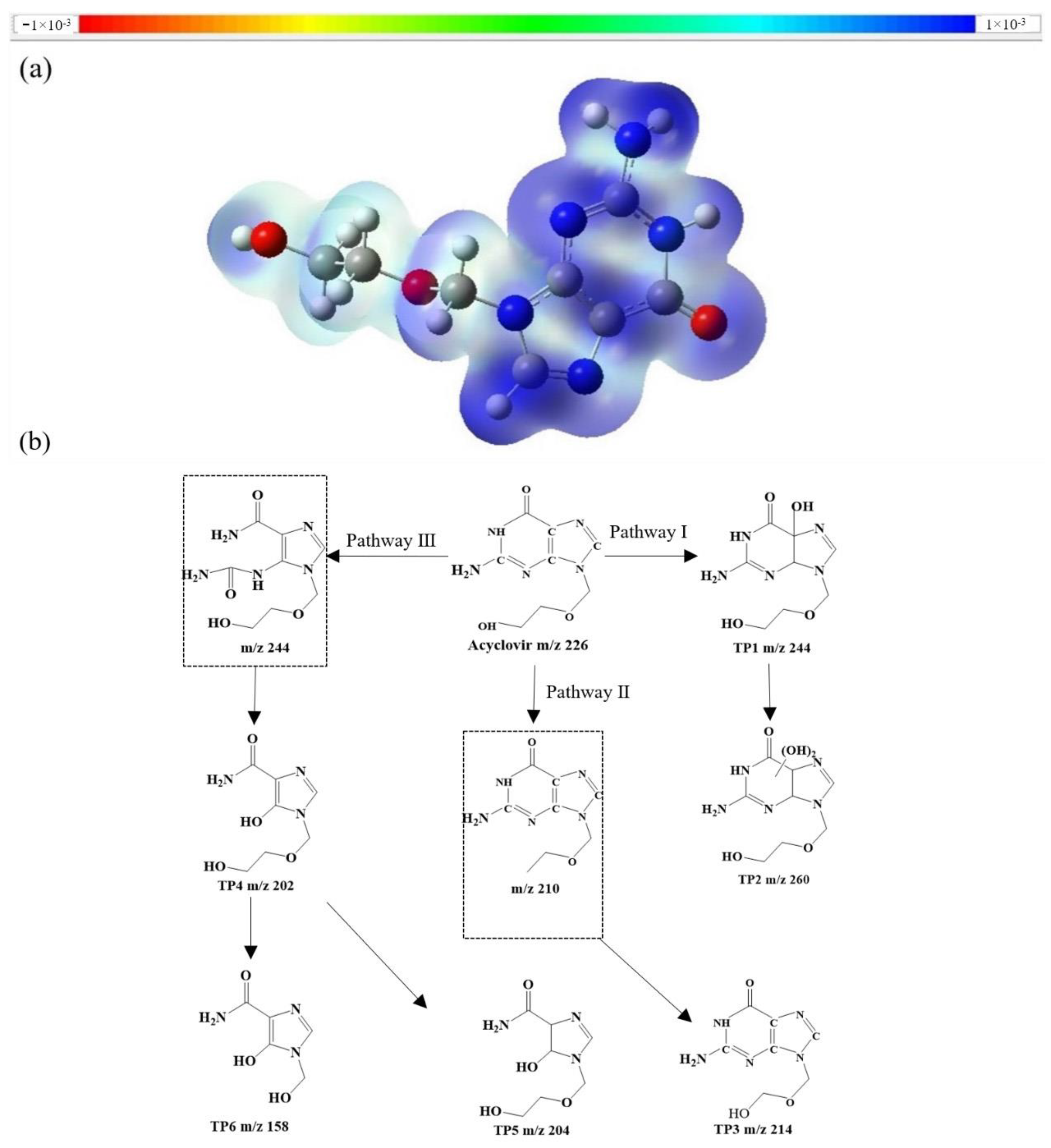
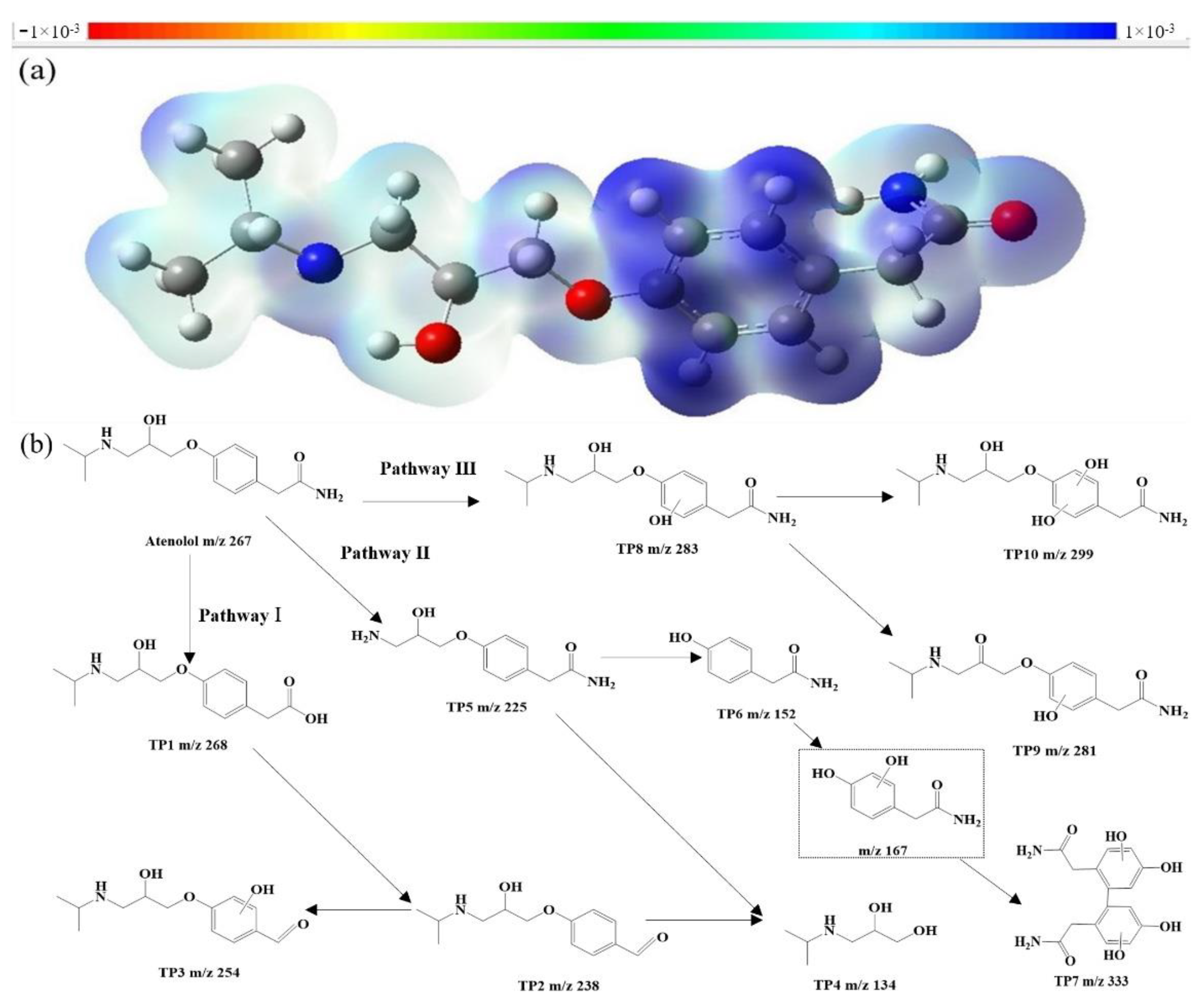
3.5. Oxidation Mechanisms and Degradation Pathways Speculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.P.; Qin, W.L.; Yuan, X.J.; Sun, L.; Pan, F.; Xia, D.S. Synergistic mechanism and degradation kinetics for atenolol elimination via integrated UV/ozone/peroxymonosulfate process. J. Hazard. Mater. 2021, 407, 124393. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Siciliano, A.; Guida, M.; Galdiero, E.; Amoresano, A.; Andreozzi, R.; Reis, N.M.; Puma, G.L.; Marotta, R. Photodegradation and ecotoxicology of acyclovir in water under UV254 and UV254/H2O2 processes. Water Res. 2017, 122, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fang, L.; Zhou, Y.C.; Zhang, T.Q.; Shao, Y. Comparison of UV/PDS and UV/H2O2 processes for the degradation of atenolol in water. J. Environ. Sci. 2013, 25, 1519–1528. [Google Scholar] [CrossRef]
- Liang, C.J.; Wang, Z.S.; Mohanty, N. Influences of carbonate and chloride ions on persulfate oxidation of trichloroethylene at 20 °C. Sci. Total. Environ. 2006, 370, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Kranina, E.I. China on the way to achieving carbon neutrality. Financ. J. 2021, 13, 51–61. [Google Scholar] [CrossRef]
- Bushukina, V.I. Specific features of renewable energy development in the world and russia. Financ. J. 2021, 13, 93–107. [Google Scholar] [CrossRef]
- Ma, X.; Chen, H.; Chen, R.; Hu, X. Direct and activated chlorine dioxide oxidation for micropollutant abatement: A review on kinetics, reactive sites, and degradation pathway. Water 2022, 14, 2028. [Google Scholar] [CrossRef]
- Parolini, M.; Pedriali, A.; Binelli, A. Application of a biomarker response index for ranking the toxicity of five pharmaceutical and personal care products (PPCPs) to the bivalve Dreissena polymorpha, Arch. Environ. Contam.Toxicol. 2013, 64, 439–447. [Google Scholar] [CrossRef]
- Lai, F.; Tian, F.X.; Xu, B.; Ye, W.K.; Gao, Y.Q.; Chen, C.; Xing, H.B.; Wang, B.; Xie, M.J.; Hu, X.J. A comparative study on the degradation of phenylurea herbicides by UV/persulfate process: Kinetics, mechanisms, energy demand and toxicity evaluation associated with DBPs. Chem. Eng. J. 2022, 428, 132088. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Gao, X.S.; Geng, J.J.; Li, S.L.; Wu, G.; Ren, H.Q. Degradation of three nonsteroidal anti-inflammatory drugs by UV/persulfate: Degradation mechanisms, efficiency in effluents disposal. Chem. Eng. J. 2019, 356, 1032–1041. [Google Scholar] [CrossRef]
- Hou, S.D.; Ling, L.; Shang, C.; Guan, Y.H.; Fang, J.Y. Degradation kinetics and pathways of haloacetonitriles by the UV/persulfate process. Chem. Eng. J. 2017, 320, 478–484. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Wu, G.; Geng, J.J.; Li, J.C.; Li, S.N.; Ren, H.Q. Kinetics and modeling of artificial sweeteners degradation in wastewater by the UV/persulfate process. Water Res. 2019, 150, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; An, N.; Shao, Y.S.; Gao, N.Y.; Du, E.; Xu, B. Experimental and simulation investigations of UV/persulfate treatment in presence of bromide: Effects on degradation kinetics, formation of brominated disinfection byproducts and bromate. Sep. Purif. Technol. 2020, 242, 116767. [Google Scholar] [CrossRef]
- Acero, J.L.; Benítez, F.J.; Real, F.J.; Rodríguez, E. Degradation of selected emerging contaminants by UV-activated persulfate: Kinetics and influence of matrix constituents. Sep. Purif. Technol. 2018, 201, 41–50. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.F.; Fang, X.H.; Zhang, L.; Li, J.J.; Xie, H.Y. Synergistic effect of photocatalytic degradation of hexabromocyclododecane in water by UV/TiO2/persulfate. Catalysts 2019, 9, 189. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Xiao, Y.J.; Zhong, Y.; Lim, T.T. Comparison of amoxicillin photodegradation in the UV/H2O2 and UV/persulfate systems: Reaction kinetics, degradation pathways, and antibacterial activity. Chem. Eng. J. 2019, 372, 420–428. [Google Scholar] [CrossRef]
- Li, M.K.; Wang, C.; Yau, M.L.; Bolton, J.R.; Qiang, Z.M. Sulfamethazine degradation in water by the VUV/UV process: Kinetics, mechanism and antibacterial activity determination based on a mini-fluidic VUV/UV photoreaction system. Water Res. 2017, 108, 348–355. [Google Scholar] [CrossRef]
- Li, M.; Li, W.T.; Bolton, J.R.; Blatchley, E.R.; Qiang, Z.M. Organic pollutant degradation in water by the vacuum-ultraviolet/ultraviolet/H2O2 process: Inhibition and enhancement roles of H2O2. Environ. Sci. Technol. 2019, 53, 912–918. [Google Scholar] [CrossRef]
- Neghi, N.; Krishnan, N.R.; Kumar, M. Analysis of metronidazole removal and micro-toxicity in photolytic systems: Effects of persulfate dosage, anions and reactor operation-mode. J. Environ. Chem. Eng. 2018, 6, 754–761. [Google Scholar] [CrossRef]
- Lin, Z.; Qin, W.L.; Sun, L.; Yuan, X.J.; Xia, D.S. Kinetics and mechanism of sulfate radical- and hydroxyl radical-induced degradation of Bisphenol A in VUV/UV/peroxymonosulfate system. J. Environ. Chem. Eng. 2020, 38, 101636. [Google Scholar] [CrossRef]
- Xiao, Y.J.; Zhang, L.F.; Zhang, W.; Lim, K.Y.; Webster, R.D.; Lim, T.T. Comparative evaluation of iodoacids removal by UV/persulfate and UV/H2O2 processes. Water Res. 2016, 102, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Lutze, H.V.; Bircher, S.; Rapp, I.; Kerlin, N.; Bakkour, R.; Geisler, M.; von Sonntag, C.; Schmidt, T.C. Degradation of chlorotriazine pesticides by sulfate radicals and the influence of organic matter. Environ. Sci. Technol. 2015, 49, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, J.; Pang, S.Y.; Guan, C.T.; Li, J.; Wang, Z.; Ma, J.; Luo, C.W. Degradation of iopamidol by three UV-based oxidation processes: Kinetics, pathways, and formation of iodinated disinfection byproducts. Chemosphere 2019, 221, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Wen, G.; Huang, T.L.; Deng, L.Y.; Ma, J. Study on enhanced ozonation degradation of para-chlorobenzoic acid by peroxymonosulfate in aqueous solution. Chem. Eng. J. 2015, 264, 399–403. [Google Scholar] [CrossRef]
- Li, M.X.; Sun, J.F.; Han, D.D.; Wei, B.; Mei, Q.; An, Z.X.; Wang, X.Y.; Cao, H.J.; Xie, J.; He, M.X. Theoretical investigation on the contribution of •OH, SO4•− and CO3•− radicals to the degradation of phenacetin in water: Mechanisms, kinetics, and toxicity evaluation. Ecotoxicol. Environ. Saf. 2020, 204, 110977. [Google Scholar] [CrossRef]
- Lee, M.; Zimmermann-Steffens, S.G.; Arey, J.S.; Fenner, K.; Gunten, U.V. Development of prediction models for the reactivity of organic compounds with ozone in aqueous solution by quantum chemical calculations: The role of delocalized and localized molecular orbitals. Environ. Sci. Technol. 2015, 49, 9925–9935. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lee, M.Y.; Wang, W.L.; Xu, Z.B.; Ye, B.; Wu, Q.Y.; Hu, H.Y. The application of UV/PS oxidation for removal of a quaternary ammonium compound of dodecyl trimethyl ammonium chloride (DTAC): The kinetics and mechanism. Sci. Total. Environ. 2019, 655, 1261–1269. [Google Scholar] [CrossRef]
- Liu, X.H.; Liu, Y.; Lu, S.Y.; Wang, Z.; Wang, Y.Q.; Zhang, G.D.; Guo, X.C.; Guo, W.; Zhang, T.T.; Xi, B.D. Degradation difference of ofloxacin and levofloxacin by UV/H2O2 and UV/PS (persulfate): Efficiency, factors and mechanism. Chem. Eng. J. 2020, 385, 123987. [Google Scholar] [CrossRef]
- Ding, X.X.; Gutierrez, L.; Croue, J.P.; Li, M.; Wang, L.J.; Wang, Y.R. Hydroxyl and sulfate radical-based oxidation of RhB dye in UV/H2O2 and UV/persulfate systems: Kinetics, mechanisms, and comparison. Chemosphere 2020, 253, 126655. [Google Scholar] [CrossRef]
- Yasmeen, H.; Zada, A.; Liu, S.X. Surface plasmon resonance electron channeled through amorphous aluminum oxide bridged ZnO coupled g-C3N4 significantly promotes charge separation for pollutants degradation under visible light. J. Photochem. Photobiol. A Chem. 2020, 400, 112681. [Google Scholar]
- Yasmeen, H.; Zada, A.; Liu, S.X. Dye loaded MnO2 and chlorine intercalated g-C3N4 coupling impart enhanced visible light photoactivities for pollutants degradation. J. Photochem. Photobiol. A Chem. 2019, 380, 111867. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zada, A.; Cui, N.; Liu, N.W.; Liu, M.H.; Yang, Y.Z.; Jiang, D.L.; Jiang, J.H.; Liu, S.Y. Synthesis of Ag loaded ZnO/BiOCl with high photocatalytic performance for the removal of antibiotic pollutants. Crystals 2021, 11, 981. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Transition metal/UV-based advanced oxidation technologies for water decontamination. Appl. Catal. B Environ. 2004, 54, 155–163. [Google Scholar] [CrossRef]
- Lescano, M.R.; Lopez, A.O.; Romero, R.L.; Zalazar, C.S. Degradation of chlorpyrifos formulation in water by the UV/H2O2 process: Lumped kinetic modelling of total organic carbon removal. J. Photoch. Photobio. A 2021, 404, 112924. [Google Scholar] [CrossRef]
- Yoon, S.H.; Jeong, S.; Lee, S. Oxidation of bisphenol A by UV/S2O82−: Comparison with UV/H2O2. Environ. Technol. 2012, 33, 123–128. [Google Scholar] [CrossRef]
- He, X.X.; Mezyk, S.P.; Michael, I.; Fatta-Kassinos, D.; Dionysiou, D.D. Degradation kinetics and mechanism of beta-lactam antibiotics by the activation of H2O2 and Na2S2O8 under UV-254nm irradiation. J. Hazard. Mater. 2014, 279, 375–383. [Google Scholar] [CrossRef]
- Guan, Y.H.; Ma, J.; Li, X.C.; Fang, J.Y.; Chen, L.W. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system. Environ. Sci. Technol. 2011, 45, 9308–9314. [Google Scholar] [CrossRef]
- Yang, Y.; Pignatello, J.J.; Ma, J.; Mitch, W.A. Comparison of halide impacts on the efficiency of contaminant degradation by sulfate and hydroxyl radical-based advanced oxidation processes (AOPs). Environ. Sci. Technol. 2014, 48, 2344–2351. [Google Scholar] [CrossRef]
- Gunten, U.V. Ozonation of drinking water: Part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 2003, 37, 1469–1487. [Google Scholar] [CrossRef]
- Lu, X.; Shao, Y.S.; Gao, N.Y.; Chen, J.X.; Zhang, Y.S.; Xiang, H.M.; Guo, Y.L. Degradation of diclofenac by UV-activated persulfate process: Kinetic studies, degradation pathways and toxicity assessments. Ecotoxicol. Environ. Saf. 2017, 141, 139–147. [Google Scholar] [CrossRef]
- Luo, C.W.; Jiang, J.; Ma, J.; Pang, S.Y.; Liu, Y.Z.; Song, Y.; Guan, C.T.; Li, J.; Jin, Y.X.; Wu, D.J. Oxidation of the odorous compound 2,4,6-trichloroanisole by UV activated persulfate: Kinetics, products, and pathways. Water Res. 2016, 96, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.L.; Lin, Z.; Dong, H.Y.; Yuan, X.J.; Qiang, Z.M.; Liu, S.; Xia, D.S. Kinetic and mechanistic insights into the abatement of clofibric acid by integrated UV/ozone/peroxydisulfate process: A modeling and theoretical study. Water Res. 2020, 186, 116336. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Fu, Y.; Shang, C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environ. Sci. Technol. 2014, 48, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.H.; Dong, W.; Ji, Y.F.; Kong, D.Y.; Huang, Q.G. Natural organic matter exposed to sulfate radicals increases its potential to form halogenated disinfection byproducts. Environ. Sci. Technol. 2016, 50, 5060–5067. [Google Scholar] [CrossRef]
- Wu, Z.H.; Chen, C.Y.; Zhu, B.Z.; Huang, C.H.; An, T.C.; Meng, F.A.; Fang, J.Y. Reactive nitrogen species are also involved in the transformation of micropollutants by the UV/Monochloramine process. Environ. Sci. Technol. 2019, 53, 11142–11152. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Chu, W. Degradation of a xanthene dye by Fe(II)-mediated activation of oxone process. J. Hazard. Mater. 2011, 186, 1455–1461. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Ghanbari, F.; Zahedi, A. Coupling electrooxidation and oxone for degradation of 2,4-Dichlorophenoxyacetic acid (2,4-D) from aqueous solutions. J. Water Process Eng. 2018, 22, 203–209. [Google Scholar] [CrossRef]
- Wang, F.G.; Wang, W.J.; Yuan, S.J.; Wang, W.; Hu, Z.H. Comparison of UV/H2O2 and UV/PS processes for the degradation of thiamphenicol in aqueous solution. J. Photoch. Photobio. A 2017, 348, 79–88. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.T.; Sun, K.; Lin, C.Y.; Ma, J.; He, M.C.; Ouyang, W. Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: A review. Chem. Eng. J. 2019, 372, 836–851. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Realization of conceptual density functional theory and information-theoretic approach in Multiwfn program. Concept. Density Funct. Theory 2022, 2, 631–647. [Google Scholar]
- Guo, H.G.; Ke, T.L.; Gao, N.Y.; Liu, Y.; Cheng, X. Enhanced degradation of aqueous norfloxacin and enrofloxacin by UV-activated persulfate: Kinetics, pathways and deactivation. Chem. Eng. J. 2017, 316, 471–480. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- An, T.C.; An, J.B.; Gao, Y.P.; Li, G.Y.; Fang, H.S.; Song, W.H. Photocatalytic degradation and mineralization mechanism and toxicity assessment of antivirus drug acyclovir: Experimental and theoretical studies. Appl. Catal. B Environ. 2015, 164, 279–287. [Google Scholar] [CrossRef]
- Miao, D.; Peng, J.B.; Zhou, X.H.; Qian, L.; Wang, M.J.; Zhai, L.; Gao, S.X. Oxidative degradation of atenolol by heat-activated persulfate: Kinetics, degradation pathways and distribution of transformation intermediates. Chemosphere 2018, 207, 174–182. [Google Scholar] [CrossRef]
- Li, M.K.; Qiang, Z.M.; Bolton, J.R.; Qu, J.H.; Li, W.T. A mini-fluidic UV photoreaction system for bench-scale photochemical studies. Environ. Sci. Technol. Lett. 2015, 2, 297–301. [Google Scholar] [CrossRef]
- Scholes, M.L.; Schuchmann, M.N.; Sonntag, C.V. Enhancement of radiation-induced base release from nucleosides in alkaline solution: Essential role of the O•− radical. Int. J. Radiat. Biol. 1992, 61, 443–449. [Google Scholar] [CrossRef]
- Yang, Y.; Pignatello, J.J.; Ma, J.; Mitch, W.A. Effect of matrix components on UV/H2O2 and UV/S2O82− advanced oxidation processes for trace organic degradation in reverse osmosis brines from municipal wastewater reuse facilities. Water Res. 2016, 89, 192–200. [Google Scholar] [CrossRef]
- Grebel, J.E.; Pignatello, J.J.; Mitch, W.A. Effect of halide ions and carbonates on organic contaminant degradation by hydroxyl radical-based advanced oxidation processes in saline waters. Environ. Sci. Technol. 2010, 44, 6822–6828. [Google Scholar] [CrossRef]
- Yu, X.Y.; Bao, Z.C.; Barker, J.R. Free radical reactions involving Cl•, Cl2•−, and SO4•− in the 248 nm Photolysis of Aqueous Solutions Containing S2O82− and Cl−. J. Phys. Chem. 2004, 108, 295–308. [Google Scholar] [CrossRef]
- Peyton, G.R. The free-radical chemistry of persulfate-based total organic carbon analyzers. Mar. Chem. 1993, 41, 91–103. [Google Scholar] [CrossRef]
- Klaning, U.K. Laser flash photolysis of HClO, ClO−, HBrO, and BrO− in aqueous solution. Reactions of Cl− and Br− atoms. Ber. Bunsenges. Phys. Chem. 1985, 89, 243–245. [Google Scholar] [CrossRef]
- Zehavi, D.; Rabani, J. Oxidation of aqueous bromide ions by hydroxyl radicals. Pulse radiolytic investigation. J. Phys. Chem. 1972, 3, 76. [Google Scholar] [CrossRef]
- Lin, M.Z.; Archirel, P.; Van-Oanh, N.T.; Muroya, Y.; Fu, H.; Yan, Y.; Nagaishi, R.; Kumagai, Y.; Katsumura, Y.; Mostafavi, M. Temperature dependent absorption spectra of Br−, Br2•−, and Br3− in aqueous solutions. J. Phys. Chem. A. 2011, 115, 4241–4247. [Google Scholar] [CrossRef] [PubMed]
- Wagner, I.; Strehlow, H. On the flash-photolysis of bromide ions in aqueous-solutions. Ber. Bunsenges. Phys. Chem. 1987, 91, 1317–1321. [Google Scholar] [CrossRef]
- Gunten, U.V.; Oliveras, Y. Advanced oxidation of bromide-containing waters: Bromate formation mechanisms. Environ. Sci. Technol. 1998, 32, 63–70. [Google Scholar] [CrossRef]
- Matthew, B.M.; Anastasio, C. A chemical probe technique for the determination of reactive halogen species in aqueous solution Part 1-bromide solutions. Atmos. Chem. Phys. 2006, 6, 2423–2437. [Google Scholar] [CrossRef]
- Neta, P.; Madhavan, V.; Zemel, H.; Fessenden, R.W. Rate constants and mechanisms of reaction of SO4•−,with aromatic compounds. J. Am. Chem. Soc. 1977, 5, 163–164. [Google Scholar] [CrossRef]
- Sutton, H.C.; Downes, M.T. Reactions of the HO2 radical in aqueous solution with bromine and related compounds. J. Chem. Soc. Faraday Trans 1 Phys. Chem. Condens. Phases 1972, 68, 1498–1507. [Google Scholar] [CrossRef]
- Heeb, M.B.; Criquet, J.; Zimmermann-Steffens, S.G.; Gunten, U.V. Oxidative treatment of bromide-containing waters: Formation of bromine and its reactions with inorganic and organic compounds—A critical review. Water Res. 2014, 48, 15–42. [Google Scholar] [CrossRef]
- Beckwith, R.C.; Wang, T.X.; Margerum, D.W. Equilibrium and kinetics of bromine hydrolysis. Inorg. Chem. 1996, 35, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Yang, Y.; Pang, S.Y.; Zhang, L.Q.; Ma, J.; Luo, C.W.; Guan, C.T.; Jiang, J. Mechanistic insight into suppression of bromate formation by dissolved organic matters in sulfate radical-based advanced oxidation processes. Chem. Eng. J. 2018, 333, 200–205. [Google Scholar] [CrossRef]
- Schwarz, H.A.; Bielski, B.H.J. Reactions of hydroperoxo and superoxide with iodine and bromine and the iodide (I2−) and iodine atom reduction potentials. J. Phys. Chem. 1986, 90, 1445–1448. [Google Scholar] [CrossRef]
- Buxton, G.V.; Dainton, F.S. The radiolysis of aqueous solutions of oxybromine compounds; the spectra and reactions of BrO and BrO2. Math. Phys. Sci. 1968, 304, 427–439. [Google Scholar]
| Radical Species (M) | PDS (mM) | pH | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.3 | 0.6 | 1.0 | 6.0 | 9.0 | 10.0 | 11.0 | |
| [•OH]ss × 10–14 | 1.00 | 3.00 | 9.83 | 28.72 | 9.44 | 12.27 | 17.72 | 17.86 |
| [SO4•–]ss × 10–13 | 1.46 | 3.39 | 6.03 | 8.38 | 6.13 | 3.83 | 2.66 | 1.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Qin, W.; Sun, L.; Dong, H.; Yuan, X.; Pan, F.; Xia, D. Insights into the Kinetics, Theoretical Model and Mechanism of Free Radical Synergistic Degradation of Micropollutants in UV/Peroxydisulfate Process. Water 2022, 14, 2811. https://doi.org/10.3390/w14182811
Liu Z, Qin W, Sun L, Dong H, Yuan X, Pan F, Xia D. Insights into the Kinetics, Theoretical Model and Mechanism of Free Radical Synergistic Degradation of Micropollutants in UV/Peroxydisulfate Process. Water. 2022; 14(18):2811. https://doi.org/10.3390/w14182811
Chicago/Turabian StyleLiu, Zhixiong, Wenlei Qin, Lei Sun, Huiyu Dong, Xiangjuan Yuan, Fei Pan, and Dongsheng Xia. 2022. "Insights into the Kinetics, Theoretical Model and Mechanism of Free Radical Synergistic Degradation of Micropollutants in UV/Peroxydisulfate Process" Water 14, no. 18: 2811. https://doi.org/10.3390/w14182811


_Dionysiou.jpg)




