Inorganic Nitrogen Uptake Characteristics of Three Typical Bloom-Forming Algae in the East China Sea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Strains and Culture Conditions
2.2. Time-Course Experiments
2.3. Nutrient Uptake Experiments
2.4. Extraction of NR and GS
2.5. Analysis of NR and GS Activities
2.6. Data Analyzing
3. Results
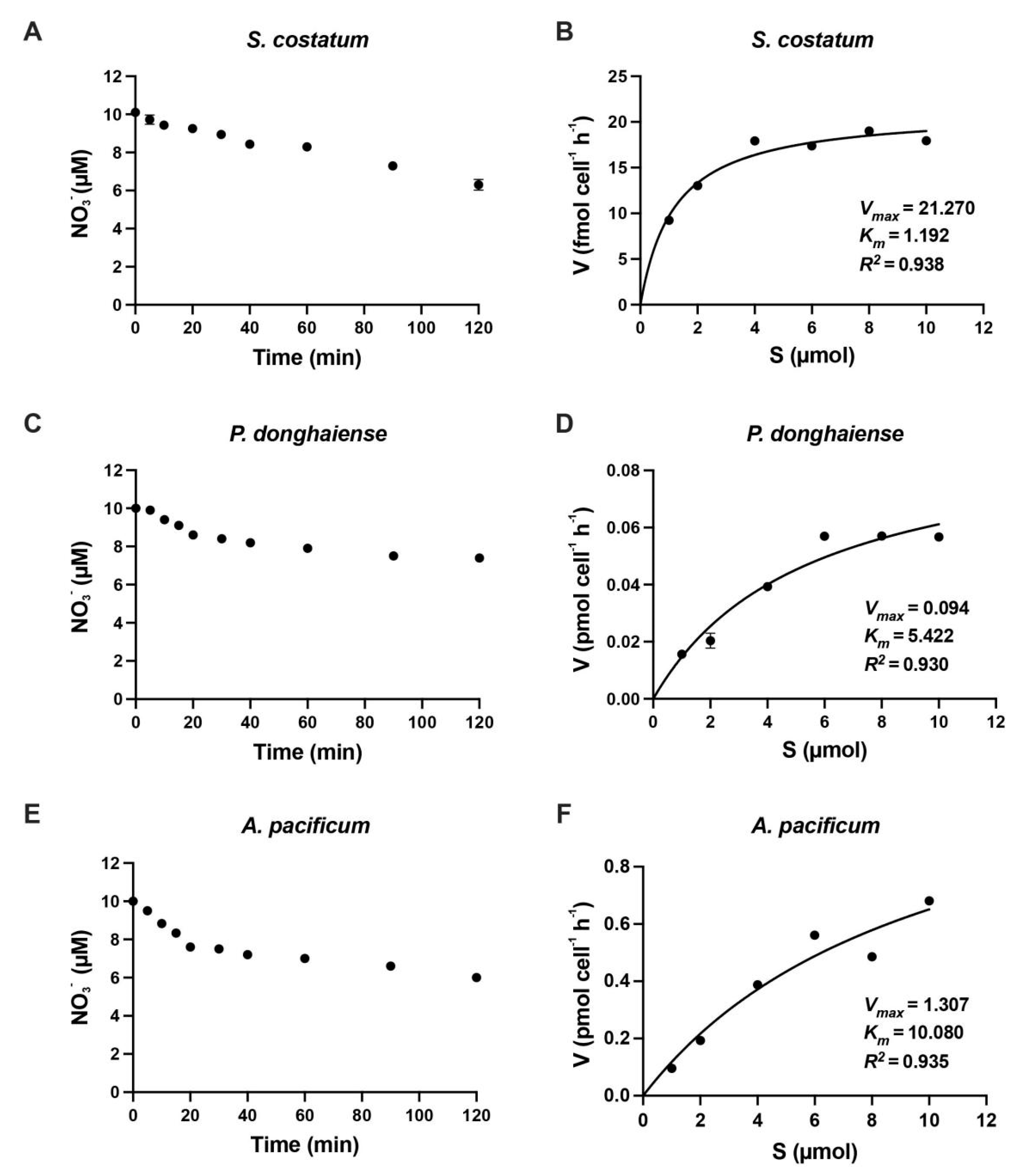
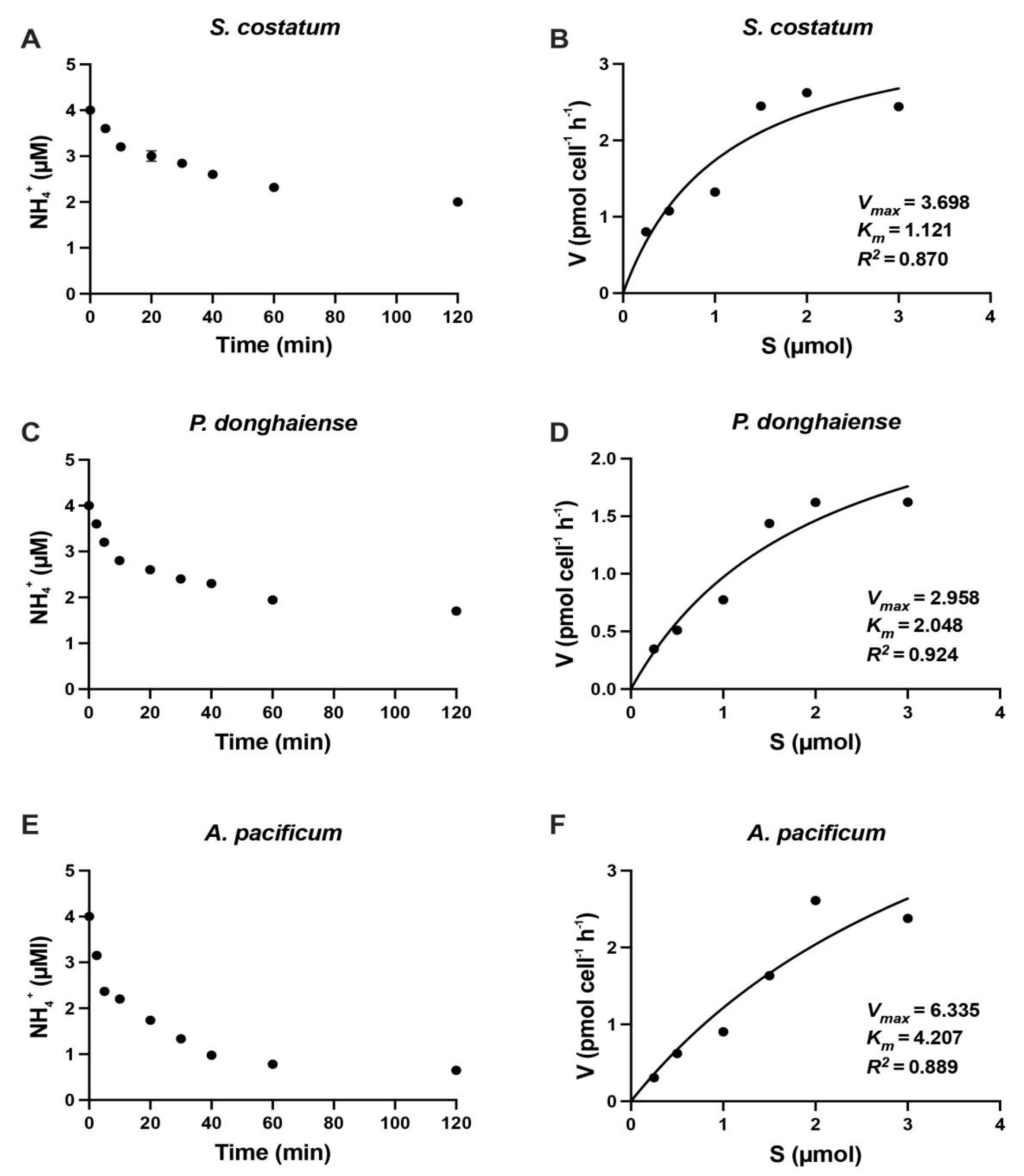
3.1. Reaction Time in N Uptake Kinetics Experiment
3.2. Kinetics of Inorganic N Uptake by S. costatum, P. donghaiense and A. pacificum
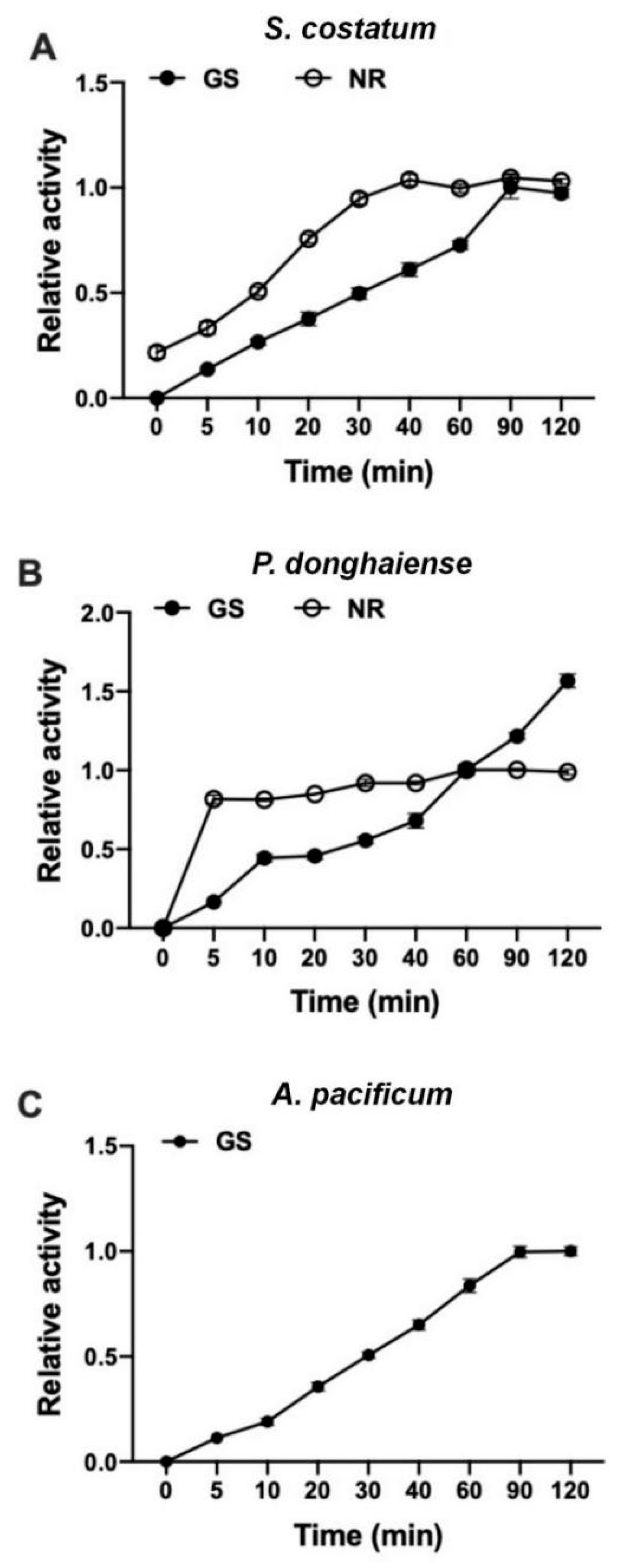
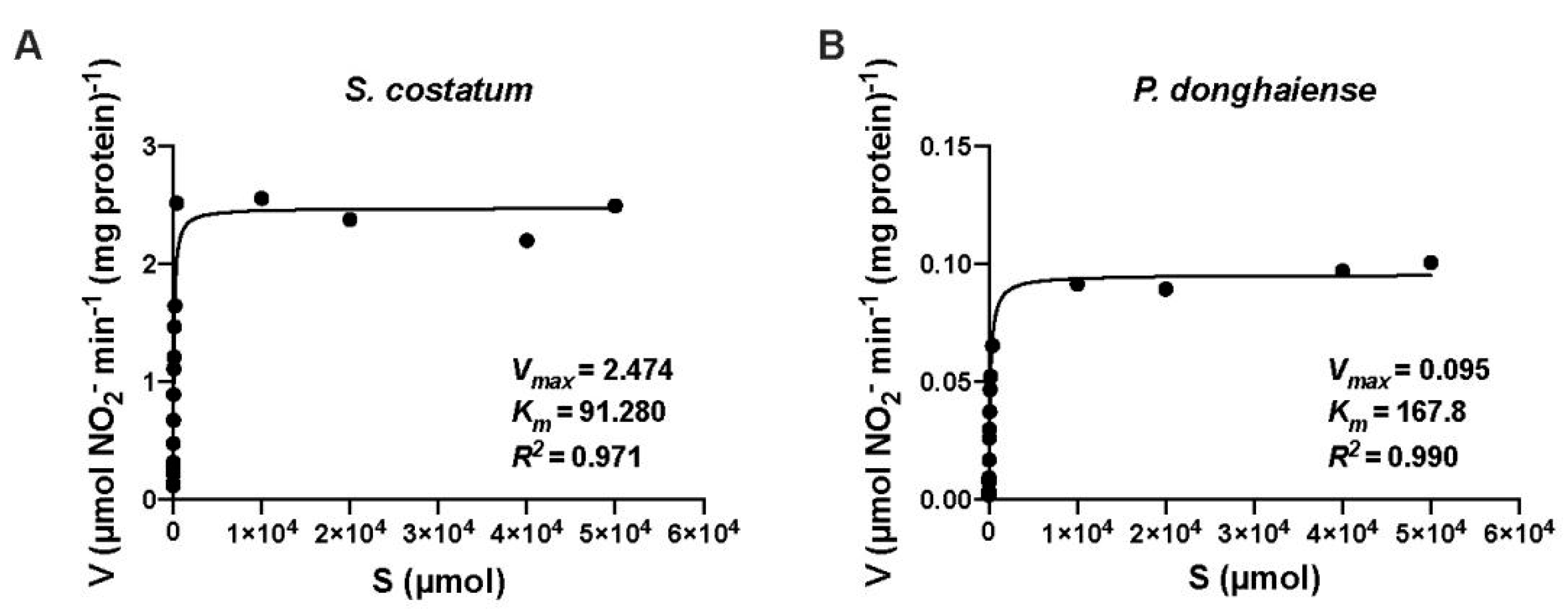
3.3. NR Kinetics of S. costatum and P. donghaiense
3.4. GS Kinetics of S. costatum, P. donghaiense and A. pacificum
4. Discussion
4.1. Uptake Kinetics of Inorganic N by S. costatum, P. donghaiense and A. pacificum
4.2. Enzymatic Kinetics of Inorganic N by S. costatum, P. donghaiense and A. pacificum
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glibert, P.M.; Berdalet, E.; Burford, M.A.; Pitcher, G.; Zhou, M.J. Global Ecology and Oceanography of Harmful Algal Blooms; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Lewitus, A.; et al. Eutrophication and harmful algal blooms: A scientifific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Killberg-Thoreson, L.; Mulholland, M.R.; Heil, C.A.; Sanderson, M.P.; O’Neil, J.M.; Bronk, D.A. Nitrogen uptake kinetics in field populations and cultured strains of Karenia brevis. Harmful Algae 2014, 38, 73–85. [Google Scholar] [CrossRef]
- Cochlan, W.P.; Bronk, D.A. Nitrogen uptake kinetics in the Ross Sea, Antarctica. Deep Sea Res. II 2001, 48, 4127–4153. [Google Scholar] [CrossRef]
- Shi, F.; Wei, X.X.; Feng, J.F.; Zhu, L. Nitrogen uptake kinetics of a diatom and model prediction analysis in different inorganic nitrogen conditions. J. Agro Environ. Sci. 2018, 37, 1833–1841. [Google Scholar]
- Lecorre, P.; Lhelguen, S.; Wafar, M. Nitrogen-source for uptake by Gyrodinium cf. aureolum in a tidal front. Limnol. Oceanogr. 1993, 38, 446–451. [Google Scholar]
- Yamamoto, T.; Oh, S.J.; Kataoka, Y. Growth and uptake kinetics for nitrate, ammonium and phosphate by the toxic dinoflagellate Gymnodinium catenatum isolated from Hiroshima Bay, Japan. Fish. Sci. 2011, 70, 108–115. [Google Scholar] [CrossRef]
- Maguer, J.F.; Helguen, S.L.; Madec, C.; Labry, C.; Corre, P.L. Nitrogen uptake and assimilation kinetics in Alexandrium minutum (Dinophyceae): Effect of N-limited growth rate on nitrate and ammonium interactions. J. Phycol. 2007, 43, 295–303. [Google Scholar] [CrossRef]
- Closset, I.; McNair, H.M.; Brzezinski, M.A.; Krause, J.W.; Thamatrakoln, K.K.; Jones, J.L. Diatom response to alterations in upwelling and nutrient dynamics associated with climate forcing in the California Current System. Limnol. Oceanogr. 2021, 66, 1578–1593. [Google Scholar] [CrossRef]
- Lomas, M.W.; Glibert, P.M. Comparisons of nitrate uptake, storage, and reduction in marine diatoms and flagellates. J. Phycol. 2000, 36, 903–913. [Google Scholar] [CrossRef]
- Dortch, Q. The interaction between ammonium and nitrate uptake in phytoplankton. Mar. Ecol. Prog. 1990, 61, 183–201. [Google Scholar] [CrossRef]
- Glibert, P.M.; Wilkerson, F.P.; Dugdale, R.C.; Raven, J.A.; Dupont, C.L.; Leavitt, P.R.; Parker, A.E.; Burkholder, J.M.; Kana, T.M. Pluses and minuses of ammonium and nitrate uptake and assimilation by phytoplankton and implications for productivity and community composition, with emphasis on nitrogen-enriched conditions. Limnol. Oceanogr. 2016, 61, 165–197. [Google Scholar] [CrossRef]
- Capone, D.G. The marine microbial nitrogen cycle. In Microbial Ecology of the Oceans; David, L.K., Ed.; John Wiley and Sons INC.: New York, NY, USA, 2008; pp. 1–6. [Google Scholar]
- Crawford, N.M. Nitrate: Nutrient and signal for plant growth. Plant Cell 1995, 7, 859–868. [Google Scholar]
- Granbom, M.; Chow, F.; Lopes, P.F.; de Oliveira, M.C.; Colepicolo, P.; de Paula, E.J.; Pedersén, M. Characterisation of nitrate reductase in the marine macroalga Kappaphycus alvarezii (Rhodophyta). Aquat. Bot. 2004, 78, 295–305. [Google Scholar] [CrossRef]
- Bender, D.; Schwarz, G. Nitrite-dependent nitric oxide synthesis by molybdenum enzymes. FEBS Lett. 2018, 592, 797. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Yan, C.; Zhang, X.Y.; Peng, W.; Geng, D.G.; Zhao, S.M.; Zhang, L.M.; Sun, Y.R. Isolation and characterization of a nitrate reductase deficient mutant of Chlorella ellipsoidea (Chlorophyta). J. Appl. Phycol. 2005, 17, 281–286. [Google Scholar] [CrossRef]
- Castro-Rodríguez, V.; García-Gutiérrez, A.; Canales, J.; Avila, C.; Kirby, E.G.; Cánovas, F.M. The glutamine synthetase gene family in populus. BMC Plant Biol. 2011, 11, 119. [Google Scholar] [CrossRef]
- Alaoui, S.; Diez, J.; Toribio, R.; Gómez-Baena, G.; Dufresne, A.; García-Fernández, J.M. Glutamine synthetase from the marine cyanobacteria Prochlorococcus spp.: Characterization, phylogeny and response to nutrient limitation. Environ. Microbiol. 2003, 5, 412–423. [Google Scholar] [CrossRef]
- Mulholland, M.R.; Lomas, M.W. Nitrogen uptake and assimilation-sciencedirect. In Nitrogen in the Marine Environment, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 303–384. [Google Scholar]
- Zhou, M.J.; Zhu, M.Y. Progress of the project “Ecology and Oceanography of Harmful Algal Blooms in China”. Adv. Earth Sci. 2006, 21, 673–679. [Google Scholar]
- Yan, T.; Zhou, M.J.; Qian, P.Y. Competition among dinoflagellate Alexandrium tamarense, raphidophyte Heterosigma carterae and diatom Skeletonema costatum under combinations of two temperatures and five salinities. J. Oceanol. Limnol. 2003, 21, 245–250. [Google Scholar]
- Li, R.X.; Zhu, M.Y.; Wang, Z.L.; Shi, X.Y.; Chen, B.Z. Mesocosm experiment on competition between two HAB species in East China Sea. Chin. Appl. Ecol. 2003, 14, 1049–1054. [Google Scholar]
- Chen, H.L.; Lu, S.H.; Zhang, C.S.; Zhu, D.D. A Survey on the red tide of Prorocentrum donghaiense in East China Sea, 2004. Ecol. Sci. 2006, 25, 226–230. [Google Scholar]
- Zhang, C.S.; Wang, J.T.; Zhu, D.D.; Shi, X.Y.; Wang, X.L. The preliminary analysis of nutrients in harmful algal blooms in the East China Sea in the spring and summer of 2005. Acta Oceanol. Sin. 2008, 30, 153–159. [Google Scholar]
- Zhou, M.J.; Shen, Z.L.; Yu, R.C. Responses of a coastal phytoplankton community to increased nutrient input from the Changjiang (Yangtze) River. Cont. Shelf Res. 2008, 28, 1483–1489. [Google Scholar] [CrossRef]
- Harrison, P.; Parslow, J.S.; Conway, H.L. Determination of nutrient uptake kinetic parameters: A comparison of methods. Mar. Ecol. Prog. Ser. 1989, 52, 301–312. [Google Scholar] [CrossRef]
- Nishikawa, T.; Tarutani, R.; Yamamoto, R. Nitrate and phosphate uptake kinetics of the harmful diatom Coscinodiscus wailesii, a causative organism in the bleaching of aquacultured Porphyra thalli. Harmful Algae 2010, 9, 563–567. [Google Scholar] [CrossRef]
- Wood, E.D.; Armstrong, F.A.J.; Richards, F.A. Determination of nitrate in sea water by cadmium-copper reduction to nitrate. J. Mar. Biol. Assoc. UK 1967, 47, 23–31. [Google Scholar] [CrossRef]
- Solórzano, L. Determination of ammonium in natural waters by the phenolhypochlorite method. Limnol. Occanogr. 1969, 14, 799–801. [Google Scholar] [CrossRef]
- Takabayashi, M.; Wilkerson, F.P.; Robertson, D. Response of glutamine synthetase gene transcription and enzyme activity to external nitrogen sources in the diatom Skeletonema costatum (bacillariophyceae)1. J. Phycol. 2005, 41, 84–94. [Google Scholar] [CrossRef]
- Matsuda, A.; Nishijima, T.; Fukami, K. Effects of nitrogenous and phosphorus nutrients on the growth of toxic dinoflagellate Alexandrium catenella. Nippon Suisan Gakk. 1999, 65, 847–855. [Google Scholar] [CrossRef]
- Collos, Y.; Gagne, C.; Laabir, M.; Vaquer, A. Nitrogenous nutrition of Alexandrium catenella (Dinophyceae) in cultures and in Thau lagoon, southern France. J. Phycol. 2004, 40, 96–103. [Google Scholar] [CrossRef]
- MacIsaac, J.J.; Grunseich, G.S.; Glover, H.E.; Clarice, M.Y. Light and nutrient limitation in Gonyaulax excavate: Nitrogen and carbon trace results. In Toxic Dinoflagellate Blooms; Elsevier: New York, NY, USA, 1979; pp. 107–110. [Google Scholar]
- Eppley, R.W.; Coatsworth, J.L.; Solorzano, L. Studies of nitrate reductase in marine phytoplankton. Limnol. Oceanogr. 1969, 14, 194–205. [Google Scholar] [CrossRef]
- Nakamura, Y. Kinetics of nitrogen- or phosphorus-limited growth and effects of growth conditions on nutrient uptake in Chattonella antiqua. J. Oceanogr. Soc. Japan 1985, 41, 381–387. [Google Scholar] [CrossRef]
- Bressler, S.L.; Ahmed, S.I. Detection of glutamine synthetase activity in marine phytoplankton: Optimization of the biosynthetic assay. Mar. Ecol. Prog. Ser. 1984, 14, 207–217. [Google Scholar] [CrossRef]
- Eppley, R.W.; Thomas, W.H. Comparison of half-saturation constants for growth and nitrate uptake of marine phytoplankton. J. Phycol. 2010, 5, 375–379. [Google Scholar] [CrossRef]
- Bonachela, J.A.; Levin, R. Dynamic model of flexible phytoplankton nutrient uptake. Proc. Natl. Acad. Sci. USA 2011, 108, 20633–20638. [Google Scholar] [CrossRef]
- Huang, K.; Feng, Q.; Zhang, Y.; Ou, L.; Qi, Y. Comparative uptake and assimilation of nitrate, ammonium, and urea by dinoflagellate Karenia mikimotoi and diatom Skeletonema costatum s.l. in the coastal waters of the East China Sea. Mar. Pollut. Bull. 2020, 155, 111200. [Google Scholar] [CrossRef]
- Ou, L.J. Ecophysiological Responses of Typical Harmful Algal Bloom Species to Phosphorus; Xiamen University: Xiamen, China, 2008. [Google Scholar]
- Liao, T.C.; Yu, H.G.; Dai, C.J.; Zhao, M. Impact of cell size effect on nutrient-phytoplankton dynamics. Complexity 2019, 2019, 8205696. [Google Scholar] [CrossRef]
- Button, D.K. Biochemical basis for whole-cell uptake kinetics: Specific affinity, oligotrophic capacity, and the meaning of the Michaelis constant. Appl. Environ. Microbiol. 1991, 57, 2033–2038. [Google Scholar] [CrossRef]
- Joseph, L.; Villareal, T.A. Nitrate reductase activity as a measure of nitrogen incorporation in Rhizosolenia formasa (H. Peragallo): Internalnitrate and diel effects. J. Exp. Mar. Biol. Ecol. 1998, 229, 159–176. [Google Scholar] [CrossRef]
- Lopes, P.F.; de Oliveira, M.C.; Colepicolo, P. Characterization and daily variation of nitrate reductase in Gracilaria tenuistipitata (Rhodophyta). Biochem. Biophys. Res. Commun. 2002, 295, 50–54. [Google Scholar] [CrossRef]
- Kim, J.; Brown, C.M.; Min, K.K.; Burrows, E.H.; Falkowski, P.G. Effect of cell cycle arrest on intermediate metabolism in the marine diatom Phaeodactylum tricornutum. Proc. Natl. Acad. Sci. USA 2017, 114, E8007–E8016. [Google Scholar] [CrossRef]
- Ramalho, C.B.; Hastings, J.W.; Colepicolo, P. Circadian oscillation of nitrate reductase activity in Gonyaulax polyedra is due to changes in cellular protein levels. Plant Physiol. 1995, 107, 225–231. [Google Scholar] [CrossRef]
- Varela, D.E.; Harrison, P.J. Effect of ammonium on nitrate utilization by Emiliania huxleyi, a coccolithophore from the oceanic northeastern Pacific. Mar. Ecol. Prog. Ser. 1999, 186, 67–74. [Google Scholar] [CrossRef]
- Serra, J.L.; Llama, M.J.; Cadenas, E. Characterization of the nitrate reductase enzyme activity in the diatom Skeletonema costatum. Plant Sci. Lett. 1978, 13, 41–48. [Google Scholar] [CrossRef]
- Gao, Y.; Smith, G.J.; Alberte, R.S. Nitrate reductase from the marine diatom Skeletonema costatum biochemical and immunological characterization. Plant Physiol. 1993, 103, 1437–1445. [Google Scholar] [CrossRef]
- Berges, J.A.; Harrison, P.J. Nitrate reductase activity quantitatively predicts the rate of nitrate incorporation under steady state light limitation: A revised assay and characterization of the enzyme in three species of marine phytoplankton. Limnol. Oceanogr. 1995, 40, 82–93. [Google Scholar] [CrossRef]
- García-Fernández, J.M.A.; López-Ruiz, J.; Alhama, J.D. Light regulation of glutamine synthetase in the green alga Monoraphidium braunii. J. Plant Physiol. 1995, 146, 577–583. [Google Scholar] [CrossRef]
- Maurin-Defossez, C.; le Gal, Y. Diel periodicity of glutamine synthetase activity during the cell cycle of Emiliania huxleyi. Plant Physiol. Biochem. 1998, 36, 233–236. [Google Scholar] [CrossRef]
| Species | KS (µM) | Vmax (pmol cell−1 h−1) | Reference |
|---|---|---|---|
| Alexandrium catenella | 7.7 | — | [33] |
| Alexandrium pacificum | 10.02 | 1.30 | Present study |
| Alexandrium catenella | 0.6–28.1 | — | [34] |
| Alexandrium tamarense | 2.84 | — | [35] |
| Alexandrium minutum | 0.22–0.28 | 0.29–0.40 | [9] |
| Gymnodinium catenatum | 7.59 | 6.48 | [8] |
| Prorocentrum donghaiense | 5.98 | 0.098 | Present study |
| Skeletonema costatum | 1.19 | 0.021 | Present study |
| Skeletonema costatum | 0.4–0.5 | 0.063 | [36] |
| Prorocentrum minimum | 5.0 | 0.102 | [11] |
| Thalassiosira weissflogii | 2.8 | 0.310 | [11] |
| Chaetoceros sp. | 3.1 | 0.024 | [11] |
| Pavlova lutheri | 22.7 | 0.021 | [11] |
| Species | KS (µM) | Vmax (pmol cell−1 h−1) | Reference |
|---|---|---|---|
| Alexandrium catenella | 3.3 | — | [33] |
| Alexandrium pacificum | 4.27 | 4.21 | Present study |
| Alexandrium catenella | 0.6–28.1 | — | [34] |
| Alexandrium minutum | 0.25–0.33 | 0.65–0.82 | [9] |
| Alexandrium tamarense | 1.49 | — | [35] |
| Gymnodinium catenatum | 33.6 | 3.37 | [8] |
| Prorocentrum donghaiense | 2.04 | 0.296 | Present study |
| Skeletonema costatum | 1.12 | 0.037 | Present study |
| Skeletonema costatum | 0.8–3.6 | — | [36] |
| Chattonella antiqua | 2.19 | 2.02 | [37] |
| Species | Low Concentration | High Concentration | Reference | ||
|---|---|---|---|---|---|
| KS (µM) | Vmax (µmol NO2− min−1 (mg protein)−1) | KS (µM) | Vmax (µmol NO2− min−1 (mg protein)−1) | ||
| Skeletonemacostatum | 82.69 | 2.26 | 91.29 | 2.4 | Present study |
| Prorocentrumdonghaiense | 168.48 | 0.29 | 168.48 | 0.29 | Present study |
| Skeletonemacostatum | 290 | — | — | — | [11] |
| Species | Low Concentration | High Concentration | Reference | ||
|---|---|---|---|---|---|
| KS (µM) | Vmax (µmol PO43− min−1 (mg protein)−1) | KS (µM) | Vmax (µmol PO43− min−1 (mg protein)−1) | ||
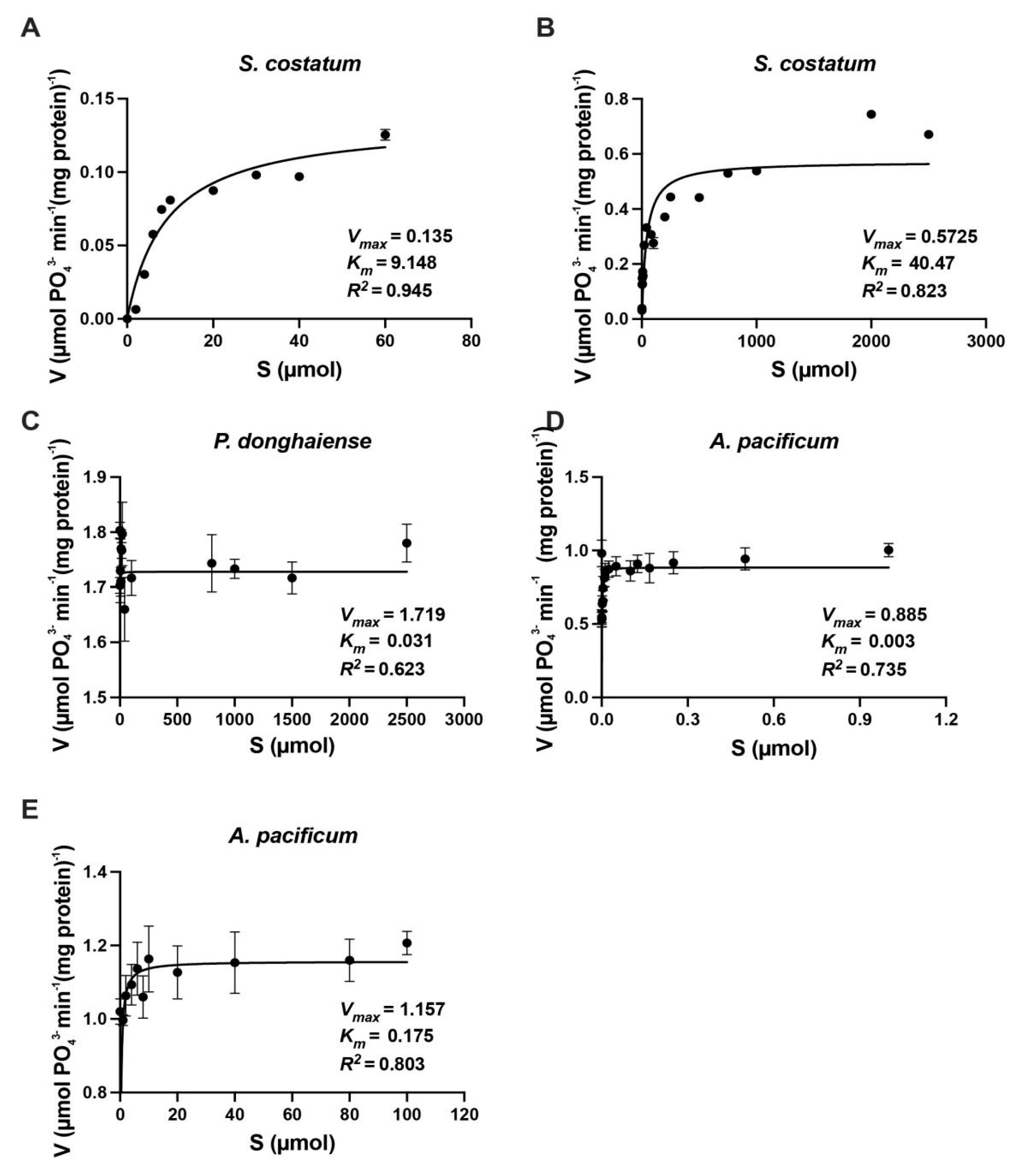
| Skeletonemacostatum | 9.30 | 0.34 | 41.1 | 0.57 | Present study |
| Prorocentrumdonghaiense | 0.04 | 0.85 | 0.04 | 0.85 | Present study |
| Alexandriumpacificum | 0.16 | 1.15 | 10.35 | 1.66 | Present study |
| Skeletonema costatum | 8.20 | 0.32 | — | — | [38] |
| Isochrysisgalbana | 8.20 | 0.22 | — | — | [38] |
| Pavlovalutheri | 1.80 | 0.39 | — | — | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, G.; Chen, H.; Gu, H.; Zhang, Y.; Li, R.; Zhang, S. Inorganic Nitrogen Uptake Characteristics of Three Typical Bloom-Forming Algae in the East China Sea. Water 2022, 14, 2580. https://doi.org/10.3390/w14162580
Ding G, Chen H, Gu H, Zhang Y, Li R, Zhang S. Inorganic Nitrogen Uptake Characteristics of Three Typical Bloom-Forming Algae in the East China Sea. Water. 2022; 14(16):2580. https://doi.org/10.3390/w14162580
Chicago/Turabian StyleDing, Guangmao, Huorong Chen, Haifeng Gu, Youquan Zhang, Rongmao Li, and Shufeng Zhang. 2022. "Inorganic Nitrogen Uptake Characteristics of Three Typical Bloom-Forming Algae in the East China Sea" Water 14, no. 16: 2580. https://doi.org/10.3390/w14162580






