Abattoir Wastewater Treatment in Anaerobic Co-Digestion with Sugar Press Mud in Batch Reactor for Improved Biogas Yield
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Sludge and Substrates
2.2. Batch Experiment Set up and Operation
2.3. Kinetic Modelling
2.4. Statistical Evaluation
2.5. Analytical Techniques
2.6. Analysis of the Substrate’s Bioenergy Conversion Capacity
3. Results and Discussion
3.1. Substrates Characteristics
3.2. Effect of ACoD on Biomethane Production
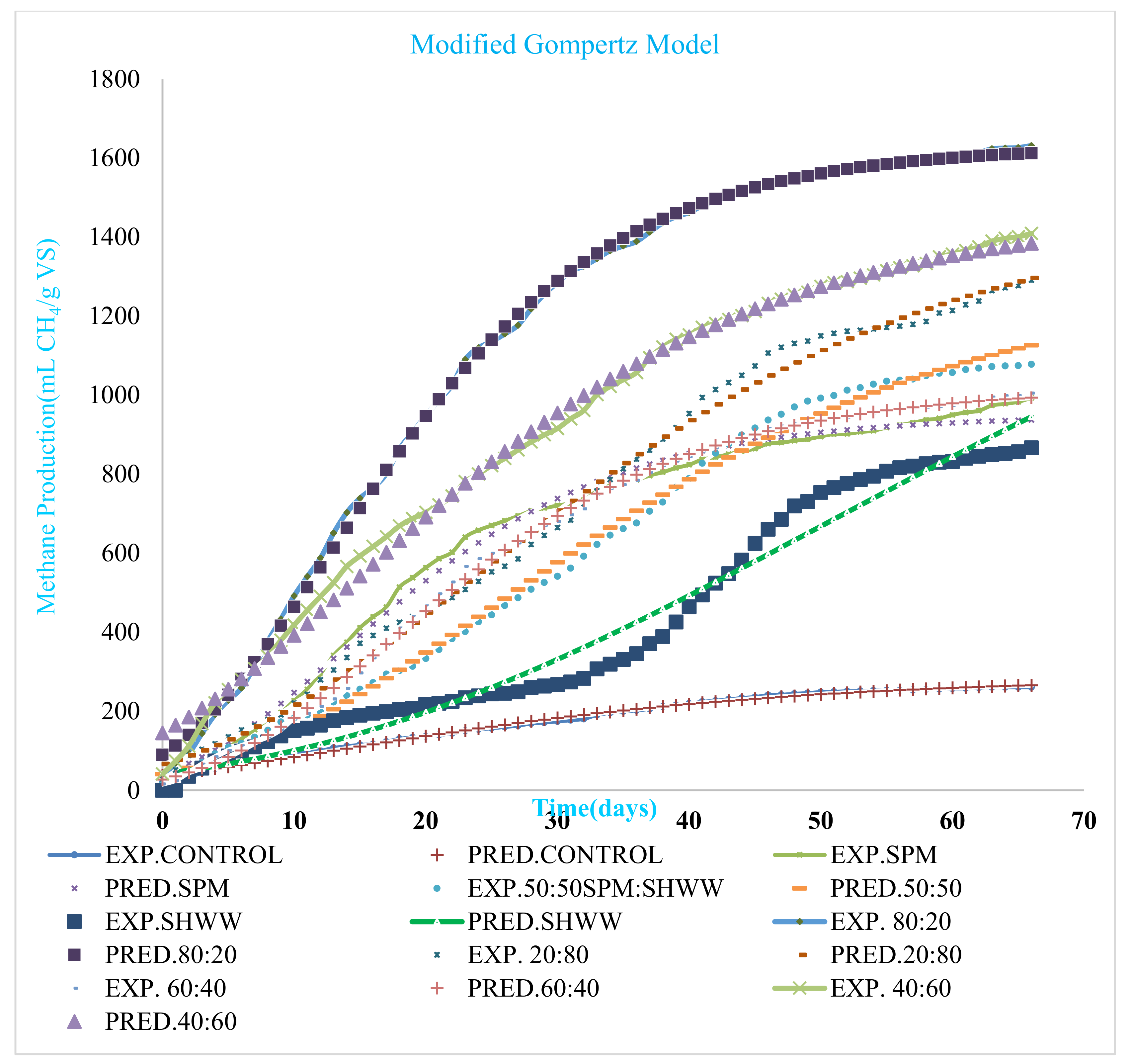
3.3. Kinetic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- e Silva, A.D.S.; Morais, N.W.S.; Coelho, M.M.H.; Pereira, E.L.; dos Santos, A.B. Potentialities of biotechnological recovery of methane, hydrogen and carboxylic acids from agro-industrial wastewaters. Bioresour. Technol. Rep. 2020, 10, 100406. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations FAO. Water for Sustainable Food and Agriculture; Food and Agriculture Organization of the United Nations FAO: Rome, Italy, 2017. [Google Scholar]
- Ritchie, H.; Roser, M. Meat and Dairy Production. OurWorldInData.org. 2017. Available online: https://ourworldindata.org/meat-production (accessed on 16 May 2020).
- Kenya Markets Trust. Kenya market trust and I-Dev international. In A Study on Meat End Market Trends in Kenya; UKaid: Nairobi, Kenya, 2019. [Google Scholar]
- Salehiyoun, A.R.; Di Maria, F.; Sharifi, M.; Norouzi, O.; Zilouei, H.; Aghbashlo, M. Anaerobic co-digestion of sewage sludge and slaughterhouse waste in existing wastewater digesters. Renew. Energy 2020, 145, 2503–2509. [Google Scholar] [CrossRef]
- Bustillo-Lecompte, C.; Mehrvar, M.; Quiñones-Bolaños, E. Slaughterhouse wastewater characterization and treatment: An economic and public health necessity of the meat processing industry in Ontario, Canada. J. Geosci. Environ. Prot. 2016, 4, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Dar, R.A.; Parmar, M.; Dar, E.A.; Sani, R.K.; Phutela, U.G. Biomethanation of agricultural residues: Potential, limitations and possible solutions. Renew. Sustain. Energy Rev. 2021, 135, 110217. [Google Scholar] [CrossRef]
- Reyes, I.P.; Díaz, J.P.; Horváth, I.S. Anaerobic biodegradation of solid substrates from agroindustrial activities—Slaughterhouse wastes and agrowastes. In Biodegradation and Bioremediation of Polluted Systems-New Advances and Technologies; IntechOpen: London, UK, 2015. [Google Scholar]
- Rahman, M.A.; Møller, H.B.; Saha, C.K.; Alam, M.M. The effect of temperature on the anaerobic co-digestion of poultry droppings and sugar mill press mud. Biofuels 2022, 13, 139–147. [Google Scholar] [CrossRef]
- Wu, W. Anaerobic co-digestion of biomass for methane production: Recent research achievements. Optimization 2007, 1, 1VS. [Google Scholar]
- González LM, L.; Reyes, I.P.; Romero, O.R. Anaerobic co-digestion of sugarcane press mud with vinasse on methane yield. Waste Manag. 2017, 68, 139–145. [Google Scholar] [CrossRef]
- Karki, R.; Chuenchart, W.; Surendra, K.C.; Shrestha, S.; Raskin, L.; Sung, S.; Hashimoto, A.; Khanal, S.K. Anaerobic co-digestion: Current status and perspectives. Bioresour. Technol. 2021, 330, 125001. [Google Scholar] [CrossRef]
- Obi, F.O.; Ugwuishiwu, B.O.; Nwakaire, J.N. Agricultural waste concept, generation, utilization and management. Niger. J. Technol. 2016, 35, 957–964. [Google Scholar] [CrossRef]
- Palatsi, J.; Viñas, M.; Guivernau, M.; Fernandez, B.; Flotats, X.J.B.T. Anaerobic digestion of slaughterhouse waste: Main process limitations and microbial community interactions. Bioresour. Technol. 2011, 102, 2219–2227. [Google Scholar] [CrossRef]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Madsen, M.; Holm-Nielsen, J.B.; Esbensen, K.H. Monitoring of anaerobic digestion processes: A review perspective. Renew. Sustain. Energy Rev. 2011, 15, 3141–3155. [Google Scholar] [CrossRef] [Green Version]
- Long, J.H.; Aziz, T.N.; Francis, I.I.I.L.; Ducoste, J.J. Anaerobic co-digestion of fat, oil, and grease FOG: A review of gas production and process limitations. Process Saf. Environ. Prot. 2012, 90, 231–245. [Google Scholar] [CrossRef]
- Rhee, C.; Kim, D.W.; Yu, S.I.; Lee, M.E.; Shin, J.; Kim, H.W.; WooChung, J.; Shin, S.G. Biogas potential assessment and characterization of Korean slaughterhouse waste for anaerobic digestion. Environ. Technol. Innov. 2021, 24, 101858. [Google Scholar] [CrossRef]
- Mugodo, K.; Magama, P.P.; Dhavu, K. Biogas production potential from agricultural and agro-processing waste in South Africa. Waste Biomass Valoriz. 2017, 8, 2383–2392. [Google Scholar] [CrossRef]
- Talha, Z.; Hamid, A.; Ding, W.; Osman, B. Biogas production from filter mud in CSTR reactor, co-digested with various substrates wastes. Agric. Environ. Sci. J. 2017, 1, 15–24. [Google Scholar]
- Alvarez, R.; Liden, G. Semi-continuous co-digestion of solid slaughterhouse waste, manure, and fruit and vegetable waste. Renew. Energy 2008, 33, 726–734. [Google Scholar] [CrossRef]
- Rouf, M.A.; Bajpai, P.K.; Jotshi, C.K. Optimization of biogas generation from press mud in batch reactor. Bangladesh J. Sci. Ind. Res. 2010, 45, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Castellucci, S.; Cocchi, S.; Allegrini, E.; Vecchione, L. Anaerobic digestion and co-digestion of slaughterhouse wastes. J. Agric. Eng. 2013, 44 (Suppl. S2), 526–530. [Google Scholar] [CrossRef]
- González LM, L.; Reyes, I.P.; Dewulf, J.; Budde, J.; Heiermann, M.; Vervaeren, H. Effect of liquid hot water pre-treatment on sugarcane press mud methane yield. Bioresour. Technol. 2014, 169, 284–290. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A review of the processes, parameters, and optimization of anaerobic digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ripoll, V.; Agabo-García, C.; Solera, R.; Perez, M. Anaerobic digestion of slaughterhouse waste in batch and anaerobic sequential batch reactors. Biomass Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- González LM, L.; Reyes, I.P.; Garciga, J.P.; Barrera, E.L.; Romero, O.R. Energetic, economic and environmental assessment for the anaerobic digestion of pretreated and codigested press mud. Waste Manag. 2020, 102, 249–259. [Google Scholar] [CrossRef]
- Kamusoko, R.; Jingura, R.M.; Parawira, W.; Sanyika, W.T. Comparison of pretreatment methods that enhance biomethane production from crop residues-a systematic review. Biofuel Res. J. 2019, 6, 1080. [Google Scholar] [CrossRef] [Green Version]
- Sakarika, M.; Stavropoulos, K.; Kopsahelis, A.; Koutra, E.; Zafiri, C.; Kornaros, M. Two-stage anaerobic digestion harnesses more energy from the co-digestion of end-of-life dairy products with agro-industrial waste compared to the single-stage process. Biochem. Eng. J. 2020, 153, 107404. [Google Scholar] [CrossRef]
- Qamar, M.O.; Farooqi, I.H.; Munshi, F.M.; Alsabhan, A.H.; Kamal, M.A.; Khan, M.A.; Alwadai, A.S. Performance of full-scale slaughterhouse effluent treatment plant SETP. J. King Saud Univ.-Sci. 2022, 34, 101891. [Google Scholar] [CrossRef]
- Cárdenas-Cleves, L.M.; Marmolejo-Rebellon, L.F.; Torres-Lozada, P. Anaerobic codigestion of sugarcane press mud with food waste: Effects on hydrolysis stage, methane yield, and synergistic effects. Int. J. Chem. Eng. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Janke, L.; Leite, A.F.; Nikolausz, M.; Radetski, C.M.; Nelles, M.; Stinner, W. Comparison of start-up strategies and process performance during semi-continuous anaerobic digestion of sugarcane filter cake co-digested with bagasse. Waste Manag. 2016, 48, 199–208. [Google Scholar] [CrossRef]
- Ma, G.; Ndegwa, P.; Harrison, J.H.; Chen, Y. Methane yields during anaerobic co-digestion of animal manure with other feedstocks: A meta-analysis. Sci. Total Environ. 2020, 728, 138224. [Google Scholar] [CrossRef]
- Mozhiarasi, V.; Speier, C.J.; Rose, P.M.B.; Weichgrebe, D.; Venkatachalam, S.S. Influence of pre-treatments and anaerobic co-digestion of slaughterhouse waste with vegetable, fruit and flower market wastes for enhanced methane production. Biomass Convers. Biorefinery 2021, 8, 1–18. [Google Scholar] [CrossRef]
- Pagés-Díaz, J.; Pereda-Reyes, I.; Sanz, J.L.; Lundin, M.; Taherzadeh, M.J.; Horváth, I.S. A comparison of process performance during the anaerobic mono-and co-digestion of slaughterhouse waste through different operational modes. J. Environ. Sci. 2018, 64, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Latifi, P.; Karrabi, M.; Danesh, S. Anaerobic co-digestion of poultry slaughterhouse wastes with sewage sludge in batch-mode bioreactors (effect of inoculum-substrate ratio and total solids). Renewable Sustainable Energy Rev. 2019, 107, 288–296. [Google Scholar] [CrossRef]
- Sounni, F.; Elgnaoui, Y.; El Bari, H.; Merzouki, M.; Benlemlih, M. Effect of mixture ratio and organic loading rate during anaerobic co-digestion of olive mill wastewater and agro-industrial wastes. Biomass Convers. Biorefinery 2021, 1–7. [Google Scholar] [CrossRef]
- Bayr, S.; Rantanen, M.; Kaparaju, P.; Rintala, J. Mesophilic and thermophilic anaerobic co-digestion of rendering plant and slaughterhouse wastes. Bioresour. Technol. 2012, 104, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Panizio, R.M.; Calado, L.F.D.C.; Lourinho, G.; de Brito, P.S.D.; Mees, J.B. Potential of biogas production in anaerobic co-digestion of Opuntia ficus-indica and slaughterhouse wastes. Waste Biomass Valorization 2020, 11, 4639–4647. [Google Scholar] [CrossRef]
- Bouallagui, H.; Rachdi, B.; Gannoun, H.; Hamdi, M. Mesophilic and thermophilic anaerobic co-digestion of abattoir wastewater and fruit and vegetable waste in anaerobic sequencing batch reactors. Biodegradation 2009, 20, 401–409. [Google Scholar] [CrossRef]
- Monou, M.; Pafitis, N.; Kythreotou, N.; Smith, S.R.; Mantzavinos, D.; Kassinos, D. Anaerobic co-digestion of potato processing wastewater with pig slurry and abattoir wastewater. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2008, 83, 1658–1663. [Google Scholar] [CrossRef]
- Hailu, A.M.; Asfaw, S.L.; Tegaye, T.A. Effect of carbon-rich-waste addition as co-substrate on the performance and stability of anaerobic digestion of abattoir wastewater without agitation. Bioresources Bioprocess. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Filer, J.; Ding, H.H.; Chang, S. Biochemical methane potential BMP assay method for anaerobic digestion research. Water 2019, 11, 921. [Google Scholar] [CrossRef] [Green Version]
- Wandera, S.M.; Qiao, W.; Algapani, D.E.; Bi, S.; Yin, D.; Qi, X.; Yueling, L.; Jacek, D.; Dong, R. Searching for possibilities to improve the performance of full scale agricultural biogas plants. Renew. Energy 2018, 116, 720–727. [Google Scholar] [CrossRef]
- Kafle, G.K.; Kim, S.H. Kinetic study of the anaerobic digestion of swine manure at mesophilic temperature: A lab scale batch operation. J. Biosyst. Eng. 2012, 37, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential BMP using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohutskyi, P.; Phan, D.; Kopachevsky, A.M.; Chow, S.; Bouwer, E.J.; Betenbaugh, M.J. Synergistic co-digestion of wastewater grown algae-bacteria polyculture biomass and cellulose to optimize carbon-to-nitrogen ratio and application of kinetic models to predict anaerobic digestion energy balance. Bioresour. Technol. 2018, 269, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Kafle, G.K.; Kim, S.H. Anaerobic treatment of apple waste with swine manure for biogas production: Batch and continuous operation. Appl. Energy 2013, 103, 61–72. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Ugwu, S.N.; Enweremadu, C.C. Biodegradability and kinetic studies on biomethane production from okra Abelmoschus esculentus waste. South Afr. J. Sci. 2019, 115, 1–5. [Google Scholar]
- Nwokolo, N.; Mukumba, P.; Obileke, K.; Enebe, M. Waste to energy: A focus on the impact of substrate type in biogas production. Processes 2020, 8, 1224. [Google Scholar] [CrossRef]
- Bamba, J.N.Y.; Almendrala1, M.C.; Caparanga1, A.R.; Doma, B.T. Effect of Biochemical Pretreatment and Nutrient Supplementation on Anaerobic Co-Digestion of Sugarcane Press Mud and Distillery Effluent. IOP Conf. Ser. Earth Environ. Sci. 2021, 801, 012001. [Google Scholar] [CrossRef]
- Jeung, J.H.; Chung, W.J.; Chang, S.W. Evaluation of anaerobic co-digestion to enhance the efficiency of livestock manure anaerobic digestion. Sustainability 2019, 11, 7170. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Fydrych, V.C.; Benítez-Olivares, G.; Meraz-Rodríguez, M.A.; Salazar-Peláez, M.L.; Fajardo-Ortiz, M.C. Methane production kinetics of pretreated slaughterhouse wastewater. Biomass Bioenergy 2019, 130, 105385. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.S.; Fonoll, X.; Peces, M.; Astals, S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Porselvam, S.; Soundara Vishal, N.; Srinivasan, S.V. Enhanced biogas yield by thermo-alkali solubilization followed by co-digestion of intestine waste from slaughterhouse with food waste. 3 Biotech 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- KC, P.; Ale, B.B. Production of Biogas from Slaughterhouse Waste in Lalitpur Sub-Metropolitan City. In Proceedings of the IOE Graduate Conference, Kirtipur, Nepal, 20–22 November 2015; pp. 143–149. [Google Scholar]
- Borowski, S.; Kubacki, P. Co-digestion of pig slaughterhouse waste with sewage sludge. Waste Manag. 2015, 40, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Selormey, G.K.; Barnes, B.; Kemausuor, F.; Darkwah, L. A review of anaerobic digestion of slaughterhouse waste: Effect of selected operational and environmental parameters on anaerobic biodegradability. Rev. Environ. Sci. Bio/Technol. 2021, 20, 1073–1086. [Google Scholar] [CrossRef]
- Guo, Z.; Usman, M.; Alsareii, S.A.; Harraz, F.A.; Al-Assiri, M.S.; Jalalah, M.; Xiangkai, L.; Salama, E.S. Synergistic ammonia and fatty acids inhibition of microbial communities during slaughterhouse waste digestion for biogas production. Bioresour. Technol. 2021, 337, 125383. [Google Scholar] [CrossRef]
- El Gnaoui, Y.; Sounni, F.; Bakraoui, M.; Karouach, F.; Benlemlih, M.; Barz, M.; El Bari, H. Anaerobic co-digestion assessment of olive mill wastewater and food waste: Effect of mixture ratio on methane production and process stability. J. Environ. Chem. Eng. 2020, 8, 103874. [Google Scholar] [CrossRef]
- Xu, F.; Wang, Z.W.; Li, Y. Predicting the methane yield of lignocellulosic biomass in mesophilic solid-state anaerobic digestion based on feedstock characteristics and process parameters. Bioresour. Technol. 2014, 173, 168–176. [Google Scholar] [CrossRef]

| Reference | Co-Digested Feedstock | Operation Conditions | Improvements |
|---|---|---|---|
| [5] | SHWW with WMS | Batch and CSTR; mesophilic temp; HRT 18d, 13.5d, 11d; OLR 1.5 kg VS/m3 d | 50% methane increase |
| [36] | Poultry SHWW with sewage sludge | Batch mode; mesophilic temp; HRT 50d, 42d | 63% VS removal; 88% COD reduction |
| [9] | Poultry droppings and SPM | Batch and CSTR; mesophilic and thermophilic temp; HRT 20d | Methane increased by 8 and 29% in contrast to AMoD of PM and PD, respectively |
| [37] | OMW with SHWW | batch and continuous ASBR; mesophilic temp; OLR 10 g COD/L/day; HRT 20d | Reactor degraded 10 g COD/L/day |
| [38] | SHWW with rendering plant | CSTR; mesophilic and thermophilic temp; 1.0 and 1.5 kg VS/m3 day OLRs; 50 d HRT | 262–572 mL CH4/g VS added |
| [39] | SHWW with OFI (75% SHWW: 25% OFI) | semi continuous; mesophilic temp; OLR 64 g VS L−1 day−1 | 57% (v/v) methane increase |
| [40] | FVW and AWW (30%FVW:70%AW) | Single-stage ASBR; mesophilic temp; 20 d, 10d HRT; 2.56 g VS l−1 day−1 OLR | 75% more methane yield |
| [41] | AWW with PPWW and/or RPS | Batch; mesophilic temp; HRT 22 d | 50 PPWW:50 pig slurry achieved 72% VS removal and 35 mL average daily methane production; 32% max methane |
| No improvement for AWW due to poor buffering and low pH | |||
| [42] | Cattle AWW with FVW (50%AWW:50%FVW) | Unstirred two-staged ASBR; mesophilic temp; semi-continuous fed | 70.26% more methane yield; 57.11% VS reduction compared to AMoD of AWW |
| Parameter | SHWW | SPM | Inoculum |
|---|---|---|---|
| TS (%) | 3.5 | 6.3 | 7.1 |
| VS (%) | 3.2 | 5.7 | 6.3 |
| Inoculum (mL) | SPM (mL) | SHWW (mL) | |
|---|---|---|---|
| Control | 80 | 0 | 0 |
| MIX 00:100 | 40 | 0 | 40 |
| Mix 100:00 | 40 | 40 | 0 |
| Mix 80:20 | 40 | 32 | 8 |
| Mix 20:80 | 40 | 8 | 32 |
| Mix 60:40 | 40 | 24 | 16 |
| MIX 40:60 | 40 | 16 | 24 |
| MIX 50:50 | 40 | 20 | 20 |
| Parameter | SPM 100:00 | SHWW 00:100 | Inoculum (Control) | MIX 80:20 | MIX 20:80 | MIX 60:40 | MIX 40:60 | MIX 50:50 |
|---|---|---|---|---|---|---|---|---|
| Mean (±) SD | Mean (±) SD | Mean (±) SD | Mean (±) SD | Mean (±) SD | Mean (±) SD | Mean (±) SD | Mean (±) SD | |
| TS (%) | 6.3 ± 0.3 (5.1 ± 0.3) | 3.5 ± 0.3 (2.4 ± 0.3) | 7.1 ± 0.3 (3.1 ± 0.3) | 6.2 ± 1.5 (4.5 ± 1.5) | 6.1 ± 0.4 (5.7 ± 0.4) | 5.98 ± 0.2 (5.8 ± 0.2) | 5.89 ± 0.8 (5.6 ± 0.8) | 6.3 ± 0.6 (5.2 ± 0.6) |
| VS (%) | 5.7 ± 0.6 (2.5 ± 0.6) | 3.2 ± 0.3 (1.5 ± 0.3) | 6.3 ± 0.3 (2.4 ± 0.3) | 4.3 ± 0.8 (1.4 ± 0.8) | 5.0 ± 0.3 (2.5 ± 0.3) | 4.2 ± 1.1 (2.4 ± 1.1) | 4.9 ± 0.6 (2.0 ± 0.6) | 3.8 ± 0.5 (1.8 ± 0.5) |
| VS/TS (%) | 90 | 91 | 90 | 70 | 82 | 70 | 83 | 60 |
| VS removal (%) | 60 | 53 | 62 | 67 | 50 | 43 | 59 | 52 |
| pH | 5.41 ± 0 (7.76 ± 0) | 8.06 ± 0 (8.34 ± 0) | 7.2 ± 0 (7.69 ± 0) | 7.2 ± 0 (8.10 ± 0) | 7.3 ± 0 (8.25 ± 0) | 7.1 ± 0 (8.21 ± 0) | 7.2 ± 0 (8.23 ± 0) | 7.3 ± 0 (8.20 ± 0) |
| TCOD (g L−1) | 7.36 ± 0 (5.16 ± 9) | 16 ± 0.1 (12 ± 6.1) | 15.0 ± 0.1 (11.0 ± 9.1) | 10.8 ± 0.1 (8.3 ± 6.1) | 12.2 ± 0.3 (9.5 ± 9.3) | 11.8 ± 0.1 (8.3 ± 8.1) | 14.6 ± 0 (11.4 ± 7) | 15.6 ± 0 (12.7 ± 4) |
| COD removal (%) | 30 | 25 | 27 | 23 | 22 | 30 | 22 | 16 |
| NH4+-N (mg L−1) | 1300 ± 3.3 (1205 ± 0.3) | 6407 ± 5.5 (4208 ± 0.5) | 1097 ± 8.7 (674 ± 0.7) | 5521 ± 7.3 (2426 ± 0.3) | 6887 ± 9.7 (2151 ± 0.7) | 6463 ± 4.6 (3841 ± 0.6) | 6323 ± 8.3 (4560 ± 0.3) | 6671 ± 6.5 (4500 ± 0.5) |
| C (%) | 27.28 ± 0.2 | 32.62 ± 0.1 | / | / | / | / | / | / |
| H (%) | 16.51 ± 0.6 | 17.88 ± 0.7 | / | / | / | / | / | / |
| O (%) | 1.37 ± 0.7 | 2.45 ± 0.4 | / | / | / | / | / | / |
| N (%) | 1.04 ± 0.4 | 3.38 ± 0.3 | / | / | / | / | / | / |
| C/N ratio | 26.23 | 9.65 | / | / | / | / | / | / |
| Control | SPM 100:00 | SHWW 00:100 | MIX 80:20 | MIX 20:80 | MIX 60:40 | MIX 40:60 | SPM: SHWW 50:50 | |
|---|---|---|---|---|---|---|---|---|
| Methane yield (mLCH4/VS) | 52.25 ± 72.3 | 198.2 ± 298.7 | 348.4 ± 285.2 | 478.4 ± 499.2 | 325.8 ± 404.7 | 302.1 ± 328.7 | 366 ± 400.8 | 357.8 ± 359.7 |
| Increase in methane yield | / | / | / | 2.10 | 1.02 | 1.17 | 1.27 | 1.31 |
| CH4 content (%) | 22 | 18 | 65 | 24 | 52 | 32 | 40 | |
| TMP (mL CH4/g VS) | / | 333.14 | 541.75 | / | / | / | / | / |
| Parameter | Control | SPM 100:00 | SPM: SHWW 50:50 | SHWW 00:100 | MIX 80:20 | MIX 20:80 | MIX 60:40 | MIX 40:60 |
|---|---|---|---|---|---|---|---|---|
| Po (mL CH4/g VS) | 289.34 | 949.06 | 1299.57 | 887 | 1631.63 | 1478.96 | 1019.73 | 1464.39 |
| Rmax (mL CH4/g VS/d) | 5.23 | 29.31 | 23.09 | 32.54 | 50.25 | 26.05 | 28.11 | 30.31 |
| λ(d) | 6.10 | 1.63 | 4.98 | 6.12 | 0.78 | 2.73 | 3.86 | 2.88 |
| R2 | 0.993 | 0.994 | 0.995 | 0.995 | 0.999 | 0.995 | 0.999 | 0.995 |
| RSME | 5.75 | 23.05 | 25.42 | 49.44 | 11.55 | 27.43 | 17.58 | 28.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anyango, B.N.; Wandera, S.M.; Raude, J.M. Abattoir Wastewater Treatment in Anaerobic Co-Digestion with Sugar Press Mud in Batch Reactor for Improved Biogas Yield. Water 2022, 14, 2571. https://doi.org/10.3390/w14162571
Anyango BN, Wandera SM, Raude JM. Abattoir Wastewater Treatment in Anaerobic Co-Digestion with Sugar Press Mud in Batch Reactor for Improved Biogas Yield. Water. 2022; 14(16):2571. https://doi.org/10.3390/w14162571
Chicago/Turabian StyleAnyango, Beatrice N., Simon M. Wandera, and James M. Raude. 2022. "Abattoir Wastewater Treatment in Anaerobic Co-Digestion with Sugar Press Mud in Batch Reactor for Improved Biogas Yield" Water 14, no. 16: 2571. https://doi.org/10.3390/w14162571
APA StyleAnyango, B. N., Wandera, S. M., & Raude, J. M. (2022). Abattoir Wastewater Treatment in Anaerobic Co-Digestion with Sugar Press Mud in Batch Reactor for Improved Biogas Yield. Water, 14(16), 2571. https://doi.org/10.3390/w14162571






