Impact of Fish Ponds on Stream Hydrology and Temperature Regime in the Context of Freshwater Pearl Mussel Conservation
Abstract
:1. Introduction
- Ponds have a significant influence on the hydrological cycle at the catchment scale, increasing flood retention during high flows and buffering low water levels during low flow conditions.
- Ponds have an impact on the temperature regime in small, cool, headwater streams, with significant increase in stream temperature through ponds effluents in summer and nearly neutral effects during winter.
- Effects on hydrological and temperature regime accumulate with increasing number of ponds draining into a stream.
2. Materials and Methods
2.1. Study Area
2.2. Hydrological Model Setup
2.3. Calibration and Validation
2.4. Temperature Measurements
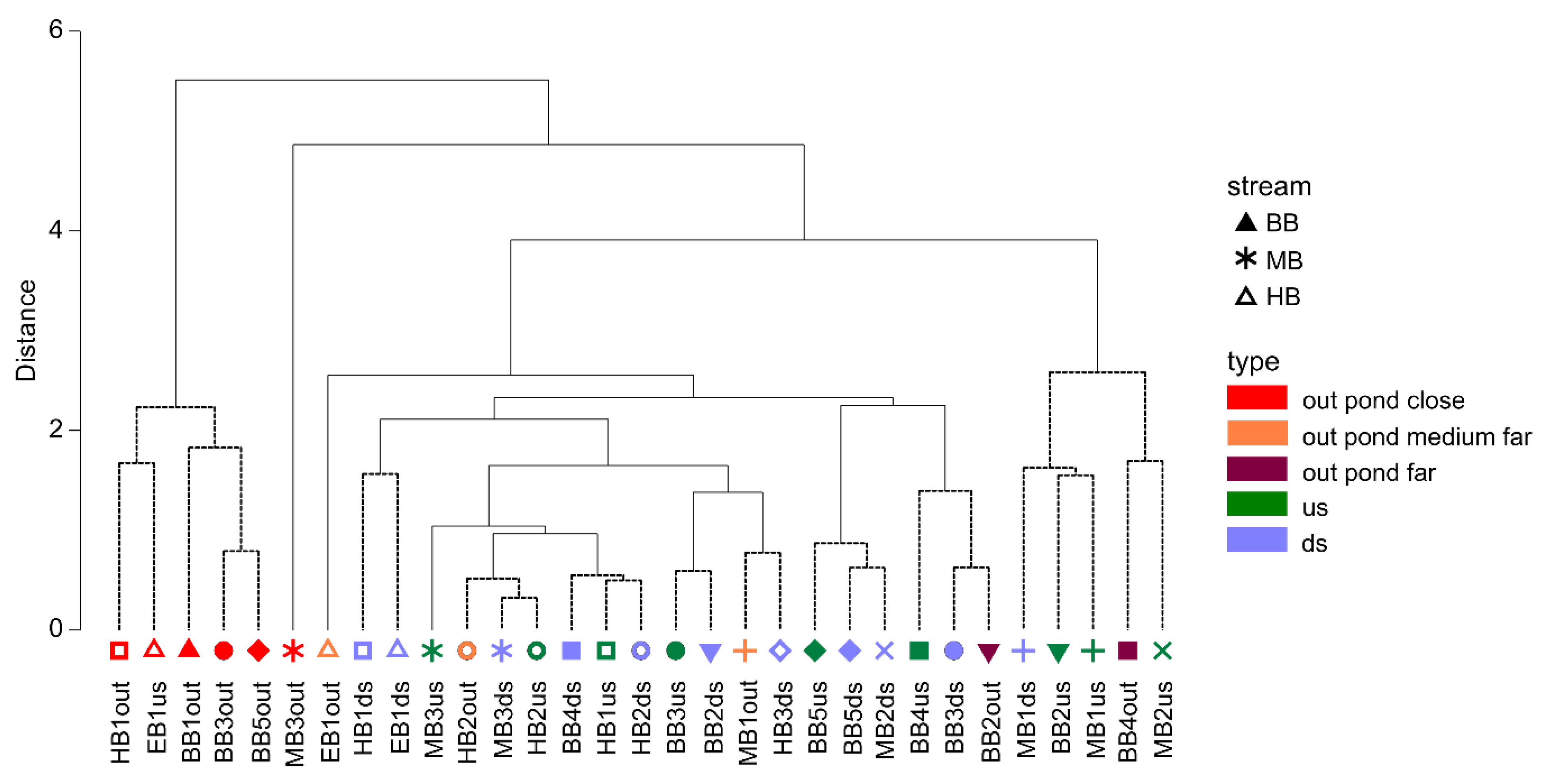
2.5. Data Analysis
3. Results
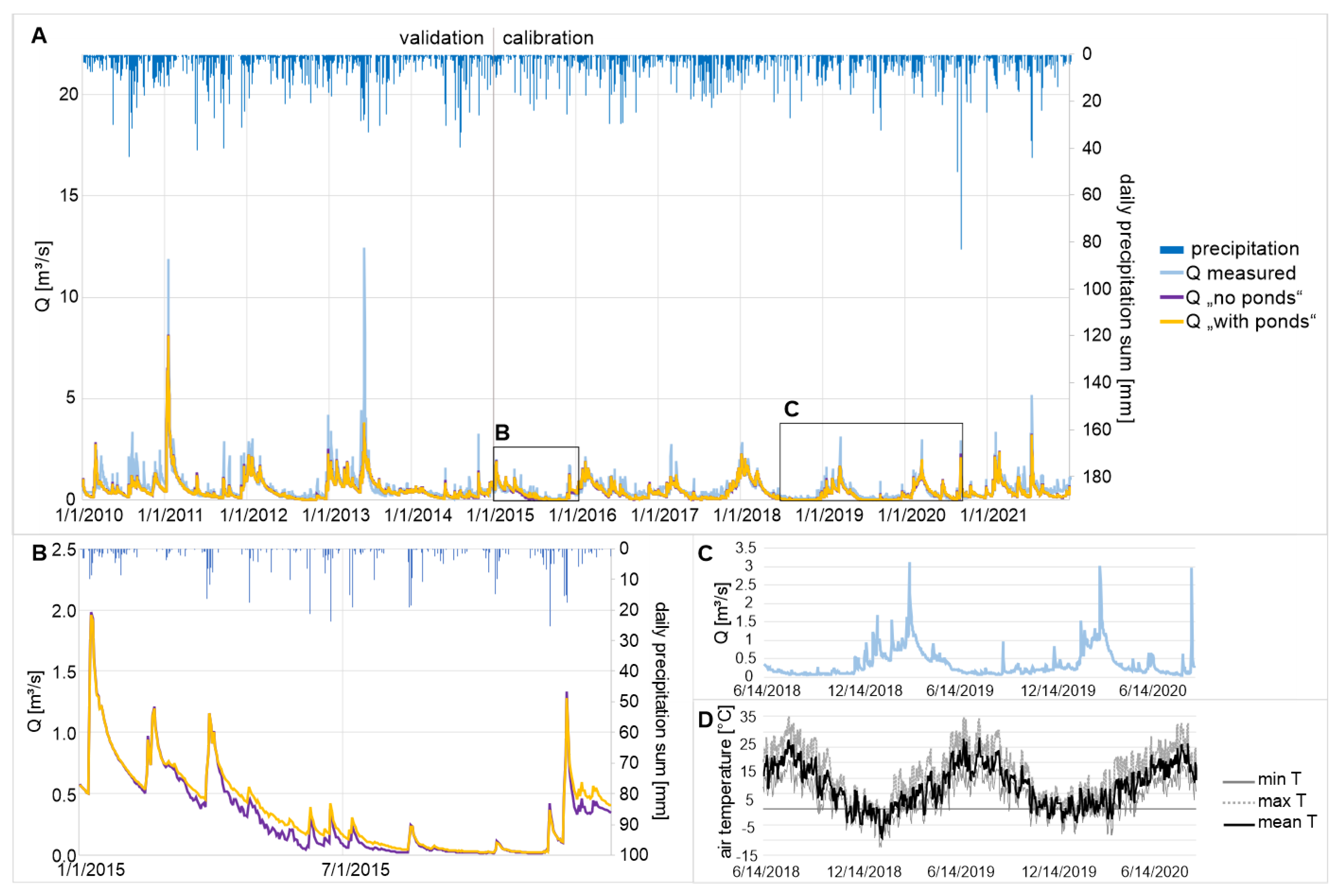
3.1. Hydrologic Regime
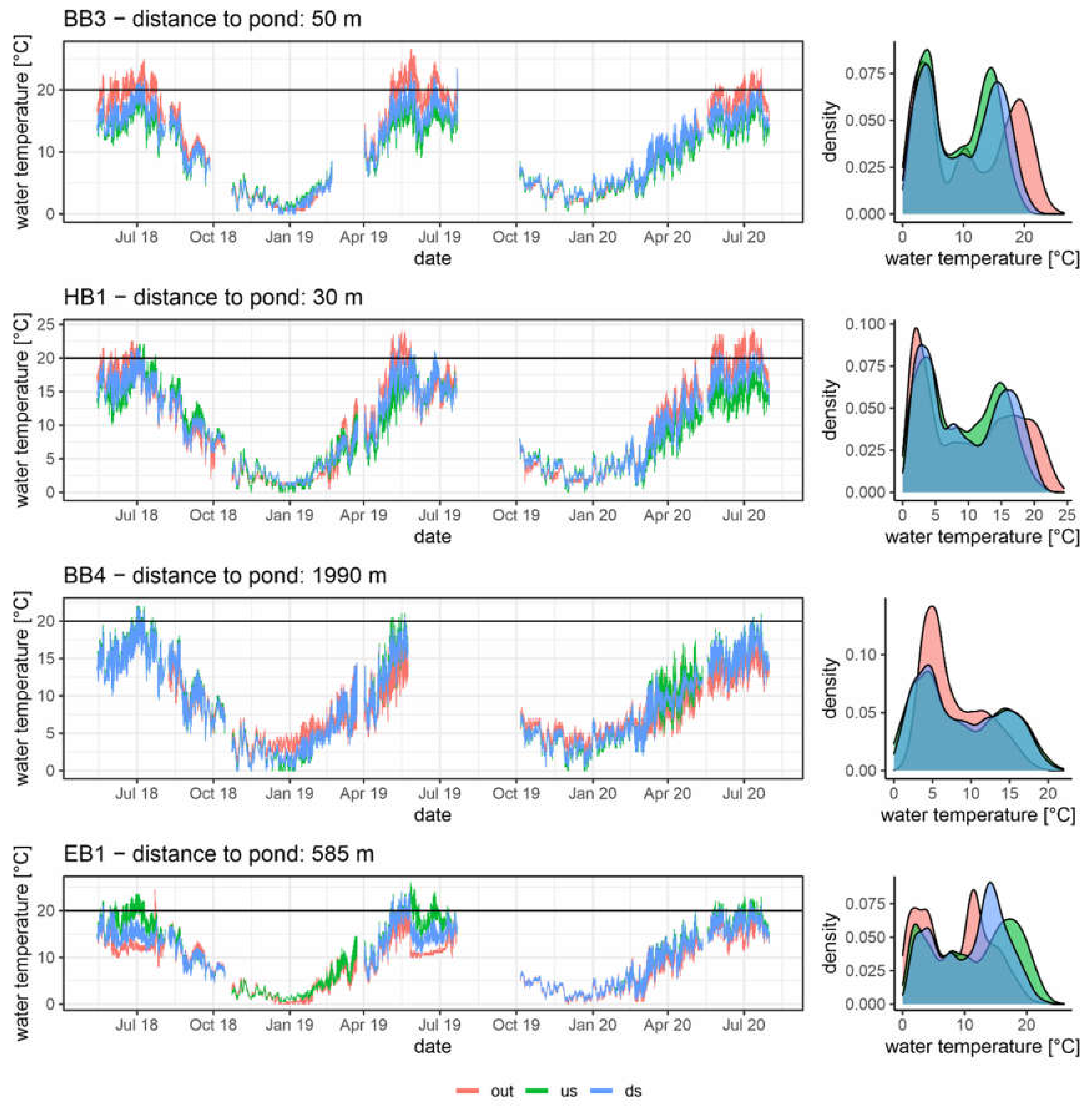
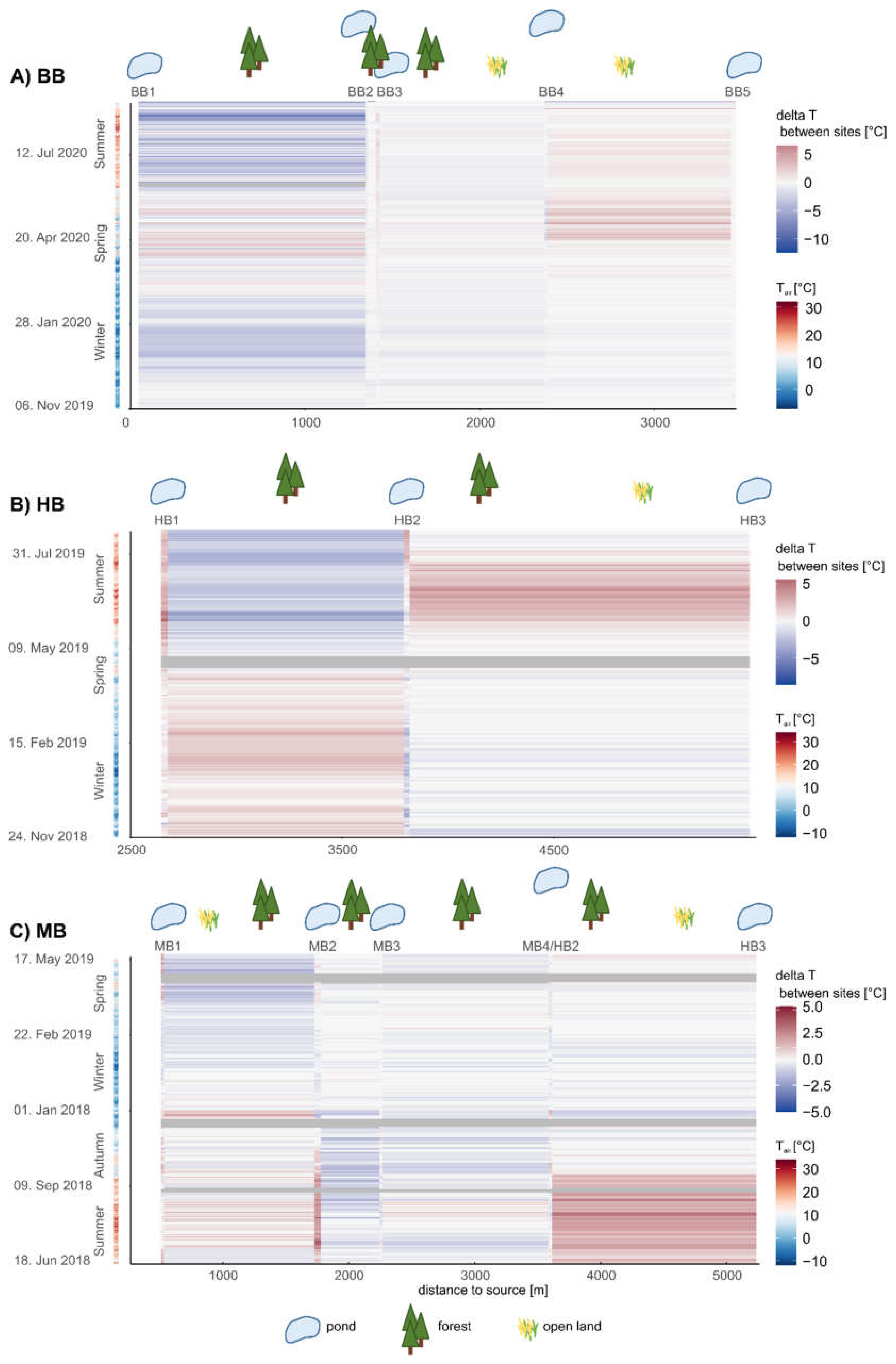
3.2. Temperature Regime
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Vliet, M.T.H.; Franssen, W.H.P.; Yearsley, J.R.; Ludwig, F.; Haddeland, I.; Lettenmaier, D.P.; Kabat, P. Global river discharge and water temperature under climate change. Glob. Environ. Chang. 2013, 23, 450–464. [Google Scholar] [CrossRef]
- Arismendi, I.; Johnson, S.L.; Dunham, J.B.; Haggerty, R.; Hockman-Wert, D. The paradox of cooling streams in a warming world: Regional climate trends do not parallel variable local trends in stream temperature in the Pacific continental United States. Geophys. Res. Lett. 2012, 39, L10401. [Google Scholar] [CrossRef]
- Casas-Mulet, R.; Pander, J.; Ryu, D.; Stewardson, M.J.; Geist, J. Unmanned Aerial Vehicle (UAV)-Based Thermal Infra-Red (TIR) and Optical Imagery Reveals Multi-Spatial Scale Controls of Cold-Water Areas Over a Groundwater-Dominated Riverscape. Front. Environ. Sci. 2020, 8, 64. [Google Scholar] [CrossRef]
- Kuhn, J.; Casas-Mulet, R.; Pander, J.; Geist, J. Assessing Stream Thermal Heterogeneity and Cold-Water Patches from UAV-Based Imagery: A Matter of Classification Methods and Metrics. Remote Sens. 2021, 13, 1379. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Wigington, P.J.; Leibowitz, S.G.; Comeleo, R.L.; Sickle, J.V. Predicting the occurrence of cold-water patches at intermittent and ephemeral tributary confluences with warm rivers. Freshw. Sci. 2015, 34, 111–124. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Liss, W.J.; Frissell, C.A. Cold water patches in warm streams: Physicochemical characteristics and the influence of shading. J. Am. Water Resour. Assoc. 2003, 39, 355–368. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Lake, P.S. Ecological effects of perturbation by drought in flowing waters. Freshw. Biol. 2003, 48, 1161–1172. [Google Scholar] [CrossRef]
- Capon, S.J.; Stewart-Koster, B.; Bunn, S.E. Future of Freshwater Ecosystems in a 1.5 °C Warmer World. Front. Environ. Sci. 2021, 9, 784642. [Google Scholar] [CrossRef]
- Auerswald, K.; Moyle, P.; Seibert, S.P.; Geist, J. HESS Opinions: Socio-economic and ecological trade-offs of flood management—Benefits of a transdisciplinary approach. Hydrol. Earth Syst. Sci. 2019, 23, 1035–1044. [Google Scholar] [CrossRef]
- Geist, J.; Auerswald, K. Synergien im Gewässer-, Boden-, Arten-und Klimaschutz am Beispiel von Flussauen. [Synergies in water, soil, species and climate protection using the example of riverine floodplains. Wasserwirtschaft 2019, 109, 11–16. [Google Scholar] [CrossRef]
- Doriean, N.J.C.; Teasdale, P.R.; Welsh, D.T.; Brooks, A.P.; Bennett, W.W. Evaluation of a simple, inexpensive, in situ sampler for measuring time-weighted average concentrations of suspended sediment in rivers and streams. Hydrol. Process. 2019, 33, 678–686. [Google Scholar] [CrossRef]
- Acreman, M.; Holden, J. How Wetlands Affect Floods. Wetlands 2013, 33, 773–786. [Google Scholar] [CrossRef]
- Khilchevskyi, V.; Grebin, V.; Zabokrytska, M.; Zhovnir, V.; Bolbot, H.; Plichko, L. Hydrographic characteristic of ponds distribution in Ukraine—Basin and regional features. J. Water Land Dev. 2020, 46, 140–145. [Google Scholar] [CrossRef]
- Ameli, A.A.; Creed, I.F. Does Wetland Location Matter When Managing Wetlands for Watershed-Scale Flood and Drought Resilience? J. Am. Water Resour. Assoc. 2019, 55, 529–542. [Google Scholar] [CrossRef]
- Cai, X.; Zeng, R.; Kang, W.H.; Song, J.; Valocchi, A.J. Strategic Planning for Drought Mitigation under Climate Change. J. Water Resour. Plan. Manag. 2015, 141, 04015004. [Google Scholar] [CrossRef]
- Akbas, A.; Freer, J.; Ozdemir, H.; Bates, P.D.; Turp, M.T. What about reservoirs? Questioning anthropogenic and climatic interferences on water availability. Hydrol. Process. 2020, 34, 5441–5455. [Google Scholar] [CrossRef]
- Al Sayah, M.J.; Nedjai, R.; Kaffas, K.; Abdallah, C.; Khouri, M. Assessing the Impact of Man–Made Ponds on Soil Erosion and Sediment Transport in Limnological Basins. Water 2019, 11, 2526. [Google Scholar] [CrossRef]
- Makhtoumi, Y.; Li, S.; Ibeanusi, V.; Chen, G. Evaluating Water Balance Variables under Land Use and Climate Projections in the Upper Choctawhatchee River Watershed, in Southeast US. Water 2020, 12, 2205. [Google Scholar] [CrossRef]
- Neupane, R.P.; Ficklin, D.L.; Knouft, J.H.; Ehsani, N.; Cibin, R. Hydrologic responses to projected climate change in ecologically diverse watersheds of the Gulf Coast, United States. Int. J. Climatol. 2018, 39, 2227–2243. [Google Scholar] [CrossRef]
- Webb, B.W.; Nobilis, F. Long-term changes in river temperature and the influence of climatic and hydrological factors. Hydrol. Sci. J. 2007, 52, 74–85. [Google Scholar] [CrossRef]
- Rau, G.C.; Andersen, M.S.; McCallum, A.M.; Acworth, R.I. Analytical methods that use natural heat as a tracer to quantify surface water–groundwater exchange, evaluated using field temperature records. Hydrogeol. J. 2010, 18, 1093–1110. [Google Scholar] [CrossRef]
- Coulter, D.P.; Sepúlveda, M.S.; Troy, C.D.; Höök, T.O. Thermal habitat quality of aquatic organisms near power plant discharges: Potential exacerbating effects of climate warming. Fish. Manag. Ecol. 2014, 21, 196–210. [Google Scholar] [CrossRef]
- Seyedhashemi, H.; Moatar, F.; Vidal, J.P.; Diamond, J.S.; Beaufort, A.; Chandesris, A.; Valette, L. Thermal signatures identify the influence of dams and ponds on stream temperature at the regional scale. Sci. Total Environ. 2021, 766, 142667. [Google Scholar] [CrossRef]
- Van Vliet, M.T.H.; Ludwig, F.; Zwolsman, J.J.G.; Weedon, G.P.; Kabat, P. Global river temperatures and sensitivity to atmospheric warming and changes in river flow. Water Resour. Res. 2011, 47, W02544. [Google Scholar] [CrossRef]
- Steel, E.A.; Beechie, T.J.; Torgersen, C.E.; Fullerton, A.H. Envisioning, Quantifying, and Managing Thermal Regimes on River Networks. Bioscience 2017, 67, 506–522. [Google Scholar] [CrossRef]
- Piatka, D.R.; Wild, R.; Hartmann, J.; Kaule, R.; Kaule, L.; Gilfedder, B.; Peiffer, S.; Geist, J.; Beierkuhnlein, C.; Barth, J.A.C. Transfer and transformations of oxygen in rivers as catchment reflectors of continental landscapes: A review. Earth-Sci. Rev. 2021, 220, 103729. [Google Scholar] [CrossRef]
- Pander, J.; Habersetzer, L.; Casas-Mulet, R.; Geist, J. Effects of Stream Thermal Variability on Macroinvertebrate Community: Emphasis on Native Versus Non-Native Gammarid Species. Front. Environ. Sci. 2022, 10, 869396. [Google Scholar] [CrossRef]
- Warren, D.R.; Robinson, J.M.; Josephson, D.C.; Sheldon, D.R.; Kraft, C.E. Elevated summer temperatures delay spawning and reduce redd construction for resident brook trout (Salvelinus fontinalis). Glob. Chang. Biol. 2012, 18, 1804–1811. [Google Scholar] [CrossRef]
- Davis, L.A.; Wagner, T.; Bartron, M.L. Spatial and temporal movement dynamics of brook Salvelinus fontinalis and brown trout Salmo trutta. Environ. Biol. Fishes 2015, 98, 2049–2065. [Google Scholar] [CrossRef]
- Bond, N.; Thomson, J.; Reich, P.; Stein, J. Using species distribution models to infer potential climate change-induced range shifts of freshwater fish in south-eastern Australia. Mar. Freshw. Res. 2011, 62, 1043–1061. [Google Scholar] [CrossRef]
- Wehrly, K.E.; Wiley, M.J.; Seelbach, P.W. Classifying Regional Variation in Thermal Regime Based on Stream Fish Community Patterns. Trans. Am. Fish. Soc. 2003, 132, 18–38. [Google Scholar] [CrossRef]
- Caissie, D. The thermal regime of rivers: A review. Freshw. Biol. 2006, 51, 1389–1406. [Google Scholar] [CrossRef]
- Maheu, A.; Poff, N.L.; St-Hilaire, A. A Classification of Stream Water Temperature Regimes in the Conterminous USA. River Res. Appl. 2016, 32, 896–906. [Google Scholar] [CrossRef]
- Souchon, Y.; Tissot, L. Synthesis of thermal tolerances of the common freshwater fish species in large Western Europe rivers. Knowl. Manag. Aquat. Ecosyst. 2012, 405, 3. [Google Scholar] [CrossRef]
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.-L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J.; Johns, T.; Krinner, G.; et al. Long-term Climate Change: Projections, Commitments and Irreversibility. In Climate Change 2013: The Physical Science Basis—Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Marteau, B.; Piégay, H.; Chandesris, A.; Michel, K.; Vaudor, L. Riparian shading mitigates warming but cannot revert thermal alteration by impoundments in lowland rivers. Earth Surf. Process. Landf. 2022, 47, 2209–2229. [Google Scholar] [CrossRef]
- Punzet, M.; Voß, F.; Kynast, E.; Bärlund, I. A Global Approach to Assess the Potential Impact of Climate Change on Stream Water Temperatures and Related In-Stream First-Order Decay Rates. J. Hydrometeorol. 2012, 13, 1052–1065. [Google Scholar] [CrossRef]
- Boon, P.J.; Cooksley, S.L.; Geist, J.; Killeen, I.J.; Moorkens, E.A.; Sime, I. Developing a standard approach for monitoring freshwater pearl mussel (Margaritifera margaritifera) populations in European rivers. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1365–1379. [Google Scholar] [CrossRef]
- Österling, E.M. Timing, growth and proportion of spawners of the threatened unionoid mussel Margaritifera margaritifera: Influence of water temperature, turbidity and mussel density. Aquat. Sci. 2015, 77, 1–8. [Google Scholar] [CrossRef]
- Geist, J. Strategies for the conservation of endangered freshwater pearl mussels (Margaritifera margaritifera L.): A synthesis of Conservation Genetics and Ecology. Hydrobiologia 2010, 644, 69–88. [Google Scholar] [CrossRef]
- Jobling, M. Temperature tolerance and the final preferendum—Rapid methods for the assessment of optimum growth temperatures. J. Fish. Biol. 1981, 19, 439–455. [Google Scholar] [CrossRef]
- Elliott, J.M.; Elliott, J.A. Temperature requirements of Atlantic salmon Salmo salar, brown trout Salmo trutta and Arctic charr Salvelinus alpinus: Predicting the effects of climate change. J. Fish. Biol. 2010, 77, 1793–1817. [Google Scholar] [CrossRef]
- Alabaster, J.S.; Downing, A. A Field and Laboratory Investigation of the Effect of Heated Effluents on Fish; Her Majesty’s Stationery Office: London, UK, 1966; Volume 6. [Google Scholar]
- Hitt, N.P.; Snook, E.L.; Massie, D.L. Brook trout use of thermal refugia and foraging habitat influenced by brown trout. Can. J. Fish. Aquat. Sci. 2017, 74, 406–418. [Google Scholar] [CrossRef]
- Hastie, L.C.; Young, M.R. Timing of spawning and glochidial release in Scottish freshwater pearl mussel (Margaritifera margaritifera) populations. Freshw. Biol. 2003, 48, 2107–2117. [Google Scholar] [CrossRef]
- Benedict, A.; Geist, J. Effects of water temperature on glochidium viability of Unio crassus and Sinanodonta woodiana: Implications for conservation, management and captive breeding. J. Molluscan Stud. 2021, 87, eyab011. [Google Scholar] [CrossRef]
- Taeubert, J.-E.; El-Nobi, G.; Geist, J. Effects of water temperature on the larval parasitic stage of the thick-shelled river mussel (Unio crassus). Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 24, 231–237. [Google Scholar] [CrossRef]
- Taeubert, J.-E.; Gum, B.; Geist, J. Variable development and excystment of freshwater pearl mussel (Margaritifera margaritifera L.) at constant temperature. Limnologica 2013, 43, 319–322. [Google Scholar] [CrossRef]
- Buddensiek, V. The culture of juvenile freshwater pearl mussels Margaritifera margaritifera L. in cages: A contribution to conservation programmes and the knowledge of habitat requirements. Biol. Conserv. 1995, 74, 33–40. [Google Scholar] [CrossRef]
- Gum, B.; Lange, M.; Geist, J. A critical reflection on the success of rearing and culturing juvenile freshwater mussels with a focus on the endangered freshwater pearl mussel (Margaritifera margaritifera L.). Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 743–751. [Google Scholar] [CrossRef]
- Moravec, V.; Markonis, Y.; Rakovec, O.; Svoboda, M.; Trnka, M.; Kumar, R.; Hanel, M. Europe under multi-year droughts: How severe was the 2014–2018 drought period? Environ. Res. Lett. 2021, 16, 034062. [Google Scholar] [CrossRef]
- Kaule, R.; Gilfedder, B.S. Groundwater Dominates Water Fluxes in a Headwater Catchment during Drought. Front. Water 2021, 3, 706932. [Google Scholar] [CrossRef]
- Sylvester, J.R. Possible effects of thermal effluents on fish: A review. Environ. Pollut. 1972, 3, 205–215. [Google Scholar] [CrossRef]
- Raptis, C.E.; van Vliet, M.T.H.; Pfister, S. Global thermal pollution of rivers from thermoelectric power plants. Environ. Res. Lett. 2016, 11, 104011. [Google Scholar] [CrossRef]
- Sinokrot, B.A.; Stefan, H.G.; McCormick, J.H.; Eaton, J.G. Modeling of climate change effects on stream temperatures and fish habitats below dams and near groundwater inputs. Clim. Chang. 1995, 30, 181–200. [Google Scholar] [CrossRef]
- Ahmad, S.K.; Hossain, F.; Holtgrieve, G.W.; Pavelsky, T.; Galelli, S. Predicting the Likely Thermal Impact of Current and Future Dams Around the World. Earths Future 2021, 9, e2020EF001916. [Google Scholar] [CrossRef]
- Zaidel, P.A.; Roy, A.H.; Houle, K.M.; Lambert, B.; Letcher, B.H.; Nislow, K.H.; Smith, C. Impacts of small dams on stream temperature. Ecol. Indic. 2021, 120, 106878. [Google Scholar] [CrossRef]
- Downing, J.A. Emerging global role of small lakes and ponds: Little things mean a lot. Limnetica 2010, 29, 9–24. [Google Scholar] [CrossRef]
- Ebel, J.D.; Lowe, W.H. Constructed Ponds and Small Stream Habitats: Hypothesized Interactions and Methods to Minimize Impacts. J. Water Resour. Prot. 2013, 5, 723–731. [Google Scholar] [CrossRef]
- EU. CORINE Land Cover CLC 2012. Available online: https://land.copernicus.eu/pan-european/corine-land-cover/clc-2012?tab=download (accessed on 11 December 2019).
- Hoess, R.; Geist, J. Effect of fish pond drainage on turbidity, suspended solids, fine sediment deposition and nutrient concentration in receiving pearl mussel streams. Environ. Pollut. 2021, 274, 116520. [Google Scholar] [CrossRef]
- Neitsch, S.L.; Arnold, J.G.; Kiniry, J.R.; Williams, J.R. Soil and Water Assessment Tool Theoretical Documentation Version 2009; Water Resources Institute: College Station, TX, USA, 2011. [Google Scholar]
- Arnold, J.G.; Moriasi, D.N.; Gassman, P.W.; Abbaspour, K.C.; White, M.J.; Srinivasan, R.; Santhi, C.; Harmel, R.D.; van Griensven, A.; Van Liew, M.W.; et al. SWAT: Model Use, Calibration, and Validation. Trans. ASABE 2012, 55, 1491–1508. [Google Scholar] [CrossRef]
- Jalowska, A.M.; Yuan, Y. Evaluation of SWAT Impoundment Modeling Methods in Water and Sediment Simulations. J. Am. Water Resour. Assoc. 2019, 55, 209–227. [Google Scholar] [CrossRef]
- Wang, X.; Yang, W.; Melesse, A.M. Using Hydrologic Equivalent Wetland Concept Within SWAT to Estimate Streamflow in Watersheds with Numerous Wetlands. Trans. ASABE 2008, 51, 55–72. [Google Scholar] [CrossRef]
- Baldan, D.; Mehdi, B.; Feldbacher, E.; Piniewski, M.; Hauer, C.; Hein, T. Assessing multi-scale effects of natural water retention measures on in-stream fine bed material deposits with a modeling cascade. J. Hydrol. 2021, 594, 125702. [Google Scholar] [CrossRef]
- Hoess, R.; Geist, J. Spatiotemporal variation of streambed quality and fine sediment deposition in five freshwater pearl mussel streams, in relation to extreme drought, strong rain and snow melt. Limnologica 2020, 85, 125833. [Google Scholar] [CrossRef]
- Rivers-Moore, N.A.; Dallas, H.F.; Morris, C. Towards setting environmental water temperature guidelines: A South African example. J. Environ. Manag. 2013, 128, 380–392. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; Volume 574. [Google Scholar]
- Bloomfield, J.P.; Gong, M.; Marchant, B.P.; Coxon, G.; Addor, N. How is Baseflow Index (BFI) impacted by water resource management practices? Hydrol. Earth Syst. Sci. 2021, 25, 5355–5379. [Google Scholar] [CrossRef]
- Chu, C.; Jones, N.E.; Mandrak, N.E.; Piggott, A.R.; Minns, C.K. The influence of air temperature, groundwater discharge, and climate change on the thermal diversity of stream fishes in southern Ontario watersheds. Can. J. Fish. Aquat. Sci. 2008, 65, 297–308. [Google Scholar] [CrossRef]
- Stanfield, L.W.; Kilgour, B.; Todd, K.; Holysh, S.; Piggott, A.; Baker, M. Estimating Summer Low-Flow in Streams in a Morainal Landscape using Spatial Hydrologic Models. Can. Water Resour. J. 2009, 34, 269–284. [Google Scholar] [CrossRef]
- World Meteorological Organization (WMO). Manual on Low Flow Estimation and Prediction; WMO: Geneva, Switzerland, 2008. [Google Scholar]
- Moriasi, D.; Gitau, M.W.; Pai, N.; Daggupati, P. Hydrologic and Water Quality Models: Performance Measures and Evaluation Criteria. Trans. ASABE 2015, 58, 1763–1785. [Google Scholar] [CrossRef]
- Riedel, T.; Nolte, C.; aus der Beek, T.; Lidtke, J.; Sures, B.; Grabner, D. Niedrigwasser, Dürre und Grundwasserneubildung—Bestandsaufnahme zur Gegenwärtigen Situation in Deutschland, den Klimaprojketionen und den Existierenden Maßnahmen und Strategien [Low Flow, Drought and Groundwater Recharge—Inventory of the Current Situation in Germany, the Climate Projects and the Existing Measures and Strategies]; German Environment Agency: Dessau-Roßlau, Germany, 2021.
- Morales, Y.; Weber, L.J.; Mynett, A.E.; Newton, T.J. Effects of substrate and hydrodynamic conditions on the formation of mussel beds in a large river. J. N. Am. Benthol. Soc. 2006, 25, 664–676. [Google Scholar] [CrossRef]
- Strayer, D.L. Use of Flow Refuges by Unionid Mussels in Rivers. J. N. Am. Benthol. Soc. 1999, 18, 468–476. [Google Scholar] [CrossRef]
- Baldan, D.; Piniewski, M.; Funk, A.; Gumpinger, C.; Flödl, P.; Höfer, S.; Hauer, C.; Hein, T. A multi-scale, integrative modeling framework for setting conservation priorities at the catchment scale for the Freshwater Pearl Mussel Margaritifera margaritifera. Sci. Total Environ. 2020, 718, 137369. [Google Scholar] [CrossRef] [PubMed]
- Hastie, L.C.; Boon, P.J.; Young, M.R. Physical microhabitat requirements of freshwater pearl mussels, Margaritifera margaritifera (L.). Hydrobiologia 2000, 429, 59–71. [Google Scholar] [CrossRef]
- Javaheri, A.; Babbar-Sebens, M. On comparison of peak flow reductions, flood inundation maps, and velocity maps in evaluating effects of restored wetlands on channel flooding. Ecol. Eng. 2014, 73, 132–145. [Google Scholar] [CrossRef]
- Babbar-Sebens, M.; Barr, R.C.; Tedesco, L.P.; Anderson, M. Spatial identification and optimization of upland wetlands in agricultural watersheds. Ecol. Eng. 2013, 52, 130–142. [Google Scholar] [CrossRef]
- Stone, N.M.; Boyd, C.E. Seepage from Fishponds; Auburn University: Auburn, AL, USA, 1989. [Google Scholar]
- Rains, M.; Leibowitz, S.; Cohen, M.; Creed, I.; Golden, H.; Jawitz, J.; Kalla, P.; Lane, C.; Lang, M.; McLaughlin, D. Geographically isolated wetlands are part of the hydrological landscape. Hydrol. Process. 2016, 30, 153–160. [Google Scholar] [CrossRef]
- Majerova, M.; Neilson, B.T.; Schmadel, N.M.; Wheaton, J.M.; Snow, C.J. Impacts of beaver dams on hydrologic and temperature regimes in a mountain stream. Hydrol. Earth Syst. Sci. 2015, 19, 3541–3556. [Google Scholar] [CrossRef]
- Juszczak, R.; Kędziora, A.; Olejnik, J. Assessment of Water Retention Capacity of Small Ponds in Wyskoć Agricultural-Forest Catchment in Western Poland. Pol. J. Environ. Stud. 2007, 16, 685–695. [Google Scholar]
- Van Buren, M.A.; Watt, W.E.; Marsalek, J.; Anderson, B.C. Thermal Balance of On-Stream Storm-Water Management Pond. J. Environ. Eng. 2000, 126, 509–517. [Google Scholar] [CrossRef]
- Balon, E.K. Origin and domestication of the wild carp, Cyprinus carpio: From Roman gourmets to the swimming flowers. Aquaculture 1995, 129, 3–48. [Google Scholar] [CrossRef]
- Pandolfo, T.J.; Kwak, T.J.; Cope, W.G. Thermal Tolerances of Freshwater Mussels and their Host Fishes: Species Interactions in a Changing Climate. Freshw. Mollusk Biol. Conserv. 2012, 15, 69–83. [Google Scholar] [CrossRef]
- Monk, W.A.; Wilbur, N.M.; Allen Curry, R.; Gagnon, R.; Faux, R.N. Linking landscape variables to cold water refugia in rivers. J. Environ. Manag. 2013, 118, 170–176. [Google Scholar] [CrossRef]
- Garner, G.; Malcolm, I.A.; Sadler, J.P.; Hannah, D.M. What causes cooling water temperature gradients in a forested stream reach? Hydrol. Earth Syst. Sci. 2014, 18, 5361–5376. [Google Scholar] [CrossRef]
- Story, A.; Moore, R.D.; Macdonald, J.S. Stream temperatures in two shaded reaches below cutblocks and logging roads: Downstream cooling linked to subsurface hydrology. Can. J. For. Res. 2003, 33, 1383–1396. [Google Scholar] [CrossRef]
- Garner, G.; Malcolm, I.A.; Sadler, J.P.; Millar, C.P.; Hannah, D.M. Inter-annual variability in the effects of riparian woodland on micro-climate, energy exchanges and water temperature of an upland Scottish stream. Hydrol. Process. 2015, 29, 1080–1095. [Google Scholar] [CrossRef]
- Garner, G.; Malcolm, I.A.; Sadler, J.P.; Hannah, D.M. The role of riparian vegetation density, channel orientation and water velocity in determining river temperature dynamics. J. Hydrol. 2017, 553, 471–485. [Google Scholar] [CrossRef]
- Imholt, C.; Gibbins, C.N.; Malcolm, I.A.; Langan, S.; Soulsby, C. Influence of riparian cover on stream temperatures and the growth of the mayfly Baetis rhodani in an upland stream. Aquat. Ecol. 2010, 44, 669–678. [Google Scholar] [CrossRef]
- Roth, T.R.; Westhoff, M.C.; Huwald, H.; Huff, J.A.; Rubin, J.F.; Barrenetxea, G.; Vetterli, M.; Parriaux, A.; Selker, J.S.; Parlange, M.B. Stream Temperature Response to Three Riparian Vegetation Scenarios by Use of a Distributed Temperature Validated Model. Environ. Sci. Technol. 2010, 44, 2072–2078. [Google Scholar] [CrossRef]
- Malcolm, I.A.; Soulsby, C.; Hannah, D.M.; Bacon, P.J.; Youngson, A.F.; Tetzlaff, D. The influence of riparian woodland on stream temperatures: Implications for the performance of juvenile salmonids. Hydrol. Process. 2008, 22, 968–979. [Google Scholar] [CrossRef]
- Dugdale, S.J.; Malcolm, I.A.; Kantola, K.; Hannah, D.M. Stream temperature under contrasting riparian forest cover: Understanding thermal dynamics and heat exchange processes. Sci. Total Environ. 2018, 610–611, 1375–1389. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Liss, W.J.; Frissell, C.A. Relationship between stream temperature, thermal refugia and rainbow trout Oncorhynchus mykiss abundance in arid-land streams in the northwestern United States. Ecol. Freshw. Fish 2001, 10, 1–10. [Google Scholar] [CrossRef]
- Elliott, J.M. Pools as refugia for brown trout during two summer droughts: Trout responses to thermal and oxygen stress. J. Fish Biol. 2000, 56, 938–948. [Google Scholar] [CrossRef]

| Parameter | Definition | Source |
|---|---|---|
| PND_FR | Fraction of subbasin area that drains into ponds (0–1) | DEM |
| PND_PSA | Surface area of ponds when filled to principal spillway [ha] | Shape file based on orthophotos |
| PND_PVOL | Volume of water stored in ponds when filled to the principal spillway [104 m3 H2O] | Surface area × depth (=0.8 m, mean depth derived from field observations) |
| PND_ESA | Surface area of ponds when filled to emergency spillway [ha] | PSA × 1.1 (derived from field observations) |
| PND_EVOL | Volume of water stored in ponds when filled to the emergency spillway [104 m3 H2O] | PSA × depth (=1.0 m, derived from field observations) |
| PND_VOL | Initial volume of water in ponds [104 m3 H2O] | =PVOL |
| PND_SED | Initial sediment concentration in pond water [mg/L] | 39 (derived from water samples from fish-free pond) |
| PND_NSED | Equilibrium sediment concentration in pond water [mg/L] | 96 (derived from water samples from stocked pond) |
| PND_K | Hydraulic conductivity through the bottom of ponds (mm/h) | 1 (after Baldan et al. [67]) |
| IFLOD1 | Beginning month of non-flood season | 0 |
| IFLOD 2 | Ending month of non-flood season | 0 |
| NDTARG | Number of days needed to reach target storage from current pond storage [d] | 5 (derived from field observations) |
| PND_D50 | Median particle diameter of sediment [µm] | 10 (default) |
| Parameter | Definition | Initial Calibration Range | Fitted Value |
|---|---|---|---|
| r__CN2.mgt | Initial SCS runoff curve number for soil moisture condition II | −0.5–0.5 | −0.302425 |
| v__ESCO.hru | Soil evaporation compensation factor of HRU | 0.0–0.5 | −0.207090 |
| r_SOL_AWC(#).sol | Available water capacity of the soil layer (#) (mm H2O/mm soil) | −1.0–0.5 | −0.404389 |
| r__SOL_BD(#).sol | Moist bulk density of the soil layer (#) (mg/m3) | 0.0–0.7 | 0.640855 |
| a__CANMX.hru | Maximum canopy storage (mm H2O) | 80–180 | 82.587418 |
| r__SOL_K(#).sol | Saturated hydraulic conductivity of the soil layer (#) (mm/h) | −0.2–0.8 | 0.755521 |
| a__GW_REVAP.gw | Groundwater “revap” coefficient | 0.00–0.18 | 0.061701 |
| a__GWQMN.gw | Threshold depth of water in the shallow aquifer required for return flow to occur (mm H2O) | −1000–2000 | −500.878265 |
| a__REVAPMN.gw | Threshold depth of water in the shallow aquifer required for “revap” or percolation to the deep aquifer to occur (mm H2O) | −750–0 | −373.519958 |
| r__SLSUBBSN.hru | Average slope length (m) | −0.5–1.0 | 0.580487 |
| a__GW_DELAY.gw | Groundwater delay time (days) | 100–350 | 41.244041 |
| a__OV_N.hru | Manning’s “n” for overland flow | 0–100 | 47.643097 |
| v__PND_K.pnd | Hydraulic conductivity through bottom of ponds (mm/h) | 0–1 | 0.602785 |
| v__ALPHA_BF.gw | Baseflow alpha factor (1/days) | 0–1 | 0.799400 |
| v__RCHRG_DP.gw | Deep aquifer percolation fraction | 0.0–0.4 | 0.231797 |
| Metrics | Relevance | |
|---|---|---|
| Magnitude | ||
| ADM_su | Average daily mean Tw in summer | Physiological response, development/growth rates, concept of degree-days |
| ADM_wi | Average daily mean Tw in winter | |
| MaxD_su | Maximum daily mean Tw in summer | Potential thermal limit for aquatic organisms |
| MaxD_wi | Maximum daily mean Tw in winter | |
| AMax_su | Average daily maximum Tw in summer | |
| AMax_wi | Average daily maximum Tw in winter | |
| MaxT | Maximum daily maximum Tw in summer | |
| Variability | ||
| Range_su | Average daily range in Tw in summer | Dial variation |
| Range_wi | Average daily range in Tw in winter | |
| Timing | ||
| Jdmax | Julian day of MaxT in summer | Possible shift in timing of life history transitions |
| Frequency | ||
| b14_5 | Number of days in summer with average daily Tw < 14.5 °C | Tw > 14.5 °C needed to achieve sufficient growth in FPM |
| a20 | Number of days in summer with maximum daily Tw > 20 °C | Host fish (brown trout) will migrate from stream reach at Tw > 21 °C |
| NSE | PBIAS | R2 | |
|---|---|---|---|
| Calibration 1 January 2015–31 December 2021 | 0.71 “good” | 3.3 “very good” | 0.71 “good” |
| Validation 1 January 2010–31 December 2014 | 0.77 “good” | 16.2 “not satisfactory” | 0.79 “good” |
| ADM [°C] | MaxD [°C] | Amax [°C] | Range [°C] | MaxT [°C] | Jdmax | b14_5 | a20 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| su | wi | su | wi | su | wi | su | wi | su | su | su | su | |
| BB1 | 19.2 | 4.4 | 22.5 | 6.7 | 20.2 | 4.6 | 1.9 | 0.5 | 23.8 | 218 | 4.0 | 44.0 |
| BB2 out | 15.9 | 2.9 | 20.1 | 5.9 | 17.6 | 3.4 | 3.2 | 1.0 | 22.3 | 209 | 17.7 | 7.7 |
| BB2 us | 13.5 | 3.0 | 16.5 | 5.6 | 14.9 | 3.5 | 2.6 | 0.9 | 17.8 | 207 | 62.0 | 0.0 |
| BB2 ds | 14.8 | 3.2 | 18.4 | 5.6 | 16.2 | 3.5 | 2.7 | 0.7 | 20.5 | 204 | 35.3 | 2.0 |
| Delta BB2 | 1.3 | 0.1 | 1.9 | 0.0 | 1.3 | 0.0 | 0.0 | −0.1 | 2.7 | −4 | −26.7 | 2.0 |
| BB3 out | 19.2 | 2.4 | 22.8 | 5.0 | 20.6 | 2.6 | 2.7 | 0.4 | 25.0 | 211 | 2.3 | 47.0 |
| BB3 us | 14.9 | 2.9 | 18.6 | 5.9 | 16.4 | 3.4 | 2.9 | 1.0 | 20.7 | 208 | 33.7 | 1.0 |
| BB3 ds | 16.2 | 2.8 | 19.5 | 5.6 | 17.8 | 3.3 | 3.0 | 0.9 | 22.2 | 229 | 15.7 | 10.3 |
| Delta BB3 | 1.3 | −0.1 | 0.9 | −0.2 | 1.4 | −0.1 | 0.1 | −0.1 | 1.5 | 22 | −18.0 | 9.3 |
| BB4 out | 13.4 | 4.2 | 16.3 | 6.4 | 15.7 | 4.9 | 4.1 | 1.4 | 19.5 | 229 | 65.0 | 0.0 |
| BB4 us | 15.9 | 2.7 | 19.3 | 6.0 | 17.8 | 3.5 | 3.8 | 1.4 | 22.2 | 226 | 19.3 | 11.3 |
| BB4 ds | 15.6 | 3.0 | 19.0 | 6.0 | 17.4 | 3.7 | 3.5 | 1.4 | 21.7 | 205 | 23.0 | 6.7 |
| Delta BB4 | −0.3 | 0.2 | −0.3 | 0.0 | −0.4 | 0.2 | −0.3 | 0.0 | −0.5 | −22 | 3.7 | −4.7 |
| BB5 out | 19.0 | 3.1 | 23.5 | 5.7 | 20.3 | 3.4 | 2.7 | 0.6 | 25.7 | 205 | 4.3 | 41.3 |
| BB5 us | 15.9 | 3.0 | 19.5 | 6.3 | 18.2 | 3.9 | 4.2 | 1.6 | 22.5 | 204 | 19.7 | 14.3 |
| BB5 ds | 16.3 | 3.0 | 19.9 | 6.1 | 18.5 | 3.8 | 4.1 | 1.5 | 23.2 | 211 | 16.7 | 18.3 |
| Delta BB5 | 0.4 | 0.0 | 0.5 | −0.1 | 0.3 | 0.0 | −0.1 | −0.1 | 0.7 | 7 | −3.0 | 4.0 |
| MB1 out | 15.6 | 2.7 | 19.1 | 5.1 | 17.2 | 3.3 | 3.0 | 1.0 | 21.0 | 215 | 27.0 | 3.0 |
| MB1 us | 12.6 | 3.5 | 15.9 | 6.2 | 14.2 | 4.3 | 3.1 | 1.5 | 17.5 | 218 | 74.0 | 0.0 |
| MB1 ds | 13.6 | 3.3 | 17.0 | 5.9 | 15.1 | 4.0 | 3.1 | 1.3 | 19.2 | 224 | 60.7 | 0.3 |
| Delta MB1 | 1.0 | −0.1 | 1.5 | −0.2 | 0.9 | −0.2 | −0.1 | −0.2 | 1.0 | 3 | −13.5 | 0.0 |
| MB2 us | 14.0 | 2.8 | 17.2 | 5.6 | 16.0 | 3.4 | 4.0 | 1.1 | 20.0 | 211 | 51.0 | 0.5 |
| MB2 ds | 15.8 | 3.0 | 19.7 | 5.8 | 18.1 | 3.7 | 4.3 | 1.3 | 23.5 | 214 | 20.7 | 15.7 |
| Delta MB2 | 2.2 | −0.2 | 3.0 | 0.0 | 2.8 | −0.1 | 0.6 | 0.1 | 4.5 | −2 | −37.5 | 20.5 |
| MB3 out | 15.8 | 1.9 | 19.4 | 5.4 | 17.2 | 2.5 | 2.6 | 1.1 | 23.0 | 154 | 21.0 | 8.0 |
| MB3 us | 15.3 | 2.5 | 18.9 | 5.8 | 17.4 | 3.2 | 4.1 | 1.3 | 21.5 | 204 | 27.7 | 7.3 |
| MB3 ds | 14.9 | 2.5 | 18.3 | 5.8 | 16.9 | 3.2 | 3.7 | 1.3 | 20.8 | 207 | 33.3 | 2.3 |
| Delta MB3 | −0.3 | 0.0 | −0.6 | 0.0 | −0.6 | 0.0 | −0.4 | 0.0 | −0.8 | 2 | 5.7 | −5.0 |
| EB1 out | 14.0 | 2.1 | 18.3 | 5.0 | 15.8 | 2.6 | 3.2 | 1.0 | 23.7 | 210 | 44.7 | 7.0 |
| EB1 us | 17.9 | 2.0 | 21.8 | 4.8 | 19.8 | 2.4 | 3.5 | 0.6 | 24.2 | 201 | 4.0 | 37.0 |
| EB1 ds | 15.8 | 2.7 | 19.9 | 5.0 | 17.7 | 3.2 | 3.3 | 1.0 | 22.3 | 186 | 23.7 | 14.0 |
| Delta EB1 | −2.1 | 0.0 | −1.9 | 0.0 | −2.1 | 0.0 | −0.2 | 0.0 | −1.8 | −15 | 19.7 | −23.0 |
| HB1 out | 18.0 | 2.1 | 22.0 | 4.8 | 19.5 | 2.4 | 3.0 | 0.5 | 24.3 | 220 | 8.7 | 35.7 |
| HB1 us | 15.3 | 2.5 | 18.7 | 5.7 | 17.1 | 3.0 | 3.5 | 1.0 | 21.0 | 206 | 27.0 | 6.0 |
| HB1 ds | 16.5 | 2.6 | 20.0 | 5.2 | 18.1 | 3.0 | 3.2 | 0.7 | 22.0 | 203 | 9.7 | 11.3 |
| Delta HB1 | 1.2 | 0.1 | 1.3 | −0.6 | 1.0 | −0.1 | −0.3 | −0.3 | 1.0 | −3 | −17.3 | 5.3 |
| HB2 out | 14.7 | 2.7 | 18.2 | 6.0 | 16.5 | 3.4 | 3.7 | 1.3 | 20.5 | 205 | 37.7 | 4.7 |
| HB2 us | 15.1 | 3.0 | 17.8 | 5.5 | 17.0 | 3.4 | 3.7 | 0.8 | 20.8 | 206 | 31.5 | 3.5 |
| HB2 ds | 15.5 | 2.7 | 18.7 | 5.7 | 17.3 | 3.2 | 3.7 | 1.1 | 20.8 | 204 | 22.3 | 5.7 |
| Delta HB2 | 0.8 | 0.2 | 1.0 | 0.4 | 0.6 | 0.2 | −0.1 | 0.1 | 0.3 | −5 | −15.5 | 3.0 |
| Delta MB4 | 0.8 | −0.1 | 0.5 | −0.2 | 0.8 | −0.1 | 0.0 | −0.1 | 0.3 | −1 | −15.3 | 1.0 |
| HB3 ds | 15.8 | 2.4 | 19.5 | 5.6 | 17.4 | 3.0 | 3.2 | 1.1 | 21.5 | 208 | 20.7 | 6.0 |
| Reach | Reach Length (km) | % Pond Area | % Forested Area | % Open Land | Total Area [ha] |
|---|---|---|---|---|---|
| BB1 | 0.77 | 12.5 | 87.5 | 0.0 | 42.49 |
| BB1-BB2 | 1.44 | 0.8 | 99.2 | 0.0 | 71.58 |
| BB2 | 0.06 | 5.2 | 94.8 | 0.0 | 114.07 |
| BB2-BB3 | 0.11 | 0.0 | 100.0 | 0.0 | 1.34 |
| BB3 | 0.02 | 16.3 | 83.7 | 0.0 | 5.51 |
| BB3-BB4 | 1.16 | 0.0 | 68.1 | 31.9 | 16.39 |
| BB4 | 0.02 | 0.1 | 47.2 | 52.7 | 62.27 |
| BB4-BB5 | 1.16 | 0.8 | 5.0 | 94.2 | 34.44 |
| BB5 | 0.03 | 12.9 | 0.0 | 87.1 | 2.73 |
| EB1 | 0.02 | 3.3 | 96.7 | 0.0 | 3.62 |
| HB1 | 0.06 | 7.1 | 92.9 | 0.0 | 12.24 |
| HB1-HB2 | 1.18 | 2.6 | 97.4 | 0.0 | 32.43 |
| HB2 | 0.03 | 50.4 | 49.6 | 0.0 | 2.03 |
| HB2-HB3 | 1.75 | 2.3 | 47.8 | 40.0 | 34.38 |
| MB1 | 0.03 | 8.7 | 8.2 | 83.0 | 9.07 |
| MB1-MB2 | 1.16 | 0.3 | 59.8 | 40.0 | 22.86 |
| MB2 | 0.16 | 7.8 | 57.2 | 35.0 | 3.17 |
| MB2-MB3 | 0.39 | 0.0 | 68.2 | 31.8 | 6.63 |
| MB3 | 0.02 | 25.4 | 74.6 | 0.0 | 1.48 |
| MB3-MB4/HB2 | 1.35 | 2.6 | 97.4 | 0.0 | 26.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoess, R.; Generali, K.A.; Kuhn, J.; Geist, J. Impact of Fish Ponds on Stream Hydrology and Temperature Regime in the Context of Freshwater Pearl Mussel Conservation. Water 2022, 14, 2490. https://doi.org/10.3390/w14162490
Hoess R, Generali KA, Kuhn J, Geist J. Impact of Fish Ponds on Stream Hydrology and Temperature Regime in the Context of Freshwater Pearl Mussel Conservation. Water. 2022; 14(16):2490. https://doi.org/10.3390/w14162490
Chicago/Turabian StyleHoess, Rebecca, Konstantina A. Generali, Johannes Kuhn, and Juergen Geist. 2022. "Impact of Fish Ponds on Stream Hydrology and Temperature Regime in the Context of Freshwater Pearl Mussel Conservation" Water 14, no. 16: 2490. https://doi.org/10.3390/w14162490
APA StyleHoess, R., Generali, K. A., Kuhn, J., & Geist, J. (2022). Impact of Fish Ponds on Stream Hydrology and Temperature Regime in the Context of Freshwater Pearl Mussel Conservation. Water, 14(16), 2490. https://doi.org/10.3390/w14162490






