Bioremediation of Raw Landfill Leachate Using Galdieria sulphuraria: An Algal-Based System for Landfill Leachate Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culturing of G. sulphuraria and Landfill Leachate Collection
2.2. Evaluation of G. sulphuraria for Nutrient Removal from Landfill Leachate and Algal Biomass Production
2.2.1. Experiment 1
2.2.2. Experiment 2
2.3. Sample Analysis
3. Results and Discussion
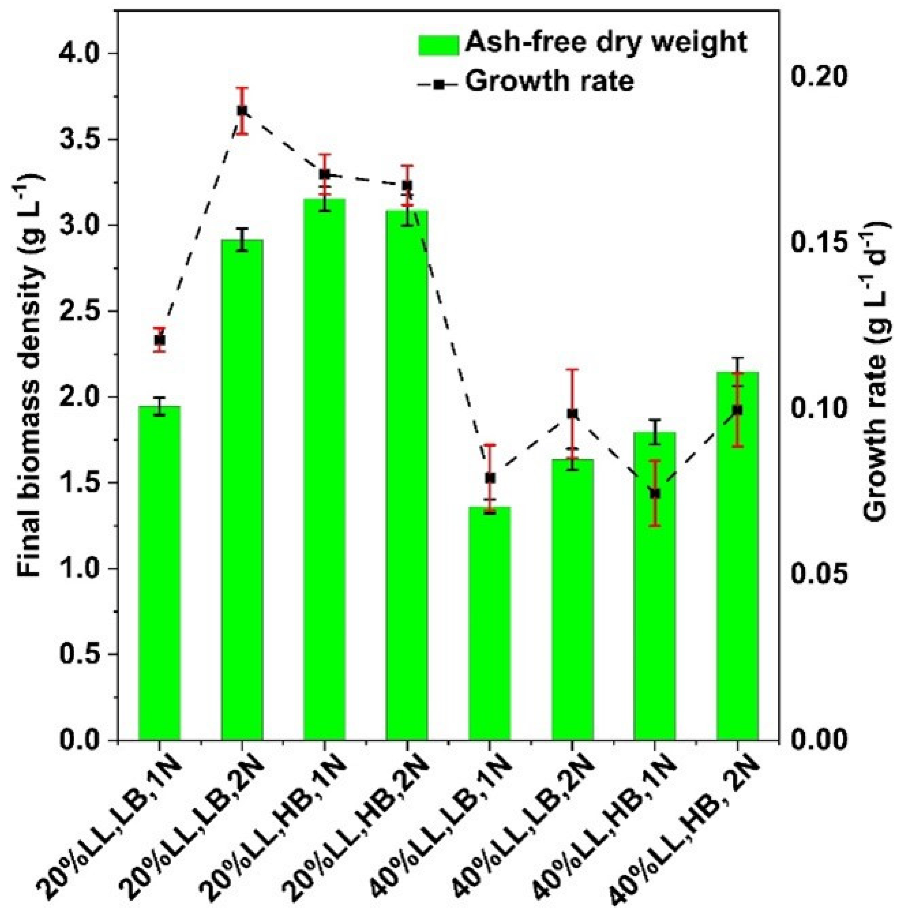
3.1. Effects of Experimental Media Compositions on the Growth of G. sulphuraria
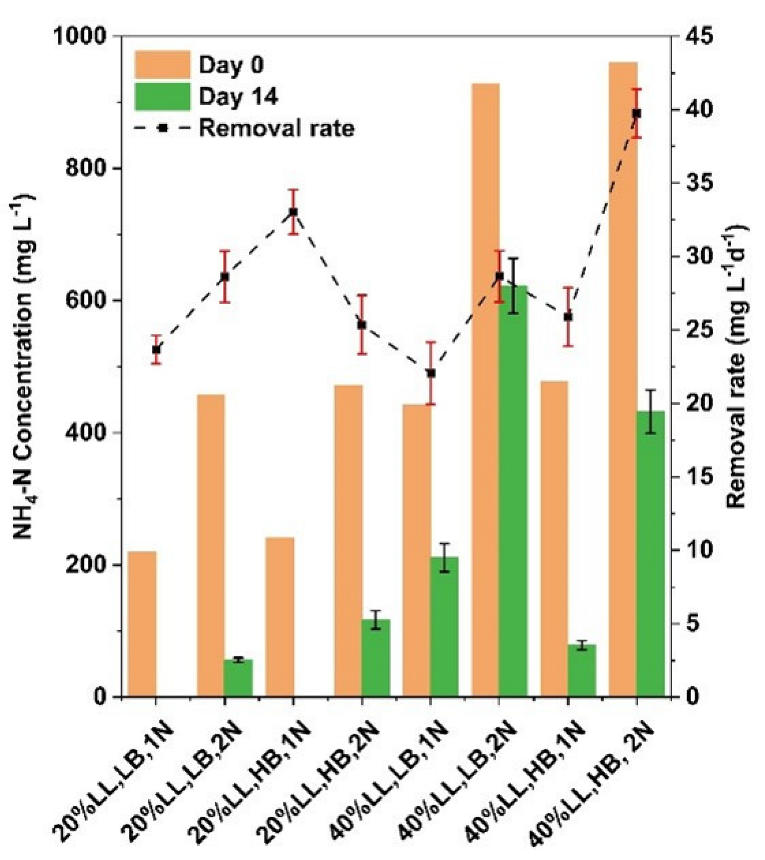
3.2. Effects of Experimental Media Compositions on Nitrogen Removal
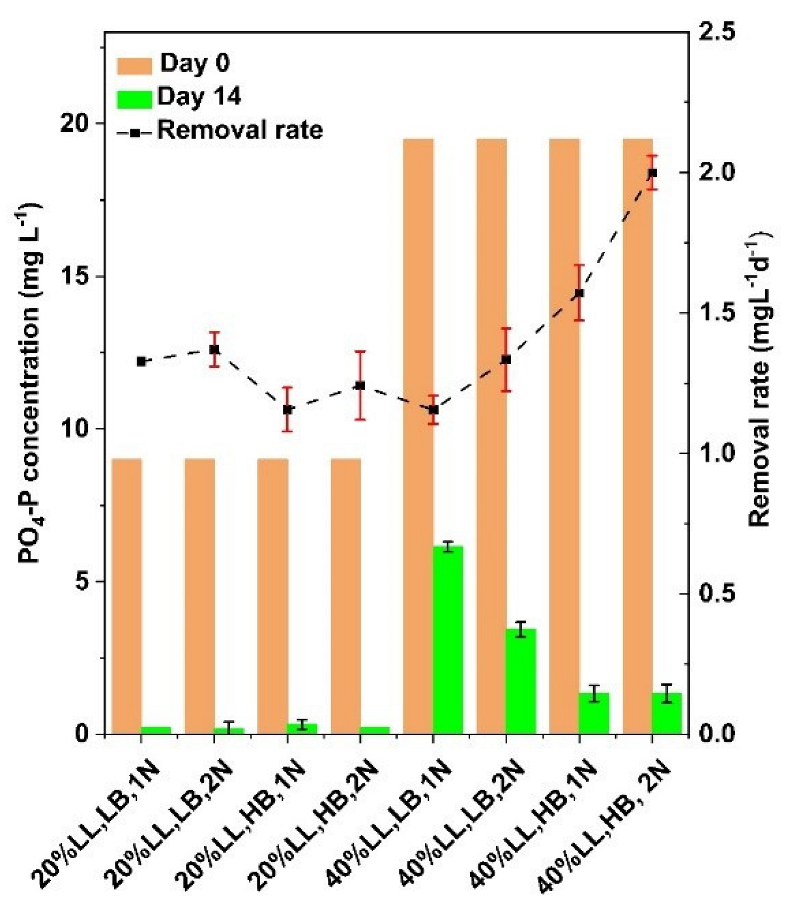
3.3. Effects of Experimental Media Compositions on Phosphate Removal
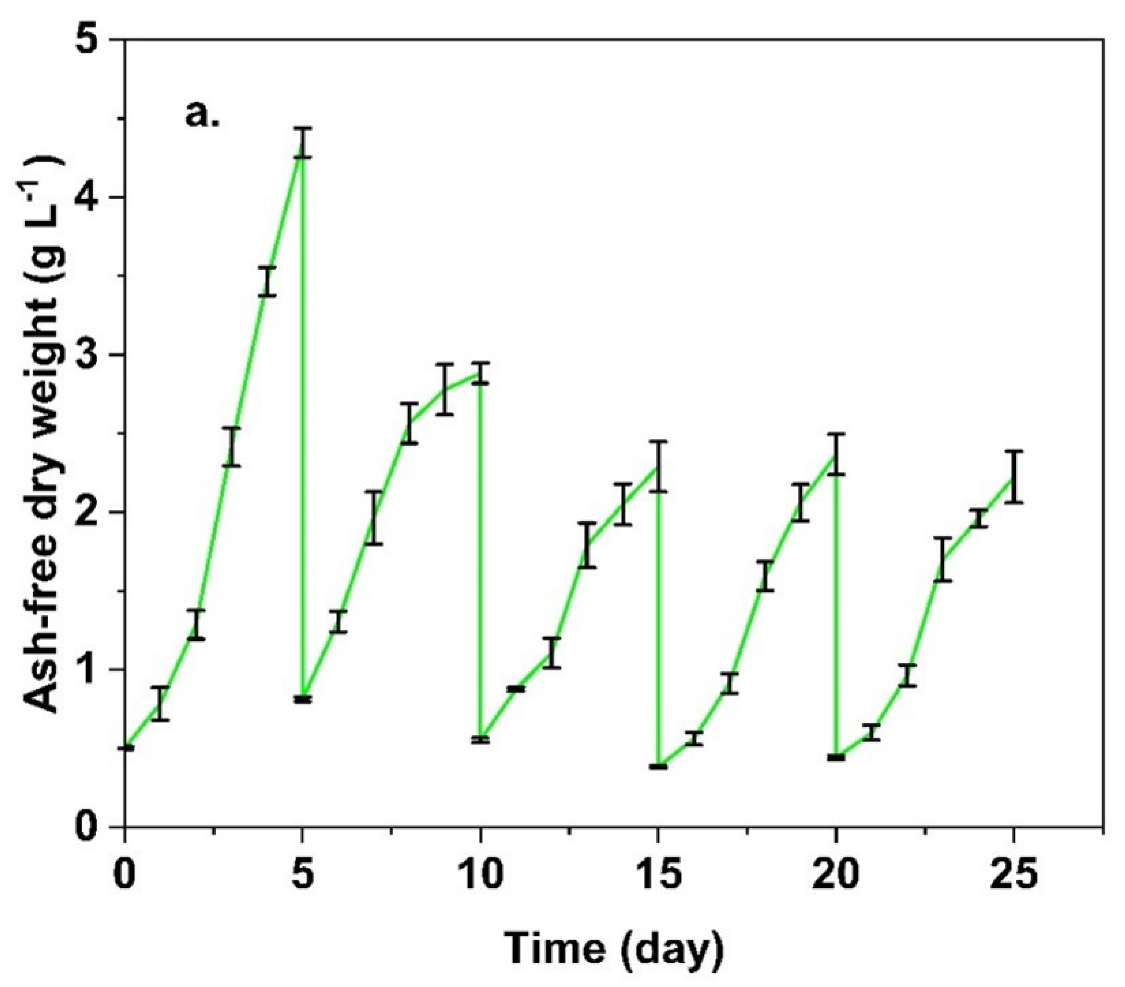
3.4. Long-Term Running Performance of Algal-Based LL Treatment System
3.5. Comparison between the Current Study and the Literature Reported for Long-Term Running
3.6. Limitations and Practical Implications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aziz, H.A.; Yusoff, M.S.; Adlan, M.N.; Adnan, N.H.; Alias, S. Physico-chemical removal of iron from semi-aerobic landfill leachate by limestone filter. Waste Manag. 2004, 24, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Dixon, K.L.; Nawaz, T.; Rahman, A.; Selvaratnam, T. Evaluation of Galdieria sulphuraria for nitrogen removal and biomass production from raw landfill leachate. Algal Res. 2021, 54, 102183. [Google Scholar] [CrossRef]
- Nawaz, T.; Rahman, A.; Pan, S.; Dixon, K.; Petri, B.; Selvaratnam, T. A Review of Landfill Leachate Treatment by Microalgae: Current Status and Future Directions. Processes 2020, 8, 384. [Google Scholar] [CrossRef] [Green Version]
- Sniffen, K.D.; Sales, C.M.; Olson, M.S. Nitrogen removal from raw landfill leachate by an algae-bacteria consortium. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2016, 73, 479–485. [Google Scholar] [CrossRef]
- Quan, X.; Hu, R.; Chang, H.; Tang, X.; Huang, X.; Cheng, C.; Zhong, N.; Yang, L. Enhancing microalgae growth and landfill leachate treatment through ozonization. J. Clean. Prod. 2020, 248, 119182. [Google Scholar] [CrossRef]
- Dogaris, I.; Loya, B.; Cox, J.; Philippidis, G. Study of landfill leachate as a sustainable source of water and nutrients for algal biofuels and bioproducts using the microalga Picochlorum oculatum in a novel scalable bioreactor. Bioresour. Technol. 2019, 282, 18–27. [Google Scholar] [CrossRef]
- Mustafa, E.-M.; Phang, S.-M.; Chu, W.-L. Use of an algal consortium of five algae in the treatment of landfill leachate using the high-rate algal pond system. J. Appl. Phycol. 2012, 24, 953–963. [Google Scholar] [CrossRef]
- Sniffen, K.D.; Sales, C.M.; Olson, M.S. The fate of nitrogen through algal treatment of landfill leachate. Algal Res. 2018, 30, 50–58. [Google Scholar] [CrossRef]
- Martins, C.L.; Fernandes, H.; Costa, R.H.R. Landfill leachate treatment as measured by nitrogen transformations in stabilization ponds. Bioresour. Technol. 2013, 147, 562–568. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Chan, G.Y.S.; Jiang, B.L.; Lan, C.Y. Use of ammoniacal nitrogen tolerant microalgae in landfill leachate treatment. Waste Manag. 2007, 27, 1376–1382. [Google Scholar] [CrossRef]
- Paskuliakova, A.; Tonry, S.; Touzet, N. Phycoremediation of landfill leachate with chlorophytes: Phosphate a limiting factor on ammonia nitrogen removal. Water Res. 2016, 99, 180–187. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Huang, S.; Qiu, D.; Schideman, L.; Chai, X.; Zhao, Y. Characterization of microalgae-bacteria consortium cultured in landfill leachate for carbon fixation and lipid production. Bioresour. Technol. 2014, 156, 322–328. [Google Scholar] [CrossRef]
- Sniffen, K.D.; Price, J.R.; Sales, C.M.; Olson, M.S. Influence of Scale on Biomass Growth and Nutrient Removal in an Algal–Bacterial Leachate Treatment System. Environ. Sci. Technol. 2017, 51, 13344–13352. [Google Scholar] [CrossRef]
- Au-Sniffen, K.D.; Au-Sales, C.M.; Au-Olson, M.S. Comparison of Scale in a Photosynthetic Reactor System for Algal Remediation of Wastewater. JoVE 2017, 121, e55256. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pegallapati, A.K.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Evaluation of a thermo-tolerant acidophilic alga, Galdieria sulphuraria, for nutrient removal from urban wastewaters. Bioresour. Technol. 2014, 156, 395–399. [Google Scholar] [CrossRef]
- Gross, W.; Heilmann, I.; Lenze, D.; Schnarrenberger, C. Biogeography of the Cyanidiaceae (Rhodophyta) based on 18S ribosomal RNA sequence data. Eur. J. Phycol. 2001, 36, 275–280. [Google Scholar] [CrossRef]
- Henkanatte-Gedera, S.M.; Selvaratnam, T.; Karbakhshravari, M.; Myint, M.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Removal of dissolved organic carbon and nutrients from urban wastewaters by Galdieria sulphuraria: Laboratory to field scale demonstration. Algal Res. 2017, 24 Pt B, 450–456. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pegallapati, A.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Lammers, P.J.; van Voorhies, W. Feasibility of algal systems for sustainable wastewater treatment. Renew. Energy 2015, 82, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Toplin, J.A.; Norris, T.B.; Lehr, C.R.; McDermott, T.R.; Castenholz, R.W. Biogeographic and phylogenetic diversity of thermoacidophilic Cyanidiales in Yellowstone National Park, Japan, and New Zealand. Appl. Environ. Microbiol. 2008, 74, 2822–2833. [Google Scholar] [CrossRef] [Green Version]
- Bakare, A.A.; Mosuro, A.A.; Osibanjo, O. Landfill leachate-induced toxicity in mice. J. Environ. Biol. 2003, 24, 429–435. [Google Scholar]
- Bialowiec, A.; Randerson, P.F. Phytotoxicity of landfill leachate on willow—Salix amygdalina L. Waste Manag. 2010, 30, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Clément, B.; Merlin, G. The contribution of ammonia and alkalinity to landfill leachate toxicity to duckweed. Sci. Total Environ. 1995, 170, 71–79. [Google Scholar] [CrossRef]
- Jamers, A.; Blust, R.; De Coen, W.; Griffin, J.L.; Jones, O.A.H. Copper toxicity in the microalga Chlamydomonas reinhardtii: An integrated approach. Biometals 2013, 26, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Quan, X.; Zhong, N.; Zhang, Z.; Lu, C.; Li, G.; Cheng, Z.; Yang, L. High-efficiency nutrients reclamation from landfill leachate by microalgae Chlorella vulgaris in membrane photobioreactor for bio-lipid production. Bioresour. Technol. 2018, 266, 374–381. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pegallapati, A.K.; Reddy, H.; Kanapathipillai, N.; Nirmalakhandan, N.; Deng, S.; Lammers, P.J. Algal biofuels from urban wastewaters: Maximizing biomass yield using nutrients recycled from hydrothermal processing of biomass. Bioresour. Technol. 2015, 182, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Selvaratnam, T.; Reddy, H.; Muppaneni, T.; Holguin, F.O.; Nirmalakhandan, N.; Lammers, P.J.; Deng, S. Optimizing energy yields from nutrient recycling using sequential hydrothermal liquefaction with Galdieria sulphuraria. Algal Res. 2015, 12, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Yuan, Q. Ammonium removal using algae–bacteria consortia: The effect of ammonium concentration, algae biomass, and light. Biodegradation 2018, 29, 105–115. [Google Scholar] [CrossRef]
- Paskuliakova, A.; Tonry, S.; Touzet, N. Microalgae isolation and selection for the treatment of landfill leachate. In Proceedings of the 13th International Conference on Modelling, Monitoring and Management of Water Pollution (WP 2016), Milan, Italy, 28–30 June 2022; pp. 65–78. [Google Scholar]
- Oesterhelt, C.; Schmalzlin, E.; Schmitt, J.M.; Lokstein, H. Regulation of photosynthesis in the unicellular acidophilic red alga Galdieria sulphuraria. Plant J. 2007, 51, 500–511. [Google Scholar] [CrossRef]
- Tighiri, H.O.; Erkurt, E.A. Biotreatment of landfill leachate by microalgae-bacteria consortium in sequencing batch mode and product utilization. Bioresour. Technol. 2019, 286, 121396. [Google Scholar] [CrossRef]

| Parameters | Unit | Landfill Leachate | Cyanidium Media |
|---|---|---|---|
| Total Solids | g L−1 | 23.97 ± 0.24 | 2.6 |
| Chemical Oxygen Demand (COD) | mg L−1 | 4827 ± 186 | 0 |
| Total Organic Carbon (TOC) | mg L−1 | 742 ± 16 | 0 |
| Biochemical Oxygen Demand (BOD5) | mg L−1 | 650 ± 10 | 0 |
| Ammoniacal Nitrogen (NH3-N) | mg L−1 | 1140 ± 30 | 280 |
| Nitrate Nitrogen (NO3-N) | mg L−1 | 7.08 ± 0.06 | 0 |
| Nitrite Nitrogen (NO-N) | mg L−1 | 0.13 ± 0.014 | 0 |
| Total Nitrogen (TN) | mg L−1 | 1320 ± 35 | 280 |
| Free Phosphate Phosphorus (PO4-P) | mg L−1 | 4.47 ± 0.12 | 61.5 |
| Total Phosphorus (TP) | mg L−1 | 18.8 ± 0.2 | 61.5 |
| Group | LL Conc. % | Biomass Density g L−1 | Nitrogen Level | Label |
|---|---|---|---|---|
| 1 | 20 | 0.25 | 1N | 20% LL, LB, 1N |
| 2 | 20 | 0.25 | 2N | 20% LL, LB, 2N |
| 3 | 20 | 0.75 | 1N | 20% LL, HB, 1N |
| 4 | 20 | 0.75 | 2N | 20% LL, HB, 2N |
| 5 | 40 | 0.25 | 1N | 40% LL, LB, 1N |
| 6 | 40 | 0.25 | 2N | 40% LL, LB, 2N |
| 7 | 40 | 0.75 | 1N | 40% LL, HB, 1N |
| 8 | 40 | 0.75 | 2N | 40% LL, HB, 2N |
| Dependent Variable | Final Biomass Density | Growth Rate | N Removal Rate | N Removal Efficiency | P Removal Rate | P Removal Efficiency |
|---|---|---|---|---|---|---|
| g L−1 | g L−1 d−1 | mg L−1 d−1 | % | mg L−1 d−1 | % | |
| Source | Sig. | Sig. | Sig. | Sig. | Sig. | Sig. |
| Corrected Model | 0.000 | 0.000 | 0.040 | 0.000 | 0.004 | 0.001 |
| Intercept | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| LLC | 0.000 | 0.000 | 0.564 | 0.000 | 0.015 | 0.000 |
| NL | 0.000 | 0.000 | 0.083 | 0.000 | 0.058 | 0.212 |
| IBD | 0.000 | 0.350 | 0.044 | 0.001 | 0.042 | 0.012 |
| LLC * NL | 0.434 | 0.434 | 0.028 | 0.302 | 0.192 | 0.317 |
| LLC * IBD | 0.246 | 0.246 | 0.371 | 0.000 | 0.001 | 0.007 |
| NL * IBD | 0.014 | 0.018 | 0.583 | 0.047 | 0.408 | 0.288 |
| LLC * NL * IBD | 0.006 | 0.005 | 0.055 | 0.753 | 0.542 | 0.235 |
| R Squared | 0.934 | 0.92 | 0.553 | 0.953 | 0.685 | 0.727 |
| Adjusted R Squared | 0.905 | 0.884 | 0.357 | 0.932 | 0.547 | 0.607 |
| Parameter | C1D0 | C1D5 | C2D0 | C2D5 | C3D0 | C3D5 | C4D0 | C4D5 | C5D0 | C5D5 |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | 2.500 | 1.780 | 2.400 | 1.790 | 2.450 | 1.740 | 2.400 | 1.910 | 2.550 | 1.920 |
| EC | 9.790 | 12.040 | 11.020 | 13.300 | 11.430 | 13.280 | 11.510 | 13.010 | 11.320 | 12.900 |
| Ag | 0.313 | 0.296 | 0.312 | 0.302 | 0.320 | 0.304 | 0.305 | 0.307 | 0.307 | 0.299 |
| Al | 0.380 | 0.390 | 0.441 | 0.472 | 0.461 | 0.521 | 0.498 | 0.490 | 0.443 | 0.465 |
| As | 5.950 | 5.650 | 6.480 | 6.270 | 6.790 | 6.430 | 6.580 | 6.450 | 6.500 | 6.380 |
| Ba | 0.020 | 0.075 | 0.026 | 0.090 | 0.025 | 0.089 | 0.022 | 0.084 | 0.035 | 0.083 |
| Be | 0.018 | 0.018 | 0.018 | 0.018 | 0.018 | 0.018 | 0.018 | 0.018 | 0.017 | 0.018 |
| Ca | 49.000 | 48.500 | 59.500 | 58.100 | 61.900 | 60.700 | 62.000 | 60.900 | 62.200 | 61.200 |
| Cd | 0.308 | 0.300 | 0.336 | 0.326 | 0.344 | 0.334 | 0.343 | 0.336 | 0.343 | 0.337 |
| Ce | 3.180 | 3.060 | 3.170 | 3.090 | 3.240 | 3.130 | 3.120 | 3.130 | 3.140 | 3.090 |
| Co | 0.345 | 0.340 | 0.355 | 0.349 | 0.359 | 0.352 | 0.356 | 0.354 | 0.355 | 0.351 |
| Cr | 0.377 | 0.375 | 0.387 | 0.384 | 0.392 | 0.387 | 0.386 | 0.388 | 0.387 | 0.386 |
| Cu | 0.495 | 0.510 | 0.499 | 0.484 | 0.486 | 0.472 | 0.474 | 0.474 | 0.477 | 0.476 |
| Fe | 0.061 | 0.371 | 0.519 | 0.568 | 0.439 | 0.535 | 0.357 | 0.475 | 0.304 | 0.495 |
| Hg | 0.656 | 2.290 | 0.605 | 1.620 | 0.558 | 1.290 | 0.502 | 0.952 | 0.472 | 0.762 |
| K | 190 | 190 | 211 | 210 | 219 | 215 | 216 | 214 | 220 | 212 |
| Li | 0.896 | 0.893 | 0.912 | 0.904 | 0.937 | 0.908 | 0.908 | 0.908 | 0.908 | 0.900 |
| Mg | 50.700 | 48.300 | 59.300 | 56.700 | 61.500 | 59.100 | 61.600 | 59.800 | 61.500 | 60.000 |
| Mn | 0.206 | 0.187 | 0.227 | 0.085 | 0.212 | 0.100 | 0.214 | 0.112 | 0.215 | 0.110 |
| Mo | 0.437 | 0.430 | 0.457 | 0.444 | 0.463 | 0.451 | 0.456 | 0.454 | 0.458 | 0.452 |
| Na | 1060 | 1100 | 1270 | 1340 | 1350 | 1380 | 1330 | 1440 | 1360 | 1340 |
| Ni | 0.406 | 0.402 | 0.419 | 0.414 | 0.424 | 0.418 | 0.420 | 0.418 | 0.419 | 0.417 |
| Pb | 1.160 | 1.130 | 1.180 | 1.160 | 1.190 | 1.170 | 1.170 | 1.170 | 1.180 | 1.160 |
| Sb | 1.940 | 1.890 | 2.110 | 2.080 | 2.180 | 2.090 | 2.110 | 2.080 | 2.120 | 2.050 |
| Se | 2.120 | 2.030 | 2.300 | 2.170 | 2.350 | 2.250 | 2.300 | 2.280 | 2.310 | 2.270 |
| Si | 5.280 | 5.040 | 6.580 | 6.220 | 6.950 | 6.520 | 7.350 | 6.570 | 6.890 | 6.480 |
| Sn | 0.717 | 0.710 | 0.746 | 0.736 | 0.757 | 0.745 | 0.756 | 0.749 | 0.754 | 0.743 |
| Sr | 0.888 | 0.882 | 1.040 | 1.030 | 1.080 | 1.060 | 1.070 | 1.060 | 1.070 | 1.070 |
| Ti | 0.272 | 0.269 | 0.273 | 0.271 | 0.275 | 0.271 | 0.271 | 0.271 | 0.272 | 0.270 |
| TI | 2.330 | 2.320 | 2.580 | 2.520 | 2.640 | 2.540 | 2.610 | 2.590 | 2.640 | 2.540 |
| V | 0.456 | 0.450 | 0.465 | 0.460 | 0.470 | 0.462 | 0.463 | 0.464 | 0.464 | 0.461 |
| Zn | BD | BD | BD | BD | BD | BD | BD | BD | BD | BD |
| NH4-N(NO3-N) | PO4-P | Biomass | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source a | Strain | Bioreactor | Dilution | Time (Cycles) | HRT | Initial Conc. | Removal Efficiency | Removal Rate | Initial Conc. | P Removal | P Removal Rate | Initial Density | Final Density | Growth Rate | ||
| days | mg L−1 | % | mg L−1 d −1 | mg L−1 | % | mg L−1 d −1 | g L−1 | g L−1 | g L−1 d −1 | |||||||
| A | algae–bacteria | 57 L tank semibatch | 5–20% | 22 weeks (22) | 21 | 5.0–90 | 29 | 3.1 | 0.3 | 0.36 | 0.008 | |||||
| B | algae–bacteria | 100 L tank semibatch | 1.70% | 8 weeks (8) | 21 | 3.1–14 | 1.02 | 0.024 | ||||||||
| 1000 L pond semibatch | 1.70% | 8 weeks (8) | 21 | 3.1–14 | 0.88 | 0.01 | ||||||||||
| C | algae–bacteria | 0.25 L flask semibatch | 0.38–3% | 7 days | 7 | 0.2–161 | 2.96 | 0.04–1.13 | 0.018 | |||||||
| 100 L tank semibatch | 0.38–3% | 52 weeks (52) | 21 | 0.2–161 | 2.68 | 0.1–1.65 | 0.004 | |||||||||
| 1000 L pond semibatch | 0.38–3% | 52 weeks (52) | 21 | 0.2–161 | 2.33 | 0.14–1.78 | 0.012 | |||||||||
| D | algae–bacteria | 20.33 m3 (4 ponds continuous) | 100% | 111 weeks | 102 | 805–1510 | 75–99 | 9.88 | ||||||||
| E | Picochlorum oculatum | 150 L horizontal bioreactor semibatch | 100%, 50% | 73 days (3) | 73.36 | 350,200 | 82 | 8.99 | 25 | 76 | 0.97 | 0.5 | 1.67 | 0.049 | ||
| F | algae–bacteria | 10 L photobioreactor semibatch | 10% | 54 days (3) | 19 | 250 | 98.3 | 13.93 | 10 | 95.53 | 0.51 | 0.34 | 2.92 | 0.142 | ||
| 10% | 54 days (3) | 19 | 250 | 99.9 | 14.36 | 10 | 99.12 | 0.54 | 1.35 | 8.24 | 0.383 | |||||
| This study | G. sulphuraria | 1 L tubular photobioreactor semibatch | 20% | 25 days (5) | 5 | 243 | 88.8 | 43.7 | 8.5 | 98.9 | 3.99 | 0.5 | 2.82 | 0.46 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvaratnam, T.; Pan, S.; Rahman, A.; Tan, M.; Kharel, H.L.; Agrawal, S.; Nawaz, T. Bioremediation of Raw Landfill Leachate Using Galdieria sulphuraria: An Algal-Based System for Landfill Leachate Treatment. Water 2022, 14, 2389. https://doi.org/10.3390/w14152389
Selvaratnam T, Pan S, Rahman A, Tan M, Kharel HL, Agrawal S, Nawaz T. Bioremediation of Raw Landfill Leachate Using Galdieria sulphuraria: An Algal-Based System for Landfill Leachate Treatment. Water. 2022; 14(15):2389. https://doi.org/10.3390/w14152389
Chicago/Turabian StyleSelvaratnam, Thinesh, Shanglei Pan, Ashiqur Rahman, Melissa Tan, Hari Lal Kharel, Saumya Agrawal, and Tabish Nawaz. 2022. "Bioremediation of Raw Landfill Leachate Using Galdieria sulphuraria: An Algal-Based System for Landfill Leachate Treatment" Water 14, no. 15: 2389. https://doi.org/10.3390/w14152389
APA StyleSelvaratnam, T., Pan, S., Rahman, A., Tan, M., Kharel, H. L., Agrawal, S., & Nawaz, T. (2022). Bioremediation of Raw Landfill Leachate Using Galdieria sulphuraria: An Algal-Based System for Landfill Leachate Treatment. Water, 14(15), 2389. https://doi.org/10.3390/w14152389









