Seasonal Differences in the Hydrochemical Characteristics of Karst Wetlands and the Associated Mechanisms in Huixian, China
Abstract
:1. Introduction
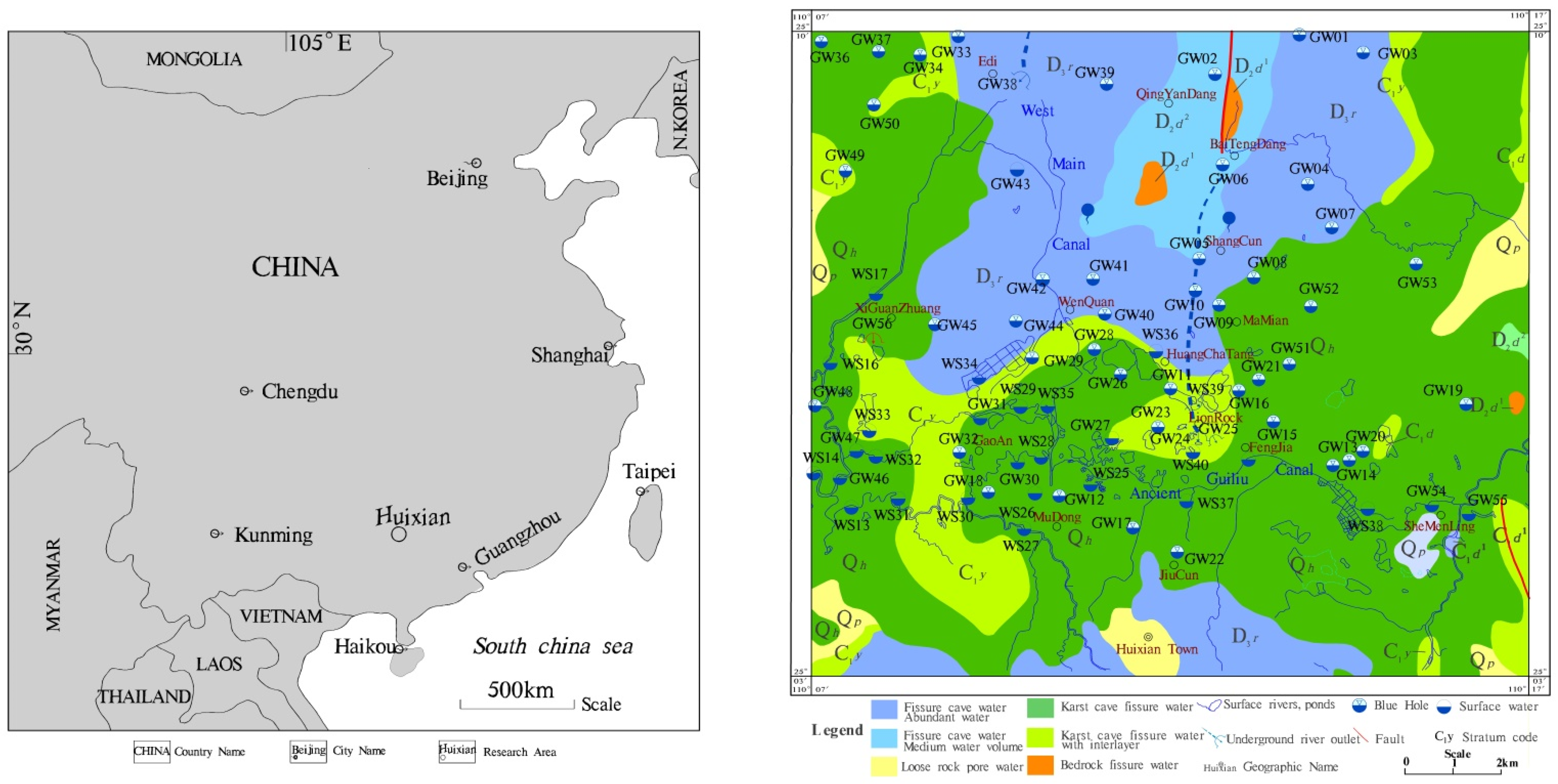
2. Study Area
3. Materials and Methods
3.1. Water Sample Collection and Testing
3.2. Methods
4. Results and Analysis
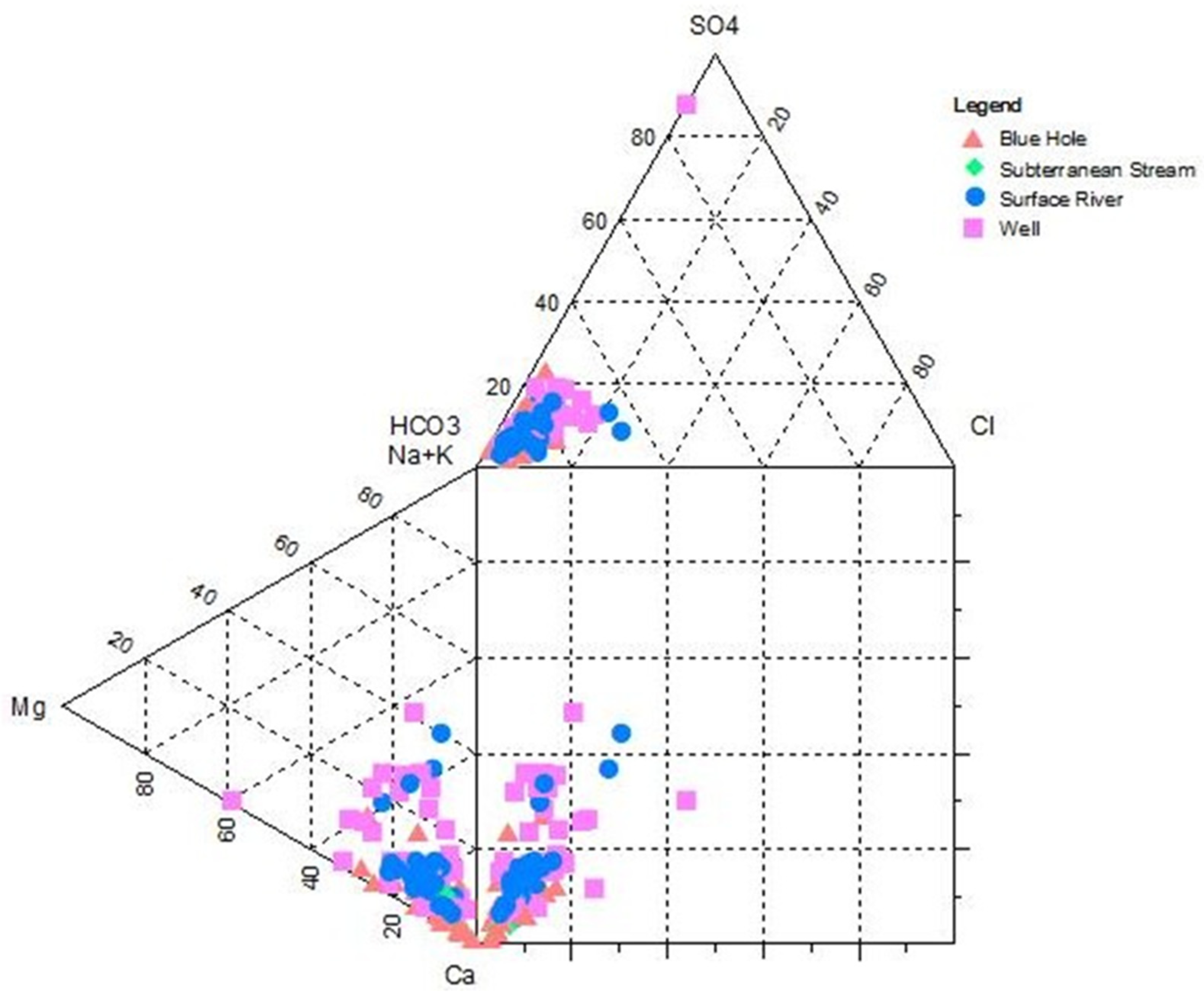
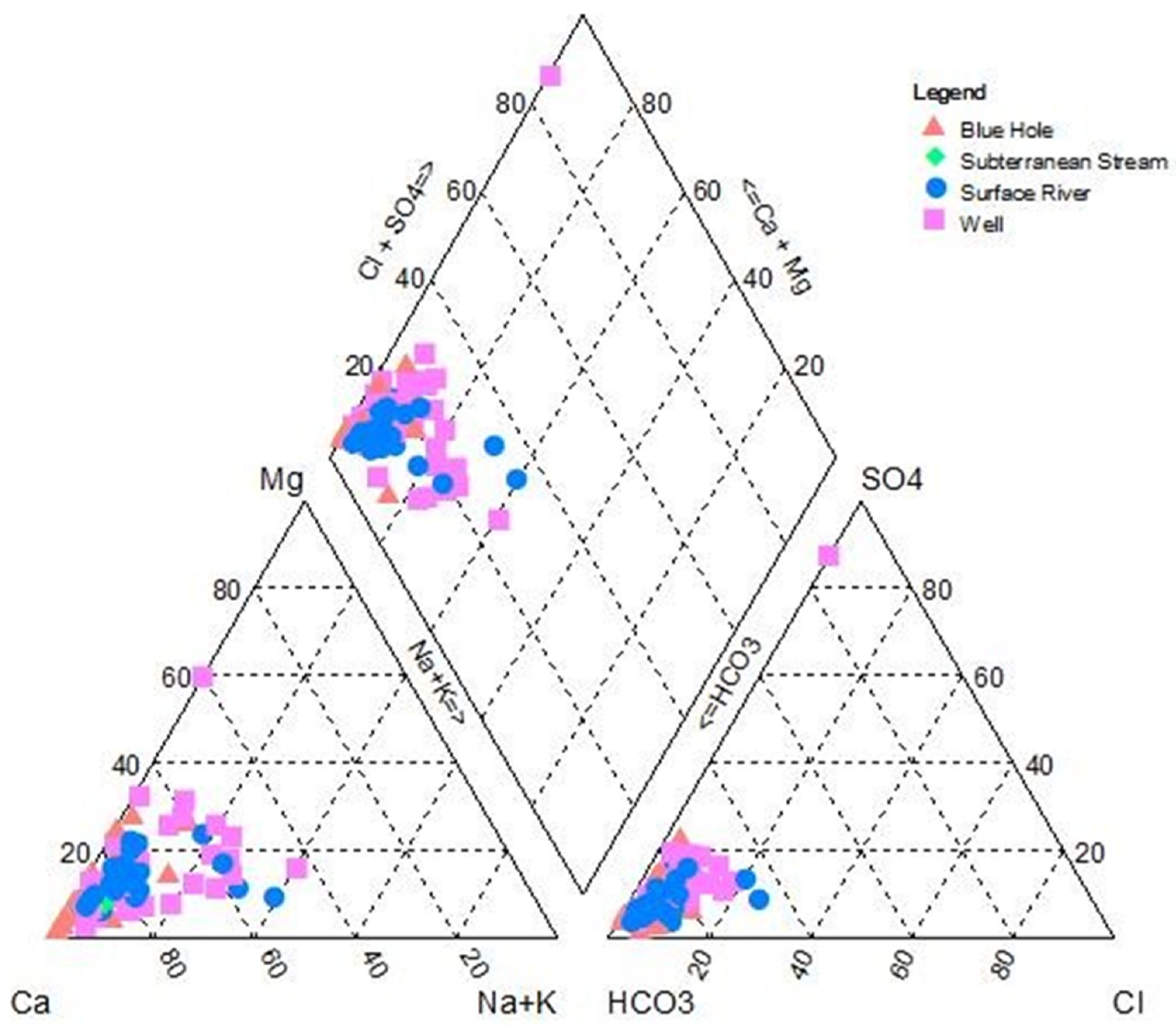
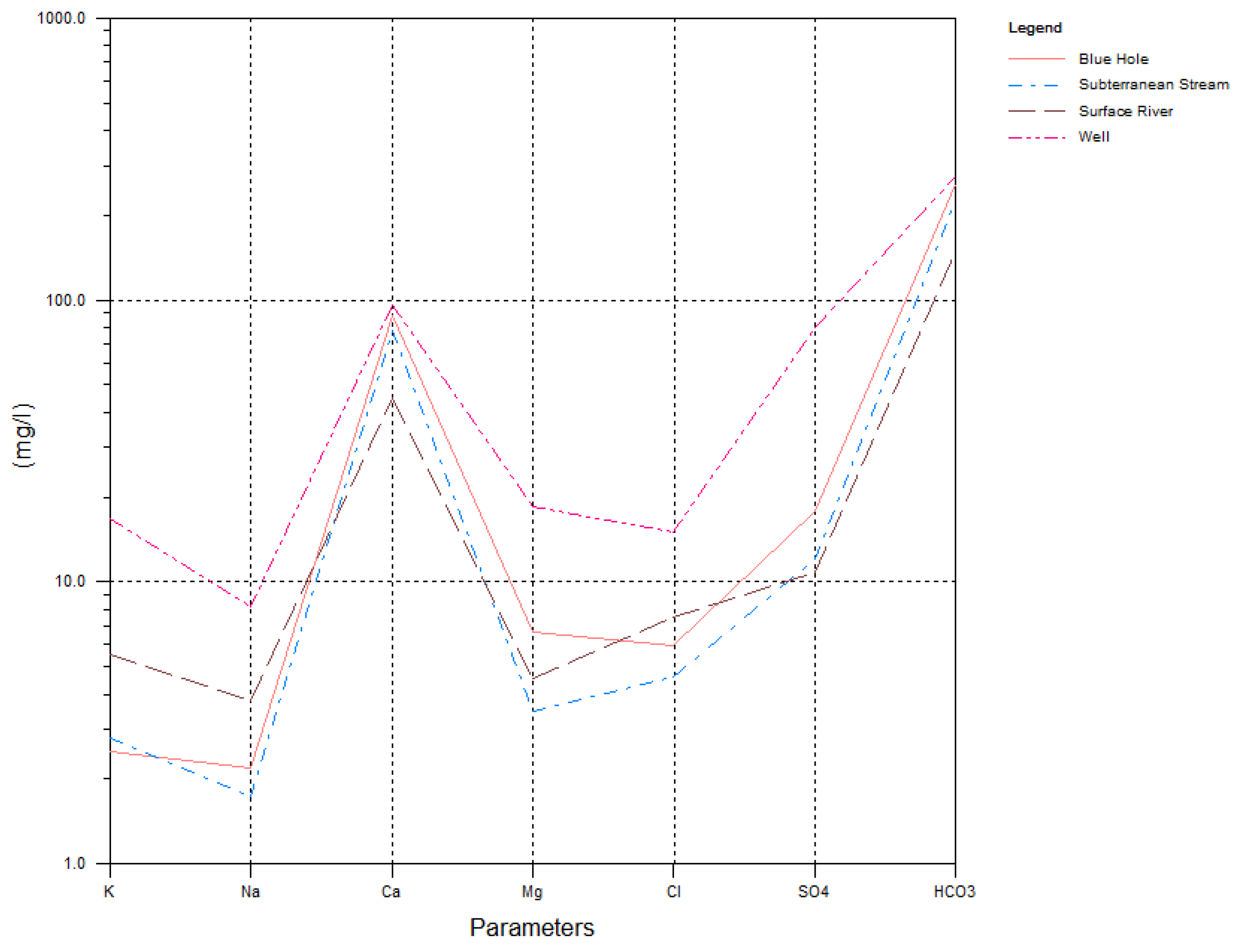
4.1. Hydrochemical Compositions of Groundwater Samples
4.2. Water-Rock Interaction
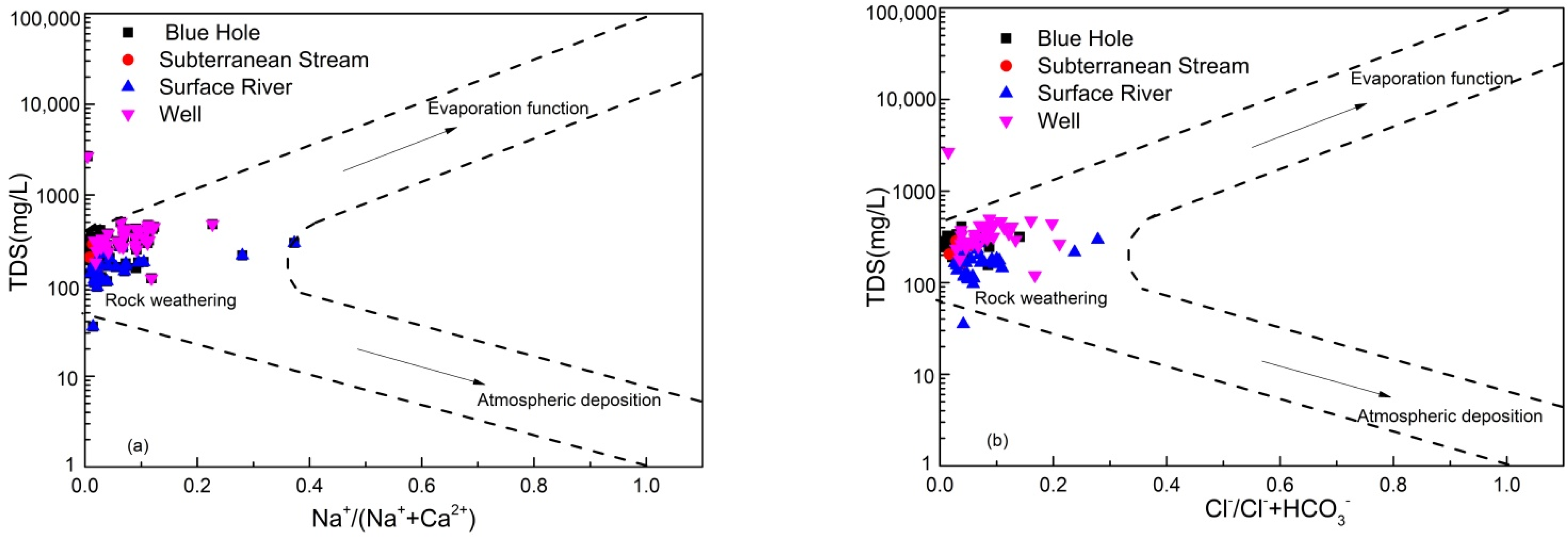
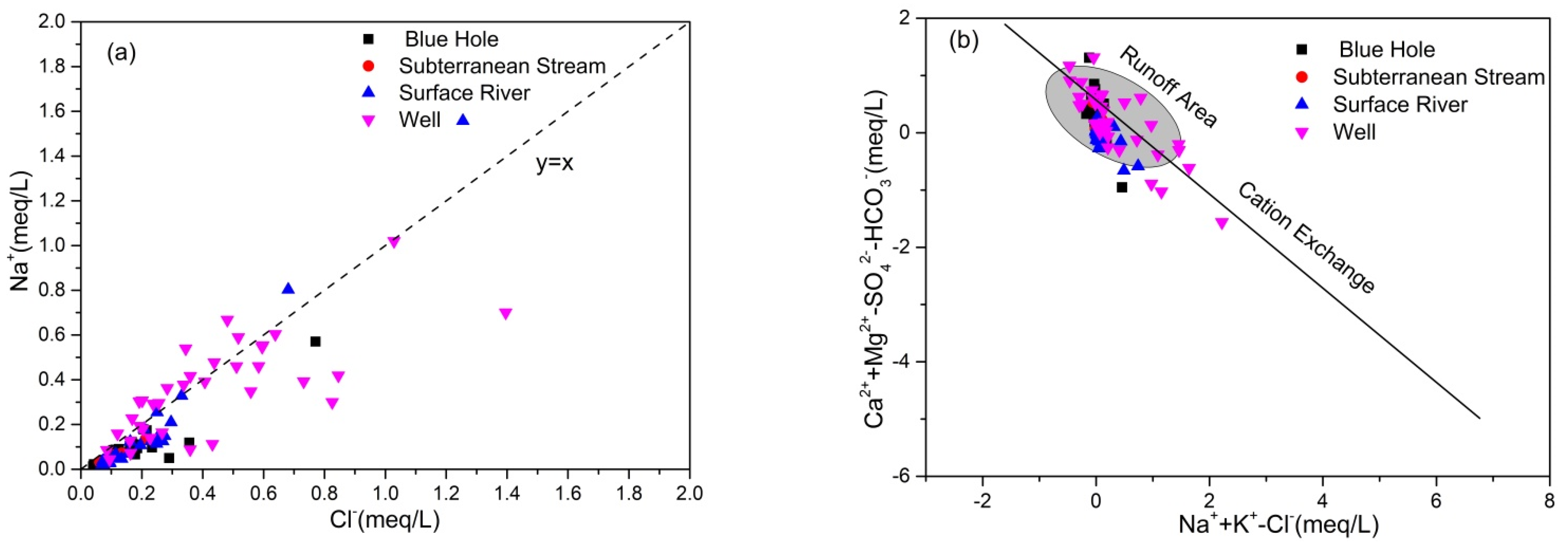
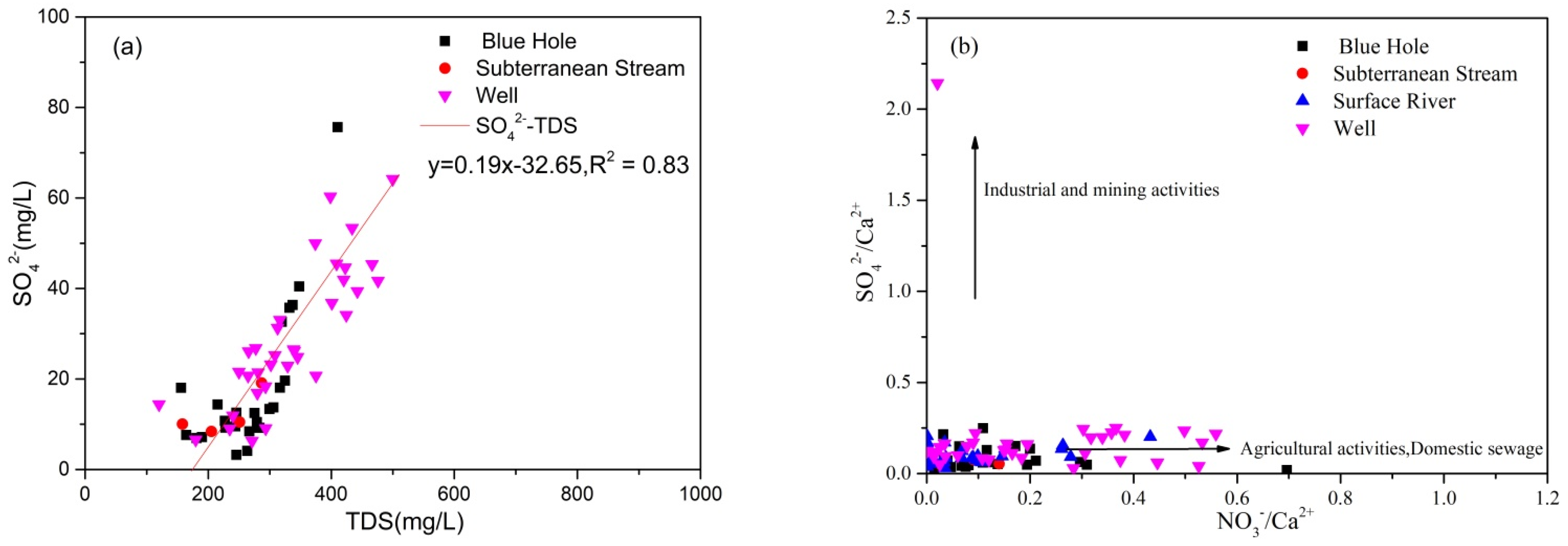
4.2.1. Ionic Ratio
4.2.2. Process of Water-Rock Interaction
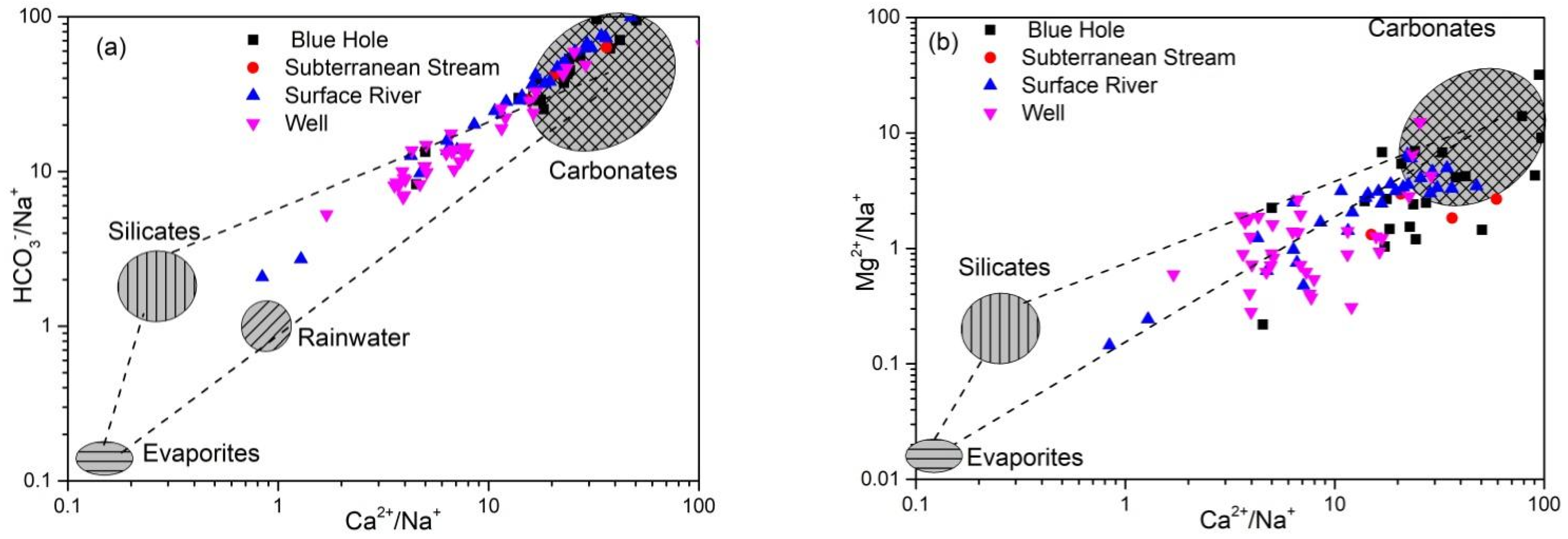
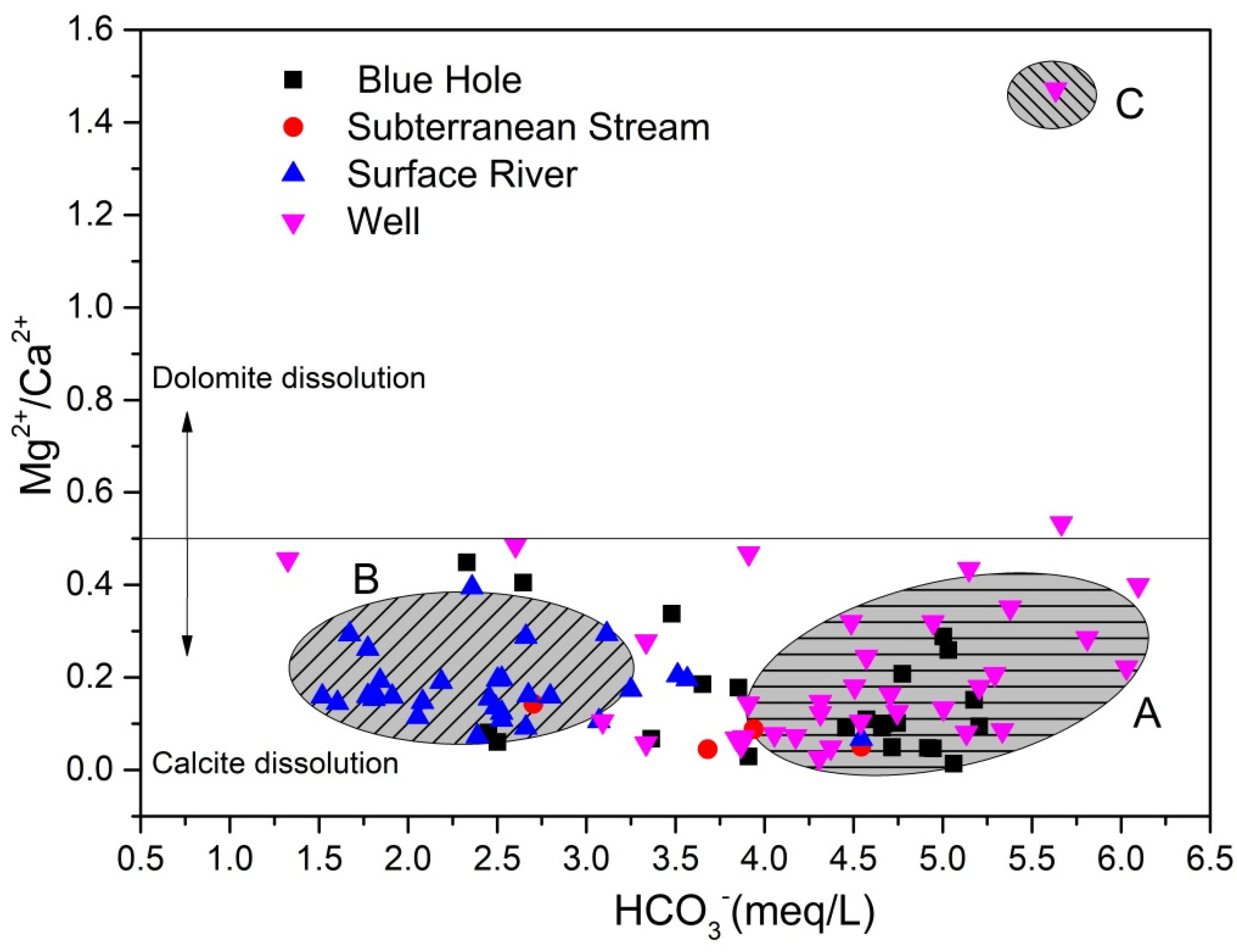
4.2.3. Sources of Ca2+, Mg2+, and HCO3−
5. Conclusions
- (1)
- The groundwater of the Huixian karst wetland was dominantly weak alkaline fresh water in which the main ions were Ca2+ and HCO3−. The contents of major ions of groundwater in the wet season exceeded that in the dry season. Groundwater Na+, Ca2+, HCO3−, and Cl− were relatively stable.
- (2)
- The influences of carbonate rock resulted in consistency in groundwater hydrochemical characteristics between the wet and dry seasons, with the dominant water type being HCO3-Ca water. The GL09 sample point was affected by upstream pyrite and strata, with SO4-Ca·Mg water distributed in both the wet and dry seasons. Overall, the highest cation concentrations of water of the Huixian karst wetland were for calcium and magnesium, whereas the lowest was for potassium and sodium; as well, the highest anion concentration was for sulfate and the lowest was for chloride.
- (3)
- The different water body types in the study area showed different degrees to which nitrogen species (NO3−, NO2−, NH4+) exceeded the Class III standards. The concentration of NO3− exceeded the standard in the study area to a higher degree than NO2− and NH4+. NO3− in groundwater typically originated from the degradation of natural organic nitrogen or humus, nitrification, and anthropogenic activities (application of chemical fertilizer and farmyard manure, industry and agriculture, domestic sewage).
- (4)
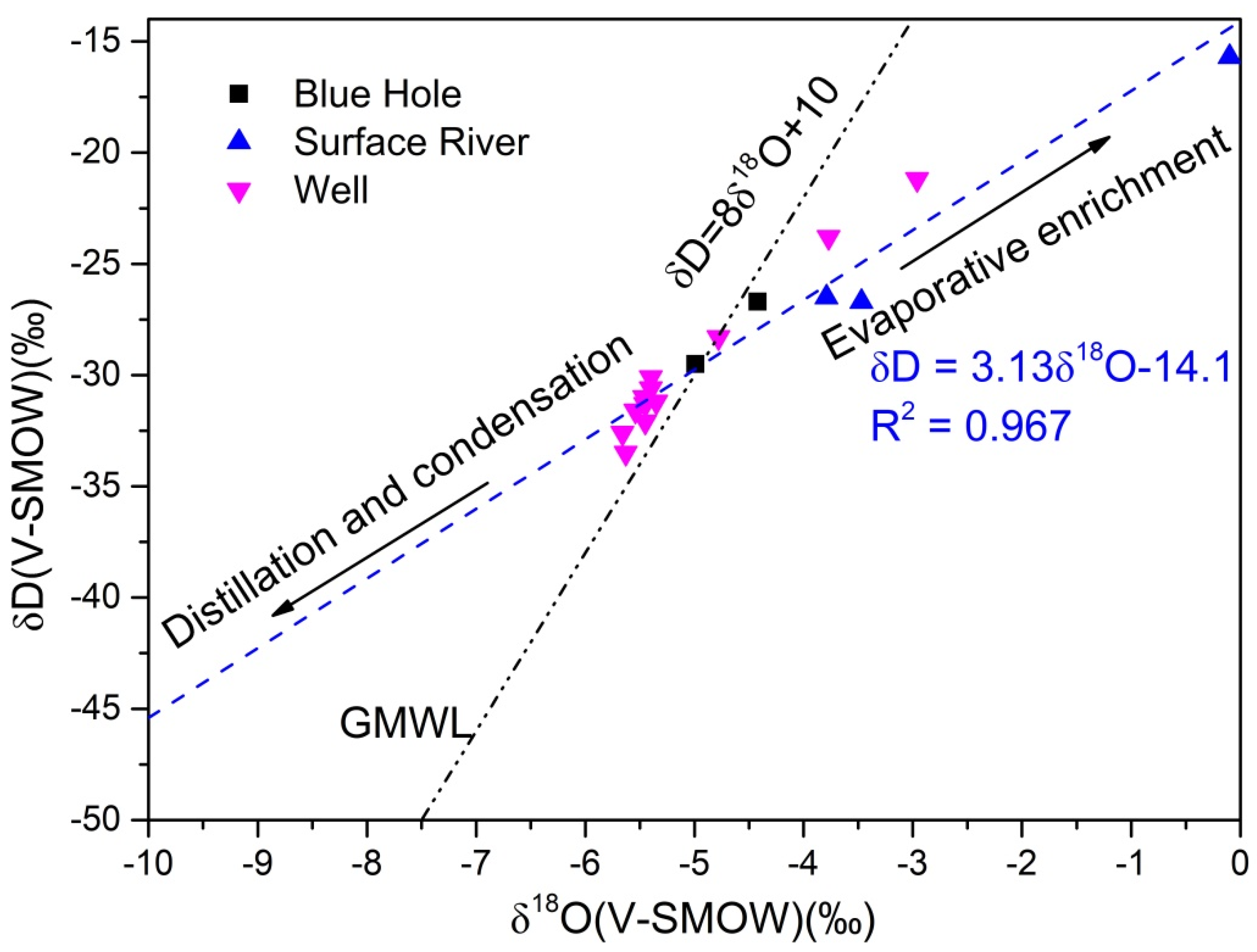
- The hydrochemical characteristics of groundwater were mainly regulated by rock weathering, with water of some shallow fissures also affected by evaporative concentration. Major ions in groundwater originated from the dissolution and precipitation of minerals from carbonate rocks, such as calcite and dolomite. The leaching of silicate and evaporite also contributed to major ions in groundwater.
- (5)
- The degree to which hydrochemical components of groundwater in the study area were regulated by water-rock interaction was consistent between the wet and dry seasons. Ca2+ and HCO3− in groundwater originated from calcite weathering, although weathering of dolomite, dolomitic limestone, and pyrite regulated the hydrochemical characteristics of some samples, resulting in high concentrations of Mg2+ and SO42− in groundwater. K+, Na+, SO42−, NO3−, and Cl− originated from precipitation, Na+ and Cl− originated from domestic sewage, whereas K+ was related to the application of potassium fertilizer in agriculture. NO3− mainly originated from chemical fertilizer.
- (6)
- The sources of recharge to surface river water and groundwater were closely related, and groundwater quality was easily affected by the surface river water quality. Groundwater was greatly affected by anthropogenic activities, among which the impacts of domestic sewage and agricultural activities exceeded that of industrial activities, particularly fertilizer application. Therefore, there should be further regulation of domestic sewage discharge and application of agricultural fertilizers to ensure sustainable use of wetland groundwater resources.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palmer, A.N. Origin and morphology of limestone caves. Geol. Soc. Am. Bull. 1991, 103, 1–21. [Google Scholar] [CrossRef]
- Kunsky, J. Thermomineral karst and caves of Zbrasov, Northern Moravia. Proc. Czechoslov. Soc. Geol. 1957, 62, 306–351. [Google Scholar]
- Dublyansky, Y.N. Hydrothermal karst in the Alpine folded region of the South of the USSR. Kras Speleol. 1980, 3, 18–36. [Google Scholar]
- Tang, C.; Jin, H.; Liang, Y. Using Isotopic and Hydrochemical Indicators to Identify Sources of Sulfate in Karst Groundwater of the Niangziguan Spring Field, China. Water 2021, 13, 390. [Google Scholar] [CrossRef]
- Ba, J.J.; Gao, F.F.; Peng, C. Characteristics of nitrate and heavy metals pollution in Huixian Wetland and its health risk assessment. Alex. Eng. J. 2022, 61, 9031–9042. [Google Scholar] [CrossRef]
- Darwish, W.S.; Sebak, M.A.; Daoud, J.R. Estimation of metal residues in oreochromis niloticus and mugil cephalus intended for human consumption in Egypt: A health risk assessment study with some reduction trials. J. Consum. Prot. Food Saf. 2019, 14, 81–91. [Google Scholar]
- Hajahmadi, Z.; Younesi, H.; Bahramifar, N.; Khakpour, H.; Pirzadeh, K. Multicomponent isotherm for biosorption of Zn(II), CO(II) and Cd(II) from ternary mixture onto pretreated dried Aspergillus niger biomass. Water Resour. Ind. 2015, 11, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Ismahene, G.; El Hadi, K.M. Assessment of heavy metal concentrations (Lead, Cadmium and Zinc) in three Crustacean species fished for in two regions of eastern Algeria. Ann. Biol. Res. 2012, 3, 2838–2842. [Google Scholar]
- Li, J.; Miao, X.Y.; Hao, Y.P. Health risk assessment of metals (Cu, Pb, Zn, Cr, Cd, As, Hg, Se) in angling fish with different lengths collected from Liuzhou, China. Int. J. Environ. Res. Public Health 2020, 17, 2192. [Google Scholar] [CrossRef] [Green Version]
- Miao, X.Y.; Hao, Y.P.; Tang, X. Analysis and health risk assessment of toxic and essential elements of the wild fish caught by anglers in Liuzhou as a large industrial city of China. Chemosphere 2020, 243, 125337. [Google Scholar] [CrossRef]
- Jia, B.; Si, J.; Xi, H.; Qin, J.A. Characterization of the Hydrochemistry and Main Controlling Factors of Lakes in the Badain Jaran Desert, China. Water 2021, 13, 2931. [Google Scholar] [CrossRef]
- Pradhan, R.M.; Behera, A.K.; Kumar, S.; Kumar, P.; Biswal, T.K. Recharge and Geochemical Evolution of Groundwater in Fractured Basement Aquifers (NW India): Insights from Environmental Isotopes (δ18O, δ2H, andδ3H) and Hydrogeochemical Studies. Water 2022, 14, 315. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Zou, S.Z. Metal Pollutions and Human Health Risks in Groundwater from Wet, Normal, and Dry Periods in the Huixian Karst Wetland, China. Huan Jing Ke Xue 2021, 42, 184–194. [Google Scholar]
- Chen, J.; Luo, M.; Ma, R.; Zhou, H.; Zou, S.; Gan, Y. Nitrate distribution under the influence of seasonal hydrodynamic changes and human activities in Huixian karst wetland, South China. J. Contam. Hydrol. 2020, 234, 103700. [Google Scholar] [CrossRef] [PubMed]
- Jakóbczyk-Karpierz, S.; Sitek, S.; Jakobsen, R.; Kowalczyk, A. Geochemical and isotopic study to determine sources and processes affecting nitrate and sulphate in groundwater influenced by intensive human activity—Carbonate aquifer Gliwice (southern Poland). Appl. Geochem. 2017, 76, 168–181. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Q. Risk assessment and seasonal variations of dissolved trace elements and heavy metals in the Upper Han River, China. J. Hazard. Mater. 2010, 181, 1051–1058. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, H.; Cai, D. The comparative study on the ecological sensitivity analysis in Huixian karst wetland, China. Procedia Environ. Sci. 2010, 2, 386–398. [Google Scholar]
- Li, Z.; Jin, Z.; Li, Q. Changes in Land Use and their Effects on Soil Properties in Huixian Karst Wetland System. Pol. J. Environ. Stud. 2017, 26, 699–707. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, L.; Xin, C.; Yu, S.; Guo, Y.; Wang, J. Impact of anthropogenic sulfate deposition via precipitation on carbonate weathering in a typical industrial city in a karst basin of southwest China: A case study in Liuzhou. Appl. Geochem. 2019, 110, 104417. [Google Scholar] [CrossRef]
- Roy, A.; Keesari, T.; Mohokar, H.; Pant, D.; Sinha, U.K.; Mendhekar, G.N. Geochemical evolution of groundwater in hard-rock aquifers of South India using statistical and modelling techniques. Hydrol. Sci. J. 2020, 65, 951–968. [Google Scholar] [CrossRef]
- Mai, Y.; Zhao, X.; Huang, G. Temporal and spatial variability of water quality in an urban wetland and the effects of season and rainfall: A case study in the Daguan Wetland, China. Environ. Monit. Assess. 2022, 194, 347. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Liu, G.; Xia, H.; Jiang, F.; Meng, Y. Influence of saline intrusion on the wetland ecosystem revealed by isotopic and hydrochemical indicators in the Yellow River Delta, China. Ecol. Indic. 2021, 133, 108422. [Google Scholar] [CrossRef]
- Ban, J.; Ling, B.; Huang, W.; Liu, X.; Peng, W.; Zhang, J. Spatiotemporal Variations in Water Flow and Quality in the Sanyang Wetland, China: Implications for Environmental Restoration. Sustainability 2021, 13, 4637. [Google Scholar] [CrossRef]
- Qin, L.T.; Pang, X.R.; Zeng, H.H.; Liang, Y.P.; Mo, L.Y.; Wang, D.Q.; Dai, J.F. Ecological and human health risk of sulfonamides in surface water and groundwater of Huixian karst wetland in Guilin, China. Sci. Total Environ. 2019, 708, 134552. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, G.; Lone, S.A.; Nisa, A.U.; Deshpande, R.D.; Padhya, V. Use of stable water isotopes to identify and estimate the sources of groundwater recharge in an alluvial aquifer of Upper Jhelum Basin (UJB), western Himalayas. Hydrol. Sci. J. 2021, 66, 2330–2339. [Google Scholar] [CrossRef]
- Mahlangu, S.; Lorentz, S.; Diamond, R.; Dippenaar, M. Surface water-groundwater interaction using tritium and stable water isotopes: A case study of Middelburg, South Africa. J. Afr. Earth Sci. 2020, 171, 103886. [Google Scholar] [CrossRef]
- Oiro, S.; Comte, J.C.; Soulsby, C.; Walraevens, K. Using stable water isotopes to identify spatio-temporal controls on groundwater recharge in two contrasting East African aquifer systems. Hydrol. Sci. J. 2018, 63, 862–877. [Google Scholar] [CrossRef]
- Sukhija, B.S.; Reddy, D.V.; Nagabhushanam, P. Isotopic fingerprints of paleoclimates during the last 30,000 years in deep confined groundwaters of Southern India. Quat. Res. 1998, 50, 252–260. [Google Scholar] [CrossRef]
- Moran, J.E.; Hudson, G.B. Using Groundwater Age and Other Isotopic Signatures to Delineate Groundwater Flow and Stratification (No. UCRL-PROC-215146); Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 2005. [Google Scholar]
- Zheng, L.; Chen, X.; Dong, X.; Wei, X.; Jiang, C.; Tang, Q. Using δ34S–SO4 and δ18O–SO4 to trace the sources of sulfate in different types of surface water from the Linhuan coal-mining subsidence area of Huaibei, China. Ecotoxicol. Environ. Saf. 2019, 181, 231–240. [Google Scholar] [CrossRef]
- Chen, X.; Sun, Y.; Huang, R. Role of hydro-geochemical functions on karst critical zone hydrology for sustainability of water resources and ecology in Southwest China. Acta Geochim. 2017, 36, 494–497. [Google Scholar] [CrossRef]
- Holland, M.; Witthüser, K.T. Geochemical characterization of karst groundwater in the cradle of humankind world heritage site. S. Afr. Environ. Geol. 2008, 57, 513–524. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Lu, Y.; Zhang, F.E.; Zhang, S.; Yin, M.; Wu, G. Identification of geochemical processes by hydrogeochemical analysis in karst aquifers of a semi-arid region, Northern China. Carbonates Evaporites 2017, 34, 297–313. [Google Scholar] [CrossRef]
- Gil-Márquez, J.M.; Andreo, B.; Mudarra, M. Combining hydrodynamics, hydrochemistry, and environmental isotopes to understand the hydrogeological functioning of evaporite-karst springs. An example from southern Spain. J. Hydrol. 2019, 576, 299–314. [Google Scholar] [CrossRef]
- Husic, A.; Fox, J.; Adams, E.; Pollock, E.; Ford, W.; Agouridis, C.; Backus, J. Quantification of nitrate fate in a karst conduit using stable isotopes and numerical modeling. Water Res. 2020, 170, 115348. [Google Scholar] [CrossRef]
- Hong Quang, N.; Tuan, V.A.; Thi Thu Hang, L.; Manh Hung, N.; Thi The, D.; Thi Dieu, D.; Duc Anh, N.; Hackney, C.R. Hydrological/Hydraulic Modeling-Based Thresholding of Multi SAR Remote Sensing Data for Flood Monitoring in Regions of the Vietnamese Lower Mekong River Basin. Water 2019, 12, 71. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.L.; Chin, K.L.; H’ng, P.S.; Rashid, U.; Maminski, M.; Khoo, P.S. Effect of pretreatment conditions on the chemical-structural characteristics of coconut and palm kernel shell: A potentially valuable precursor for eco-efficient activated carbon production. Environ. Technol. Innov. 2021, 21, 101309. [Google Scholar] [CrossRef]
- Li, T.; Liu, B.; Bi, X.; Wu, J.; Zhang, Y.; Feng, Y. Size and chemical characteristics of particles emitted from typical rural biomass cookstoves in North China. Atmos. Res. 2021, 249, 105295. [Google Scholar] [CrossRef]
- Hasan, O.; Miko, S.; Ilijani, N.; Brunovi´c, D.; Dedi´c, Ž.; Miko, M.Š.; Peh, Z. Discrimination of topsoil environments in a karst landscape: An outcome of a geochemical mapping campaign. Geochem. Trans. 2020, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Pan, X.; Zeng, J.; Yuan, D. Contaminant sources and processes affecting spring water quality in a typical karst basin (Hongjiadu Basin, SW China): Insights provided by hydrochemical and isotopic data. Environ. Sci. Pollut. Res. 2019, 26, 31354–31367. [Google Scholar] [CrossRef] [PubMed]
- Boumaiza, L.; Chesnaux, R.; Drias, T.; Walter, J.; Huneau, F.; Garel, E.; Knoeller, K.; Stumpp, C. Identifying groundwater degradation sources in a Mediterranean coastal area experiencing significant multi-origin stresses. Sci. Total Environ. 2020, 746, 141203. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, M.; Awawdeh, M.; Matiatos, I.; Al-Ajlouni, A.; Al-Mughaid, H. Identification and apportionment of nitrate sources in the phreatic aquifers in Northern Jordan using a dual isotope method (δ15N and δ18O of NO3-). Groundw. Sustain. Dev. 2020, 12, 100505. [Google Scholar] [CrossRef]
- GB/T14848. 2017 Standard for Groundwater Quality. General Administration of Quality Supervision, Inspection and Quarantine of the P.R. China, Standardization Administration of the P.R. China: Beijing, China, 2017.
- Santana, N.A.; Jacques, R.J.S.; Antoniolli, Z.I.; Martínez-Cordeiro, H.; Domínguez, J. Changes in the chemical and biological characteristics of grape marc vermicompost during a two-year production period. Appl. Soil Ecol. 2020, 154, 103587. [Google Scholar] [CrossRef]
- Yue, F.J.; Li, S.L.; Waldron, S.; Wang, Z.J.; Oliver, D.M.; Chen, X.; Liu, C.Q. Rainfall and conduit drainage combine to accelerate nitrate loss from a karst agroecosystem: Insights from stable isotope tracing and high-frequency nitrate sensing. Water Res. 2020, 186, 116388. [Google Scholar] [CrossRef] [PubMed]
- Banda, J.F.; Lu, Y.; Hao, C.; Pei, L.; Du, Z.; Zhang, Y.; Wei, P.; Dong, H. The Effects of Salinity and pH on Microbial Community Diversity and Distribution Pattern in the Brines of Soda Lakes in Badain Jaran Desert, China. Geomicrobiol. J. 2020, 37, 1–12. [Google Scholar] [CrossRef]
- Jin, K.; Rao, W.; Zhang, W.; Zheng, F.; Wang, S. Strontium isotopic and hydrochemical characteristics of shallow groundwater and lake water in the Badain Jaran Desert, North China. Isot. Environ. Health Stud. 2021, 57, 516–534. [Google Scholar] [CrossRef]
- Yuan, R.; Wang, M.; Wang, S.; Song, X. Water transfer imposes hydrochemical impacts on groundwater by altering the interaction of groundwater and surface water. J. Hydrol. 2020, 583, 124617. [Google Scholar] [CrossRef]


| Type | Parameter | pH | Mass Concentrations of Components/mg·L−1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | NO3− | NO2− | NH4+ | CODMn | TDS | |||
| Surface river water samples (n = 50) | mean | 7.34 | 5.56 | 3.79 | 44.89 | 4.55 | 7.49 | 10.83 | 149.75 | 7.20 | 0.20 | 0.80 | 3.30 | 160.76 |
| maximum | 7.63 | 22.45 | 35.82 | 93.21 | 9.52 | 44.55 | 23.93 | 277.18 | 27.63 | 1.23 | 5.26 | 9.60 | 295.73 | |
| minimum | 6.61 0.22 | 1.36 4.98 | 0.49 | 27.74 | 1.99 | 2.46 | 3.43 | 92.60 | 0.88 | 0.00 | nd | 0.77 | 35.29 | |
| standard deviation | 6.96 | 14.31 | 1.98 | 8.26 | 6.19 | 41.57 | 6.94 | 0.39 | 1.45 | 1.83 | 53.14 | |||
| Coefficient of variation | 0.03 | 0.90 | 1.84 | 0.32 | 0.44 | 1.10 | 0.57 | 0.28 | 0.96 | 1.92 | 1.82 | 0.55 | 0.33 | |
| Subterranean stream samples (n = 4) | mean | 7.26 | 2.81 | 1.73 | 78.12 | 3.49 | 4.59 | 12.01 | 226.75 | 11.86 | 0.96 | 1.93 | 1.51 | 225.26 |
| maximum | 7.35 | 7.14 | 3.09 | 104.25 | 4.50 | 7.52 | 19.12 | 277.18 | 17.53 | 1.90 | 1.93 | 2.23 | 286.63 | |
| minimum | 7.13 | 0.34 | 0.73 | 52.58 | 2.03 | 2.20 | 8.40 | 164.91 | 3.35 | 0.02 | nd | 0.83 | 158.03 | |
| standard deviation | 0.11 | 3.12 | 0.99 | 21.21 | 1.13 | 2.22 | 4.82 | 46.75 | 7.50 | 1.33 | nd | 0.70 | 55.83 | |
| Coefficient of variation | 0.01 | 1.11 | 0.57 | 0.27 | 0.32 | 0.48 | 0.40 | 0.21 | 0.63 | 1.38 | nd | 0.47 | 0.25 | |
| Blue hole water samples (n = 24) | mean | 7.43 | 2.51 | 2.18 | 88.17 | 6.62 | 5.97 | 17.89 | 256.33 | 17.70 | 0.41 | 2.15 | 4.50 | 269.21 |
| maximum | 7.82 | 27.28 | 13.11 | 126.10 | 15.69 | 27.37 | 75.63 | 317.53 | 76.50 | 1.56 | 12.96 | 10.96 | 410.45 | |
| minimum | 7.08 | 0.05 | 0.29 | 34.83 | 0.89 | 1.43 | 3.22 | 142.10 | 1.45 | 0.00 | nd | 0.83 | 156.02 | |
| standard deviation | 0.20 | 5.68 | 2.56 | 23.83 | 4.19 | 5.36 | 16.14 | 57.58 | 16.28 | 0.67 | 4.46 | 5.61 | 42.72 | |
| Coefficient of variation | 0.03 | 2.26 | 1.17 | 0.27 | 0.63 | 0.90 | 0.90 | 0.22 | 0.92 | 1.64 | 2.07 | 1.25 | 0.16 | |
| Well water samples (n = 35) | mean | 7.39 | 16.87 | 8.16 | 95.75 | 18.57 | 15.05 | 80.38 | 273.40 | 28.43 | 0.21 | 0.04 | 0.05 | 455.53 |
| maximum | 7.72 | 86.90 | 23.46 | 352.68 | 311.31 | 49.55 | 1813.50 | 371.86 | 74.74 | 1.04 | 0.14 | 0.83 | 2674.07 | |
| minimum | 7.08 | 0.12 | 1.01 | 24.45 | 1.43 | 3.02 | 6.28 | 80.84 | 1.92 | 0.00 | 0.02 | nd | 120.03 | |
| standard deviation | 0.14 | 21.61 | 4.95 | 49.64 | 51.35 | 10.20 | 301.93 | 60.92 | 23.03 | 0.35 | 0.03 | nd | 404.64 | |
| Coefficient of variation | 0.02 | 1.28 | 0.61 | 0.52 | 2.76 | 0.68 | 3.76 | 0.22 | 0.81 | 1.68 | 0.81 | nd | 0.89 | |
| Exceeding rate/% | 0 | — | 0 | — | — | 0 | 1.1 | — | 0 | 0 | 9.7 | 0 | 0.1 | |
| Quality standards of groundwater | 6.5–8.5 | — | 200 | — | — | 250 | 250 | — | 88.57 | 3.29 | 0.64 | 3.0 | 1000 | |
| Hygienic standard for drinking water | 6.5–8.5 | — | 200 | — | — | 300. | 300 | — | 88.57 | — | 0.64 | 5.0 | 1500 | |
| Sample No. | Sample Category | δD(V-SMOW)‰ | STD | C.V | δ18O(V-SMOW)‰ | STD | C.V |
|---|---|---|---|---|---|---|---|
| GL03 | well water | −23.8 | 1.28 | 0.03 | −3.77 | 0.13 | 0.02 |
| GL05 | well water | −33.5 | 2.20 | 0.05 | −5.63 | 0.37 | 0.05 |
| GL08 | well water | −31.2 | 2.30 | 0.06 | −5.35 | 0.29 | 0.04 |
| GL09 | well water | −30.1 | 0.79 | 0.02 | −5.40 | 0.12 | 0.02 |
| HX05 | well water | −31.3 | 1.65 | 0.03 | −5.45 | 0.07 | 0.009 |
| HX06 | well water | −21.2 | 0.84 | −0.02 | −2.96 | 0.11 | 0.01 |
| HX10 | well water | −32.1 | 3.19 | 0.11 | −5.45 | 0.31 | −0.08 |
| HX11 | well water | −28.3 | 1.58 | 0.12 | −4.78 | 0.32 | 0.13 |
| HX13 | well water | −30.6 | 2.94 | 0.07 | −5.40 | 0.15 | 0.07 |
| HX15 | well water | −31.6 | 3.37 | −0.01 | −5.54 | 0.16 | −0.01 |
| HX16 | well water | −31.0 | 1.76 | 0.06 | −5.46 | 0.24 | 0.05 |
| HX18 | well water | −32.6 | 1.17 | 0.03 | −5.66 | 0.22 | 0.04 |
| HX14 | blue hole water | −26.7 | 1.21 | 0.08 | −4.42 | 0.19 | 0.07 |
| HX17 | blue hole water | −25.7 | 1.19 | 0.04 | −4.46 | 0.27 | 0.11 |
| HX23 | blue hole water | −29.5 | 1.38 | 0.06 | −4.99 | 0.15 | 0.08 |
| HX40 | blue hole water | −31.3 | 2.33 | 0.07 | −5.47 | 0.24 | 0.18 |
| HX41 | blue hole water | −28.9 | 0.69 | −0.01 | −3.89 | 0.12 | 0.01 |
| HX01 | Surface river | −15.7 | 1.51 | 0.09 | −0.10 | 0.16 | 0.03 |
| HX12 | Surface river | −26.5 | 1.58 | 0.07 | −3.79 | 0.22 | 0.04 |
| HX21 | Surface river | −26.7 | 1.79 | 0.02 | −3.47 | 0.36 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ba, J.; Dan, Y.; Luo, F.; Tang, C.; Peng, C. Seasonal Differences in the Hydrochemical Characteristics of Karst Wetlands and the Associated Mechanisms in Huixian, China. Water 2022, 14, 2362. https://doi.org/10.3390/w14152362
Ba J, Dan Y, Luo F, Tang C, Peng C. Seasonal Differences in the Hydrochemical Characteristics of Karst Wetlands and the Associated Mechanisms in Huixian, China. Water. 2022; 14(15):2362. https://doi.org/10.3390/w14152362
Chicago/Turabian StyleBa, Junjie, Yong Dan, Fei Luo, Chunlei Tang, and Cong Peng. 2022. "Seasonal Differences in the Hydrochemical Characteristics of Karst Wetlands and the Associated Mechanisms in Huixian, China" Water 14, no. 15: 2362. https://doi.org/10.3390/w14152362
APA StyleBa, J., Dan, Y., Luo, F., Tang, C., & Peng, C. (2022). Seasonal Differences in the Hydrochemical Characteristics of Karst Wetlands and the Associated Mechanisms in Huixian, China. Water, 14(15), 2362. https://doi.org/10.3390/w14152362





