Enhanced Removal of Bordeaux B and Red G Dyes Used in Alpaca Wool Dying from Water Using Iron-Modified Activated Carbon
Abstract
1. Introduction
2. Methods
2.1. Reagents
2.2. Methods
2.2.1. Quantum Calculation Details
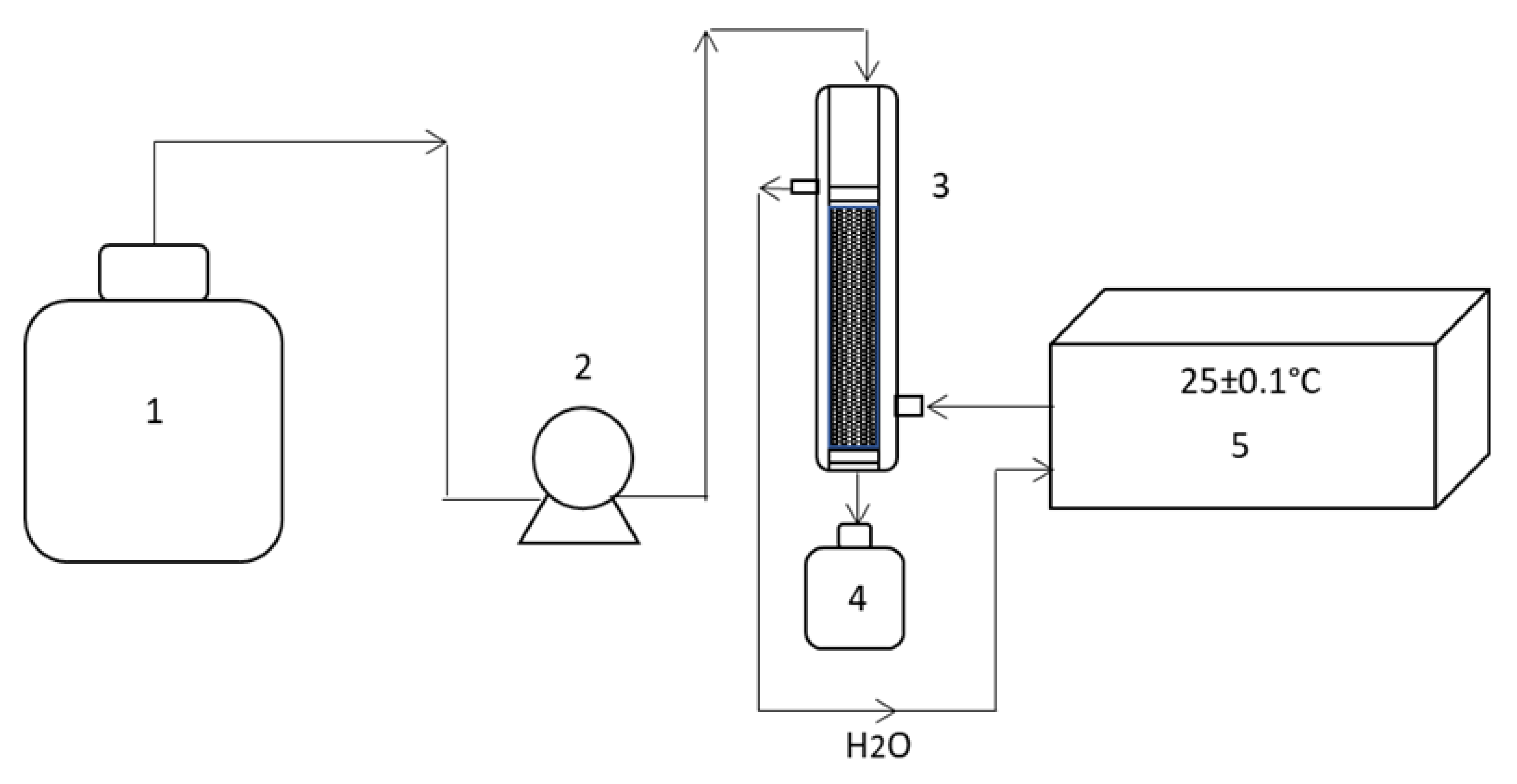
2.2.2. Treatment of the Dye Solution in a Continuous-Flow Column with Activated Carbon
Apparent Density and Ash Content of Activated Carbon on Dry Basis
pH Activated Carbon
Preparation of Activated Carbon and Packing of the Column
Column Packing
Packed-Bed Column Operation
Estimation of the Adsorption Column Design Parameters
Kinetic Models
- = Thomas rate constant, mL/mg.min;
- , mg/g;
- V = volume of solution, mL.
- , min−1;
- τ = time required for 50% adsorbate breakthrough or time when Ct/Co = 0.5, min.
3. Results and Discussion
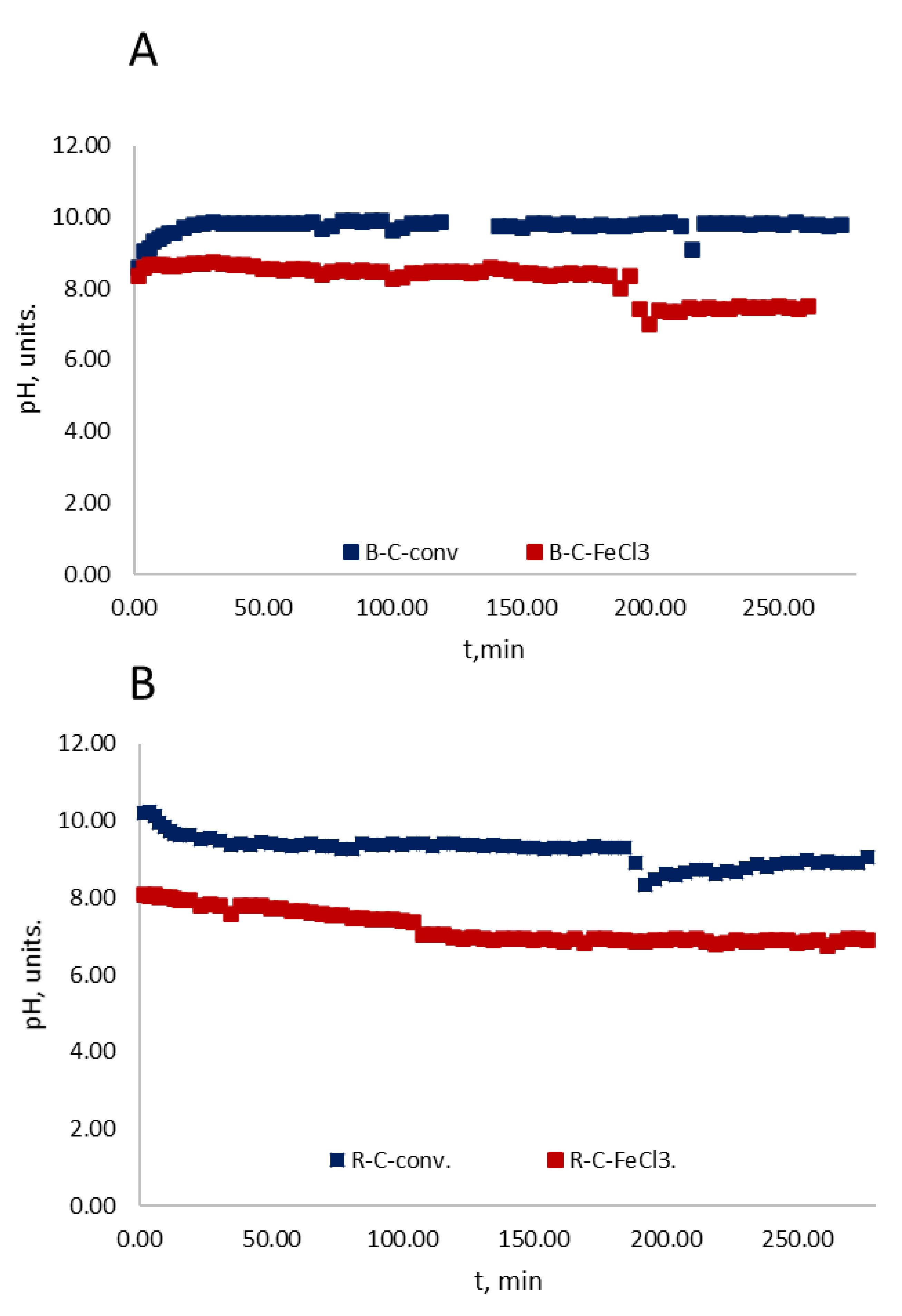
3.1. Correlation Analysis of pH with Respect to the Concentration of the Azo Dyes
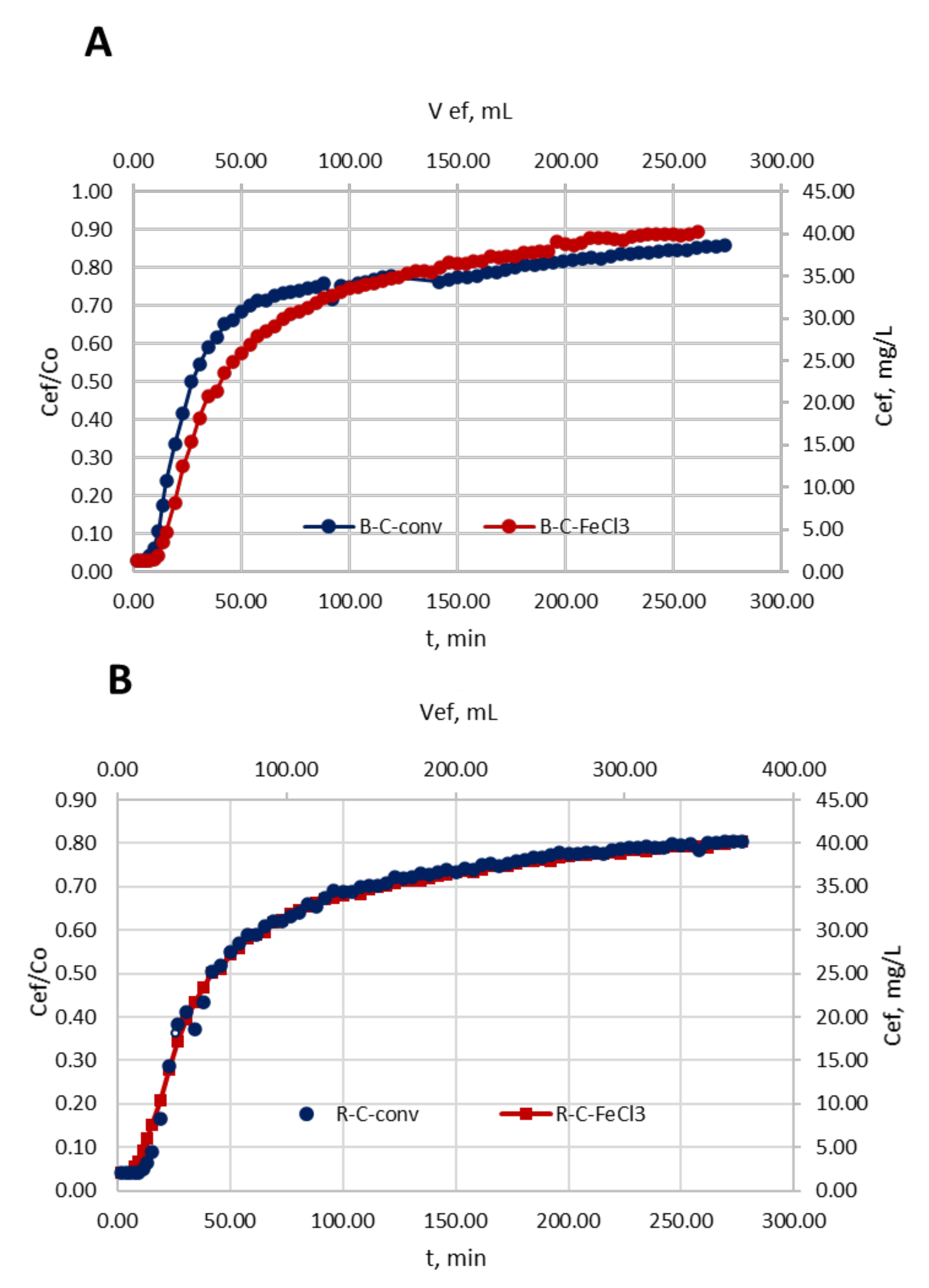
3.2. Treatment in a Fixed-Bed Column with Continuous-Flow Activated Carbon
3.2.1. General Qualitative and Quantitative Physicochemical Characteristics of the Adsorbent
3.2.2. Treatment in a Fixed-Bed Column with Continuous-Flow Activated Carbon: Breakthrough Curve and Mathematic Models
3.2.3. Economic Study
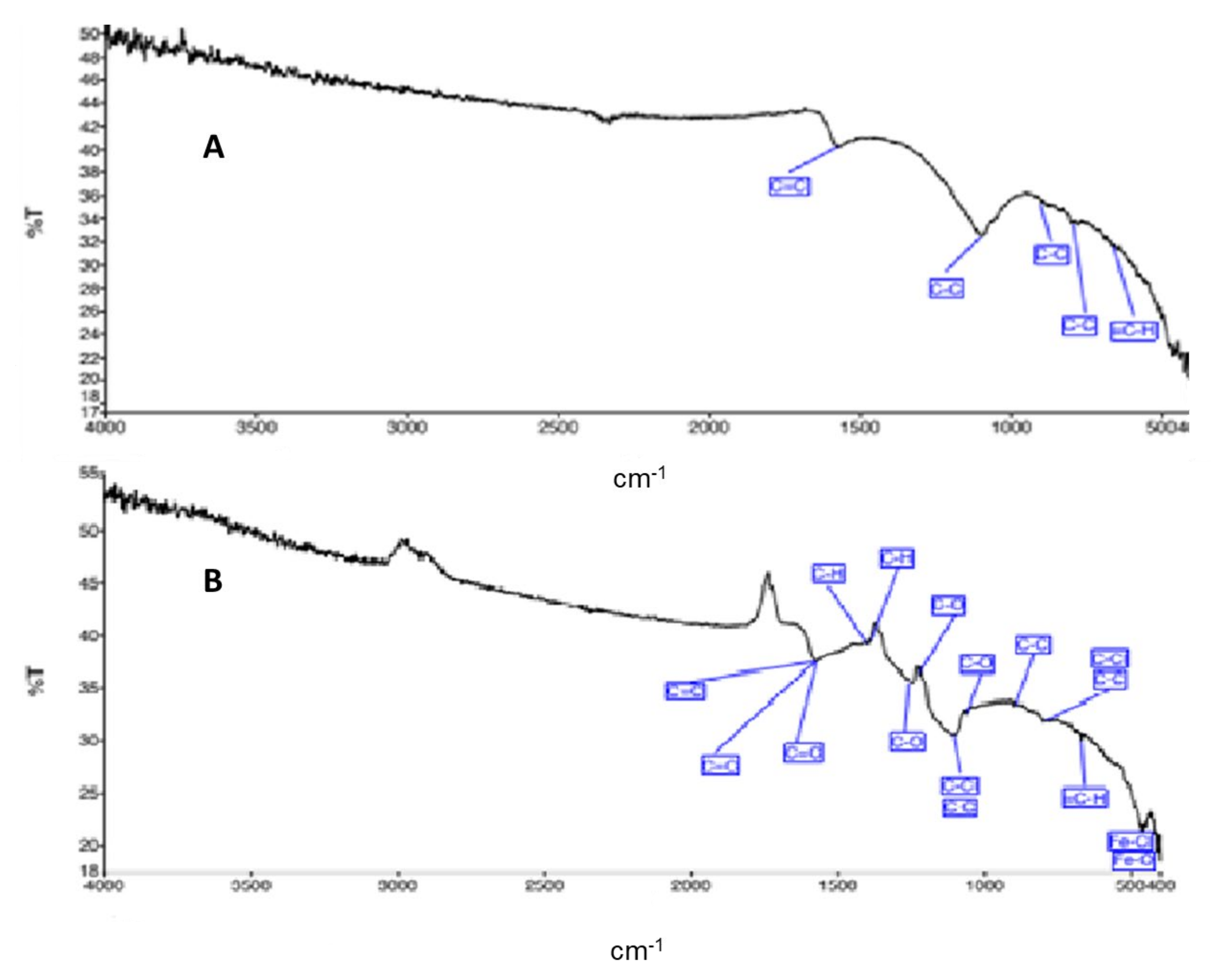
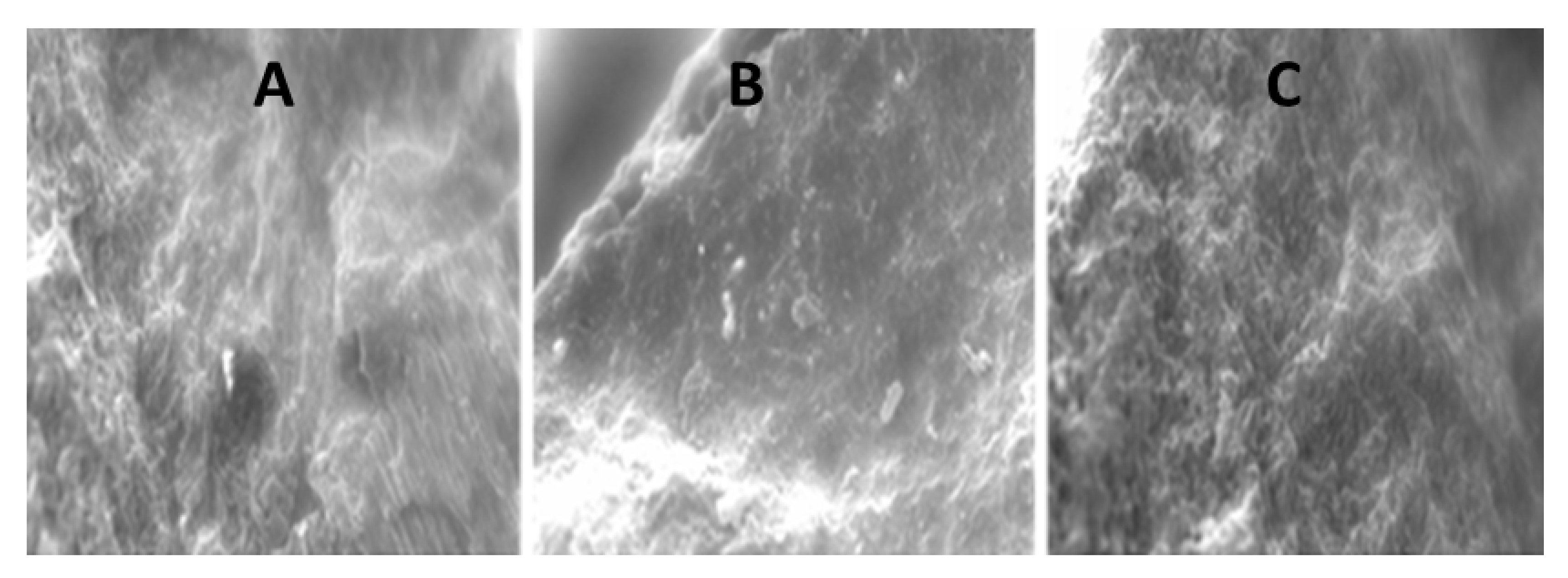
3.2.4. SEM Analysis
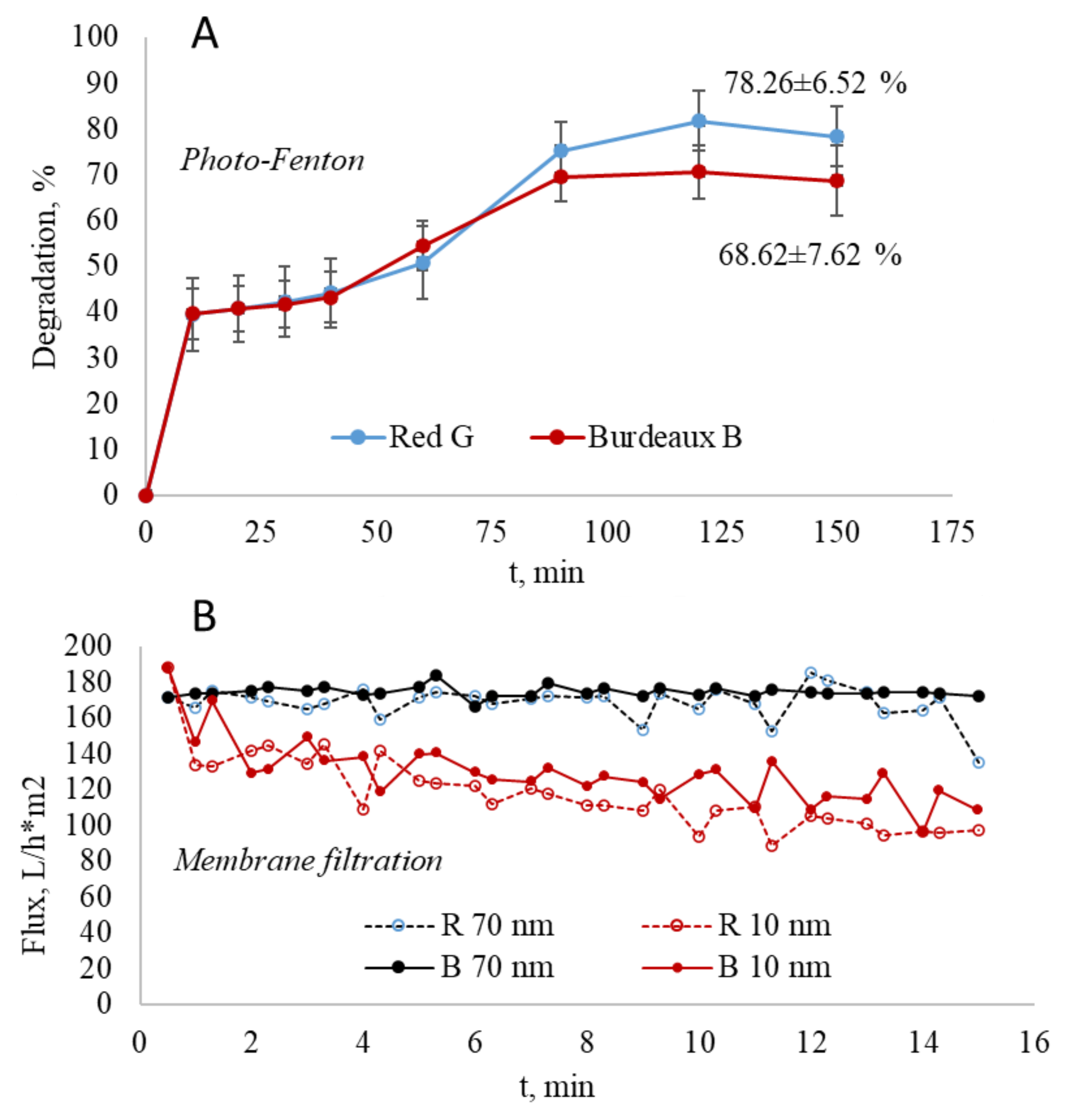
3.3. Other Alternative Processes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, L.; Ji, Y.; Liu, X.; Mu, L.; Zhu, J. Sorption Mechanism of Organic Dyes on a Novel Self-Nitrogen-Doped Porous Graphite Biochar: Coupling DFT Calculations with Experiments. Chem. Eng. Sci. 2021, 242, 116739. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Karim, M.N.; Nitun, N.A.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic Performance Assessment of GO and Ag Co-Synthesized TiO2 Nanocomposite for the Removal of Methyl Orange Dye under Solar Irradiation. Environ. Technol. Innov. 2021, 22, 101537. [Google Scholar] [CrossRef]
- Ayisha Sidiqua, M.; Priya, V.S. Removal of Yellow Dye Using Composite Binded Adsorbent Developed Using Natural Clay and Activated Carbon from Sapindus Seed. Biocatal. Agric. Biotechnol. 2021, 33, 101965. [Google Scholar] [CrossRef]
- Serrano-Martínez, A.; Mercader-Ros, M.T.; Martínez-Alcalá, I.; Lucas-Abellán, C.; Gabaldón, J.A.; Gómez-López, V.M. Degradation and Toxicity Evaluation of Azo Dye Direct Red 83:1 by an Advanced Oxidation Process Driven by Pulsed Light. J. Water Process Eng. 2020, 37, 101530. [Google Scholar] [CrossRef]
- Leng, L.; Li, W.; Chen, J.; Leng, S.; Chen, J.; Wei, L.; Peng, H.; Li, J.; Zhou, W.; Huang, H. Co-Culture of Fungi-Microalgae Consortium for Wastewater Treatment: A Review. Bioresour. Technol. 2021, 330, 125008. [Google Scholar] [CrossRef]
- Radhika, R.; Jayalatha, T.; Jacob, S.; Rajeev, R.; George, B.K. Adsorption Performance of Packed Bed Column for the Removal of Perchlorate Using Modified Activated Carbon. Process Saf. Environ. Prot. 2018, 117, 350–362. [Google Scholar] [CrossRef]
- Ajmani, A.; Patra, C.; Subbiah, S.; Narayanasamy, S. Packed Bed Column Studies of Hexavalent Chromium Adsorption by Zinc Chloride Activated Carbon Synthesized from Phanera Vahlii Fruit Biomass. J. Environ. Chem. Eng. 2020, 8, 103825. [Google Scholar] [CrossRef]
- Lei, B.; Liu, B.; Zhang, H.; Yan, L.; Xie, H.; Zhou, G. CuO-Modified Activated Carbon for the Improvement of Toluene Removal in Air. J. Environ. Sci. 2020, 88, 122–132. [Google Scholar] [CrossRef]
- Gholamiyan, S.; Hamzehloo, M.; Farrokhnia, A. RSM Optimized Adsorptive Removal of Erythromycin Using Magnetic Activated Carbon: Adsorption Isotherm, Kinetic Modeling and Thermodynamic Studies. Sustain. Chem. Pharm. 2020, 17, 100309. [Google Scholar] [CrossRef]
- Lin, S.; Zou, C.; Liang, H.; Peng, H.; Liao, Y. The Effective Removal of Nickel Ions from Aqueous Solution onto Magnetic Multi-Walled Carbon Nanotubes Modified by β-Cyclodextrin. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126544. [Google Scholar] [CrossRef]
- Barjasteh-Askari, F.; Davoudi, M.; Dolatabadi, M.; Ahmadzadeh, S. Iron-Modified Activated Carbon Derived from Agro-Waste for Enhanced Dye Removal from Aqueous Solutions. Heliyon 2021, 7, e07191. [Google Scholar] [CrossRef] [PubMed]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange-Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Pereira, I.C.; Carvalho, K.Q.; Passig, F.H.; Ferreira, R.C.; Rizzo-Domingues, R.C.P.; Hoppen, M.I.; Macioski, G.; Nagalli, A.; Perretto, F. Thermal and Thermal-Acid Treated Sewage Sludge for the Removal of Dye Reactive Red 120: Characteristics, Kinetics, Isotherms, Thermodynamics and Response Surface Methodology Design. J. Environ. Chem. Eng. 2018, 6, 7233–7246. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Shao, X.; Ma, J.; Tian, G. Properties of Magnetic Carbon Nanomaterials and Application in Removal Organic Dyes. Chemosphere 2018, 207, 377–384. [Google Scholar] [CrossRef]
- Castillo-Cervantes, J.N.; Gómora-Herrera, D.R.; Navarrete-Bolaños, J.; Likhanova, N.V.; Olivares-Xometl, O.; Lijanova, I.V. A Complete In-Situ Analysis of UV–Vis and 2D-FTIR Spectra of the Molecular Interaction between RO16 (Azo Dye) and Synthesized Ammonium-Based Ionic Liquids. Sep. Purif. Technol. 2021, 254, 117652. [Google Scholar] [CrossRef]
- Ferreira, W.H.; Silva, L.G.A.; Pereira, B.C.S.; Gouvêa, R.F.; Andrade, C.T. Adsorption and Visible-Light Photocatalytic Performance of a Graphene Derivative for Methylene Blue Degradation. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100373. [Google Scholar] [CrossRef]
- Hassan, A.F.; Elhadidy, H.; Abdel-Mohsen, A.M. Adsorption and Photocatalytic Detoxification of Diazinon Using Iron and Nanotitania Modified Activated Carbons. J. Taiwan Inst. Chem. Eng. 2017, 75, 299–306. [Google Scholar] [CrossRef]
- ASTM D2854-70; ASTM Standard Test Method for Apparent Density of Activated Carbon 1. ASTM International: West Conshohocken, PA, USA, 2000.
- ASTM D3838-05; ASTM Standard Test Method for PH of Activated Carbon Method. ASTM International: West Conshohocken, PA, USA, 2004; pp. 1–3.
- Yin, C.Y.; Aroua, M.K.; Daud, W.M.A.W. Fixed-Bed Adsorption of Metal Ions from Aqueous Solution on Polyethyleneimine-Impregnated Palm Shell Activated Carbon. Chem. Eng. J. 2009, 148, 8–14. [Google Scholar] [CrossRef][Green Version]
- Singh, T.S.; Pant, K.K. Experimental and Modelling Studies on Fixed Bed Adsorption of As(III) Ions from Aqueous Solution. Sep. Purif. Technol. 2006, 48, 288–296. [Google Scholar] [CrossRef]
- Iheanacho, O.C.; Nwabanne, J.T.; Obi, C.C.; Onu, C.E. Packed Bed Column Adsorption of Phenol onto Corn Cob Activated Carbon: Linear and Nonlinear Kinetics Modeling. S. Afr. J. Chem. Eng. 2021, 36, 80–93. [Google Scholar] [CrossRef]
- Madan, S.S.; De, B.S.; Wasewar, K.L. Adsorption Performance of Packed Bed Column for Benzylformic Acid Removal Using CaO2 Nanoparticles. Chem. Data Collect. 2019, 23, 100267. [Google Scholar] [CrossRef]
- Chittoo, B.S.; Sutherland, C. Column Breakthrough Studies for the Removal and Recovery of Phosphate by Lime-Iron Sludge: Modeling and Optimization Using Artificial Neural Network and Adaptive Neuro-Fuzzy Inference System. Chin. J. Chem. Eng. 2020, 28, 1847–1859. [Google Scholar] [CrossRef]
- Patel, H. Fixed-Bed Column Adsorption Study: A Comprehensive Review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef]
- Malkoc, E.; Nuhoglu, Y.; Abali, Y. Cr(VI) Adsorption by Waste Acorn of Quercus Ithaburensis in Fixed Beds: Prediction of Breakthrough Curves. Chem. Eng. J. 2006, 119, 61–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, X.; Zhang, P.; Gao, H.; Ou, C.; Kong, X. Production of Activated Carbons from Four Wastes via One-Step Activation and Their Applications in Pb2+ Adsorption: Insight of Ash Content. Chemosphere 2020, 245, 125587. [Google Scholar] [CrossRef] [PubMed]
- Vacca, V.J.; Colina, G.; Rincón, N.; Díaz, A.; Behling, E.; Marín, J.; Chacín, E.; Fernández, N. Adsorption for the Removal of Phenolic Compounds from the Effluent of a Biologic Reactor [Adsorción Para La Remoción de Compuestos Fenólicos Presentes En El Efluente de Un Reactor Biológico]. Rev. Tec. Fac. Ing. Univ. Zulia 2012, 35, 252–260. [Google Scholar]
- Bai, S.; Li, J.; Ding, W.; Chen, S.; Ya, R. Removal of Boron by a Modified Resin in Fixed Bed Column: Breakthrough Curve Analysis Using Dynamic Adsorption Models and Artificial Neural Network Model. Chemosphere 2022, 296, 134021. [Google Scholar] [CrossRef] [PubMed]
- Kumari, U.; Mishra, A.; Siddiqi, H.; Meikap, B.C. Effective Defluoridation of Industrial Wastewater by Using Acid Modified Alumina in Fixed-Bed Adsorption Column: Experimental and Breakthrough Curves Analysis. J. Clean. Prod. 2021, 279, 123645. [Google Scholar] [CrossRef]
- Simsek, E.B.; Beker, U.; Senkal, B.F. Predicting the Dynamics and Performance of Selective Polymeric Resins in a Fixed Bed System for Boron Removal. Desalination 2014, 349, 39–50. [Google Scholar] [CrossRef]
- Lakshmipathy, R.; Sarada, N.C. Metal Ion Free Watermelon (Citrullus Lanatus) Rind as Adsorbent for the Removal of Lead and Copper Ions from Aqueous Solution. Desalination Water Treat. 2016, 57, 15362–15372. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Dutta, S.; Saxena, V.K. A Review on Biosorptive Removal of Dyes and Heavy Metals from Wastewater Using Watermelon Rind as Biosorbent. Environ. Adv. 2020, 2, 100007. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A Critical Review of Biosorption of Dyes, Heavy Metals and Metalloids from Wastewater as an Efficient and Green Process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Usman, M.A.; Khan, A.Y. Selective Adsorption of Anionic Dye from Wastewater Using Polyethyleneimine Based Macroporous Sponge: Batch and Continuous Studies. J. Hazard. Mater. 2022, 428, 128238. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, G.; Subhapriya, P.; Dhanapal, V.; Dineshkumar, G.; Venkateswaran, V. Dye Removal Kinetics and Adsorption Studies of Activated Carbon Derived from the Stems of Phyllanthus Reticulatus. Mater. Today Proc. 2021, 45, 7934–7938. [Google Scholar] [CrossRef]
- Zhang, Z.; Chuang, Y.H.; Huang, N.; Mitch, W.A. Predicting the Contribution of Chloramines to Contaminant Decay during Ultraviolet/Hydrogen Peroxide Advanced Oxidation Process Treatment for Potable Reuse. Environ. Sci. Technol. 2019, 53, 4416–4425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, H.; Zhu, L.; Wang, G.; Zeng, Z.; Li, X. High Flux and High Selectivity Thin-Film Composite Membranes Based on Ultrathin Polyethylene Porous Substrates for Continuous Removal of Anionic Dyes. J. Environ. Chem. Eng. 2022, 10, 107202. [Google Scholar] [CrossRef]
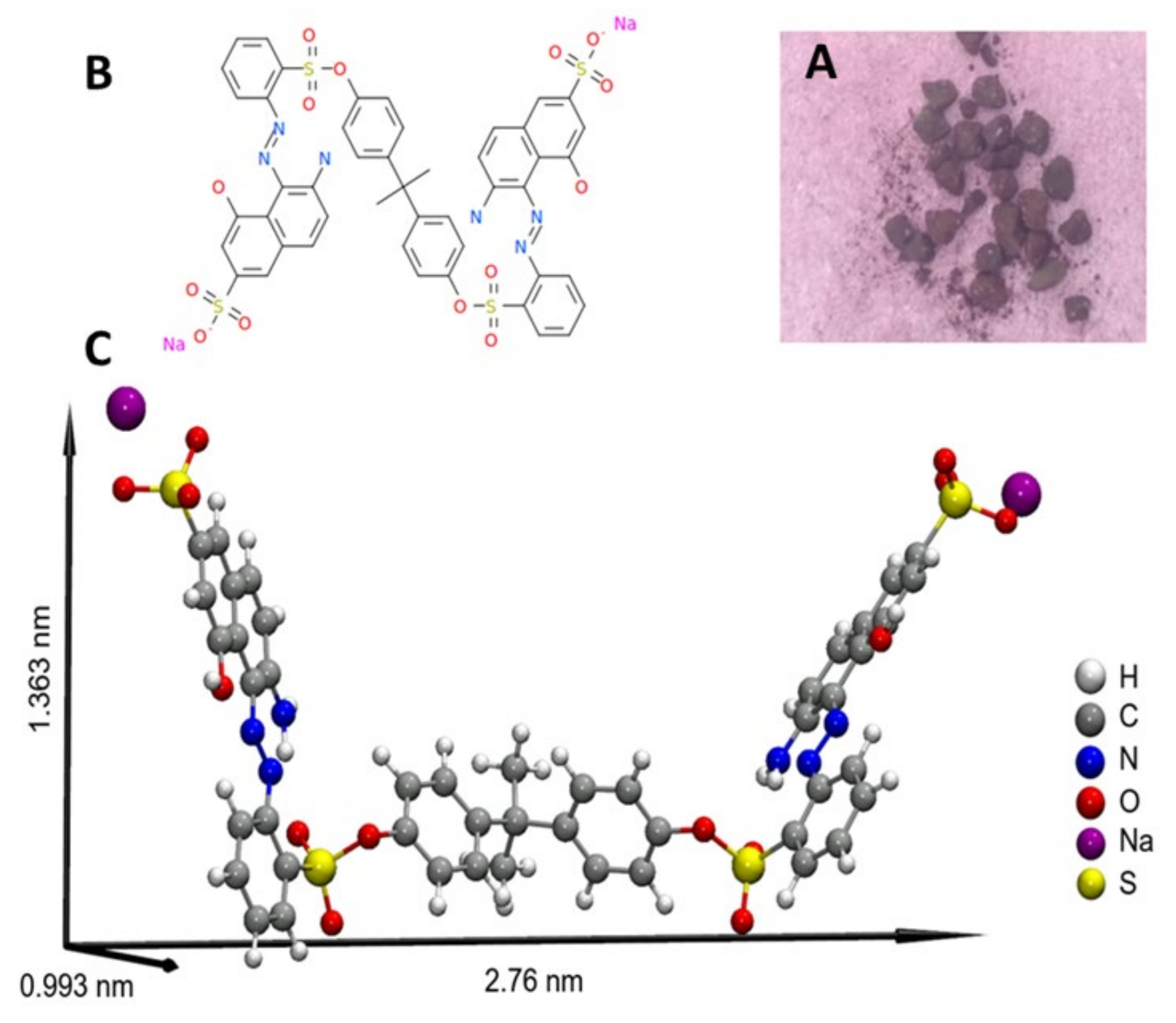
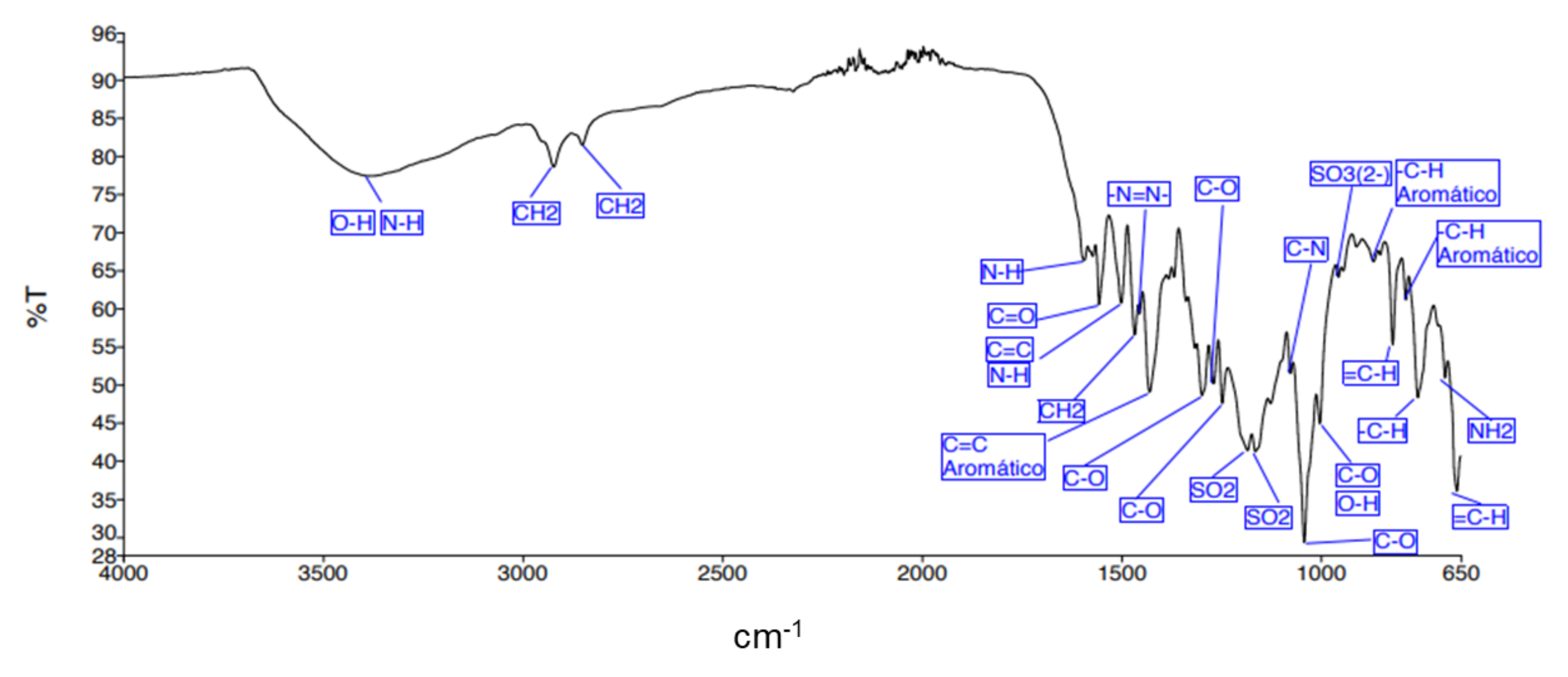
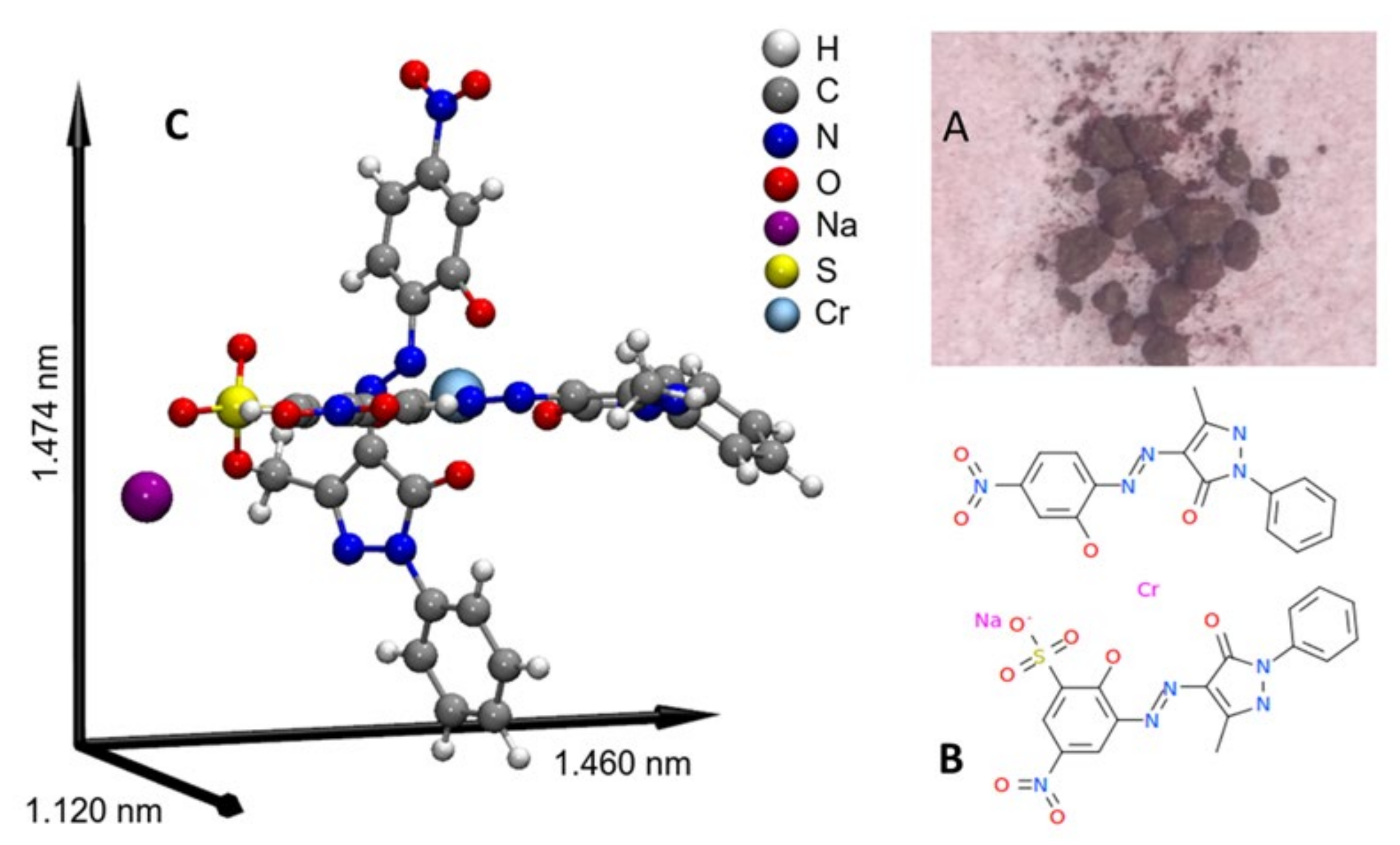
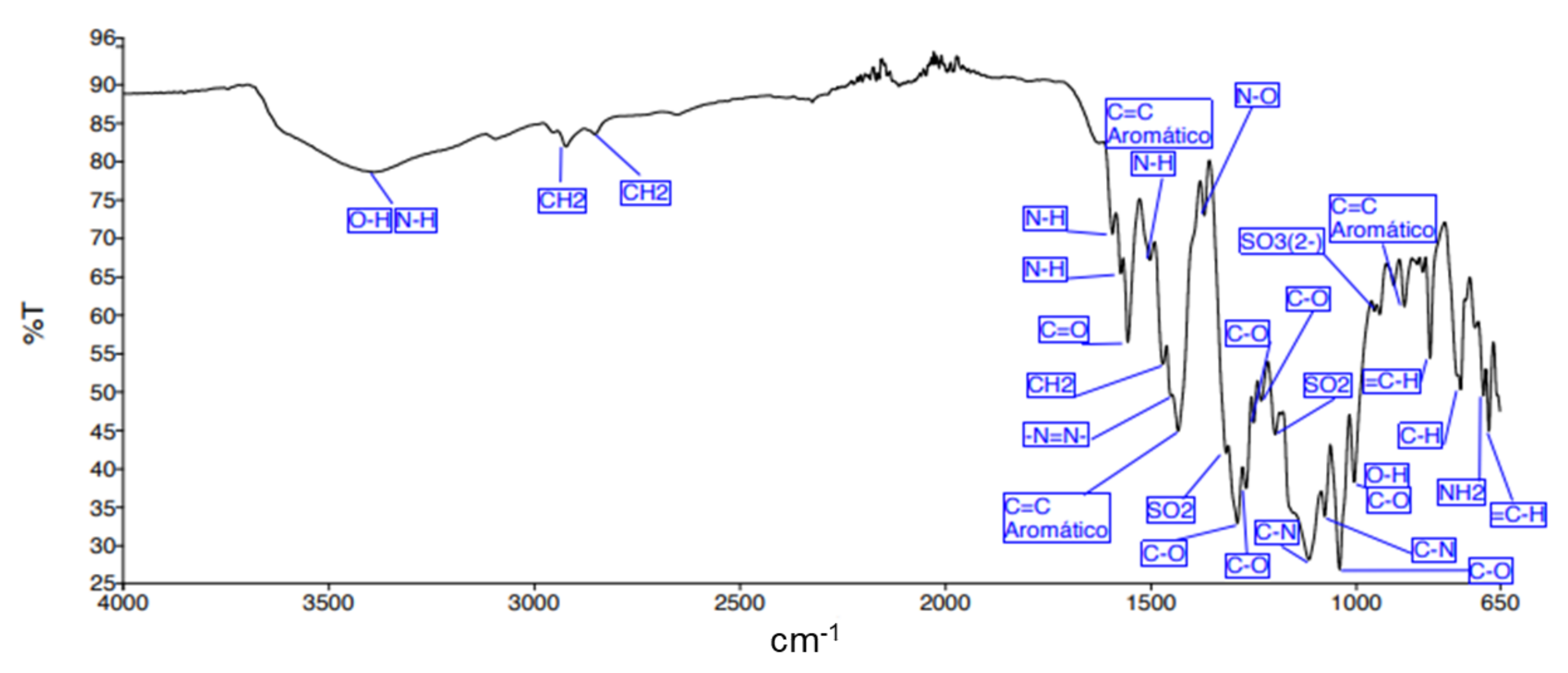
| Adsorbent | tb/min | ts/min | LZTM/Cm | qtotal/mg | qeq/mg · g−1 | Mtotal, mg | RT/% |
|---|---|---|---|---|---|---|---|
| C-conv | 7.69 | 136.54 | 7.26 | 5.86 ± 1.29 | 1.17 ± 0.26 | 11.02 ± 5.19 | 56.78 ± 15.07 |
| 10.56 | 198.05 | 8.43 | 7.93 ± 0.80 | 1.59 ± 0.16 | 13.98 ± 3.01 | 57.43 ± 6.66 |
| TM | |||
|---|---|---|---|
| C-conv | 237.88 | 0.0058 | 0.9842 |
| 216.21 | 0.0080 | 0.9799 | |
| YNM | |||
| C-conv | 17.97 | 0.2989 | 0.9794 |
| 16.38 | 0.4068 | 0.9799 |
| Adsorbent | tb/min | ts/min | LZTM/cm | qtotal/mg | qeq/mg · g−1 | Mtotal, mg | RT/% |
|---|---|---|---|---|---|---|---|
| C-conv | 11.54 | 238.46 | 8.19 | 11.07 ± 0.13 | 2.21 ± 0.03 | 16.30 ± 0.00 | 67.87 ± 0.78 |
| 10.54 | 250.00 | 8.06 | 11.99 ± 0.90 | 2.44 ± 0.24 | 17.44 ± 0.64 | 68.70 ± 2.63 |
| TM | |||
|---|---|---|---|
| C-conv | 338.46 | 0.0037 | 0.9944 |
| 329.42 | 0.0030 | 0.9925 | |
| YNM | |||
| C-conv | 28.74 | 0.1690 | 0.9944 |
| 26.63 | 0.1481 | 0.9975 | |
| TM | |||
| C-conv | 237.88 | 0.0058 | 0.9842 |
| 216.21 | 0.0080 | 0.9799 | |
| YNM | |||
| C-conv | 17.97 | 0.2989 | 0.9794 |
| 16.38 | 0.4068 | 0.9799 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colina Andrade, G.J.; Vilca Quilla, J.M.; Terán Hilares, R.; Tejada Meza, K.; Mogrovejo Valdivia, A.C.; Aguilar-Pineda, J.A.; Cárdenas García, J.D.; Pacheco Tanaka, D.A. Enhanced Removal of Bordeaux B and Red G Dyes Used in Alpaca Wool Dying from Water Using Iron-Modified Activated Carbon. Water 2022, 14, 2321. https://doi.org/10.3390/w14152321
Colina Andrade GJ, Vilca Quilla JM, Terán Hilares R, Tejada Meza K, Mogrovejo Valdivia AC, Aguilar-Pineda JA, Cárdenas García JD, Pacheco Tanaka DA. Enhanced Removal of Bordeaux B and Red G Dyes Used in Alpaca Wool Dying from Water Using Iron-Modified Activated Carbon. Water. 2022; 14(15):2321. https://doi.org/10.3390/w14152321
Chicago/Turabian StyleColina Andrade, Gilberto J., Jessica M. Vilca Quilla, Ruly Terán Hilares, Kevin Tejada Meza, Alejandra C. Mogrovejo Valdivia, Jorge A. Aguilar-Pineda, Jaime D. Cárdenas García, and David A. Pacheco Tanaka. 2022. "Enhanced Removal of Bordeaux B and Red G Dyes Used in Alpaca Wool Dying from Water Using Iron-Modified Activated Carbon" Water 14, no. 15: 2321. https://doi.org/10.3390/w14152321
APA StyleColina Andrade, G. J., Vilca Quilla, J. M., Terán Hilares, R., Tejada Meza, K., Mogrovejo Valdivia, A. C., Aguilar-Pineda, J. A., Cárdenas García, J. D., & Pacheco Tanaka, D. A. (2022). Enhanced Removal of Bordeaux B and Red G Dyes Used in Alpaca Wool Dying from Water Using Iron-Modified Activated Carbon. Water, 14(15), 2321. https://doi.org/10.3390/w14152321







