Abstract
Recently, ClO2-based oxidation has attracted increasing attention to micropollutant abatement, due to high oxidation potential, low disinfection byproduct (DBPs) formation, and easy technical implementation. However, the kinetics, reactive sites, activation methods, and degradation pathways involved are not fully understood. Therefore, we reviewed current literature on ClO2-based oxidation in micropollutant abatement. In direct ClO2 oxidation, the reactions of micropollutants with ClO2 followed second-order reaction kinetics (kapp = 10−3–106 M−1 s−1 at neutral pH). The kapp depends significantly on the molecular structures of the micropollutant and solution pH. The reactive sites of micropollutants start with certain functional groups with the highest electron densities including piperazine, sulfonyl amido, amino, aniline, pyrazolone, phenol groups, urea group, etc. The one-electron transfer was the dominant micropollutant degradation pathway, followed by indirect oxidation by superoxide anion radical (O2•−) or hydroxyl radical (•OH). In UV-activated ClO2 oxidation, the reactions of micropollutants followed the pseudo-first-order reaction kinetics with the rates of 1.3 × 10−4–12.9 s−1 at pH 7.0. Their degradation pathways include direct ClO2 oxidation, direct UV photolysis, ozonation, •OH-involved reaction, and reactive chlorine species (RCS)-involved reaction. Finally, we identified the research gaps and provided recommendations for further research. Therefore, this review gives a critical evaluation of ClO2-based oxidation in micropollutant abatement, and provides recommendations for further research.
1. Introduction
The micropollutants also known as contaminants of emerging concerns (CECs) are comprised of various anthropogenic and natural compounds, such as pharmaceuticals and personal care products (PPCPs), endocrine disruptors, and pesticides [1,2]. Their presence in natural and engineered systems, even at trace concentrations (ng L−1 to μg L−1), has attracted significant attention because of their toxic, persistent, bioaccumulative properties [3]. Due to increased industrialization and urbanization, many micropollutants are widely used, and eventually end up in different types of wastewaters. Unfortunately, traditional wastewater treatment plants (WWTPs) are not explicitly designed for micropollutant abatement, resulting in WWTPs being one of the significant sources of micropollutants in surface water [4]. Until now, various techniques have been proposed for micropollutant abatement in WWTPs, including activated carbon/biochar adsorption [5], advanced oxidation processes (AOPs) [6,7], and membrane filtration [8]. AOPs have attracted growing attention among these techniques because of their simple operation, high removal efficiency, and rapid oxidation.
AOPs enable an approach combining two individual processes of disinfection and decontamination with improved cost-effectiveness [9,10]. Chlorine Dioxide (ClO2) is a green disinfectant/oxidant and was listed as an A1-level, safe, and efficient disinfectant by the World Health Organization (WHO) [11]. It has been prevalently used as a drinking water disinfectant alternative to chlorine (Cl2) due to its effectiveness in pathogen inactivation and limited formation of halogenated disinfection byproducts (DBPs), such as trihalomethanes (THMs) and haloacetic acids (HAAs) [12]. In addition, the products of ClO2 disinfection/oxidation consist 50–70% of chlorite (ClO2−) and 30–50% of chlorate (ClO3−) and chloride (Cl−) [13,14]. Controlling the levels of these ClO2 residuals is critical for successfully implementing ClO2 disinfection/oxidation.
Recently, ClO2-based oxidation for micropollutant abatement has attracted increased attention due to its advantages of strong oxidation, low DBPs formation, and easy technical implementation. ClO2 can effectively oxidize micropollutants with electron-rich functional groups such as aniline, phenolic, aromatic, and tertiary amine groups [15,16]. ClO2 is typically transformed into ClO2− through a single-electron oxidation process [17]. Recent studies used external energy to activate ClO2 to produce reactive species, resulting in improved micropollutant abatement. For example, the excellent performance of the co-exposure of ClO2 and ultraviolet radiation (UV) was reported in micropollutant abatement due to the high yield of reactive species [18,19].
ClO2-based oxidation includes direct and activated ClO2 oxidations. Though studies on ClO2-based oxidation, especially on the direct ClO2 oxidation, have been increasing over the past decade, there is still a limited understanding of these processes on micropollutant abatement, such as their kinetics, reactive sites, activation methods, and degradation pathways. The existing review about ClO2 primarily focused on the reaction with (in)organic compounds in water treatment [17], pathogenic microbe inactivation in water treatment [20], antimicrobial food packaging [21], disposable ClO2 wipes [22], and postharvest handling and food storage [23]. To the best of our knowledge, there is no comprehensive review on ClO2-based oxidation in micropollutant abatement. Therefore, providing a comprehensive review of this technology is crucial for future research and application. In this review, we emphatically discussed (1) ClO2 properties; (2) reaction kinetics, reactive sites, and degradation pathways in the directed ClO2 oxidation; and (3) reaction kinetics and degradation pathways in the UV-activated ClO2 oxidation.
2. ClO2 Physicochemical Properties
ClO2 is a green-yellowish gas and has a pungent odor similar to Cl2. It is one of the few compounds in nature that exist almost entirely as monomeric free radicals due to a single unpaired electron [24]. The molecular weight and the standard oxidation state of Cl atoms in ClO2 are 67.46 and +4, respectively. ClO2 has a boiling point of 11 °C, a melting point of −59 °C, a density of 1.64 g mL−1 (liquid) at 0 °C [25], a water solubility of 3.0 g L−1 at 25 °C [17], and pKa value of 3.0. ClO2 is strongly soluble in water and does not hydrolyze to any appreciable extent but remains in solution as a dissolved gas [26]. ClO2 in aqueous solutions is quite stable when protected from light and kept cool, well-sealed, and slightly acidified (pH = 6). The ultraviolet absorption spectrum of ClO2 solutions has broadband with a peak at 359 nm and a molar extinction coefficient of ~1250 M−1 cm−1 [27].
ClO2 has a relatively short half-life and is highly volatile and explosive under concentrations of >10% in the air [28]. ClO2 solution under concentrations of <~10 g L−1 will not produce sufficiently high vapor pressure for an explosive hazard. In water treatment practice, the concentrations of concentrated ClO2 solution rarely exceed 4 g L−1. Furthermore, ClO2 cannot be compressed, stored, or transported under pressure and must be generated on-site [29]. Compared with the electrolysis method, the chemical method is more mature for ClO2 production, which refers to the reactions of sodium chlorite (NaClO2) or sodium chlorate (NaClO3) with Cl2, hydrochloric acid (HCl), or peroxydisulfate (H2S2O8) (Equations (1)–(3)). The reaction of NaClO2 with an acid, such as HCl, has become an increasingly common method for ClO2 production due to the operational difficulty and safety concerns of handling Cl2 gas. Noted, to produce the same mass weight of ClO2, hydrochloric-based ClO2 production (Equation (2)) uses 1.25 times more NaClO2 than chlorine-based (Equation (1)) or peroxydisulfate-based (Equation (3)) ClO2 production.
2NaClO2 + Cl2 → 2ClO2 + 2NaCl
5NaClO2 + 4HCl → 4ClO2 + 5NaCl + 2H2O
2NaClO2 + 4Na2S2O8 → 2ClO2 + 2Na2SO4
In the water, ClO2 reacts first with other compounds to form ClO2− through a one-electron transfer reaction (Equation (4)), with the redox potential of 0.936 V [30]. The second reaction of the formed ClO2− transforming to Cl− by gaining four electrons does not occur readily, due to the low reactivity of ClO2− (Equation (5)). In practice, fast oxidation predominates, and therefore, ClO2− will be the significant byproduct during ClO2 disinfection/oxidation [31]. ClO3− will be another byproduct because of its presence in proprietary solutions of ClO2. ClO2 accepts five electrons when thoroughly reduced to Cl−, while Cl2 accepts two electrons from the oxidation compounds (Equations (6) and (7)). Therefore, the oxidative capacity of ClO2 is also approximately 2.5 times of Cl2 on a weight basis.
ClO2 + e− → ClO2−
ClO2− + 2H2O + 4e− → Cl− + 4OH
ClO2 + 2H2O + 5e− → Cl− + 4OH−
Cl2 + 2e− → 2Cl−
3. Direct ClO2 Oxidation
3.1. Reaction Kinetics
The reaction kinetics between ClO2 and micropollutants can be well described by second-order kinetic models (Equations (8) and (9)), referring to a first-order model in ClO2 concentration and a first-order model in micropollutant concentration [32,33].
where MP is an organic micropollutant; kapp is the apparent second-order rate constant for the overall reaction; [ClO2] and [MP]tot is the ClO2 and MP concentration, respectively.
ClO2 + MP → product
The reaction kinetics of antibiotics with ClO2 depends on their molecular structures and pH. The kapp of antibiotics ranged from 1.2 to 1.3 × 106 M−1 s−1 at neutral pH, with a general order of tetracyclines (105–106) > triclosan (104–105) > sulfonamides (103–104) > macrolides (101–102) > fluoroquinolones (1–101) (Table 1). There is the aniline moiety in tetracyclines and sulfonamides and phenol moiety in triclosan, but the alkyl amine moiety in macrolides and fluoroquinolones. The results suggested that antibiotics with the aniline and phenol groups may be more vulnerable to ClO2 attack than those with the alkyl amine. Furthermore, enrofloxacin and ofloxacin with tertiary amines on piperazine moieties reacted faster with ClO2 than other fluoroquinolones with secondary amines on piperazine moieties [34]. However, the reactivity of the dimethylamino group in tetracycline to ClO2 is higher than that of trimethylamine but lower than that of N,N-dimethylaniline [35]. The kapp of antibiotics was related to pH as well. A kinetic study demonstrated that the kapp of ciprofloxacin (belonging to fluoroquinolones) increased by more than three orders of magnitude from pH 4.48 to 9.55 [34]. Similarly, the kapp increased by more than 4 to 6 and 1.6 to 2.2 orders of magnitude from pH 2.5 to 10.5 and from 4.0 to 9.5 for the ClO2 oxidation of tetracyclines and sulfonamides, respectively [35,36]. The large variation with pH could be attributed to the varying reactivity of antibiotic acid-base species toward ClO2. An increase in pH led to a larger fraction of the deprotonated species (A−), thus facilitating the reaction of antibiotics with ClO2. Similar trends were also observed in fluoroquinolones [34] and tetracyclines [35], indicating that the deprotonation of these antibiotics as pH increases considerably favors their oxidation by ClO2.

Table 1.
Second-order rate constants (kapp) in the reaction of ClO2 with micropollutants.
In addition, the direct ClO2 oxidation was applied for degrading other PPCPs such as antipyretic analgesics, β-blockers, antiepileptics, psychostimulants, antineoplastics, and lipid regulator. The kapp of antipyretic analgesics ranged from 4.8 × 10−1 to 2.1 × 105 M−1 s−1 at neutral pH (Table 1). Among them, aminopyrine, diclofenac, and acetaminophen had the highest kapp due to the tertiary amine, aniline, and phenol moiety in their molecule, respectively. The second highest kapp was observed in propylphenazone with the heterocyclic amine moiety and naproxen with substituted benzene moiety. The remaining studied PPCPs were less reactivity towards ClO2 (Table 1). These results implied that ClO2 is a highly selective oxidant with respect to micropollutants with specific functional groups such as aniline, phenolic moieties, the second and tertiary amine, heterocyclic amine, aromatic nucleus.
3.2. Reactive Sites
Reactive sites of micropollutants during ClO2 oxidation are determined by functional groups with the highest electron densities due to the one-electron transfer mechanism. As for PPCPs, the main reactive sites include piperazine group, sulfonyl amido group, amino group, aniline group, pyrazolone group and phenol group (Table 2). The N4 atom in the piperazine ring of fluoroquinolones was the specific site to be attacked by ClO2 [34]. Similarly, He et al. [43,44] found the tertiary N4 amines and the secondary N4 amines with the highest 2FEDHOMO2 value in the piperazinyl group as the most vulnerable sites in the reactions between ClO2 and the fluoroquinolones of fleroxacin and enoxacin. The sulfonyl amido-nitrogen of sulfonamides could be the main reaction site toward ClO2 [36]. The reaction of three representative β-lactam antibiotics with ClO2 starts with a single-electron transfer from the lone electron pair of the amino group to ClO2 [45]. ClO2 reacts with tetracyclines predominantly in the unprotonated dimethylamino group and deprotonated phenolic-diketone group [35]. Furthermore, the reactive site of triclosan was the phenol group during ClO2 oxidation [37]. For antipyretic analgesics, the aniline group in diclofenac was the reactive site and acted as the electron-rich moieties during ClO2 oxidation [37]. The N2 atom on the pyrazolone ring of antipyrine was vulnerable under the electrophilic reaction of ClO2 due to its high electron cloud density [39]. However, the C=C double bond on the pyrazolone ring of isopropylphenazone and aminopyrine were the most reactive sites toward ClO2 [40].
The main reactive sites of pesticides are the urea group and aromatic benzene ring of phenylurea and sulfonylurea herbicides, the sulfur center of ametryn and methiocarb, the amide group and the phosphinothioyl group of organophosphorus pesticides. For example, the primary attack on two phenylurea herbicides of diuron and chlortoluron by ClO2 might be the electron-rich nitrogen atom on the ureic side-chain [46]. However, the aromatic benzene ring of isoproturon is vulnerable to the attack of ClO2 [47]. Additionally, the degradation of two sulfonylurea herbicides of nicosulfuron and thifensulfuron methyl started with an attack on the urea groups by ClO2 [48]. The main reactive site of ametryn herbicide and methiocarb pesticide during ClO2 oxidation was the sulfur center in their molecules [47,49]. ClO2 oxidation of two organophosphorus pesticides started with an attack on the amide group of azamethiphos and the phosphinothioyl group of dimethoate [16].

Table 2.
Reactive sites and degradation pathways based on the intermediate products in the reaction of ClO2 with micropollutants.
Table 2.
Reactive sites and degradation pathways based on the intermediate products in the reaction of ClO2 with micropollutants.
| Micropollutants | Molecular Structure | Reactive Sites | Pathways | References |
|---|---|---|---|---|
| β-lactam antibiotics | ||||
| Amoxicillin | 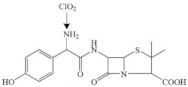 | amino group | pathway: β-lactam ring breaking | [45] |
| Cefadroxil | 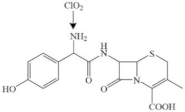 | |||
| fluoroquinolones | ||||
| Ciprofloxacin | 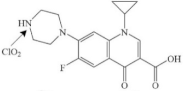 | piperazine’s N4 atom | pathway: dealkylation, hydroxylation and intramolecular ring closure at the piperazine moiety, and the quinolone ring mostly intact | [34] |
| Norfloxacin | 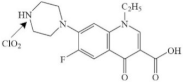 | |||
| Enoxacin | 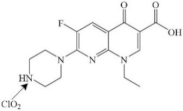 | pathway: piperazine group cleavage, the decarboxylating quinolone ring, and hydroxylation | [44] | |
| Fleroxacin | 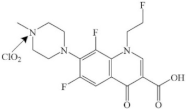 | pathway: the cleavage of the piperazine ring and the decarboxylation and chlorination of the quinolone ring | [32] | |
| sulfonamides | ||||
| Sulfamethoxazole | 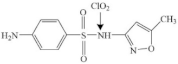 | sulfonyl amido-nitrogen | pathway: breakage of S-N and C-S bonds and hydroxylation of aniline group | [36] |
| tetracyclines | ||||
| Tetracycline | 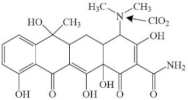 | dimethylamino groups | pathway: (hydr)oxylation and breakage of tetracycline molecules | [35] |
| triclosan | ||||
| Triclosan | 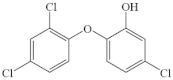 | phenol group a | pathway: the closure of the phenolic ring, chlorination of the phenolic ring and cleavage of the ether bond | [38] |
| antipyretic analgesics | ||||
| Diclofenac | 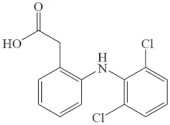 | aniline group a | pathway: decarboxylation, hydroxylation and chlorination of the phenylacetic acid moiety, and further C-N bond cleavage | [50] |
| Antipyrine | 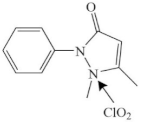 | pyrazolone’s N2 atom | pathway: chlorination substitution, ring-opening reaction and de-carbonyl reaction of the pyrazolone ring | [39] |
| Iso-propylphenazone | 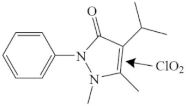 | pyrazolone’s C=C | pathway: C=C cleavage, ring opening reaction and de-carbonyl reaction of the pyrazolone ring | [40] |
| Aminopyrine | 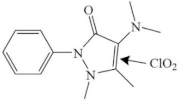 | |||
| antidepressant | ||||
| Venlafaxine | 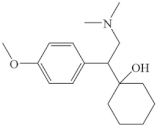 | - | pathway: dehydration, demethylation and cleavage of the molecular structure | [51] |
| phenylurea herbicides | ||||
| Fenuron | 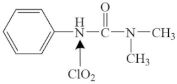 | urea group | pathway: electrophilic substitution and cleavage of the urea group products: a chloro-quinone product and an urea derivative | [52] |
| Isoproturon | 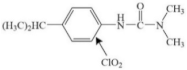 | aromatic benzene ring | pathway: aromatic-ring hydroxylated substituted derivatives | [47] |
| Chlortoluron | 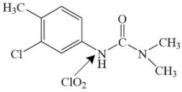 | nitrogen atom on the ureic side-chain | pathway: radical intermediates formation, hydroxylation reactions and cleavage of the N–C bond on the ureic side-chain | [46] |
| Diuron | 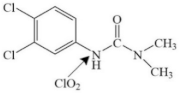 | pathway: hydroxylation reactions and cleavage of the N–C bond on the ureic side-chain, dechloridation of the benzene ring | ||
| sulfonylurea herbicides | ||||
| Nicosulfuron | 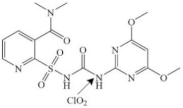 | urea group | pathway: the urea group breaking | [48] |
| herbicide | ||||
| Ametryn | 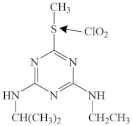 | sulfur center | pathway: oxidation reactiveproduct: the sulphoxide derivative | [47] |
| carbamate pesticides | ||||
| Methiocarb | 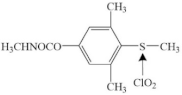 | sulfur center | pathway: oxidation reaction products: methiocarb sulfoxidemethiocarb sulfone | [49] |
| organophosphorus pesticides | ||||
| Azamethiphos | 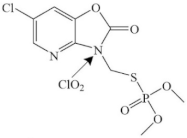 | amide group | pathway: the breaking of amide group or S–C bond | [16] |
| Dimethoate | 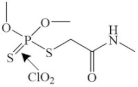 | the phosphinothioyl group | pathway: the breaking of S–C bond, oxidation of the phosphinothioyl group | |
a [37]; -: not available.
3.3. Degradation Pathways
One-electron transfer was the dominant degradation pathway in the direct ClO2 oxidation of micropollutants, followed by indirect oxidation by oxygen species such as superoxide anion radical (O2•−) or hydroxyl radical (•OH) (Figure 1). In detail, the one-electron transfer oxidation pathway refers to: (1) ClO2 attacks the atom of the micropollutant with the highest electron density or the strongest electron-donating and takes away an electron from the atom to make micropollutant forming a radical intermediate (MP•), and is thus reduced to ClO2− (Equation (10); it is the rate-determining step) and (2) MP• combines with another ClO2 to form degradation products by undergoing molecular rearrangement and binding to itself or ClO2 (Equation (11)). During the direct ClO2 oxidization of MP•, the oxidant is initially reduced to ClO, then to HClO, and subsequently to Cl−. Therefore, four tentative routes occur through the oxygen transfer process and potentially contribute to direct ClO2 oxidization of micropollutants (Equations (12)–(15)), which were confirmed by the identification of decarbonyl-MP, hydroxyl-MP, chloro-MP, and even ring rupture of MP [38,53,54].
MP + ClO2 → MP• + ClO2−
MP• + ClO2 → products
MP• + ClO2 → MP − OH + ClO
MP• + ClO2 → decarbonyl − MP + ClO
MP• + H+ + ClO → MP+ + HOCl
MP• + HOCl → MP − Cl + H2O
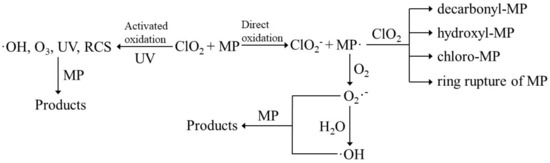
Figure 1.
The degradation pathway of direct and activated ClO2 oxidation for micropollutant abatement. RCS: Cl•, ClO• and Cl2•−; MP: micropollutant.
ClO2 oxidation of PPCPs mainly led to the ring-opening reaction, dealkylation, decarboxylation, hydroxylation, and chlorination. The cleavage of the β-lactam ring in the molecules of penicillin, amoxicillin, and cefadroxil was observed in the ClO2 oxidation of β-lactam antibiotics [45]. ClO2 oxidation of fluoroquinolones, ciprofloxacin, and norfloxacin, led to dealkylation, hydroxylation, and intramolecular ring closure at the piperazine moiety but left the quinolone ring mostly intact [34]. Similarly, the primary and initial step in the ClO2 oxidation of enoxacin and fleroxacin was the cleavage of the piperazine ring [32,44]. The decarboxylation and hydroxylation or chlorination of the quinolone ring occurred in enoxacin and fleroxacin, whereas the quinolone ring was unreactive of ciprofloxacin and norfloxacin. The cleavage of S−N and C−S bonds and the hydroxylation of aniline moiety were the major degradation pathways of sulfamethoxazole [36]. Furthermore, (hydr)oxylation and breakage of tetracycline were observed during the ClO2 oxidation [35]. The pyrazolone ring-opening reaction caused by C=C double bond cleavage and further de-carbonyl reaction were the main degradation pathways of three antipyretic analgesics of antipyrine, isopropylphenazone, and aminopyrine [39,40]. ClO2 oxidation of triclosan involved the cleavage of the ether link, chlorination of the phenolic ring, and ring closure [38]. The transformation pathways of venlafaxine included dehydration, demethylation, and cleavage of the molecule during ClO2 oxidation [51].
ClO2 reacts with commonly used phenylurea herbicides and sulfonylurea herbicides predominantly by the cleavage of the urea group and hydroxylated substitutes of the aromatic ring. ClO2 oxidation of phenylurea herbicides of chlortoluron and diuron was subjected to steps including radical intermediates formation, hydroxylation reactions, and cleavage of the N−C bond on the ureic side-chain [46]. Isoproturon, a phenylurea-derivative, reacts with ClO2 to form aromatic-ring hydroxylated substituted derivatives [47]. The urea group in sulfonylurea herbicide of nicosulfuron reacts firstly with ClO2, resulting in breaking one bond and forming two degradation products of 2-(Nformylsulfamoyl)-N,N-dimethylnicotinamide and 4,6-dimethoxypyrimidin-2-amine [48].
Additionally, ClO2 oxidation of other pesticides with the sulfur or phosphinothioyl center in their molecules led to sulfoxide and sulfone, ring rupture, hydroxylation, and decarbonyl products. Ametryn (R-S-CH3) reacted with ClO2 forming the sulfoxide derivative (R-SO-CH3) [47]. Similarly, during the ClO2 oxidation of the carbamate pesticide of methiocarb (MC), methiocarb sulfoxide and methiocarb sulfone were generated by losing HClO2 and HOCl from the intermediate adduct of MC-ClO2-OH, respectively, which was first formed around the sulfur center of methiocarb [49]. Pergal, et al. [16] studied the ClO2 oxidation of two organophosphorus pesticides and found the successive attack on the amide group and the sulfide group in azamethiphos, leading to the break of the amide group and S–C bond, and the hydroxylation of the phosphinothioyl and then decarbonyl in dimethoate.
The indirect oxidation pathway of ClO2 with micropollutants includes (1) the formation of O2•− by concurrently transferring an electron from MP• to dissolved oxygen in solution (Equation (16)), (2) the reaction of O2•− with water with the formation of •OH (Equation (17)), and (3) the degradation of micropollutant by the formed O2•−/•OH (Equation (18)). For example, two major degradation pathways of diclofenac (DCF) were proposed as (1) direct ClO2 oxidation through one-electron transfer and (2) indirect O2•− oxidation by concurrently transferring an electron from DCF• to dissolved oxygen [33].
MP• + O2 → MP+ + O2•−
O2•− + H2O → •OH
MP + O2•−/•OH → products
4. UV-Activated ClO2 Oxidation
4.1. Reaction Kinetics
The micropollutant abatements were generally enhanced by combining ClO2 with shortwave ultraviolet light (UVC), which follows the pseudo-first-order reaction kinetics with the pseudo-first-order rates (kobs) of 1.3 × 10−4–9.8 × 10−3 s−1 at pH 7.0 (Table 3). For example, more than 99% of triclosan was degraded under co-exposure to UVC irradiation and ClO2 [55]. Four micropollutants (i.e., trimethoprim, iopromide, caffeine, and ciprofloxacin) were degraded by 14.4–100.0% in UVC/ClO2, with the corresponding kobs following an order of ciprofloxacin (9.8 × 10−3 s−1) > iopromide (1.2 × 10−3 s−1) > trimethoprim (5.7 × 10−4 s−1) > caffeine (1.3 × 10−4 s−1) [18]. The degradation of these four micropollutants was accelerated in UVC/ClO2, compared to direct ClO2 oxidation or UVC photolysis. Ye et al. [56] reported that 95% flumequine was degraded (kobs = 2.7 × 10−3 s−1) in UVC/ClO2 AOP, and its degradation rate gradually increased with ClO2 dosage and UV intensity, but decreased as pH ascended. Though the combination of UVC and ClO2 enhanced the micropollutant abatement via generating more reactive species (e.g., •OH and chlorine radical (Cl•)), they are less effective than the well-documented UVC/H2O2, UVC/Cl2, and UVC/NH2Cl AOPs under the same initial oxidant dosages. For example, Tian et al. [57] compared the combination of UVC with different oxidants (i.e., Cl2, NH2Cl, ClO2, and H2O2) in the degradation of iopamidol and reported the kobs following the order of UVC/Cl2 (1.9 × 10−2 s−1) > UVC/H2O2 (1.3 × 10−2 s−1) > UVC/NH2Cl (1.0 × 10−2 s−1) > UVC/ClO2 (4.4 × 10−3 s−1) under the same conditions.

Table 3.
Summary of research studying the removal of micropollutants by the UV-activated ClO2 oxidation.
A novel UVC/ClO2− AOP was proposed to remove both ClO2− residue and micropollutants in water. UV photolysis after ClO2 disinfection can effectively eliminate both ClO2− and carbamazepine by •OH and reactive chlorine species (RCS) generated from UVC/ClO2− [58]. The •OH generated from UVC/ ClO2− (Equations (19)–(21)) reacts with not only carbamazepine, but also ClO2− to generate ClO2 (Equation (22)), which subsequently is activated by UV radiation to produce RCS (i.e., Cl• and chlorine oxide radicals (ClO•)). As the products of UVC/ClO2−, Cl− and ClO3− presented the decreasing and increasing yield, respectively, with the increasing ClO2− dosage [58]. Furthermore, Su et al., [59] developed a combined ClO2− photocatalysis technique for the degradation and detoxification of norfloxacin by dosing ClO2− in a visible light photocatalytic system. The degradation rate of norfloxacin in the combined ClO2− photocatalysis system was faster than the single photocatalytic system or the single chlorite system under irradiation. The regenerated ClO2− can be retransformed into ClO2 based on the ClO2−/ClO2 dynamic interchange mechanism [59].
photocatalysts → photocatalysts (h+ + e−)
O2 + e− → O2•−
O2•− + H2O → •OH
•OH + ClO2− → ClO2 + OH− k = 6.3 × 109 M−1 s−1
However, UVC/ClO2 AOP suffer from several drawbacks: (1) low absorption of ClO2 in the UVC range with the molar absorption coefficients of 60.7 M−1 cm−1 [19]; (2) high energy demand from UVC irradiation and low energy efficiency of low-pressure mercury ultraviolet (LPUV) lamps [19]; and (3) more emitted photons of UVC irradiation absorbed by background matrix components [27]. To address these issues, an emerging AOP combining longwave ultraviolet light (UVA) with ClO2 (UVA/ClO2) was proposed as an alternative to UVC/ClO2 AOP because of the high molar absorptivity for ClO2 at UVA wavelengths (ε359 nm = 1250 M−1 cm−1), reduced photon absorption by background matrix components at 365 nm, and high energy efficiency of UVA-LEDs. Recently, a novel UVA/ClO2 AOP was proposed as an alternative to UVC/H2O2 AOP [27]. Furthermore, the novel UVA/ClO2 AOP was conducted for the degradation of 19 micropollutants with the degradation percentages of 7 to 100% and the corresponding kobs of 3.8 × 10−4–12.9 s−1 (Table 3) [19]. They also suggested that compared to UVC/Cl2, UVA/ClO2 AOP produced similar or higher levels of reactive species at similar oxidant dosages, required lower energy input, and formed lower Cl-DBPs.
4.2. Degradation Pathways
The associated radical chemistry of UV photolysis of ClO2 is rather complicated, as demonstrated in Equations (23)–(32). Studies have reported that ClO2 has strong absorption bands in the near-ultraviolet region, and photoexcitation of ClO2 can lead to the breaking of the O−ClO bond by two active product channels. ClO• and oxygen atoms (O(3P)) [18] or excited state oxygen (1O2) [56] were proposed as the primary photo-fragments formed through the O−ClO bond breakage (Equation (23)). As for another channel, Cl• and dissolved oxygen (O2) were also observed from ClO2 photolysis (Equation (24)) [27]. The generated product radicals ClO•, O(3P)/1O2, and Cl• from ClO2 photolysis can undergo distinct chain reactions to generate secondary reactive species. ClO• reacts rapidly with H2O/HO− to yield free chlorine (HOCl/OCl−) (Equations (25) and (26)). O(3P) reacts rapidly with O2 to produce ozone (O3) (Equation (27)). The reactions of Cl• with Cl−, H2O/HO−, or HOCl/OCl− can form dichlorine radical anions (Cl2•−) (Equation (28)), HO• (Equations (29) and (30)), or ClO• (Equations (31) and (32)) [18].
ClO2 + hv → ClO• + O(3P) / 2ClO2 + hv → 2ClO• + 1O2
ClO2 + hv → ClOO → Cl• + O2
2ClO• + H2O → HOCl + HClO2 k = 2.5 × 109 M−1 s−1
2ClO• + HO− → OCl− + HClO2 k = 2.5 × 109 M−1 s−1
O(3P) + O2 → O3 k = 4 × 109 M−1 s−1
Cl• + Cl− → Cl2•− k = 8.5 × 109 M−1 s−1
Cl• + HO− → ClOH•− k = 1.8 × 1010 M−1 s−1
ClOH•− → HO• + Cl− k = 6.1 × 109 M−1 s−1
Cl• + HOCl → ClO• + H+ + Cl− k = 3.0 × 109 M−1 s−1
Cl• + OCl− → ClO• + Cl− k = 8.3 × 109 M−1 s−1
The degradation pathways of micropollutants in UV-activated ClO2 oxidation include direct ClO2 oxidation as discussed in Section 3.3, direct UV photolysis, ozonation, •OH-involved reactions, and RCS-involved reactions (i.e., Cl•, ClO• and Cl2•−) (Figure 1). Kong et al. [18] investigated the degradation pathways of four micropollutants of trimethoprim, iopromide, caffeine, and ciprofloxacin, with diverse chemical characteristics (i.e., caffeine bears electron-deficient moieties; trimethoprim and ciprofloxacin bear electron-rich moieties; iopromide bears photolabile moieties), during UVC/ClO2. The degradation of caffeine was mainly caused by Cl• (66.5%) and •OH (33.5%), whereas the degradation of trimethoprim, iopromide, and ciprofloxacin were mainly contributed by ClO2 oxidation (72.2%), UVC photolysis (87.1%), in situ formed free chlorine (84.3%), respectively (Table 4). The degradation of flumequine in UVC/ClO2 was contributed by 11.37% UV photolysis, 14.72% 1O2, 19.79% •OH, and 54.12% RCS (i.e., Cl•, ClO• and Cl2•−) (Table 4) [56]. The reaction pathways for the major species in UVA/ClO2 AOP was recently well-summarized by Chuang et al. [27], which generates secondary reactive species such as •OH, Cl2•−, and O3 with relatively high and stable concentrations. Additionally, the contribution of reactive species on the removal of 19 micropollutants followed an order of O3 > ClO• > HO• > Cl• and their concentrations were ∼10−7, ∼10−13, ∼10−14, and ∼10−15 M, respectively, in UVA/ClO2 at a ClO2 dosage of 5 mg L−1 and a UV fluence of 47.5 mJ cm−2 (Table 4) [19].

Table 4.
The contribution of reactive species for micropollutant abatement in UV/ClO2 process.
5. Research Gap and Future Research
Compared to UVC/ClO2 AOP, UVA/ClO2 AOP is practically promising for micropollutant abatement due to high absorption coefficients of ClO2 in the UVA range, reduced photon absorption by the background matrix components, and high energy efficiency of UVA-LEDs. However, up until now, reports regarding degradation efficiency, degradation products, degradation pathways, reactive species, influencing factors (e.g., ClO2 concentration, UV intensity, pH, and water matrices), and DBP formation are still limited during micropollutant abatement by UVA/ClO2. Although the formation of halogenated DBPs during ClO2-based oxidation is limited, inorganic products (i.e., ClO2−, ClO3−, and Cl−) might be a new concern when this technology is used in micropollutant abatement in water. Studies reported that the inorganic products were comprised of 50 to 70% of ClO2− and 30 to 50% of ClO3− and Cl− in direct ClO2 oxidation. However, research regarding the yield of ClO2−, ClO3−, and Cl− in activated ClO2 oxidation and influencing factors (e.g., ClO2 dosage, pH, activation methods, and water matrices) on inorganic products in both direct ClO2 oxidation and activated ClO2 oxidation remain unclear. Recently, studies have reported that carbamazepine and norfloxacin were degraded by •OH and RCS generated from a novel UVC/ClO2− system, providing a possibility of “killing two birds with one stone”: eliminating both ClO2− residue and micropollutants. However, up until now, degradation efficiency, degradation products, degradation pathways, influencing factors (e.g., ClO2− concentration, UV intensity, pH, and water matrixes), reactive species, formation of organic or inorganic DBPs, and ClO2−/ClO2 dynamic interchange are still limited.
Author Contributions
Writing—original draft preparation, X.M.; writing—review and editing, X.M., H.C., X.H. and R.C.; validation, X.M. and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the National Natural Science Foundation of China (52100200), the United States Department of Agriculture-National Institute of Food and Agriculture (USDA-NIFA, 2020-67019-31022, 2021-67019-33682, and 2022-67019-37177), Gansu Provincial Youth Science and Technology Planning Project (20JR10RA109) and the Scientific Research Ability Promotion plan of Young Teachers from Northwest Normal University (NWNU-LKQN2019-31).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lado Ribeiro, A.R.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of water matrix on the removal of micropollutants by advanced oxidation technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef] [Green Version]
- Gorito, A.M.; Pesqueira, J.F.J.R.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Nunes, O.C.; Almeida, C.M.R.; Silva, A.M.T. Ozone-based water treatment (O3, O3/UV, O3/H2O2) for removal of organic micropollutants, bacteria inactivation and regrowth prevention. J. Environ. Chem. Eng. 2021, 9, 105315. [Google Scholar] [CrossRef]
- Borrull, J.; Colom, A.; Fabregas, J.; Borrull, F.; Pocurull, E. Presence, behaviour and removal of selected organic micropollutants through drinking water treatment. Chemosphere 2021, 276, 130023. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Kadrispahic, H.; Koustrup Jørgensen, M.; Brøndum Berg, S.; Thornberg, D.; Mielczarek, A.T.; Bester, K. Removal of micropollutants in a ceramic membrane bioreactor for the post-treatment of municipal wastewater. Chem. Eng. J. 2022, 427, 131458. [Google Scholar] [CrossRef]
- Xu, L.; Wu, C.; Chai, C.; Cao, S.; Bai, X.; Ma, K.; Jin, X.; Shi, X.; Jin, P. Adsorption of micropollutants from wastewater using iron and nitrogen co-doped biochar: Performance, kinetics and mechanism studies. J. Hazard. Mater. 2022, 424, 127606. [Google Scholar] [CrossRef]
- Kiejza, D.; Kotowska, U.; Polińska, W.; Karpińska, J. Peracids—New oxidants in advanced oxidation processes: The use of peracetic acid, peroxymonosulfate, and persulfate salts in the removal of organic micropollutants of emerging concern—A review. Sci. Total Environ. 2021, 790, 148195. [Google Scholar] [CrossRef]
- Mukherjee, A.; Mullick, A.; Moulik, S.; Roy, A. Oxidative degradation of emerging micropollutants induced by rotational hydrodynamic cavitating device: A case study with ciprofloxacin. J. Environ. Chem. Eng. 2021, 9, 105652. [Google Scholar] [CrossRef]
- Tagliavini, M.; Schäfer, A.I. Removal of steroid micropollutants by polymer-based spherical activated carbon (PBSAC) assisted membrane filtration. J. Hazard. Mater. 2018, 353, 514–521. [Google Scholar] [CrossRef]
- Ao, X.W.; Eloranta, J.; Huang, C.H.; Santoro, D.; Sun, W.J.; Lu, Z.D.; Li, C. Peracetic acid-based advanced oxidation processes for decontamination and disinfection of water: A review. Water Res. 2021, 188, 116479. [Google Scholar] [CrossRef]
- Malvestiti, J.A.; Cruz-Alcalde, A.; López-Vinent, N.; Dantas, R.F.; Sans, C. Catalytic ozonation by metal ions for municipal wastewater disinfection and simulataneous micropollutants removal. Appl. Catal. B-Environ. 2019, 259, 118104. [Google Scholar] [CrossRef]
- Mei, L.; Shilong, T.; Jin, S.; Xizhuo, W.; Jianxin, C.; Shouqiang, L.; Xia, G.; Jiachun, T. Effects of chlorine dioxide on morphology and ultrastructure of Fusarium sulphureum and its virulence to potato tubers. Int. J. Agric. Biol. Eng. 2017, 10, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Zhang, X.; Li, W.; Jiang, J. Low chlorine impurity might be beneficial in chlorine dioxide disinfection. Water Res. 2021, 188, 116520. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y. Roles of Sulfites in Reverse Osmosis (RO) Plants and Adverse Effects in RO Operation. Membranes 2022, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Rougé, V.; Allard, S.; Croué, J.P.; von Gunten, U. In-situ formation of free chlorine during ClO2 treatment: Implications on the formation of disinfection by-products. Environ. Sci. Technol. 2018, 52, 13421–13429. [Google Scholar] [CrossRef]
- Huber, M.M.; Korhonen, S.; Ternes, T.A.; von Gunten, U. Oxidation of pharmaceuticals during water treatment with chlorine dioxide. Water Res. 2005, 39, 3607–3617. [Google Scholar] [CrossRef]
- Pergal, M.V.; Kodranov, I.D.; Dojcinovic, B.; Avdin, V.V.; Stankovic, D.M.; Petkovic, B.B.; Manojlovic, D.D. Evaluation of azamethiphos and dimethoate degradation using chlorine dioxide during water treatment. Environ. Sci. Pollut. Res. Int. 2020, 27, 27147–27160. [Google Scholar] [CrossRef]
- Gan, W.H.; Ge, Y.X.; Zhong, Y.; Yang, X. The reactions of chlorine dioxide with inorganic and organic compounds in water treatment: Kinetics and mechanisms. Environ. Sci. Water Res. 2020, 6, 2287–2312. [Google Scholar] [CrossRef]
- Kong, Q.; Fan, M.; Yin, R.; Zhang, X.; Lei, Y.; Shang, C.; Yang, X. Micropollutant abatement and byproduct formation during the co-exposure of chlorine dioxide (ClO2) and UVC radiation. J. Hazard. Mater. 2021, 419, 126424. [Google Scholar] [CrossRef]
- Peng, J.; Yin, R.; Yang, X.; Shang, C. A Novel UVA/ClO2 Advanced Oxidation Process for the Degradation of Micropollutants in Water. Environ. Sci. Technol. 2022, 56, 1257–1266. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, X.; Shu, L.; Yang, X. Kinetics and Mechanisms of Virus Inactivation by Chlorine Dioxide in Water Treatment: A Review. Bull. Environ. Contam. Toxicol. 2021, 106, 560–567. [Google Scholar] [CrossRef]
- Singh, S.; Maji, P.K.; Lee, Y.S.; Gaikwad, K.K. Applications of gaseous chlorine dioxide for antimicrobial food packaging: A review. Environ. Chem. Lett. 2020, 19, 253–270. [Google Scholar] [CrossRef]
- Tofanelli, M.; Capriotti, V.; Saraniti, C.; Marcuzzo, A.V.; Boscolo-Rizzo, P.; Tirelli, G. Disposable chlorine dioxide wipes for high-level disinfection in the ENT department: A systematic review. Am. J. Otolaryngol. 2020, 41, 102415. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Baldwin, E.; Bai, J. Applications of gaseous chlorine dioxide on postharvest handling and storage of fruits and vegetables—A review. Food Control 2019, 95, 18–26. [Google Scholar] [CrossRef]
- McCarthy, W.P.; O’Callaghan, T.F.; Danahar, M.; Gleeson, D.; O’Connor, C.; Fenelon, M.A.; Tobin, J.T. Chlorate and Other Oxychlorine Contaminants Within the Dairy Supply Chain. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1561–1575. [Google Scholar] [CrossRef] [Green Version]
- Kessler, S.J. Shelf Life Extension of Fresh Strawberries Packaged in Clamshells with Chlorine Dioxide Generating Sachets. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2020. [Google Scholar]
- Champ, T.B.; Jang, J.H.; Lee, J.L.; Wu, G.; Reynolds, M.A.; Abu-Omar, M.M. Lignin-Derived Non-Heme Iron and Manganese Complexes: Catalysts for the On-Demand Production of Chlorine Dioxide in Water under Mild Conditions. Inorg. Chem. 2021, 60, 2905–2913. [Google Scholar] [CrossRef]
- Chuang, Y.H.; Wu, K.L.; Lin, W.C.; Shi, H.J. Photolysis of Chlorine Dioxide under UVA Irradiation: Radical Formation, Application in Treating Micropollutants, Formation of Disinfection Byproducts, and Toxicity under Scenarios Relevant to Potable Reuse and Drinking Water. Environ. Sci. Technol. 2022, 56, 2593–2604. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Sadeghi, K.; Seo, J. Controlled self-release of ClO2 as an encapsulated antimicrobial agent for smart packaging. Innov. Food Sci. Emerg. 2021, 74, 102802. [Google Scholar] [CrossRef]
- Trinh, V.M.; Yuan, M.H.; Chen, Y.H.; Wu, C.Y.; Kang, S.C.; Chiang, P.C.; Hsiao, T.C.; Huang, H.P.; Zhao, Y.L.; Lin, J.F.; et al. Chlorine dioxide gas generation using rotating packed bed for air disinfection in a hospital. J. Clean. Prod. 2021, 320, 128885. [Google Scholar] [CrossRef]
- Mao, Q.; Li, Q.; Li, H.; Yuan, S.; Zhang, J. Oxidative paraben removal with chlorine dioxide: Reaction kinetics and mechanism. Sep. Purif. Technol. 2020, 237, 116327. [Google Scholar] [CrossRef]
- Hupperich, K.; Mutke, X.A.M.; Abdighahroudi, M.S.; Jütte, M.; Schmidt, T.C.; Lutze, H.V. Reaction of chlorine dioxide with organic matter—Formation of inorganic products. Environ. Sci. Water Res. 2020, 6, 2597–2606. [Google Scholar] [CrossRef]
- He, G.; Zhang, T.; Li, Y.; Li, J.; Chen, F.; Hu, J.; Dong, F. Comparison of fleroxacin oxidation by chlorine and chlorine dioxide: Kinetics, mechanism and halogenated DBPs formation. Chemosphere 2022, 286, 131585. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Xie, Y.; Ni, T.; Liu, G. Oxidative removal of diclofenac by chlorine dioxide: Reaction kinetics and mechanism. Chem. Eng. J. 2015, 279, 409–415. [Google Scholar] [CrossRef]
- Wang, P.; He, Y.L.; Huang, C.H. Oxidation of fluoroquinolone antibiotics and structurally related amines by chlorine dioxide: Reaction kinetics, product and pathway evaluation. Water Res. 2010, 44, 5989–5998. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; He, Y.L.; Huang, C.H. Reactions of tetracycline antibiotics with chlorine dioxide and free chlorine. Water Res. 2011, 45, 1838–1846. [Google Scholar] [CrossRef]
- Ben, W.W.; Shi, Y.W.; Li, W.W.; Zhang, Y.; Qiang, Z.M. Oxidation of sulfonamide antibiotics by chlorine dioxide in water: Kinetics and reaction pathways. Chem. Eng. J. 2017, 327, 743–750. [Google Scholar] [CrossRef]
- Lee, Y.; von Gunten, U. Quantitative structure-activity relationships (QSARs) for the transformation of organic micropollutants during oxidative water treatment. Water Res. 2012, 46, 6177–6195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.S.; Yu, J.W.; Chen, W.Z.; Ma, X.Y.; Li, G.X.; Chen, G.Y.; Deng, J. Degradation of triclosan by chlorine dioxide: Reaction mechanism,2,4-dichlorophenol accumulation and toxicity evaluation. Chemosphere 2018, 207, 449–456. [Google Scholar] [CrossRef]
- Jia, X.H.; Feng, L.; Liu, Y.Z.; Zhang, L.Q. Oxidation of antipyrine by chlorine dioxide: Reaction kinetics and degradation pathway. Chem. Eng. J. 2017, 309, 646–654. [Google Scholar] [CrossRef]
- Jia, X.H.; Feng, L.; Liu, Y.Z.; Zhang, L.Q. Degradation behaviors and genetic toxicity variations of pyrazolone pharmaceuticals during chlorine dioxide disinfection process. Chem. Eng. J. 2018, 345, 156–164. [Google Scholar] [CrossRef]
- Lee, Y.; von Gunten, U. Oxidative transformation of micropollutants during municipal wastewater treatment: Comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrate VI, and ozone) and non-selective oxidants (hydroxyl radical). Water Res. 2010, 44, 555–566. [Google Scholar] [CrossRef]
- Terhalle, J.; Kaiser, P.; Jutte, M.; Buss, J.; Yasar, S.; Marks, R.; Uhlmann, H.; Schmidt, T.C.; Lutze, H.V. Chlorine dioxide—Pollutant transformation and formation of hypochlorous acid as a secondary oxidant. Environ. Sci. Technol. 2018, 52, 9964–9971. [Google Scholar] [CrossRef]
- He, G.L.; Zhang, T.Q.; Zheng, F.F.; Li, C.; Zhang, Q.Z.; Dong, F.L.; Huang, Y. Reaction of fleroxacin with chlorine and chlorine dioxide in drinking water distribution systems: Kinetics, transformation mechanisms and toxicity evaluations. Chem. Eng. J. 2019, 374, 1191–1203. [Google Scholar] [CrossRef]
- He, G.; Zhang, T.; Zhang, Q.; Dong, F.; Wang, Y. Characterization of enoxacin (ENO) during ClO2 disinfection in water distribution system: Kinetics, byproducts, toxicity evaluation and halogenated disinfection byproducts (DBPs) formation potential. Chemosphere 2021, 283, 131251. [Google Scholar] [CrossRef] [PubMed]
- Navalon, S.; Alvaro, M.; Garcia, H. Reaction of chlorine dioxide with emergent water pollutants: Product study of the reaction of three beta-lactam antibiotics with ClO2. Water Res. 2008, 42, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.X.; Xu, B.; Zhang, T.Y.; Gao, N.Y. Degradation of phenylurea herbicides by chlorine dioxide and formation of disinfection by-products during subsequent chlor(am)ination. Chem. Eng. J. 2014, 258, 210–217. [Google Scholar] [CrossRef]
- Lopez, A.; Mascolo, G.; Tiravanti, G.; Passino, R. Degradation of herbicides (ametryn and isoproturon) during water disinfection by means of two oxidants (hypochlorite and chlorine dioxide). Water Sci. Technol. 1997, 35, 129–136. [Google Scholar] [CrossRef]
- Pergal, M.V.; Kodranov, I.D.; Pergal, M.M.; Dojčinović, B.P.; Stanković, D.M.; Petković, B.B.; Manojlović, D.D. Assessment of Degradation of Sulfonylurea Herbicides in Water by Chlorine Dioxide. Water Air Soil Poll. 2018, 229, 287. [Google Scholar] [CrossRef]
- Tian, F.; Qiang, Z.; Liu, C.; Zhang, T.; Dong, B. Kinetics and mechanism for methiocarb degradation by chlorine dioxide in aqueous solution. Chemosphere 2010, 79, 646–651. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Liu, G.; Xie, Y. Oxidation of diclofenac by aqueous chlorine dioxide: Identification of major disinfection byproducts and toxicity evaluation. Sci. Total Environ. 2014, 473–474, 437–445. [Google Scholar] [CrossRef]
- Lv, J.; Ou, C.; Fu, M.; Xu, Z. Characteristics and transformation pathways of venlafaxine degradation during disinfection processes using free chlorine and chlorine dioxide. Chemosphere 2021, 276, 130147. [Google Scholar] [CrossRef]
- Benitez, F.J.; Beltran-Heredia, J.; Gonzalez, T.; Lara, P. Oxidation of Fenuron by chlorine dioxide. J. Environ. Sci. Health A 1992, 27, 643–662. [Google Scholar] [CrossRef]
- Ali, O.A.; Tarek, S.J. Removal of polycyclic aromatic hydrocarbons from Ismailia Canal water by chlorine, chlorine dioxide and ozone. Desalin. Water Treat. 2009, 1, 289–298. [Google Scholar] [CrossRef]
- Hey, G.; Grabic, R.; Ledin, A.; la Cour Jansen, J.; Andersen, H.R. Oxidation of pharmaceuticals by chlorine dioxide in biologically treated wastewater. Chem. Eng. J. 2012, 185–186, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.S.; Cai, H.W.; Li, G.X.; Chen, G.Y.; Ma, X.Y.; He, W.L. Degradation behavior of triclosan by co-exposure to chlorine dioxide and UV irradiation: Influencing factors and toxicity changes. Environ. Sci. Pollut. Res. Int. 2018, 25, 9391–9401. [Google Scholar] [CrossRef]
- Ye, W.K.; Tian, F.X.; Xu, B.; Zhao, D.S.; Ye, J.; Wang, B.; Lai, F.; Tan, Y.J.; Hu, X.J. Insights into the enhanced degradation of flumequine by UV/ClO2 integrated process: Kinetics, mechanisms and DBPs-related toxicity in post-disinfection. Sep. Purif. Technol. 2022, 280, 119846. [Google Scholar] [CrossRef]
- Tian, F.X.; Ye, W.K.; Xu, B.; Hu, X.J.; Ma, S.X.; Lai, F.; Gao, Y.Q.; Xing, H.B.; Xia, W.H.; Wang, B. Comparison of UV-induced AOPs (UV/Cl2, UV/NH2Cl, UV/ClO2 and UV/H2O2 ) in the degradation of iopamidol: Kinetics, energy requirements and DBPs-related toxicity in sequential disinfection processes. Chem. Eng. J. 2020, 398, 125570. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Bu, L.; Zhu, S.; Zhang, W.; Zhou, S.; Gao, N. Simultaneous removal of chlorite and contaminants of emerging concern under UV photolysis: Hydroxyl radicals vs. chlorate formation. Water Res. 2021, 190, 116708. [Google Scholar] [CrossRef]
- Su, R.; Huang, L.; Li, N.; Li, L.; Jin, B.; Zhou, W.; Gao, B.; Yue, Q.; Li, Q. Chlorine dioxide radicals triggered by chlorite under visible-light irradiation for enhanced degradation and detoxification of norfloxacin antibiotic: Radical mechanism and toxicity evaluation. Chem. Eng. J. 2021, 414, 128768. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).