Mitochondrial Lineage Diversity and Phylogeography of Daphnia (Daphnia) (Crustacea: Cladocera) in North-East Russia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethic Statement
2.2. Sampling and Used Material
2.3. DNA Sequencing
2.4. Phylogenetic Analyses
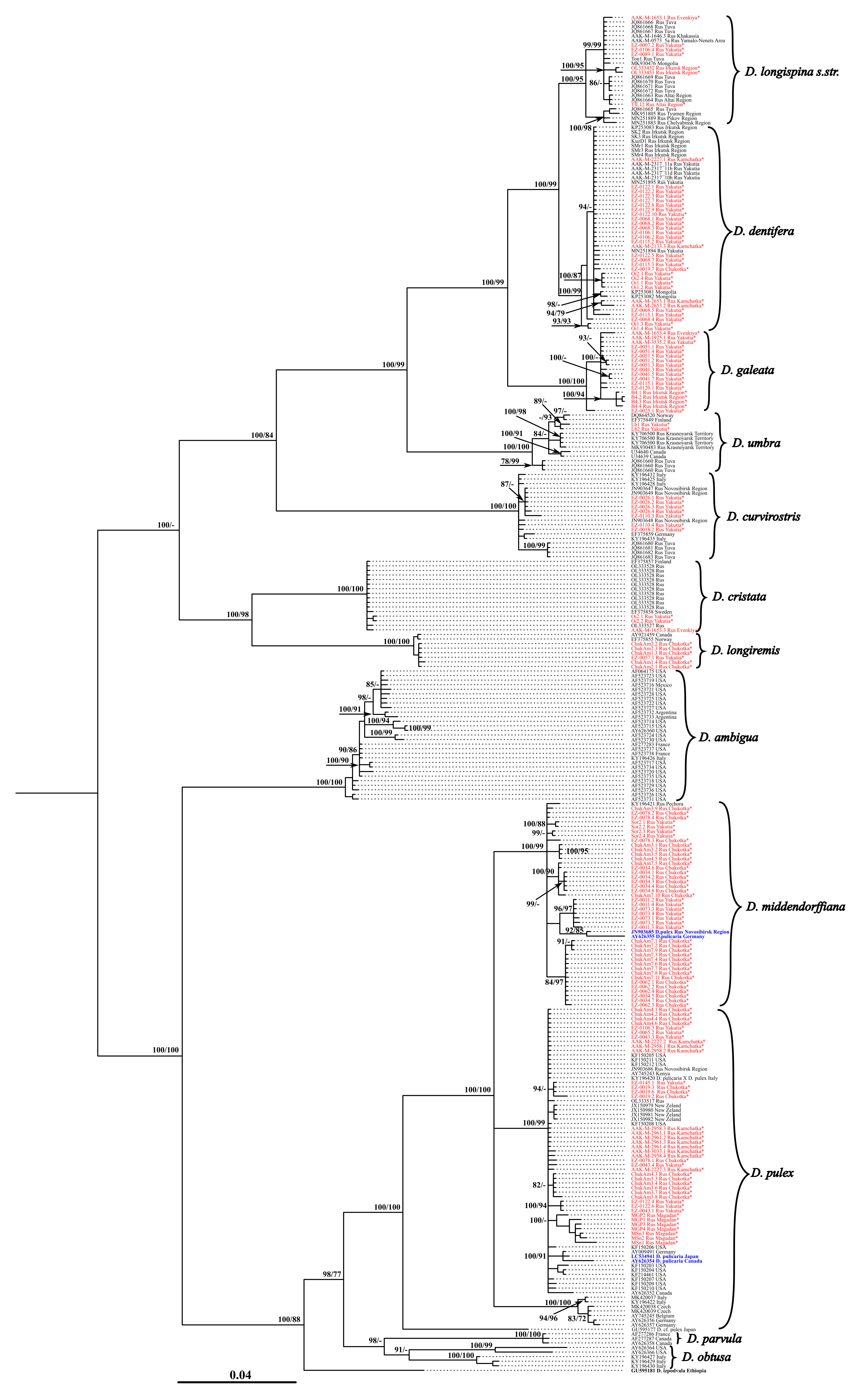
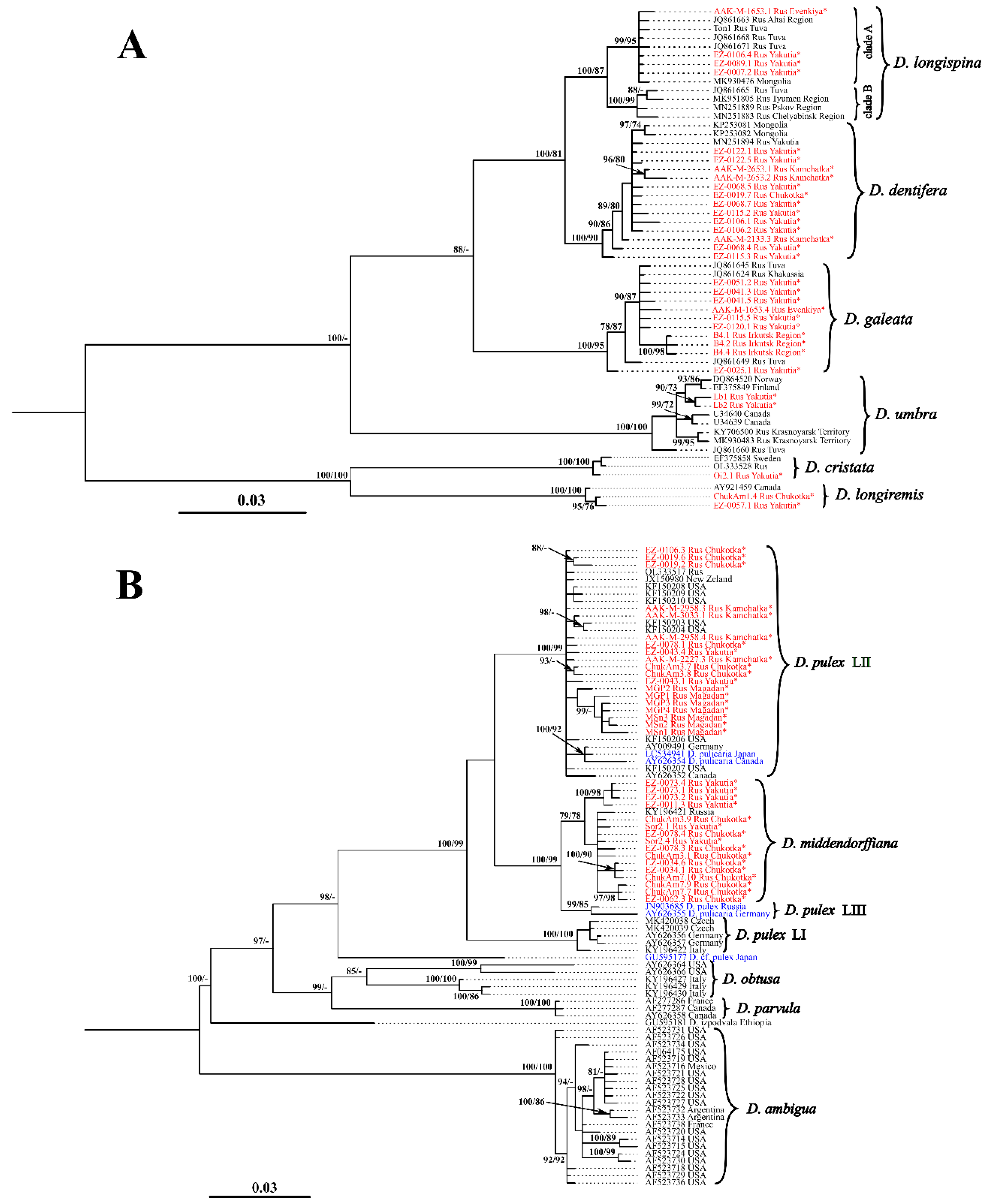
3. Results
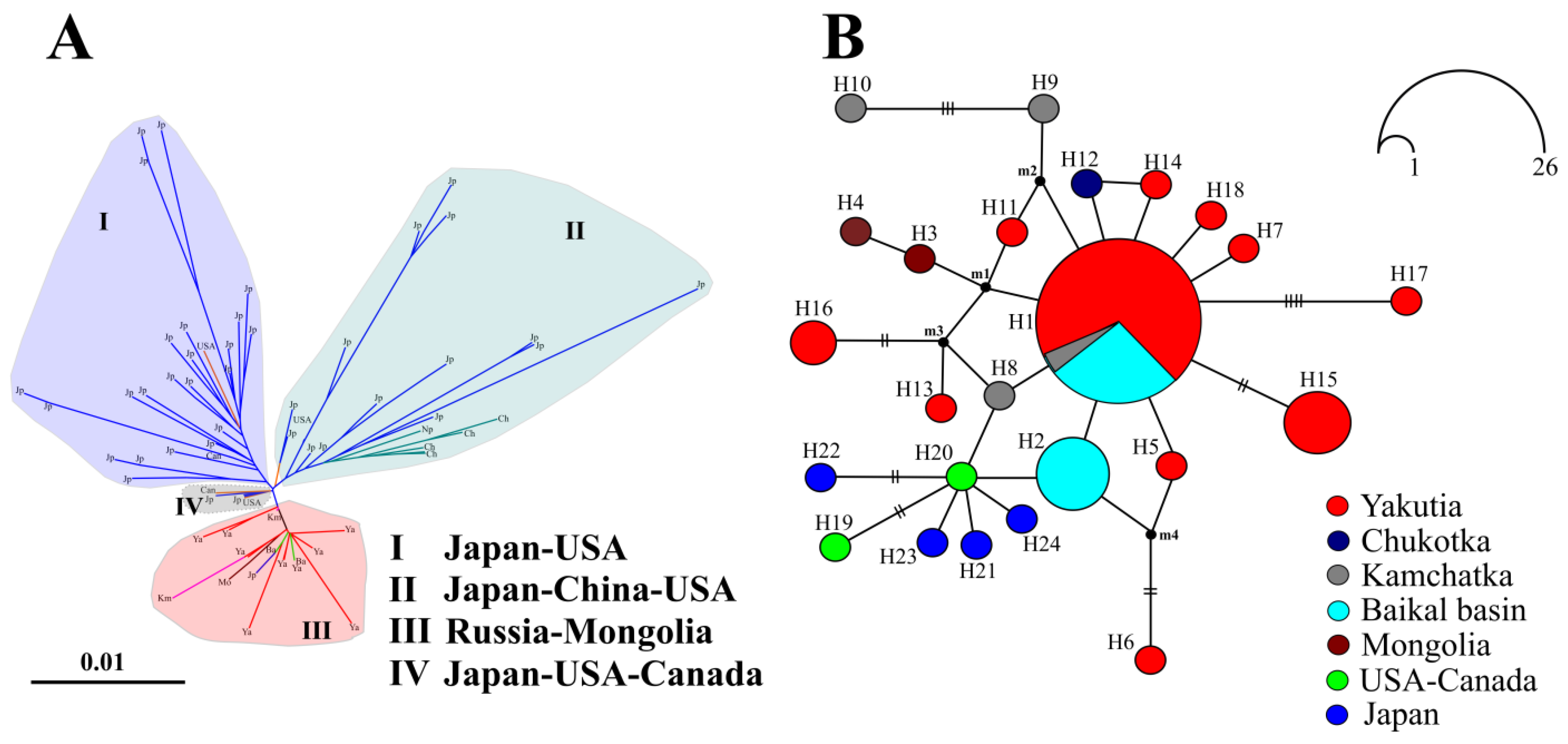
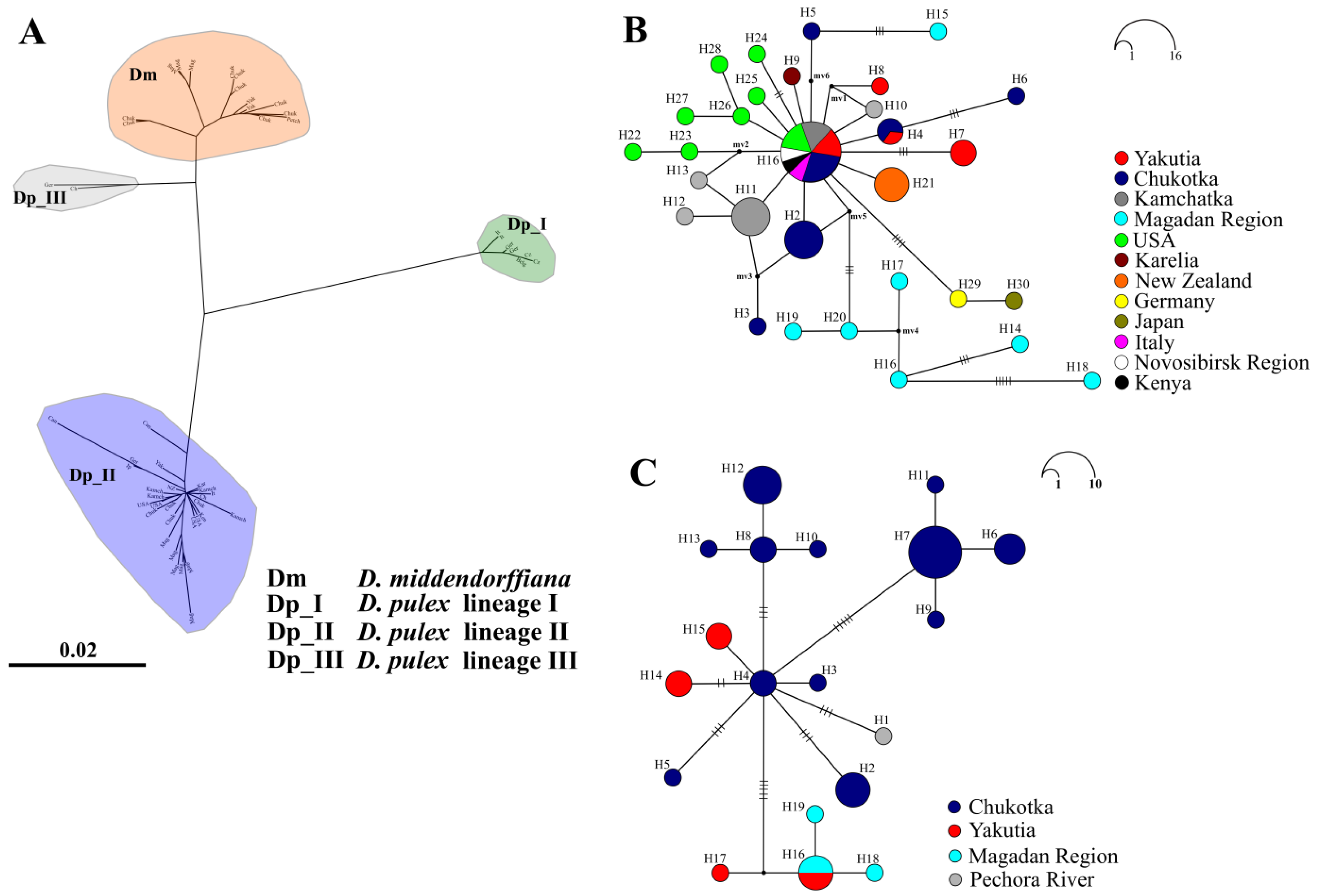
3.1. Mitochondrial Phylogeny and Haplotypes Distribution
- (1)
- D. longispina O.F. Müller, 1776 s.str.;
- (2)
- D. dentifera Forbes, 1893;
- (3)
- D. galeata Sars, 1863;
- (4)
- D. umbra Taylor, Hebert & Colbourne, 1996;
- (5)
- D. curvirostris Eylmann, 1887;
- (6)
- D. cristata Sars, 1862;
- (7)
- D. longiremis Sars, 1862;
- (8)
- D. middendorffiana Fischer, 1851;
- (9)
- D. pulex Leydig, 1860 s.l.
3.2. Mitochondrial Polymorphism, Evolutionary Distances and Neutrality Tests
4. Discussion
4.1. Comments of Revealed Species and Phylogroups
4.2. Demographic History: Preliminary Data on Mitochondrial DNA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Harrison, I.; Abell, R.; Darwall, W.; Thieme, M.L.; Tickner, D.; Timboe, I. The freshwater biodiversity crisis. Science 2018, 362, 1369. [Google Scholar] [CrossRef] [PubMed]
- Maasri, A.; Jähnig, S.C.; Adamescu, M.C.; Adrian, R.; Baigun, C.; Baird, D.J.; Batista-Morales, A.; Bonada, N.; Brown, L.E.; Cai, Q.; et al. A global agenda for advancing freshwater biodiversity research. Ecol. Lett. 2022, 25, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Vinarski, M.V.; Bolotov, I.N.; Aksenova, O.V.; Babushkin, E.S.; Bespalaya, Y.V.; Makhrov, A.A.; Nekhaev, I.O.; Vikhrev, I.V. Freshwater Mollusca of the Circumpolar Arctic: A review on their taxonomy, diversity and biogeography. Hydrobiologia 2021, 848, 2891–2918. [Google Scholar] [CrossRef]
- Goedkoop, W.; Culp, J.M.; Christensen, T.; Christoffersen, K.S.; Fefilova, E.; Guðbergsson, G.; Lárusson, K.F.; Liljaniemi, P.; Novichkova, A.A.; Ólafsson, J.S.; et al. Improving the framework for assessment of ecological change in the Arctic: A circumpolar synthesis of freshwater biodiversity. Freshw. Biol. 2022, 67, 210–223. [Google Scholar] [CrossRef]
- Lento, J.; Goedkoop, W.; Culp, J.; Christoffersen, K.S.; Lárusson, K.F.; Fefilova, E.; Guðbergsson, G.; Liljaniemi, P.; ÓLafsson, J.S.; Sandøy, S.; et al. State of the Arctic Freshwater Biodiversity; Consvervation of Arctic Flora and Fauna: Akureyri, Iceland, 2019; ISBN 978-9935-431-77-6. [Google Scholar]
- Hultén, E. Flora of the Aleutian Islands and Westernmost Alaska Peninsula with Notes on the Flora of Commander Islands; Bokforlags Aktiebolaget Thule: Stockholm, Sweden, 1937. [Google Scholar]
- Hopkins, D.M.; Matthews, J.V.; Schweger, C.E.; Young, S.V. (Eds.) Paleoecology of Beringia; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Stamford, M.D.; Taylor, E.B. Phylogeographical lineages of Arctic grayling (Thymallus arcticus) in North America: Divergence, origins and affinities with Eurasian Thymallus. Mol. Ecol. 2004, 13, 1533–1549. [Google Scholar] [CrossRef]
- Campbell, M.A.; Lopéz, J.A. Mitochondrial phylogeography of a Beringian relict: The endemic freshwater genus of blackfish Dallia (Esociformes). J. Fish Biol. 2014, 84, 523–538. [Google Scholar] [CrossRef]
- Cox, A.J.; Hebert, P.D. Colonization, extinction, and phylogeographic patterning in a freshwater crustacean. Mol. Ecol. 2001, 10, 371–386. [Google Scholar] [CrossRef]
- Provan, J.; Bennett, K.D. Phylogeographic insights into cryptic glacial refugia. Trends Ecol. Evol. 2008, 23, 564–571. [Google Scholar] [CrossRef]
- Dumont, H.J.; Negrea, Ș. Branchiopoda; Backhuys: Leiden, The Netherlands, 2002; ISBN 9789057821127. [Google Scholar]
- Sobakina, I.G.; Sokolova, B.A.; Solomonov, N.M. Modern structure of zooplankton in deltas of Lena river during the autumn period. Izv. Samar. Nauchnogo Cent. Ross. Akad. Nauk. 2009, 11, 347–349. [Google Scholar]
- Streletskaya, E.A. Review of the fauna of Rotatoria, Cladocera, and Copepoda of the basin of the Anadyr’ River. Contemp. Probl. Ecol. 2010, 3, 469–480. [Google Scholar] [CrossRef]
- Sobakina, I.G. Zooplankton of lower Yana River. Ekol. Ross. Na Puti K Innov. 2013, 7, 103–106. [Google Scholar]
- Klimovsky, A.I.; Bekker, E.I.; Korovchinsky, N.M.; Kotov, A. Cladocera (Crustacea, Branchiopoda) of Central Yakutia 1. Some representatives of the families Sididae, Daphniidae and Ophryoxidae. Zool. Zhurnal 2015, 94, 882–898. [Google Scholar] [CrossRef]
- Klimovsky, A.I.; Bekker, E.I.; Sinev, A.Y.; Korovchinsky, N.M.; Smirnov, N.N.; Kotov, A. Cladocera (Crustacea, Branchiopoda) of Central Yakutia. 4. Taxonomical-faunistic and zoogeographical analysis. Zool. Zhurnal 2015, 94, 1367–1378. [Google Scholar]
- Klimovsky, A.I.; Kotov, A.A. Cladocera (Crustacea, Branchiopoda) of Central Yakutia 3. Taxa from the Chydorus sphaericus s.l. species group (Anomopoda, Chydoridae). Zool. Zhurnal 2015, 94, 1257–1267. [Google Scholar]
- Klimovsky, A.I.; Sinev, A.Y.; Bekker, E.I.; Kotov, A.A. Cladocera (Crustacea, Branchiopoda) of Central Yakutia 2. Some representatives of the families Bosminidae, Eurycercidae and Chydoridae. Zool. Zhurnal 2015, 94, 1009–1022. [Google Scholar]
- Novichkova, A.A.; Chertoprud, E.S. The freshwater crustaceans (Cladocera: Copepoda) of Bering Island (Commander Islands, Russian Far East): Species richness and taxocene structure. J. Nat. Hist. 2016, 50, 1357–1368. [Google Scholar] [CrossRef]
- Novichkova, A.A.; Azovsky, A.I. Factors affecting regional diversity and distribution of freshwater microcrustaceans (Cladocera, Copepoda) at high latitudes. Polar Biol. 2017, 40, 185–198. [Google Scholar] [CrossRef]
- Trofimova, T.P.; Zhirkov, I.I.; Zhirkov, K.I.; Sobakina, I.G.; Ivanov, K.P. Recent state of the lakes of the Yana River basin. Vest. North-East. Fed. Univ. Earth Sci. 2018, 2, 32–40. [Google Scholar]
- Chertoprud, E.S.; Novichkova, A.A. Crustaceans in the meiobenthos and plankton of the thermokarst lakes and polygonal ponds in the Lena River Delta (Northern Yakutia, Russia): Species composition and factors regulating assemblage structures. Water 2021, 13, 1936. [Google Scholar] [CrossRef]
- Kotov, A.A.; Karabanov, D.P.; Bekker, E.I.; Neretina, T.V.; Taylor, D.J. Phylogeography of the Chydorus sphaericus Group (Cladocera: Chydoridae) in the Northern Palearctic. PLoS ONE 2016, 11, e0168711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotov, A.A.; Garibian, P.G.; Bekker, E.I.; Taylor, D.J.; Karabanov, D.P. A new species group from the Daphnia curvirostris species complex (Cladocera: Anomopoda) from the eastern Palaearctic: Taxonomy, phylogeny and phylogeography. Zool. J. Linn. Soc. 2021, 191, 772–822. [Google Scholar] [CrossRef]
- Bekker, E.I.; Karabanov, D.P.; Galimov, Y.R.; Haag, C.R.; Neretina, T.V.; Kotov, A.A. Phylogeography of Daphnia magna Straus (Crustacea: Cladocera) in Northern Eurasia: Evidence for a deep longitudinal split between mitochondrial lineages. PLoS ONE 2018, 13, e0194045. [Google Scholar] [CrossRef] [PubMed]
- Bekker, E.I.; Kotov, A.A.; Taylor, D.J. A revision of the subgenus Eurycercus (Eurycercus) Baird, 1843 emend. nov. (Cladocera: Eurycercidae) in the Holarctic with the description of a new species from Alaska. Zootaxa 2012, 3206, 1–40. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Hebert, P.D.N.; Kotov, A.A.; Cristescu, M.E. The noncosmopolitanism paradigm of freshwater zooplankton: Insights from the global phylogeography of the predatory cladoceran Polyphemus pediculus (Linnaeus, 1761) (Crustacea, Onychopoda). Mol. Ecol. 2009, 18, 5161–5179. [Google Scholar] [CrossRef]
- Garibian, P.G.; Neretina, A.N.; Taylor, D.J.; Kotov, A.A. Partial revision of the neustonic genus Scapholeberis Schoedler, 1858 (Crustacea: Cladocera): Decoding of the barcoding results. PeerJ 2020, 8, e10410. [Google Scholar] [CrossRef]
- Haney, R.A.; Taylor, D.J. Testing paleolimnological predictions with molecular data: The origins of Holarctic Eubosmina. J. Evol. Biol. 2003, 16, 871–882. [Google Scholar] [CrossRef] [Green Version]
- Belyaeva, M.; Taylor, D.J. Cryptic species within the Chydorus sphaericus species complex (Crustacea: Cladocera) revealed by molecular markers and sexual stage morphology. Mol. Phylogenet. Evol. 2009, 50, 534–546. [Google Scholar] [CrossRef]
- Adamowicz, S.J.; Petrusek, A.; Colbourne, J.K.; Hebert, P.D.N.; Witt, J.D.S. The scale of divergence: A phylogenetic appraisal of intercontinental allopatric speciation in a passively dispersed freshwater zooplankton genus. Mol. Phylogenet. Evol. 2009, 50, 423–436. [Google Scholar] [CrossRef]
- Petrusek, A.; Hobaek, A.; Nilssen, J.P.; Skage, M.; Černý, M.; Brede, N.; Schwenk, K. A taxonomic reappraisal of the European Daphnia longispina complex (Crustacea, Cladocera, Anomopoda). Zool. Scr. 2008, 37, 507–519. [Google Scholar] [CrossRef]
- Schwenk, K.; Posada, D.; Hebert, P.D. Molecular systematics of European Hyalodaphnia: The role of contemporary hybridization in ancient species. Proc. Biol. Sci. 2000, 267, 1833–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, D.J.; Finston, T.L.; Hebert, P.D.N. Biogeography of a widespread freshwater crustacean: Pseudocongruence and cryptic endemism in the North American Daphnia laevis complex. Evolution 1998, 52, 1648–1670. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Taylor, D.J. Quaternary diversification in a sexual Holarctic zooplankter, Daphnia galeata. Mol. Ecol. 2007, 16, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Taylor, D.J. Mature habitats associated with genetic divergence despite strong dispersal ability in an arthropod. BMC Evol. Biol. 2007, 7, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weider, L.J.; Hobaek, A.; Colbourne, J.K.; Crease, T.J.; Dufresne, F.; Hebert, P.D.N. Holarctic phylogeography of an asexual species complex. I. Mitochondrial DNA variation in Arctic Daphnia. Evolution 1999, 53, 777–792. [Google Scholar] [CrossRef]
- Colbourne, J.K.; Crease, T.J.; Weider, L.J.; Hebert, P.D.N.; Duferesne, F.; Hobaek, A. Phylogenetics and evolution of a circumarctic species complex (Cladocera: Daphnia pulex). Biol. J. Linn. Soc. 1998, 65, 347–365. [Google Scholar] [CrossRef]
- Crease, T.J.; Omilian, A.R.; Costanzo, K.S.; Taylor, D.J. Transcontinental phylogeography of the Daphnia pulex species complex. PLoS ONE 2012, 7, e46620. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.J.; Connelly, S.J.; Kotov, A.A. The Intercontinental phylogeography of neustonic daphniids. Sci. Rep. 2020, 10, 1818. [Google Scholar] [CrossRef]
- Korovchinsky, N.M.; Kotov, A.A.; Sinev, A.Y.; Neretina, A.N.; Garibian, P.G. Water Fleas (Crustacea: Cladocera) of North Eurasia. Vol. 2.; KMK Press: Moscow, Russia, 2021. [Google Scholar]
- Zuykova, E.I.; Bochkarev, N.A.; Katokhin, A.V. Identification of the Daphnia species (Crustacea: Cladocera) in the lakes of the Ob and Yenisei River basins: Morphological and molecular phylogenetic approaches. Hydrobiologia 2013, 715, 135–150. [Google Scholar] [CrossRef]
- Zuykova, E.I.; Simonov, E.P.; Bochkarev, N.A.; Taylor, D.J.; Kotov, A.A. Resolution of the Daphnia umbra problem (Crustacea: Cladocera) using an integrated taxonomic approach. Zool. J. Linn. Soc. 2018, 184, 969–998. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Tavaré, S.; Miura, R.M. Lectures on mathematics in the life sciences. Lect. Math. Life Sci. 1986, 17, 57–86. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [Green Version]
- Helaers, R.; Milinkovitch, M.C. MetaPIGA v2.0: Maximum likelihood large phylogeny estimation using the metapopulation genetic algorithm and other stochastic heuristics. BMC Bioinform. 2010, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandelt, H.J.; Forster, P.; Rohl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.X.; Li, W.H. Statistical tests of neutrality of mutations. Genetics 1993, 133, 693–709. [Google Scholar] [CrossRef]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Zuykova, E.I.; Bochkarev, N.A.; Taylor, D.J.; Kotov, A.A. Unexpected endemism in the Daphnia longispina complex (Crustacea: Cladocera) in Southern Siberia. PLoS ONE 2019, 14, e0221527. [Google Scholar] [CrossRef] [Green Version]
- Zuykova, E.I.; Bochkarev, N.A.; Katokhin, A.V. Molecular genetic identification and phylogeny of Daphnia species (Crustacea, Cladocera) from water bodies of Lake Chany basin. Genetika 2013, 49, 235–243. [Google Scholar] [CrossRef]
- Zuykova, E.I.; Bochkarev, N.A.; Kotov, A.A. Specific and Genetic Structure of the Daphnia longispina s. l. Complex (Cladocera, Daphniidae) in Water Bodies of Southern Siberia. Biol. Bull. Russ. Acad. Sci. 2021, 48, 880–891. [Google Scholar] [CrossRef]
- Nilssen, J.P.; Hobæk, A.; Petrusek, A.; Skage, M. Restoring Daphnia lacustris G.O. Sars, 1862 (Crustacea, Anomopoda): A cryptic species in the Daphnia longispina group. Hydrobiologia 2007, 594, 5–17. [Google Scholar] [CrossRef]
- Jeffery, N.W.; Elías-Gutiérrez, M.; Adamowicz, S.J. Species diversity and phylogeographical affinities of the Branchiopoda (Crustacea) of Churchill, Manitoba, Canada. PLoS ONE 2011, 6, e18364. [Google Scholar] [CrossRef] [PubMed]
- Weider, L.J.; Hobæk, A. Postglacial dispersal, glacial refugia, and clonal structure in Russian/Siberian populations of the arctic Daphnia pulex complex. Heredity 1997, 78, 363–372. [Google Scholar] [CrossRef]
- Tsugeki, N.K.; Honjo, M.N.; Kuwae, M. Interspecific variation in ephippial size between Daphnia galeata and D. pulicaria in Lake Biwa, Japan. Limnology 2021, 22, 197–207. [Google Scholar] [CrossRef]
- Taylor, D.J.; Hebert, P.D.; Colbourne, J.K. Phylogenetics and evolution of the Daphnia longispina group (Crustacea) based on 12S rDNA sequence and allozyme variation. Mol. Phylogenet. Evol. 1996, 5, 495–510. [Google Scholar] [CrossRef] [Green Version]
- Penton, E.H.; Crease, T.J. Evolution of the transposable element Pokey in the ribosomal DNA of species in the subgenus Daphnia (Crustacea: Cladocera). Mol. Biol. Evol. 2004, 21, 1727–1739. [Google Scholar] [CrossRef] [Green Version]
- Alonso, M. Crustacea: Branchiopoda, Fauna Iberica; Consejo Superior de Investigaciones Cientificas: Madrid, Spain, 1996; ISBN 978-8400075712. [Google Scholar]
- Novichkova, A.A.; Kotov, A.A.; Chertoprud, E.S. Freshwater crustaceans of Bykovsky Peninsula and neighboring territory (Northern Yakutia, Russia). Arthropoda Sel. 2020, 29, 1–12. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Hann, B.J. Patterns in the composition of Arctic tundra pond microcrustacean communities. Can. J. Fish. Aquat. Sci. 1986, 43, 1416–1425. [Google Scholar] [CrossRef]
- Rautio, M. Community structure of crustacean zooplankton in subarctic ponds—Effects of altitude and physical heterogeneity. Ecography 1998, 21, 327–335. [Google Scholar] [CrossRef]
- Pinel-Alloul, B.; Patoine, A.; Marty, J. Multi-scale and multi-system perspectives of zooplankton structure and function in Canadian freshwaters. Can. J. Fish. Aquat. Sci. 2021, 78, 1543–1562. [Google Scholar] [CrossRef]
- Schartau, A.K.; Mariash, H.L.; Christoffersen, K.S.; Bogan, D.; Dubovskaya, O.P.; Fefilova, E.B.; Hayden, B.; Ingvason, H.R.; Ivanova, E.A.; Kononova, O.N.; et al. First circumpolar assessment of Arctic freshwater phytoplankton and zooplankton diversity: Spatial patterns and environmental factors. Freshw. Biol. 2021, 67, 141–158. [Google Scholar] [CrossRef]
- Dimante-Deimantovica, I.; Walseng, B.; Chertoprud, E.; Novichkova, A. New and previously known species of Copepoda and Cladocera (Crustacea) from Svalbard, Norway—Who are they and where do they come from? Fauna Norv. 2018, 38, 18–29. [Google Scholar] [CrossRef]
- Marková, S.; Maurone, C.; Racchetti, E.; Bartoli, M.; Rossi, V. Daphnia diversity in water bodies of the Po River Basin. J. Limnol. 2017, 76, 261–271. [Google Scholar] [CrossRef] [Green Version]
- Dubovskaya, O.P.; Kotov, A.A.; Korovchinsky, N.M.; Smirnov, N.N.; Sinev, A.Y. Zooplankton of lakes in the spurs of the Putorana Plateau and adjacent territories (North of Krasnoyarsk Krai). Contemp. Probl. Ecol. 2010, 3, 401–434. [Google Scholar] [CrossRef]
- Fefilova, E.B.; Kononova, O.N.; Dubovskaya, O.P.; Khokhlova, L.G. The current state of zooplankton in the lake system of Bol’shezemel’skaya tundra. Inland Water Biol. 2012, 5, 333–341. [Google Scholar] [CrossRef]
- Fefilova, E.; Dubovskaya, O.; Kononova, O.; Frolova, L.; Abramova, E.; Nigamatzyanova, G. Data on taxa composition of freshwater zooplankton and meiobenthos across Arctic regions of Russia. Data Brief 2021, 36, 107112. [Google Scholar] [CrossRef]
- Neretina, A.N.; Gololobova, M.A.; Neplyukhina, A.A.; Zharov, A.A.; Rogers, C.D.; Horne, D.J.; Protopopov, A.V.; Kotov, A.A. Crustacean remains from the Yuka mammoth raise questions about non-analogue freshwater communities in the Beringian region during the Pleistocene. Sci. Rep. 2020, 10, 859. [Google Scholar] [CrossRef] [Green Version]
- Avise, J.C. Phylogeography: The History and Formation of Species; Harvard University Press: Cambridge, MA, USA, 1999; ISBN 978-0674666382. [Google Scholar]
- Ishida, S.; Takahashi, A.; Matsushima, N.; Yokoyama, J.; Makino, W.; Urabe, J.; Kawata, M. The long-term consequences of hybridization between the two Daphnia species, D. galeata and D. dentifera, in mature habitats. BMC Evol. Biol. 2011, 11, 209. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Petrusek, A.; Wolinska, J.; Gieβler, S.; Zhong, Y.; Yang, Z.; Hu, W.; Yin, M. Diversity of the Daphnia longispina species complex in Chinese lakes: A DNA taxonomy approach. J. Plankton Res. 2015, 37, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Möst, M.; Petrusek, A.; Sommaruga, R.; Juračka, P.J.; Slusarczyk, M.; Manca, M.; Spaak, P. At the edge and on the top: Molecular identification and ecology of Daphnia dentifera and D. longispina in high-altitude Asian lakes. Hydrobiologia 2013, 715, 51–62. [Google Scholar] [CrossRef]
- Karabanov, D.P.; Bekker, E.I.; Kotov, A.A. Underestimated consequences of biological invasions in phylogeographic reconstructions as seen in Daphnia magna (Crustacea, Cladocera). Zool. Zhurnal 2020, 99, 1232–1241. [Google Scholar] [CrossRef]
- Mergeay, J.; Verschuren, D.; De Meester, L. Invasion of an asexual American water flea clone throughout Africa and rapid displacement of a native sibling species. Proc. Biol. Sci. 2006, 273, 2839–2844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duggan, I.; Robinson, K.; Burns, C.; Banks, J.; Hogg, I. Identifying invertebrate invasions using morphological and molecular analyses: North American Daphnia ‘pulex’ in New Zealand fresh waters. AI 2012, 7, 585–590. [Google Scholar] [CrossRef] [Green Version]
- Ye, Z.; Williams, E.; Zhao, C.; Burns, C.W.; Lynch, M. The rapid, mass invasion of New Zealand by North American Daphnia “pulex”. Limnol. Oceanogr. 2021, 66, 2672–2683. [Google Scholar] [CrossRef]

| Within Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.3 ± 0.1 | 0.5 | 0.7 | 1.0 | 1.3 | 1.5 | 1.5 | 1.5 | 1.6 | 1.6 | 1.6 | 1.7 | 1.6 | 1.5 | 1.6 | |
| 2 | 0.7 ± 0.2 | 2.0 | 0.7 | 1.0 | 1.2 | 1.5 | 1.5 | 1.5 | 1.6 | 1.6 | 1.6 | 1.7 | 1.6 | 1.5 | 1.6 | |
| 3 | 0.3 ± 0.1 | 3.5 | 3.5 | 1.1 | 1.2 | 1.5 | 1.5 | 1.6 | 1.6 | 1.6 | 1.6 | 1.7 | 1.6 | 1.5 | 1.6 | |
| 4 | 0.6 ± 0.2 | 7.1 | 7.9 | 7.8 | 1.3 | 1.6 | 1.5 | 1.5 | 1.6 | 1.6 | 1.6 | 1.7 | 1.7 | 1.5 | 1.6 | |
| 5 | 1.1 ± 0.3 | 12.7 | 12.9 | 12.4 | 13.3 | 1.4 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.7 | 1.7 | 1.6 | 1.6 | |
| 6 | 0.9 ± 0.2 | 18.2 | 17.7 | 18.1 | 18.6 | 18.2 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.7 | 1.7 | 1.5 | 1.6 | |
| 7 | 0.1 ± 0.1 | 17.3 | 17.8 | 17.6 | 17.1 | 18.5 | 18.2 | 1.3 | 1.6 | 1.5 | 1.6 | 1.6 | 1.6 | 1.4 | 1.4 | |
| 8 | 0.1 ± 0.1 | 18.8 | 18.5 | 19.4 | 18.1 | 18.9 | 19.2 | 11.9 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.5 | 1.6 | |
| 9 | 1.2 ± 0.2 | 22.3 | 22.7 | 22.6 | 21.6 | 21.2 | 21.7 | 19.8 | 19.3 | 0.9 | 1.0 | 0.6 | 1.4 | 1.2 | 1.4 | |
| 10 | 1.0 ± 0.1 | 23.0 | 23.7 | 23.1 | 22.2 | 21.7 | 22.7 | 19.5 | 20.7 | 5.9 | 1.0 | 0.6 | 1.4 | 1.3 | 1.4 | |
| 11 | 0.4 ± 0.2 | 23.1 | 23.6 | 23.1 | 22.5 | 22.6 | 22.7 | 19.8 | 20.7 | 7.4 | 6.9 | 0.9 | 1.3 | 1.3 | 1.5 | |
| 12 | 1.9 ± 0.5 | 23.8 | 24.3 | 24.1 | 22.4 | 22.1 | 23.0 | 21.1 | 20.3 | 3.2 | 6.2 | 7.6 | 1.5 | 1.2 | 1.4 | |
| 13 | 0.2 ± 0.2 | 22.7 | 23.1 | 23.1 | 23.8 | 24.1 | 21.3 | 20.5 | 20.0 | 14.0 | 14.3 | 12.6 | 15.0 | 1.3 | 1.4 | |
| 14 | 6.3 ± 0.7 | 22.0 | 22.2 | 22.2 | 21.7 | 22.5 | 19.1 | 18.1 | 18.7 | 12.7 | 13.0 | 13.1 | 13.4 | 11.8 | 1.5 | |
| 15 | 1.3 ± 0.2 | 21.5 | 22.1 | 22.7 | 22.5 | 21.7 | 21.7 | 18.1 | 20.2 | 14.9 | 16.2 | 17.1 | 16.1 | 17.2 | 16.1 |
| Species | n | h | S | Hd ± st.d. | π ± st.d. | Tajima’s D | Fu’s FS |
|---|---|---|---|---|---|---|---|
| D. longispina s.str. clade A | 20 | 8 | 10 | 0.805 ± 0.070 | 0.0029 ± 0.0007 | −1.421 | −2.851 * |
| D. longispina s.str. clade B | 4 | 4 | 8 | 1.000 ± 0.177 | 0.0073 ± 0.0016 | −0.446 | −0.768 |
| D. dentifera | 45 | 16 | 22 | 0.665 ± 0.080 | 0.0032 ± 0.0006 | −2.087 ** | −9.913 ** |
| D. galeata | 18 | 11 | 20 | 0.915 ± 0.050 | 0.0064 ± 0.0011 | −1.462 | −3.550 * |
| D. umbra | 9 | 5 | 12 | 0.833 ± 0.098 | 0.0095 ± 0.0013 | 1.045 | 1.386 |
| D. curvirostris | 15 | 8 | 21 | 0.848 ± 0.071 | 0.0103 ± 0.0016 | −0.377 | 0.278 |
| D. cristata | 16 | 5 | 4 | 0.533 ± 0.142 | 0.0011 ± 0.0003 | 0 | 1.061 |
| D. longiremis | 8 | 3 | 2 | 0.607 ± 0.164 | 0.0012 ± 0.0004 | 1.826 | 13.378 |
| D. middendorffiana | 43 | 17 | 34 | 0.907 ± 0.029 | 0.0115 ± 0.0006 | −0.113 | −0.047 |
| D. pulex | 73 | 40 | 130 | 0.941 ± 0.019 | 0.0231 ± 0.0045 | −2.293 ** | −5.486 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuykova, E.I.; Sleptzova, L.P.; Bochkarev, N.A.; Kuchko, Y.A.; Sheveleva, N.G.; Zakharov, E.S.; Pestryakova, L.A.; Kotov, A.A. Mitochondrial Lineage Diversity and Phylogeography of Daphnia (Daphnia) (Crustacea: Cladocera) in North-East Russia. Water 2022, 14, 1946. https://doi.org/10.3390/w14121946
Zuykova EI, Sleptzova LP, Bochkarev NA, Kuchko YA, Sheveleva NG, Zakharov ES, Pestryakova LA, Kotov AA. Mitochondrial Lineage Diversity and Phylogeography of Daphnia (Daphnia) (Crustacea: Cladocera) in North-East Russia. Water. 2022; 14(12):1946. https://doi.org/10.3390/w14121946
Chicago/Turabian StyleZuykova, Elena I., Lana P. Sleptzova, Nikolai A. Bochkarev, Yaroslav A. Kuchko, Natalia G. Sheveleva, Evgeny S. Zakharov, Lyudmila A. Pestryakova, and Alexey A. Kotov. 2022. "Mitochondrial Lineage Diversity and Phylogeography of Daphnia (Daphnia) (Crustacea: Cladocera) in North-East Russia" Water 14, no. 12: 1946. https://doi.org/10.3390/w14121946







