Potential Use of Aquatic Vascular Plants to Control Cyanobacterial Blooms: A Review
Abstract
1. Introduction
2. Mechanisms of Aquatic Vascular Plants’ Influence on Cyanobacterial Blooms
3. The Main Groups of Allelopathically Active Substances of Aquatic Vascular Plants with Algicidal Action
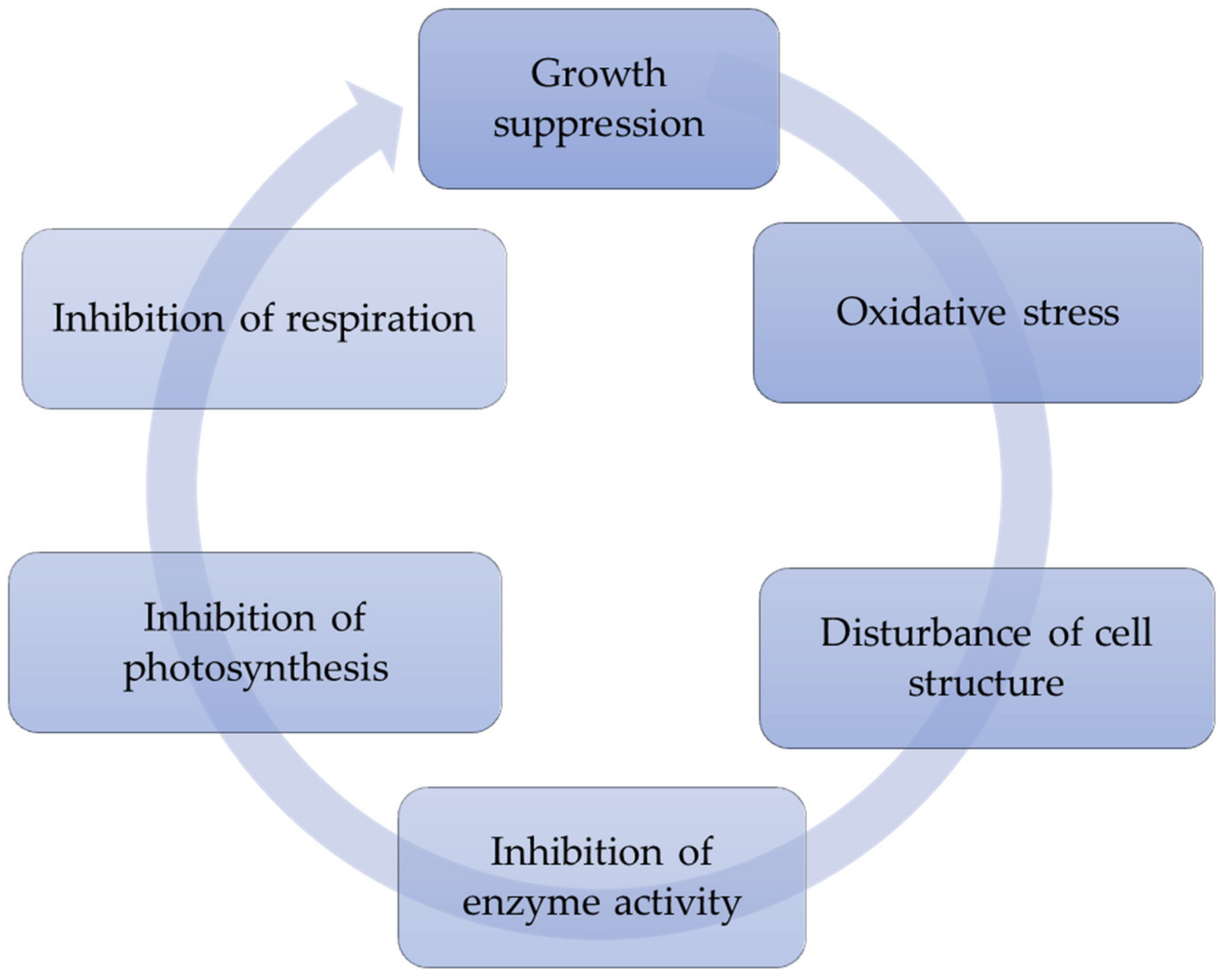
4. Physiological and Biochemical Mechanisms of Allelopathic Effect of Aquatic Vascular Plants on Cyanobacteria
4.1. Disturbance of Cell Structure
4.2. Oxidative Stress
4.3. Inhibition of Extracellular Enzyme Activity
4.4. Inhibition of Photosynthesis and Respiration
5. Factors Affecting the Allelopathic Activity of Aquatic Vascular Plants
5.1. Biotic Factors
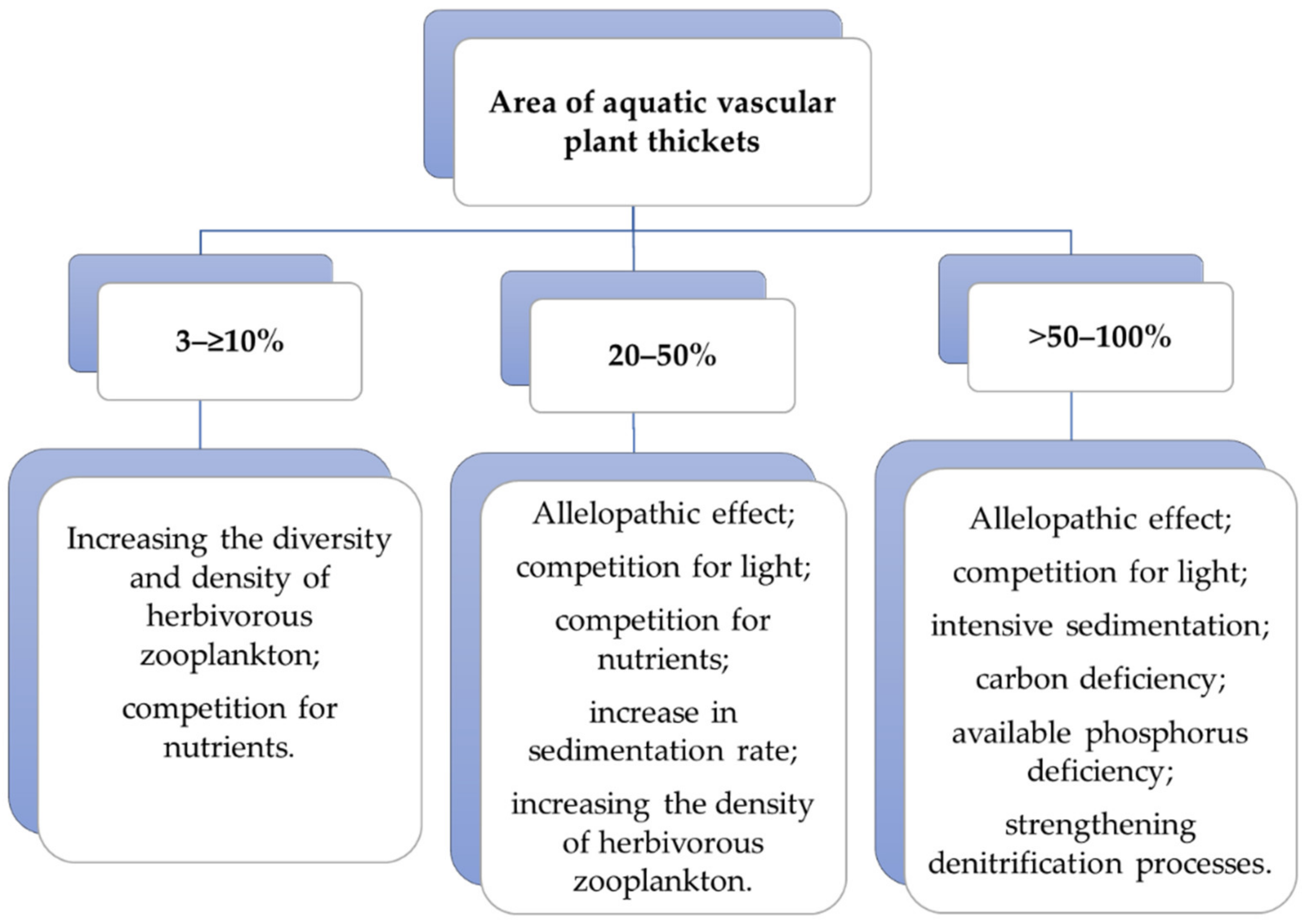
5.1.1. Area and Density of Overgrown Areas of Aquatic Vascular Plants
5.1.2. Species-Specific Features and Growth Stage of Plants
5.1.3. Concentration of Allelopathically Active Compounds and Exposure Duration
5.2. Abiotic Factors
5.2.1. Hydrological Regime
5.2.2. Physicochemical Conditions
6. The Advantages and Considerations of Using Aquatic Vascular Plants to Control Harmful Cyanobacterial Blooms
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Stroom, J.M.; Kardinaal, W.E.A. How to combat cyanobacterial blooms: Strategy toward preventive lake restoration and reactive control measures. Aquat. Ecol. 2016, 50, 541–576. [Google Scholar] [CrossRef]
- Kirpenko, N.I.; Krot, Y.G.; Usenko, O.M. Surface waters “Blooms”—Fundamental and applied aspects. Hydrobiol. J. 2019, 55, 18–30. [Google Scholar] [CrossRef]
- Kirpenko, N.I.; Krot, Y.G.; Usenko, O.M. Toxicological aspects of the surface water: “Blooms” (a Review). Hydrobiol. J. 2020, 56, 3–16. [Google Scholar] [CrossRef]
- Kurashov, E.; Krylova, J.; Protopopova, E. The Use of Allelochemicals of Aquatic Macrophytes to Suppress the Development of Cyanobacterial “Blooms”. In Plankton Communities; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Tazart, Z.; Manganelli, M.; Scardala, S.; Buratti, F.M.; Di Gregorio, F.N.; Douma, M.; Mouhri, K.; Testai, E.; Loudiki, M. Remediation strategies to control toxic cyanobacterial blooms: Effects of macrophyte aqueous extracts on Microcystis aeruginosa (growth, toxin production and oxidative stress response) and on bacterial ectoenzymatic activities. Microorganisms 2021, 9, 1782. [Google Scholar] [CrossRef]
- Burford, M.A.; Beardall, J.; Willis, A.; Orr, P.T.; Magalhaes, V.F.; Rangel, L.M.; Azevedo, S.M.F.O.E.; Neilan, B.A. Understanding the winning strategies used by the bloom-forming cyanobacterium Cylindrospermopsis raciborskii. Harmful Algae 2016, 54, 44–53. [Google Scholar] [CrossRef]
- Kurmayer, R.; Deng, L.; Entfellner, E. Role of toxic and bioactive secondary metabolites in colonization and bloom formation by filamentous cyanobacteria Planktothrix. Harmful Algae 2016, 54, 69–86. [Google Scholar] [CrossRef]
- Li, X.; Dreher, T.W.; Li, R. An overview of diversity, occurrence, genetics and toxin production of bloom-forming Dolichospermum (Anabaena) species. Harmful Algae 2016, 54, 54–68. [Google Scholar] [CrossRef]
- Greenfield, D.I.; Duquette, A.; Goodson, A.; Keppler, C.J.; Williams, S.H.; Brock, L.M.; Stackley, K.D.; White, D.; Wilde, S.B. The effects of three chemical algaecides on cell numbers and toxin content of the cyanobacteria Microcystis aeruginosa and anabaenopsis sp. J. Environ. Manag. 2014, 54, 1110–1120. [Google Scholar] [CrossRef]
- Abeynayaka, H.D.L.; Asaeda, T.; Tanaka, K.; Atsuzawa, K.; Kaneko, Y.; Nishda, H.; Inada, S. An alternative method to improve the settleability of gas-vacuolated cyanobacteria by collapsing gas vesicles. Water Supply 2016, 16, 1552–1560. [Google Scholar] [CrossRef]
- Tan, K.; Huang, Z.; Ji, R.; Qiu, Y.; Wang, Z.; Liu, J. A review of allelopathy on microalgae. Microbiology 2019, 165, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Romanenko, V.D.; Sakevich, A.I.; Usenko, O.M. Higher aquatic plants as the factor limiting water bloom caused by Cyanobacteria. Rate NASU 2005, 8, 174–177. [Google Scholar]
- Romanenko, V.D.; Sakevich, A.I.; Usenko, O.M. On the mechanism of action of easily oxidizable phenols on photosynthetic activity of algae. Hydrobiol. J. 2006, 42, 82–92. [Google Scholar] [CrossRef]
- Usenko, O.M.; Guseynova, V.P.; Sakevich, A.I. Peculiarities of the influence of polyphenols on algae under conditions of changes in pH of the environment. Hydrobiol. J. 2008, 44, 37–44. [Google Scholar] [CrossRef]
- Kirpenko, N.I.; Usenko, O.M. Influence of higher aquatic plants on microalgae (a Review). Hydrobiol. J. 2013, 49, 57–74. [Google Scholar] [CrossRef]
- Tazart, Z.; Douma, M.; Tebaa, L.; Loudiki, M. Use of macrophytes allelopathy in the biocontrol of harmful Microcystis aeruginosa blooms. Water Supply 2018, 19, 245–253. [Google Scholar] [CrossRef]
- Van Donk, E.; van de Bund, W.J. Impact of submerged macrophytes including charophytes on phyto- and zooplankton communities: Allelopathy versus other mechanisms. Aquat. Bot. 2002, 72, 261–274. [Google Scholar] [CrossRef]
- Scheffer, M. The story of some shallow lakes. In Ecology of Shallow Lakes; Springer: Dordrecht, The Netherlands, 2004; pp. 1–19. [Google Scholar]
- Thomaz, S.M.; Cunha, E.R. The role of macrophytes in habitat structuring in aquatic ecosystems: Methods of measurement, causes and consequences on animal assemblages’ composition and biodiversity. Acta Limnol. Bras. 2010, 22, 218–236. [Google Scholar] [CrossRef]
- Hilt, S.; Gross, E.M. Can allelopathically active submerged macrophytes stabilise clear-water states in shallow lakes? Basic Appl. Ecol. 2008, 9, 422–432. [Google Scholar] [CrossRef]
- Essien, A.I.; Udoinim, M.M.; Akpan, G.D.; Effiong, J.O. Aquatic macrophytes and Limnological implications on fisheries resources: A case study of Obio Akpa Stream in Akwa Ibom State, Nigeria. Int. J. Water Soil Res. 2012, 3, 15–21. [Google Scholar]
- Henriot, C.P.; Cuenot, Q.; Levrey, L.H.; Loup, C.; Chiarello, L.; Masclaux, H.; Bornette, G. Relationships between key functional traits of the waterlily Nuphar lutea and wetland nutrient content. Peer J. 2019, 7, e7861. [Google Scholar] [CrossRef] [PubMed]
- Blindow, I.; Hargeby, A.; Andersson, G. Seasonal changes of mechanisms maintaining clear water in a shallow lake with abundant Chara vegetation. Aquat. Bot. 2002, 72, 315–334. [Google Scholar] [CrossRef]
- Meijer, M.L.; Jeppesen, E.; Van Donk, E.; Moss, B.; Scheffer, M.; Lammens, E.; van Nes, E.; Van Berkum, J.A.; De Jong, G.J.; Faafeng, B.A.; et al. Long-term responses to fish-stock reduction in small shallow lakes: Interpretation of five-year results of four biomanipulation cases in The Netherlands and Denmark. Hydrobiologia 1994, 275–276, 457–466. [Google Scholar] [CrossRef]
- Pokorný, J.; Květ, J.; Ondok, J.; Toul, Z.; Ostrý, I. Production-ecological analysis of a plant community dominated by Elodea canadensis michx. Aquat. Bot. 1984, 19, 263–292. [Google Scholar] [CrossRef]
- Ozimek, T.; Gulati, R.D.; van Donk, E. Can macrophytes be useful in biomanipulation of lakes? The Lake Zwemlust example. Hydrobiologia 1990, 200–201, 399–407. [Google Scholar] [CrossRef]
- Soloviy, K.; Malovanyy, M. Freshwater ecosystem macrophytes and microphytes: Development, environmental problems, usage as raw material. Review. Environ. Probl. 2019, 4, 115–124. [Google Scholar] [CrossRef]
- Wu, M.Y.; Wu, J. In-vitro investigations on ultrasonic control of water chestnut. J. Aquat. Plant Manag. 2007, 45, 76–83. [Google Scholar]
- Cunha, D.G.F.; Bottino, F.; do Carmo Calijuri, M. Can free-floating and emerged macrophytes influence the density and diversity of phytoplankton in subtropical reservoirs? Lake Reserv. Manag. 2012, 28, 255–264. [Google Scholar] [CrossRef]
- Kiviat, E. Water chestnut (Trapa natans) In Exotic Plants with Identified Detrimental Impacts on Wildlife Habitats; New York State Cornell University: New York, NY, USA, 1987; pp. 31–38. [Google Scholar]
- Kiviat, E. Under the spreading water-chestnut. News Hudsonia 1993, 9, 1–6. [Google Scholar]
- Groth, A.T.; Lovett-Doust, L.; Lovett-Doust, J. Population density and module demography in Trapa natans (Trapaceae), an annual, clonal aquatic macrophyte. Am. J. Bot. 1996, 83, 1406–1415. [Google Scholar] [CrossRef]
- Mironga, J.M.; Mathooko, J.M.; Onywere, S.M. The effect of water Hyacinth (Eichhornia Crassipes) infestation on phytoplankton productivity in Lake Naivasha and the status of control. J. Environ. Sci. Eng. 2011, 5, 1252–1260. [Google Scholar]
- Van Donk, E.; Gulati, R.D.; Iedema, A.; Meulemans, J.T. Macrophyte-related shifts in the nitrogen and phosphorus contents of the different trophic levels in a biomanipulated shallow lake. Hydrobiologia 1993, 251, 19–26. [Google Scholar] [CrossRef]
- Barko, J.W.; James, W.F. Effects of submerged aquatic macrophytes on nutrient dynamics, sedimentation, and resuspension. In The Structuring Role of Submerged Macrophytes in Lakes; Springer: New York, NY, USA, 1998; pp. 197–214. [Google Scholar] [CrossRef]
- Seto, M.; Takamura, N.; Iwasa, Y. Individual and combined suppressive effects of submerged and floating-leaved macrophytes on algal blooms. J. Theor. Biol. 2013, 319, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Samal, K.; Kar, S.; Trivedi, S.; Upadhyay, S. Assessing the impact of vegetation coverage ratio in a floating water treatment bed of Pistia stratiotes. SN Appl. Sci. 2021, 3, 120. [Google Scholar] [CrossRef]
- Blindow, I. The composition and density of epiphyton on several species of submerged macrophytes—The neutral substrate hypothesis tested. Aquat. Bot. 1987, 29, 157–168. [Google Scholar] [CrossRef]
- Weisner, S.; Eriksson, G.; Granéli, W.; Leonardson, L. Influence of macrophytes on nitrate removal in wetlands. Ambio 1994, 23, 363–366. [Google Scholar]
- Koschel, R.; Benndorf, J.; Proft, G.; Recknagel, F. Calcite precipitation as a natural control mechanism of eutrophication. Arch. Hydrobiol. 1983, 98, 380–408. [Google Scholar]
- Reynolds, C.S. The Ecology of Freshwater Phytoplankton; Cambridge University Press: Cambridge, UK, 1984; p. 384. [Google Scholar]
- Smith, R.V.; Peat, A. Comparative structure of the gas-vacuoles of blue-green algae. Archiv. Mikrobiol. 1967, 57, 111–122. [Google Scholar] [CrossRef]
- Walsby, A.E. The gas vacuoles of blue-green algae. Sci. Am. 1977, 237, 90–97. [Google Scholar] [CrossRef]
- Walsby, A.E.; Avery, A.; Schanz, F. The critical pressures of gas vesicles in Planktorhrix rubescens in relation tothe depth of winter mixing in Lake Zürich, Switzerland. J. Plankton Res. 1998, 20, 1357–1375. [Google Scholar] [CrossRef]
- Timms, R.M.; Moss, B. Prevention of growth of potentially dense phytoplankton populations by zooplankton grazing, in the presence of zooplanktivorous fish, in a shallow wetland ecosystem. Limnol. Oceanogr. 1984, 29, 472–486. [Google Scholar] [CrossRef]
- Lauridsen, T.; Pedersen, L.J.; Jeppesen, E.; Sønergaard, M. The importance of macrophyte bed size for cladoceran composition and horizontal migration in a shallow lake. J. Plankton Res. 1996, 18, 2283–2294. [Google Scholar] [CrossRef]
- Basińska, A.; Kuczyńska-Kippen, N. Differentiated macrophyte types as a habitat for rotifers in small mid-forest water bodies. Biologia 2009, 64, 1100–1107. [Google Scholar] [CrossRef][Green Version]
- Basu, B.K.; Kalff, J.; Pinel-Alloul, B. The influence of macrophyte beds on plankton communities and their export from fluvial lakes in the St Lawrence River. Freshw. Biol. 2000, 45, 373–382. [Google Scholar] [CrossRef]
- Manatunge, J.; Asaeda, T.; Priyadarshana, T. The influence of structural complexity on fish-zooplankton interactions: A study using artificial submerged macrophytes. Environ. Biol. Fish. 2000, 58, 425–438. [Google Scholar] [CrossRef]
- Kuczynska-Kippen, N. Zooplankton structure in architecturally differentiated macrophyte habitats of shallow lakes in the Wielkopolska Region, Poland. Oceanol. Hydrobiol. Stud. 2006, 35, 179–191. [Google Scholar]
- Konovets, I.M.; Usenko, O.M.; Mardarevich, M.G.; Kudryavtseva, D.O. Influence of phenolcarbonic acids on Daphnia magna Straus fertility. Hydrobiol. J. 2021, 57, 80–88. [Google Scholar] [CrossRef]
- Kuczynska-Kippen, N.; Nagengast, B. The impact of the architecture of macrophytes on the spatial structure of zooplankton of the Wielkowiejskie lake. Rocz. AR Pozn. CCCLIV. 2003, 6, 121–129. [Google Scholar]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; p. 422. [Google Scholar]
- Usenko, O.M.; Sakevich, A.I. Allelopathic influence of higher aquatic plants on the functional activity of plankton algae. Hydrobiol. J. 2005, 41, 54–66. [Google Scholar] [CrossRef]
- Fink, P. Ecological functions of volatile organic compounds in aquatic systems. Mar. Freshw. Behav. Physiol. 2007, 40, 155–168. [Google Scholar] [CrossRef]
- Sakevych, O.I.; Usenko, O.M. Allelopathy in Hydroecosystems; Logos: Kiev, Ukraine, 2008; p. 342. (In Ukrainian) [Google Scholar]
- Zhu, X.; Dao, G.; Tao, Y.; Zhan, X.; Hu, H. A review on control of harmful algal blooms by plant-derived allelochemicals. J. Hazard. Mater. 2021, 401, 123403. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yin, Y.; Kang, L.; Feng, L.; Liu, Y.; Du, Z.; Tian, Y.; Zhang, L. A review: Application of allelochemicals in water ecological restoration—Algal inhibition. Chemosphere 2021, 267, 128869. [Google Scholar] [CrossRef] [PubMed]
- Ren, S. Allelopathy in Sustainable Agriculture and Forestry; Springer: New York, NY, USA, 2008; p. 426. [Google Scholar]
- Zhang, T.T.; Zheng, C.Y.; He, M.; Wu, A.P.; Nie, L.W. Inhibition on algae of fatty acids and the structure-effect relationship. China Environ. Sci. 2009, 29, 274–279. [Google Scholar]
- Gao, Y.N.; Dong, J.; Fu, Q.Q.; Wang, Y.P.; Chen, C.; Li, J.H.; Li, R.; Zhou, C.J. Allelopathic effects of submerged macrophytes on phytoplankton. Allelopath. J. 2017, 40, 01–22. [Google Scholar] [CrossRef]
- Usenko, O.M.; Sakevych, O.I. Resistance of Algae to Biologically Active Substances; Logos: Kiev, Ukraine, 2010; p. 192. (In Ukrainian) [Google Scholar]
- Nakai, S.; Inoue, Y.; Hosomi, M. Algal growth inhibition effects and inducement modes by plant-producing phenols. Water Res. 2001, 35, 1855–1859. [Google Scholar] [CrossRef]
- Li, F.M.; Hu, H.Y. Isolation and characterization of a novel antialgal allelochemical from Phragmites communis. Appl. Environ. Microbiol. 2005, 71, 6545–6553. [Google Scholar] [CrossRef]
- Nakai, S.; Zou, G.; Okuda, T.; Nishijima, W.; Hosomi, M.; Okada, M. Polyphenols and fatty acids responsible for anti-cyanobacterial allelopathic effects of submerged macrophyte Myriophyllum spicatum. Water Sci. Technol. 2012, 66, 993–999. [Google Scholar] [CrossRef]
- Nakai, S.; Inoue, Y.; Hosomi, M.; Murakami, A. Myriophyllum spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa. Water Res. 2000, 34, 3026–3032. [Google Scholar] [CrossRef]
- Usenko, O.M. Comparison studies on the content of phenols and quinones in the phytomass of higher aquatic plants under natural conditions. Hydrobiol. J. 2012, 48, 73–80. [Google Scholar] [CrossRef]
- Usenko, O.M. Endo- and exogenous phenolcarboxylic acids Trapa natans L. and Nuphar lutea L. TNPU Ser. Biol. 2015, 3–4, 675–678. (In Ukrainian) [Google Scholar]
- Planas, D.; Sarhan, F.; Dube, L.; Godmaire, H.; Cadieux, C. Ecological significance of phenolic compounds of Myriophyllum spicatum. Verh. Internat. Verein. Limnol. 1981, 21, 1492–1496. [Google Scholar] [CrossRef]
- Saito, K.; Matsumoto, M.; Sekine, T.; Murakoshi, I.; Morisaki, N.; Iwasaki, S. Inhibitory substances from Myriophyllum brasiliense on growth of blue-green algae. J. Nat. Prod. 1989, 52, 1221–1226. [Google Scholar] [CrossRef]
- Gross, E.M. Seasonal and spatial dynamics of allelochemicals in the submersed macrophyte Myriophyllum spicatum L. SIL Proc. 2000, 27, 2116–2119. [Google Scholar] [CrossRef][Green Version]
- Usenko, O.M.; Konovets, I.M. Analysis of pheolcarbonic acids content in phytomass of higher aquatic plants. Hydrobiol. J. 2014, 50, 47–60. [Google Scholar] [CrossRef]
- Usenko, O.M.; Konovets, I.N.; Tarashchuk, O.S.; Gorbunova, Z.N. Phenolcarbonic acids of the submerged aquatic plants and their effect on phytoepiphyton structure. Hydrobiol. J. 2019, 55, 55–64. [Google Scholar] [CrossRef]
- Bi, X.; Dai, W.; Zhang, S.; Pang, Y.; Xing, K.Z.; Zhang, D.J. Inhibition of photosynthesis related gene expression by berberine in Microcystis aeruginosa. Allelopath. J. 2014, 34, 335–343. [Google Scholar]
- Canton, M.C.; Holguin, F.O.; Boeing, W.J. Alkaloid gramine to control algal invaders: Algae inhibition and gramine persistence. Bioresour. Technol. Rep. 2019, 7, 100304. [Google Scholar] [CrossRef]
- Balanda, O.V.; Medved, V.A.; Sakevich, A.I. Alkaloids of Nuphar lutea (L.) Smith. and their influence on the vital activity of cyanobacteria and algae. Hydrobiol. J. 2004, 40, 108–120. [Google Scholar] [CrossRef]
- Usenko, O.M.; Balanda, O.V. Terpene compounds of water plants and their impact on the functional activity of hydrobionts. Visn. Lviv Univ. Ser. Biol. 2013, 62, 216–226. (In Ukrainian) [Google Scholar]
- Hu, H.; Hong, Y. Algal-bloom control by allelopathy of aquatic macrophytes—A review. Front. Environ. Sci. Eng. China 2008, 2, 421–438. [Google Scholar] [CrossRef]
- Palamarchuk, V.D. Influence of phenol acids of hydrophytes on the development of plankton algae. Int. J. Algae 2002, 4, 20–28. [Google Scholar] [CrossRef]
- Mohamed, Z.A. Macrophytes-cyanobacteria allelopathic interactions and their implications for water resources management—A review. Limnologica 2017, 63, 122–132. [Google Scholar] [CrossRef]
- Körner, S.; Nicklisch, A. Allelopathic growth inhibition of selected phytoplankton species by submerged macrophytes. J. Phycol. 2002, 38, 862–871. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, B.; Wang, J.; Gao, Y.; Wu, Z. Study on the mechanism of allelopathic influence on cyanobacteria and chlorophytes by submerged macrophyte (Myriophyllum spicatum) and its secretion. Aquat. Toxicol. 2010, 98, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Aliotta, G.; Molinaro, A.; Monaco, P.; Pinto, G.; Previtera, L. Three biologically active phenylpropanoid glucosides from Myriophyllum verticillatum. Phytochemistry 1992, 31, 109–111. [Google Scholar] [CrossRef]
- Hilt, S.; Ghobrial, M.; Gross, E.M. In situ allelopathic potential of Myriophyllum verticillatum (Haloragaceae) against selected phytoplankton species. J. Phycol. 2006, 42, 1189–1198. [Google Scholar] [CrossRef]
- Erhard, D.; Gross, E.M. Allelopathic activity of Elodea canadensis and Elodea nuttallii against epiphytes and phytoplankton. Aquat. Bot. 2006, 85, 203–211. [Google Scholar] [CrossRef]
- Sun, W.H.; Yu, S.W.; Yang, S.Y.; Zhao, B.W.; Yu, Z.W.; Wu, H.L.; Huang, S.Y.; Tang, C.S. Allelochemicals from root exudates of water hyacinth (Eichhornis crassipes). Acta Photophysiol. Sin. 1993, 19, 92–96. [Google Scholar]
- Shanab, S.M.M.; Shalaby, E.A.; Lightfoot, D.A.; El-Shemy, H.A. Allelopathic effects of water hyacinth Eichhornia crassipes. PLoS ONE 2010, 5, e13200. [Google Scholar] [CrossRef]
- Gross, E.M.; Erhard, D.; Iványi, E. Allelopathic activity of Ceratophyllum demersum L. and Najas marina ssp. intermedia (Wolfgang) Casper. Hydrobiologia 2003, 506, 583–589. [Google Scholar] [CrossRef]
- Wu, X.; Wu, H.; Chen, J.; Ye, J. Effects of allelochemical extracted from water lettuce (Pistia stratiotes Linn.) on the growth, microcystin production and release of Microcystis aeruginosa. Environ. Sci. Pollut. Res. 2013, 20, 8192–8201. [Google Scholar] [CrossRef] [PubMed]
- Aliotta, G.; Monaco, P.; Pinto, G.; Pollio, A.; Previtera, L. Potential allelochemicals from Pistia stratiotes L. J. Chem. Ecol. 1991, 17, 2223–2234. [Google Scholar] [CrossRef] [PubMed]
- Waridel, P.; Wolfender, J.L.; Lachavanne, J.B.; Hostettmann, K. ent-Labdane diterpenes from the aquatic plant Potamogeton pectinatus. Phytochemistry 2003, 64, 1309–1317. [Google Scholar] [CrossRef]
- Cangiano, T.; DellaGreca, M.; Fiorentino, A.; Isidori, M.; Monaco, P.; Zarrelli, A. Lactone diterpenes from the aquatic plant Potamogeton natans. Phytochemistry 2001, 56, 469–473. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, W.; Yang, X.; Zhang, T. Inhibition effect on Microcystis aeruginosa PCC7806 as well as separation and identification of algicidal substances isolated from Salvinia natans (L.). All. J. Hyg. Res. 2016, 45, 442–447. [Google Scholar]
- Zhou, S.; Nakai, S.; Hosomi, M.; Sezaki, Y.; Tominaga, M. Allelopathic growth inhibition of cyanobacteria by reed. Allelopath. J. 2006, 18, 277–285. [Google Scholar]
- Zhang, T.T.; Hu, W.; Zhang, D. Allelopathic effect of Typha Angustifolia L. on phytoplankton. Adv. Mat. Res. 2011, 383–390, 3724–3728. [Google Scholar] [CrossRef]
- Kang, P.G.; Hong, J.; Kim, E.; Kim, B. Effects of extracts of reed and cattail on the growth of a cyanobacterium, Microcystis aeruginosa. J. Freshw. Ecol. 2020, 35, 123–134. [Google Scholar] [CrossRef]
- Aliotta, G.; Greca, M.D.; Monaco, P.; Pinto, G.; Pollio, A.; Previtera, L. In vitro algal growth inhibition by phytotoxins of Typha latifolia L. J. Chem. Ecol. 1990, 16, 2637–2646. [Google Scholar] [CrossRef]
- Wang, H.Q.; Liang, F.; Qiao, N.; Dong, J.X.; Zhang, L.Y.; Guo, Y.F. Chemical composition of volatile oil from two emergent plants and their algae inhibition activity. Pol. J. Environ. Stud. 2014, 6, 2371–2374. [Google Scholar]
- Leu, E.; Krieger-Liszkay, A.; Goussias, C.; Gross, E.M. Polyphenolic allelochemicals from the aquatic angiosperm Myriophyllum spicatum L. inhibit photosystem II. Plant Physiol. 2002, 130, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Li, F.M.; Hu, H.Y.; Chong, Y.X.; Men, Y.J.; Guo, M.T. Effects of allelochemical EMA isolated from Phragmites communis on algal cell membrane lipid and ultrastructure. Huan Jing Ke Xue 2007, 28, 1534–1538. [Google Scholar] [PubMed]
- Lourenção, A.; Mecina, G.F.; Cordeiro-Araújo, M.K.; Bittencourt-Oliveira, M.C.; Chia, M.A.; Bronzel-Júnior, J.L.; Granero, F.O.; Silva, L.P.; da Silva, R.M.G. Characterization of allelochemicals from Pistia stratiotes extracts and their effects on the growth and physiology of Microcystis aeruginosa. Environ. Sci. Pollut. Res. 2021, 28, 57248–57259. [Google Scholar] [CrossRef] [PubMed]
- Nakai, S.; Asaoka, S.; Okuda, T.; Nishijima, W. Growth inhibition of Microcystis aeruginosa by allelopathic compounds originally isolated from Myriophyllum spicatum: Temperature and light efects and evidence of possible major mechanisms. J. Chem. Eng. Jpn. 2014, 47, 488–493. [Google Scholar] [CrossRef]
- Nakai, S.; Yamada, S.; Hosomi, M. Anti-cyanobacterial fatty acids released from Myriophyllum spicatum. Hydrobiologia 2005, 543, 71–78. [Google Scholar] [CrossRef]
- Ni, L.; Rong, S.; Gu, G.; Hu, L.; Wang, P.; Li, D.; Yue, F.; Wang, N.; Wu, H.; Li, S. Inhibitory effect and mechanism of linoleic acid sustained-release microspheres on Microcystis aeruginosa at different growth phases. Chemosphere 2018, 212, 654–661. [Google Scholar] [CrossRef]
- Li, F.M.; Hu, H.Y.; Chong, Y.X.; Men, Y.J.; Guo, M.T.I. Influence of EMA isolated from Phragmites communis on physiological characters of Microcystis aeruginosa. J. Environ. Sci. 2007, 27, 377–381. [Google Scholar]
- Gao, Y.N.; Ge, F.J.; Zhang, L.P.; He, Y.; Lu, Z.Y.; Zhang, Y.Y.; Liu, B.Y.; Zhou, Q.H.; Wu, Z.B. Enhanced toxicity to the cyanobacterium Microcystis aeruginosa by low-dosage repeated exposure to the allelochemical N-phenyl-1-naphthylamine. Chemosphere 2017, 174, 732–738. [Google Scholar] [CrossRef]
- Wang, H.Q.; Zhu, H.J.; Zhang, L.Y.; Xue, W.J.; Yuan, B. Identification of antialgal compounds from the aquatic plant Elodea nuttallii. Allelopath. J. 2014, 34, 207–213. [Google Scholar]
- Xin, L.; Hong-Ying, H.; Jia, Y.; Yin-Hu, W. Enhancement effect of ethyl-2-methyl acetoacetate on triacylglycerols production by a freshwater microalga, Scenedesmus sp. LX1. Bioresour. Technol. 2010, 101, 9819–9821. [Google Scholar] [CrossRef]
- Shao, J.; Wu, Z.; Yu, G.; Peng, X.; Li, R. Allelopathic mechanism of pyrogallol to Microcystis aeruginosa PCC7806 (Cyanobacteria): From views of gene expression and antioxidant system. Chemosphere 2009, 75, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Shi, J.; Yang, S. The effect of pyrogallic acid on growth, oxidative stress, and gene expression in Cylindrospermopsis raciborskii (Cyanobacteria). Ecotoxicology 2013, 22, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Romanenko, V.D.; Usenko, O.M.; Sakevich, A.I. Metabolic mechanisms of relationship between higher aquatic plants and water bloom forming blue-green algae. Hydrobiol. J. 2005, 41, 43–55. [Google Scholar] [CrossRef]
- Gross, E.M. Allelopathy of aquatic autotrophs. Crit. Rev. Plant Sci. 2003, 22, 313–339. [Google Scholar] [CrossRef]
- Zhang, T.T.; Zheng, C.Y.; Hu, W.; Xu, W.W.; Wang, H.F. The allelopathy and allelopathic mechanism of phenolic acids on toxic Microcystis aeruginosa. J. Appl. Phycol. 2010, 22, 71–77. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants; John Wiley and Sons: Hoboken, NJ, USA, 2015; p. 1280. [Google Scholar]
- Gopal, B.; Goel, U. Competition and allelopathy in aquatic plant communities. Bot. Rev. 1993, 59, 155–210. [Google Scholar] [CrossRef]
- Ruggiero, A.; Solimini, A.G.; Carchini, G. Nutrient and chlorophyll a temporal patterns in eutrophic mountain ponds with contrasting macrophyte coverage. Hydrobiologia 2003, 506–509, 657–663. [Google Scholar] [CrossRef]
- Canfield, D.E., Jr.; Shireman, J.V.; Colle, D.E.; Haller, W.T.; Watkins, C.E., II; Maceina, M.J. Prediction of chlorophyll a concentrations in florida lakes: Importance of aquatic macrophytes. Can. J. Fish. Aquat. Sci. 1984, 41, 497–501. [Google Scholar] [CrossRef]
- Nakai, S.; Inoue, Y.; Hosomi, M.; Murakami, A. Growth inhibition of blue-green algae by allelopathic effects of macrophytes. Water Sci. Technol. 1999, 39, 47–53. [Google Scholar] [CrossRef]
- Mulderij, G.; Mooij, W.M.; Smolders, A.J.P.; Donk, E.V. Allelopathic inhibition of phytoplankton by exudates from Stratiotes aloides. Aquat. Bot. 2005, 82, 284–296. [Google Scholar] [CrossRef]
- Kogan, S.I.; Chinnova, G.A. Relations between Ceratophyllum demersum (L.) and some blue-green algae. Hydrobiol. J. 1972, 8, 21–27. [Google Scholar]
- Amorim, C.A.; Ulisses, C.; Moura, A.N. Biometric and physiological responses of Egeria densa Planch. cultivated with toxic and non-toxic strains of Microcystis. Aquat. Toxicol. 2017, 191, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.A.; Moura-Falcão, R.H.D.; Valença, C.R.; Souza, V.R.D.; Moura, A.D.N. Allelopathic effects of the aquatic macrophyte Ceratophyllum demersum L. on phytoplankton species: Contrasting effects between cyanobacteria and chlorophytes. Acta Limnol. Bras. 2019, 31, 1–10. [Google Scholar] [CrossRef]
- Mulderij, G.; Van Donk, E.; Roelofs, J.G.M. Differential sensitivity of green algae to allelopathic substances from Chara. Hydrobiologia 2003, 491, 261–271. [Google Scholar] [CrossRef]
- Kurashov, E.A.; Krylov, Y.V.; Chernova, A.M.; Mytrukova, G.G. Component composition of volatile low-molecular organic substances Nuphar lutea (Nymphaeaceae) at the beginning of the growing season. Water Chem. Ecol. 2013, 5, 67–80. (In Ukrainian) [Google Scholar]
- Westlake, D.F. Some effects of low-velocity currents on the metabolism of aquatic macrophytes. J. Exp. Bot. 1967, 18, 187–205. [Google Scholar] [CrossRef]
- Marshall, E.J.P.; Westlake, D.F. Water velocities around water plants in chalk streams. Folia Geobot. 1990, 25, 279–289. [Google Scholar] [CrossRef]
- Riis, T.; Biggs, B.J.F. Hydrologic and hydraulic control of macrophyte establishment and performance in streams. Limnol. Oceanogr. 2003, 48, 1488–1497. [Google Scholar] [CrossRef]
- Franklin, P.; Dunbar, M.; Whitehead, P. Flow controls on lowland river macrophytes: A review. Sci. Total Environ. 2008, 400, 369–378. [Google Scholar] [CrossRef]
- Madsen, J.D.; Chambers, P.A.; James, W.F.; Koch, E.W.; Westlake, D.F. The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia 2001, 444, 71–84. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Academic Press: San Diego, CA, USA, 2001; p. 1006. [Google Scholar] [CrossRef]
- Sakevich, A.I.; Usenko, O.M. Phenol compounds in the water of the Dnieper reservoirs. Hydrobiol. J. 2003, 39, 37–47. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Sánchez-Moreiras, A.; González, L. Ecophysiological approach in allelopathy. Crit. Rev. Plant. Sci. 1999, 18, 577–608. [Google Scholar] [CrossRef]
- Maberly, S.C.; Spence, D.H.N. Photosynthesis and photorespiration in freshwater organisms: Amphibious plants. Aquat. Bot. 1989, 34, 267–286. [Google Scholar] [CrossRef]
- Arthaud, F.; Vallod, D.; Robin, J.; Bornette, G. Eutrophication and drought disturbance shape functional diversity and life-history traits of aquatic plants in shallow lakes. Aquat. Sci. 2011, 74, 471–481. [Google Scholar] [CrossRef]
- Fitzgerald, G.P. Some factors in the competition or antagonism among bacteria, algae and aquatie weeds. J. Phycol. 1969, 5, 351. [Google Scholar] [CrossRef] [PubMed]
- Mjelde, M.; Faafeng, B. Ceratophyllum demersum hampers phytoplankton development in some small Norwegian lakes over a wide range of phosphorus concentrations and geographical latitude. Freshw. Biol. 1997, 37, 355–365. [Google Scholar] [CrossRef]
- Bornette, G.; Puijalon, S. Macrophytes: Ecology of aquatic plants. In Encyclopedia of Life Sciences; John Wiley and Sons Ltd.: Chichester, UK, 2009; pp. 1–9. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Brunel, S. Assessing and managing the current and future pest risk from water hyacinth, (Eichhornia crassipes), an invasive aquatic plant threatening the environment and water security. PLoS ONE 2016, 11, e0120054. [Google Scholar] [CrossRef]
- Santamaría, L.; van Vierssen, W. Photosynthetic temperature responses of fresh- and brackish-water macrophytes: A review. Aquat. Bot. 1997, 58, 135–150. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Z.; Chen, D.; Zhang, J.; Yang, W.; Jin, Y. Allelopathic effects of Eichhornia crassipes on the growth of Microcystis aeruginosa. J. Agric. Sci. Technol. 2012, 2, 1400–1406. [Google Scholar]
- Wu, X.X.; Zhang, Z.Y.; Jin, Y.G. Physiological mechanism of Eichhornia crassipes in inhibiting the growth of Microcytis aeruginosa. Russ. J. Plant Physiol. 2019, 66, 433–439. [Google Scholar] [CrossRef]
- Meerhoff, M.; Mazzeo, N.; Moss, B.; Rodríguez-Gallego, L. The structuring role of free-floating versus submerged plants in a subtropical shallow lake. Aquat Ecol. 2003, 37, 377–391. [Google Scholar] [CrossRef]
- Gross, E.M.; Hilt, S.; Lombardo, P.; Mulderij, G. Searching for allelopathic effects of submerged macrophytes on phytoplankton—state of the art and open questions. Hydrobiologia 2007, 584, 77–88. [Google Scholar] [CrossRef]
- Wu, Z.B.; Zhang, Y.Y.; Liu, B.Y. Allelopathy of Aquatic Macrophytes on Phytoplankton; Science Press: Beijing, China, 2016; pp. 44–80. [Google Scholar]
- Erhard, D.; Gross, E.M. Do environmental factors influence composition of potential allelochemicals in the submersed freshwater macrophyte Elodea nuttallii (Hydrocharitaceae)? Verh. Int. Verein. Limnol. 2005, 29, 287–291. [Google Scholar] [CrossRef][Green Version]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.d.P.; Abrahão, J. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, X.; Lin, F.; Grossart, H.P.; Nie, Z.; Sun, L.; Xu, C.; Shi, J. Continuous-release beads of natural allelochemicals for the long-term control of cyanobacterial growth: Preparation, release dynamics and inhibitory effects. Water Res. 2016, 95, 113–123. [Google Scholar] [CrossRef]
- Ni, L.; Acharya, K.; Ren, G.; Li, S.; Li, Y.; Li, Y. Preparation and characterization of anti-algal sustained-release granules and their inhibitory effects on algae. Chemosphere 2013, 91, 608–615. [Google Scholar] [CrossRef]
- Brundu, G.; Stinca, A.; Angius, L.; Bonanomi, G.; Celesti-Grapow, L.; D’Auria, G.; Griffo, R.; Migliozzi, A.; Motti, R.; Spigno, P. Pistia stratiotes L. and Eichhornia crassipes (Mart.) Solms.: Emerging invasive alien hydrophytes in Campania and Sardinia (Italy). EPPO Bull. 2012, 42, 568–579. [Google Scholar] [CrossRef]
- Yang, X.; Chen, S.; Zhang, R. Utilization of two invasive free-floating aquatic plants (Pistia stratiotes and Eichhornia crassipes) as sorbents for oil removal. Environ. Sci. Pollut. Res. 2014, 21, 781–786. [Google Scholar] [CrossRef]
- Ayanda, O.I.; Ajayi, T.; Asuwaju, F.P. Eichhornia crassipes (Mart.) Solms: Uses, Challenges, Threats, and Prospects. Sci. World J. 2020, 2020, 3452172. [Google Scholar] [CrossRef]
- Petr, T. Interactions between Fish and Aquatic Macrophytes in Inland Waters—A Review; FAO Fisheries Technical Paper; FAO: Rome, Italy, 2000; p. 185. [Google Scholar]
- Egertson, C.J.; Downing, J.A. Relationship of fish catch and composition to water quality in a suite of agriculturally eutrophic lakes. Can. J. Fish. Aquat. Sci. 2004, 61, 1784–1796. [Google Scholar] [CrossRef]

| Macrophyte Species | Allelopathically Active Compounds | Phytoplankton Species, Subjected to Inhibitory Effects | Literature Source |
| Hydrophytes | |||
| Spiked water-milfoil (Myriophyllum spicatum L.) | Tannins (telimagrandin II); Phenolic acids (gallic, ellagic, pyrogallic); Flavonoids ((+)-catechin); Fatty acids (nonanoic, oleic, petroselinic) | Limnothrix redekei (Goor) Meffert, Microcystis aeruginosa (Kützing) Kützing, Planktothrix agardhii (Gomont) Anagnostidis and Komárek, | [66,82,83] |
| Whorled water-milfoil (Myriophyllum verticillatum L.) | Phenylpropanoids (α-asarone) | Limnothrix redekei, Microcystis aeruginosa | [84,85] |
| Water caltrop (Trapa natans L.) | Phenolic acids (benzoic, p-hydroxybenzoic, salicylic, cinnamic, α-resorcylic, protocatechuic, coumaric, vanillic, gallic, caffeic, ferulic, syringic, ß-resorcylic and sinapinic) | Microcystis aeruginosa, phytoplankton in general | [5,69] |
| Yellow waterlily (Nuphar lutea (L.) Sm.) | Fatty acids (hexadecanoic acid, tetradecanoic acid); Esters of phthalic acid (dibutyl phthalate, diisobutyl phthalate) | Microcystis aeruginosa | [5] |
| Canadian waterweed (Elodea canadensis Michx.) | Flavonoids (luteolin 7-O-diglucuronide, apigenin 7-O-diglucuronide, chrysoeriol 7-O-diglucuronide) | Microcystis aeruginosa | [81,86] |
| Common water hyacinth (Pontederia crassipes Mart. = Eichhornia crassipes Mart.) | Amines (N-phenyl-1-naphthylamine, N-phenyl-2-naphthylamine); Fatty acids (linoleic acid) | Microcystis aeruginosa, Microcystis sp., green microalgae | [87,88] |
| Coontail (Ceratophyllum demersum L.) | Esters of carboxylic acids (3-hydroxy-2,2,4-trimethylpentyl ester of 2-methylpropanoic acid); Ethers of phthalic acid (dibutyl phthalate) | Aphanizomenon flosaquae Ralfs ex Bornet and Flahault, Microcystis aeruginosa, Pseudanabaena limnetica (Lemmermann) Komarek, Oscillatoria tenuis C. Agardh ex Gomont, green microalgae | [5,82,89] |
| Water lettuce (Pistia stratiotes L.) | Phenylpropanoids (α-asarone); Fatty acids (linoleic acid, γ-linolenic acid) | Microcystis aeruginosa, Synechococcus leopoliensis (Raciborski) Komárek, phytoplankton in general | [90,91] |
| Sago pondweed (Potamogeton pectinatus L.) | Terpenoids (lactone diterpenes) | Microcystis aeruginosa, Oscillatoria tenuis, green microalgae | [5,79,92] |
| Floating pondweed (Potamogeton natans L.) | Terpenoids (lactone diterpenes, furan diterpenes) | Microcystis aeruginosa, green microalgae | [93] |
| Floating fern (Salvinia natans L.) | Ethers of phthalic acid (dibutyl phthalate) | Microcystis aeruginosa | [94] |
| Helophytes (partly submerged plants) | |||
| Common reed (Phragmites communis Trin. (=Phragmites australis (Cav.) Trin. ex Steud)) | Phenolic acids (p-coumaric acid, ferulic acid, vanillic acid, syringic acid, caffeic acid, gallic acid); Fatty acids (tetradecanoic acid, palmitic acid, nonanoic acid and stearic acid) | Microcystis aeruginosa, Chlorella pyrenoidosa H.Chick | [65,95] |
| Narrowleaf cattail (Typha angustifolia L.) | Phenolic acids and their derivatives (syringic acid, isoferulic acid) | Dolichospermum flosaquae, Microcystis aeruginosa, | [96,97] |
| Broadleaf cattail (Typha latifolia L.) | Fatty acids (linoleic acid, α-linolenic acid) | Dolichospermum flosaquae, Microcystis aeruginosa, Chlorella vulgaris Beijerinck, Chlorella pyrenoidosa | [79,98,99] |
| Allelopathically Active Compounds | Structural | Mode of Action |
|---|---|---|
| Polyphenols | ||
| α-Asarone |  | Inhibition of respiration, inhibition of growth [79] |
| Gallic acid | 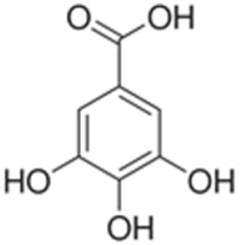 | Inhibition of photosystem II (PSII), reduction of chlorophyll content, oxidative stress [12,58,103] |
| Pyrogallic acid | 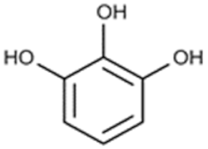 | |
| Ferulic acid | 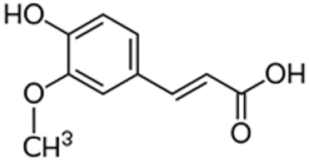 | Reduction of membrane potential, reduction of chlorophyll content [58] |
| Telimagrandin II | 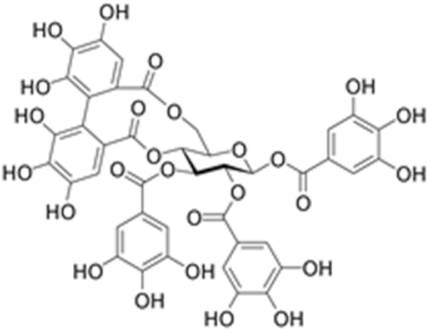 | Inhibition of photosystem II (PSII); inhibition of alkaline phosphatase activity [79,103] |
| Fatty acids/esters | ||
| Nonanoic acid | 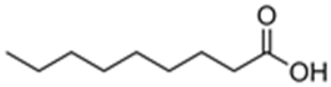 | Inhibition of oxygen evolution, inhibition of growth [103,104] |
| Linoleic acid |  | Oxidative stress, reduction of chlorophyll content, blocking the transport of electrons [58,105] |
| α-Linolenic acid |  | Inhibition of growth [58] |
| Ethyl 2-methylacetoacetate | 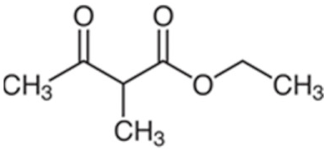 | Oxidative stress, changes in the structure of cell membranes, inhibition of growth [12,16,106] |
| Nitrogen-containing compounds | ||
| N-phenyl-1-naphthylamine |  | Oxidative stress, inhibition of growth [107] |
| Terpenoids | ||
| β-Ionone | 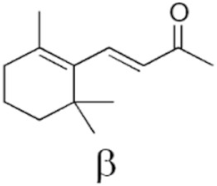 | Distortion of thylakoids [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nezbrytska, I.; Usenko, O.; Konovets, I.; Leontieva, T.; Abramiuk, I.; Goncharova, M.; Bilous, O. Potential Use of Aquatic Vascular Plants to Control Cyanobacterial Blooms: A Review. Water 2022, 14, 1727. https://doi.org/10.3390/w14111727
Nezbrytska I, Usenko O, Konovets I, Leontieva T, Abramiuk I, Goncharova M, Bilous O. Potential Use of Aquatic Vascular Plants to Control Cyanobacterial Blooms: A Review. Water. 2022; 14(11):1727. https://doi.org/10.3390/w14111727
Chicago/Turabian StyleNezbrytska, Inna, Oleg Usenko, Igor Konovets, Tetiana Leontieva, Igor Abramiuk, Mariia Goncharova, and Olena Bilous. 2022. "Potential Use of Aquatic Vascular Plants to Control Cyanobacterial Blooms: A Review" Water 14, no. 11: 1727. https://doi.org/10.3390/w14111727
APA StyleNezbrytska, I., Usenko, O., Konovets, I., Leontieva, T., Abramiuk, I., Goncharova, M., & Bilous, O. (2022). Potential Use of Aquatic Vascular Plants to Control Cyanobacterial Blooms: A Review. Water, 14(11), 1727. https://doi.org/10.3390/w14111727







