Characterization of Trophic Structure of Fish Assemblages in the East and South Seas of Korea Based on C and N Stable Isotope Ratios
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Sample Collection and Processing
2.3. Stable Isotope Analyses
2.4. Data Analyses
3. Results
3.1. Community Structure of Fish Assemblages
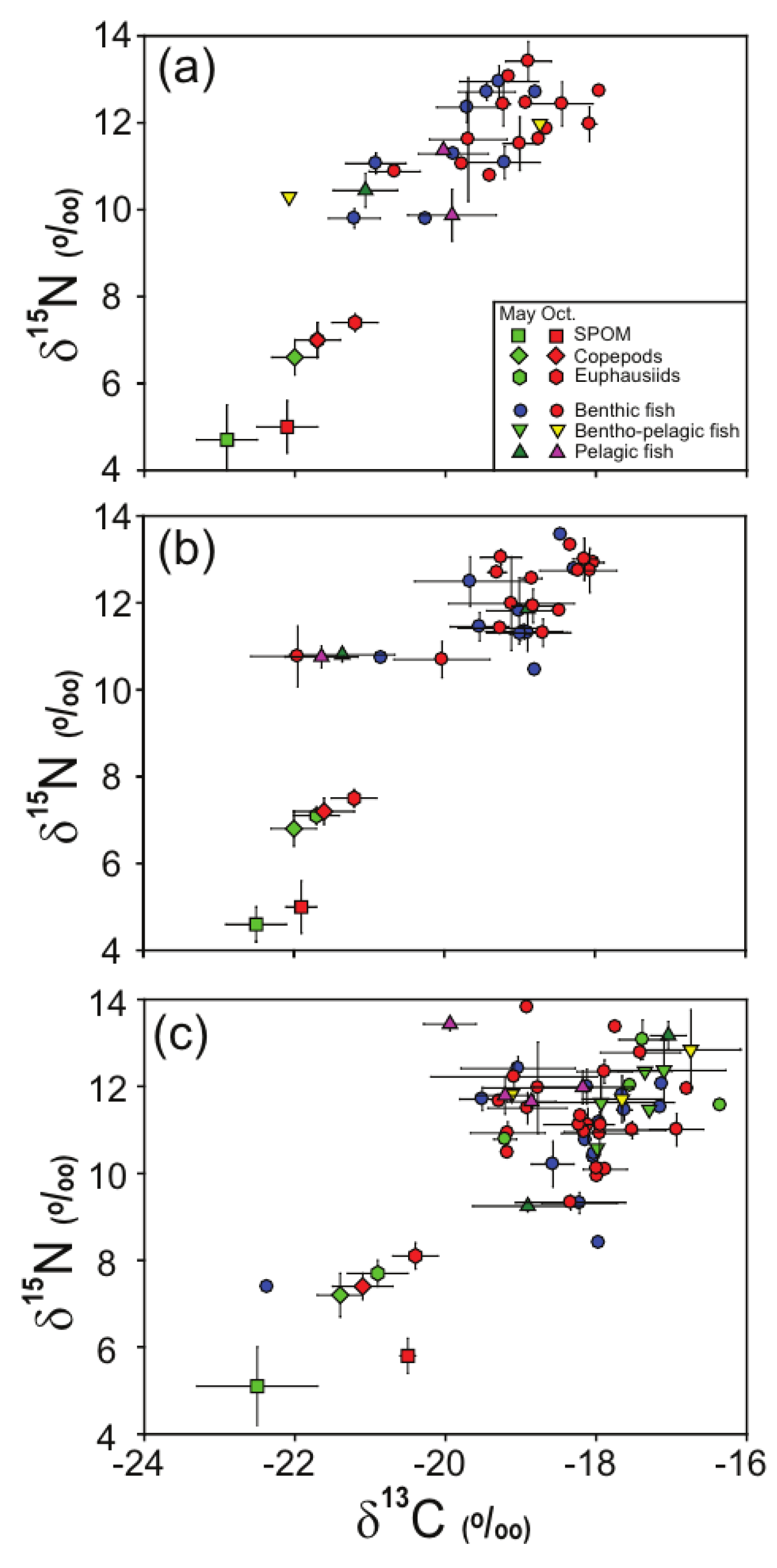
3.2. Stable Isotope Values of SPOM and Zooplankton
3.3. Stable Isotope Values of Fish Assemblages
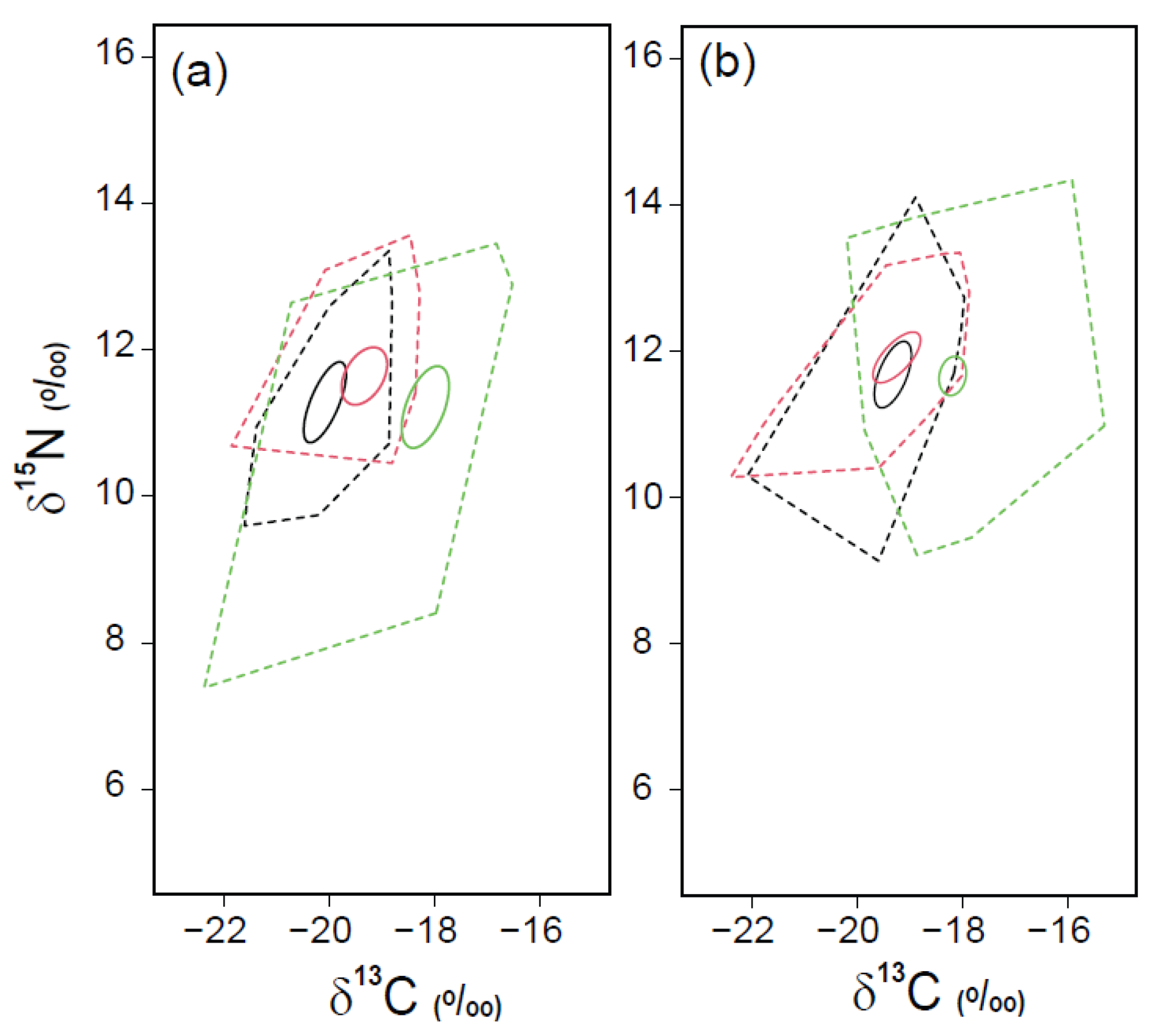
3.4. Trophic Positions and Isotopic Niche Areas of Fish Assemblages
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLusky, D.S.; Elliott, M. The Estuarine Ecosystem: Ecology Threats and Management; Oxford University Press: New York, NY, USA, 2004; p. 214. [Google Scholar]
- Akin, S.; Winemiller, K.O.; Gelwick, F.P. Seasonal and spatial variations in fish and macrocrustacean assemblage structure in Mad Island Marsh estuary, Texas. Estuar. Coast. Shelf Sci. 2003, 57, 269–282. [Google Scholar] [CrossRef]
- Costanza, R.; d’Arge, R.; de Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Naeem, S.; Limburg, K.; Paruelo, J.; O’Neill, R.V.; et al. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Dunson, W.A.; Travis, J. The role of abiotic factors in community organization. Am. Nat. 1991, 138, 1067–1091. [Google Scholar] [CrossRef]
- Thompson, R.M.; Brose, U.; Dunne, J.A.; Hall, R.O., Jr.; Hladyz, S.; Kitching, R.L.; Martinez, N.D.; Rantala, H.; Romanuk, T.N.; Stouffer, D.B.; et al. Food webs: Reconciling the structure and function of biodiversity. Trends Ecol. Evol. 2012, 27, 689–697. [Google Scholar] [CrossRef]
- McMeans, B.C.; McCann, K.S.; Humphries, M.; Rooney, N.; Fisk, A.T. Food web structure in temporally-forced ecosystems. Trends Ecol. Evol. 2015, 30, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Willis, T.V.; Wilson, K.A.; Johnson, B.J. Diets and stable isotope derived food web structure of fishes from the inshore Gulf of Maine. Estuar. Coast. 2017, 40, 889–904. [Google Scholar] [CrossRef]
- Johnson, C.R.; Seinen, I. Selection for restraint in competitive ability in spatial competition systems. Proc. R. Soc. Lond. B Biol. Sci. 2002, 269, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, C.-A.; Giraldo, C.; Cresson, P.; Ernande, B.; Travers-Trolet, M.; Rouquette, M.; Denamiel, M.; Lefebvre, S. Plasticity of trophic interactions in fish assemblages results in temporal stability of benthic-pelagic couplings. Mar. Environ. Res. 2021, 170, 105412. [Google Scholar] [CrossRef]
- Garrison, L.P.; Link, J.S. Dietary guild structure of the fish community in the Northeast United States continental shelf ecosystem. Mar. Ecol. Prog. Ser. 2000, 202, 231–240. [Google Scholar] [CrossRef]
- Pace, M.L.; Cole, J.J.; Carpenter, S.R.; Kitchell, J.F. Trophic cascades revealed in diverse ecosystems. Trends Ecol. Evol. 1999, 14, 483–488. [Google Scholar] [CrossRef]
- Park, T.H.; Lee, C.I.; Kang, C.K.; Kwak, J.H.; Lee, S.H.; Park, H.J. Seasonal variation in food web structure and fish community composition in the East/Japan sea. Estuar. Coast. 2020, 43, 615–629. [Google Scholar] [CrossRef]
- Pasquaud, S.; Elie, P.; Jeantet, C.; Billy, I.; Martinez, P.; Girardin, M. A preliminary investigation of the fish food web in the Gironde estuary France, using dietary and stable isotope analyses. Estuar. Coast. Shelf Sci. 2008, 78, 267–279. [Google Scholar] [CrossRef]
- Last, P.R.; White, W.T.; Gledhill, D.C.; Hobday, A.J.; Brown, R.; Edgar, G.J.; Pecl, G. Long-term shifts in abundance and distribution of a temperate fish fauna: A response to climate change and fishing practices. Glob. Ecol. Biogeogr. 2011, 20, 58–72. [Google Scholar] [CrossRef]
- Cury, P.; Shin, Y.J.; Planque, B.; Durant, J.; Fromentin, J.M.; Kramer-Schadt, S.; Stenseth, N.; Travers, M.; Grimm, V. Ecosystem oceanography for global change in fisheries. Trends Ecol. Evol. 2008, 23, 338–346. [Google Scholar] [CrossRef]
- Baker, R.; Fujiwara, M.; Minello, T.J. Juvenile growth and mortality effects on white shrimp Litopenaeus setiferus population dynamics in the northern Gulf of Mexico. Fish. Res. 2014, 155, 74–82. [Google Scholar] [CrossRef]
- Hertz, E.; Robinson, J.P.; Trudel, M.; Mazumder, A.; Baum, J.K. Estimation of predator-prey mass ratios using stable isotopes: Sources of errors. Mar. Ecol. Prog. Ser. 2014, 516, 1–6. [Google Scholar] [CrossRef]
- Michener, R.H.; Schell, D.M. Stable isotope ratios as tracers in marine aquatic food webs. In Stable Isotopes in Ecology and Environmental Science; Lajtha, K., Michener, R.H., Eds.; Blackwell Scientific: Oxford, UK, 1994; pp. 138–157. [Google Scholar]
- Boecklen, W.J.; Yarnes, C.T.; Cook, B.A.; James, A.C. On the use of stable isotopes in trophic ecology. Annu. Rev. Ecol. Syst. 2011, 42, 411–440. [Google Scholar] [CrossRef]
- Layman, C.A.; Araujo, M.S.; Boucek, R.; Hammerschlag-Peyer, C.M.; Harrison, E.; Jud, Z.R.; Post, D.M. Applying stable isotopes to examine food-web structure: An overview of analytical tools. Biol. Rev. 2012, 87, 545–562. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- Fry, B.; Sherr, E.B. δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contrib. Mar. Sci. 1984, 27, 13–47. [Google Scholar]
- Vander Zanden, M.J.; Rasmussen, J.B. Variation in 15N and 13C trophic fractionation: Implications for aquatic food web studies. Limnol. Oceanogr. 2001, 46, 2061–2066. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Jung, H.K.; Rahman, S.M.; Kang, C.-K.; Park, S.-Y.; Lee, S.H.; Park, H.J.; Kim, H.-W.; Lee, C.I. The influence of climate regime shifts on the marine environment and ecosystems in the East Asian Marginal Seas and their mechanisms. Deep. Res. Part II Top. Stud. Oceanogr. 2017, 143, 110–120. [Google Scholar] [CrossRef]
- Tian, Y.; Kidokoro, H.; Watanabe, T. Long-term changes in the fish community structure from the Tsushima warm current region of the Japan/East Sea with an emphasis on the impacts of fishing and climate regime shift over the last four decades. Prog. Oceanogr. 2006, 68, 217–237. [Google Scholar] [CrossRef]
- Lee, J.C. Water mass distribution and currents in the vicinity of the Hupo Bank in summer 2010. Korean J. Fish. Aquat. Sci. 2016, 49, 61–73. [Google Scholar]
- Sweeting, C.J.; Polunin, N.V.C.; Jennings, S. Effects of chemical lipid extraction and arithmetic lipid correction on stable isotope ratios of fish tissues. Rapid Commun. Mass Spectrom. 2006, 20, 595–601. [Google Scholar] [CrossRef]
- Post, D.M.; Layman, C.A.; Arrington, D.A.; Takimoto, G.; Quattrochi, J.; Montaña, C.G. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef]
- Anderson, M.J.; Clarke, K.R.; Gorley, R.N. PERMANOVA+ for Primer: Guide to Software and Statistical Methods; University of Auckland and PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Layman, C.A.; Arrington, D.A.; Montaña, C.G.; Post, D.M. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 2007, 88, 42–48. [Google Scholar] [CrossRef]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef]
- Han, K.H. Fish fauna in the south sea of Korea. In Current and Preservarion of Korean Fishes; Ichthyol Soc Korea Symp: Seoul, Korea, 2003; pp. 37–76. [Google Scholar]
- Choi, K.H.; Han, M.H.; Kang, C.K.; Park, J.M.; Choi, J.H.; Park, J.H.; Lee, C.I. Seasonal variations in species composition of fish assemblage collected by trammel net in coastal waters of the East Sea. J. Korean Soc. Fish. Ocean. Technol. 2012, 48, 415–427. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, Y.G.; Park, J.Y.; Kim, J.K.; Ryu, J.H.; Kang, C.B.; Park, J.H. Comparison of Fish Species Composition Collected by Set Net at Hupo in Gyeong-Sang-Buk-Do, and Jangho in Gang-Won-Do, Korea. Korean J. Fish. Aquat. Sci. 2014, 47, 424–430. [Google Scholar]
- Moon, D.Y.; Jeong, H.G.; Myoung, J.-G.; Choi, J.H.; Kwun, H.J.; Back, J.W.; Hong, S.Y.; Kim, S.Y. Fish Species Collected by the Fish Collection Project from the Southern Sea of Korea during 2010–2012. Korean J. Fish. Aquat. Sci. 2015, 48, 507–528. [Google Scholar]
- Lee, T.W. Seasonal variation in species composition of demersal fish in the coastal water off Uljin and Hupo in the East Sea of Korea in 2002. Korean J. Ichthyol. 2011, 23, 187–197. [Google Scholar]
- Sohn, M.H.; Park, J.H.; Yoon, B.S.; Choi, Y.M.; Kim, J.K. Species composition and community structure of demersal fish caught by a danish seine fishery in the coastal waters of the middle and southern East Sea, Korea. Korean J. Fish. Aquat. Sci. 2015, 48, 529–541. [Google Scholar]
- Song, S.H.; Jeong, J.M.; Lee, S.H.; Kim, D.H. Species composition and community structure of fish by shrimp beam trawl between Sacheon Bay and coastal waters off Namhae, Korea. J. Korean Soc. Fish. Technol. 2019, 55, 217–232. [Google Scholar] [CrossRef]
- Stephens, J.S.; Hose, J.E.; Love, M.S. Fish assemblages as indicators of environmental change in nearshore environments. In Marine Organisms as Indicators; Springer: New York, NY, USA, 1988. [Google Scholar]
- Pankhurst, N.W.; Munday, P.L. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 2011, 62, 1015–1026. [Google Scholar] [CrossRef]
- Cifuentes, L.A.; Sharp, J.H.; Fogel, M.L. Stable carbon and nitrogen isotope biogeochemistry in the Delaware Estuary. Limnol. Oceanogr. 1988, 33, 1102–1115. [Google Scholar] [CrossRef]
- Goering, J.; Alexander, B.; Haubenstock, N. Seasonal variability of stable carbon and nitrogen isotope ratios of organisms in a N. Pacific bay. Estuar. Coast Shelf. Sci. 1990, 30, 239–260. [Google Scholar] [CrossRef]
- Fry, B. 13C/12C fractionation by marine diatoms. Mar. Ecol. Prog. Ser. 1996, 134, 283–294. [Google Scholar] [CrossRef]
- Kang, C.K.; Choy, E.J.; Hur, Y.B.; Myeong, J.I. Isotopic evidence of particle size-dependent food partitioning in cocultured sea squirt Halocynthia roretzi and Pacific oyster Crassostrea gigas. Aquat. Biol. 2009, 6, 289–302. [Google Scholar] [CrossRef][Green Version]
- Davenport, S.R.; Bax, N.J. A trophic study of a marine ecosystem off southeastern Australia using stable isotopes of carbon and nitrogen. Can. J. Fish. Aquat. Sci. 2002, 59, 514–530. [Google Scholar] [CrossRef]
- Boyle, M.D.; Ebert, D.A.; Cailliet, G.M. Stable isotope analysis of a deep-sea benthic-fish assemblage: Evidence of an enriched benthic food web. J. Fish Biol. 2012, 80, 1485–1507. [Google Scholar] [CrossRef] [PubMed]
- Galván, D.E.; Sweeting, C.J.; Reid, W.D.K. Power of stable isotope techniques to detect size-based feeding in marine fishes. Mar. Ecol. Prog. Ser. 2010, 407, 271–278. [Google Scholar] [CrossRef]
- Elliott, M.; Whitfield, A.K.; Potter, I.C.; Blaber, S.J.; Cyrus, D.P.; Nordlie, F.G.; Harrison, T.D. The guild approach to categorizing estuarine fish assemblages: A global review. Fish Fish. 2007, 8, 241–268. [Google Scholar] [CrossRef]
- Gay, M.; Bao, M.; MacKenzie, K.; Pascual, S.; Buchmann, K.; Bourgau, O.; Levsen, A. Infection levels and species diversity of ascaridoid nematodes in Atlantic cod, Gadus morhua, are correlated with geographic area and fish size. Fish. Res. 2017, 202, 90–102. [Google Scholar] [CrossRef]
- Rigolet, C.; Thiébaut, É.; Brind’Amour, A.; Dubois, S.F. Investigating isotopic functional indices to reveal changes in the structure and functioning of benthic communities. Funct. Ecol. 2015, 29, 1350–1360. [Google Scholar] [CrossRef]
- Cherel, Y.; Hobson, K.A.; Guinet, C.; Vanpe, C. Stable isotopes document seasonal changes in trophic niches and winter foraging individual specialization in diving predators from the Southern Ocean. J. Anim. Ecol. 2007, 76, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Włodarska-Kowalczuk, M.; Aune, M.; Michel, L.N.; Zaborska, A.; Legeżyńska, J. Is the trophic diversity of marine benthic consumers decoupled from taxonomic and functional trait diversity? Isotopic niches of Arctic communities. Limnol. Oceanogr. 2019, 64, 2140–2151. [Google Scholar] [CrossRef]
- Kingsbury, K.M.; Gillanders, B.M.; Booth, D.J.; Nagelkerken, I. Trophic niche segregation allows range-extending coral reef fishes to co-exist with temperate species under climate change. Glob. Chang. Biol. 2020, 26, 721–733. [Google Scholar] [CrossRef] [PubMed]

| Month | Water Temperature (°C) | Salinity | Chl a (µg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surface | Bottom | Surface | Bottom | Surface | ||||||
| May | October | May | October | May | October | May | October | May | October | |
| St. A | 15.3 | 16.2 | 3.0 | 15.7 | 34.5 | 32.7 | 34.0 | 34.0 | 1.4 | 0.3 |
| St. B | 14.9 | 16.5 | 1.0 | 16.6 | 32.4 | 32.6 | 34.0 | 34.0 | 0.7 | 0.7 |
| St. C | 20.3 | 24.7 | 14.8 | 24.5 | 33.9 | 33.8 | 34.5 | 34.4 | 1.0 | 0.6 |
| Species Name | May | October | ||||
|---|---|---|---|---|---|---|
| St. A | St. B | St. C | St. A | St. B | St. C | |
| Total species number | 16 | 12 | 47 | 19 | 19 | 36 |
| Total individuals | 8995 | 27,906 | 17,067 | 61,053 | 13,715 | 40,800 |
| Richness (R) | 1.65 | 1.08 | 4.72 | 1.63 | 1.89 | 3.30 |
| Evenness (J) | 0.80 | 0.56 | 0.70 | 0.44 | 0.57 | 0.45 |
| Diversity (H′) | 2.21 | 1.40 | 2.69 | 1.31 | 1.67 | 1.62 |
| Potential Food Source | May | October | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| δ13C | δ15N | δ13C | δ15N | |||||||
| n | Mean | SD | Mean | SD | n | Mean | SD | Mean | SD | |
| St. A | ||||||||||
| SPOM | 5 | −22.9 | 0.4 | 4.7 | 0.8 | 5 | −22.1 | 0.4 | 5.0 | 0.6 |
| Copepods | 4 | −22.0 | 0.3 | 6.6 | 0.4 | 4 | −21.7 | 0.3 | 7.0 | 0.4 |
| Euphausiids | 4 | −21.7 | 0.3 | 7.0 | 0.4 | 4 | −21.2 | 0.3 | 7.4 | 0.2 |
| St. B | ||||||||||
| SPOM | 5 | −22.5 | 0.4 | 4.6 | 0.4 | 5 | −21.9 | 0.2 | 5.0 | 0.6 |
| Copepods | 4 | −22.0 | 0.3 | 6.8 | 0.4 | 4 | −21.6 | 0.4 | 7.2 | 0.3 |
| Euphausiids | 4 | −21.7 | 0.3 | 7.1 | 0.2 | 4 | −21.2 | 0.3 | 7.5 | 0.2 |
| St. C | ||||||||||
| SPOM | 5 | −22.5 | 0.8 | 5.1 | 0.9 | 5 | −20.5 | 0.1 | 5.8 | 0.4 |
| Copepods | 4 | −21.4 | 0.3 | 7.2 | 0.5 | 4 | −21.1 | 0.4 | 7.4 | 0.3 |
| Euphausiids | 4 | −20.9 | 0.4 | 7.7 | 0.3 | 4 | −20.4 | 0.3 | 8.1 | 0.3 |
| PERMANOVA test | Season | Site | Interaction | |||||||
| pseudo-F | p | pseudo-F | p | pseudo-F | p | |||||
| SPOM | 15.70 | 0.001 | 7.18 | 0.004 | 1.31 | 0.311 | ||||
| Copepods | 5.42 | 0.029 | 5.47 | 0.012 | 0.21 | 0.896 | ||||
| Euphausiids | 14.32 | 0.001 | 15.25 | 0.001 | Negative | |||||
| Species Name | St. A | St. B | St. C | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | δ13C | δ15N | TP | n | δ13C | δ15N | TP | n | δ13C | δ15N | TP | |||||||
| Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | |||||||
| Fish | ||||||||||||||||||
| Ammodytes personatus (b) | 3 | −20.3 | 0.1 | 9.8 | 0.0 | 2.9 | ||||||||||||
| Anisarchus macrops (b) | 2 | −19.5 | 0.4 | 11.4 | 0.3 | 3.4 | ||||||||||||
| Arctoscopus japonicus (b) | 1 | −20.8 | 10.7 | 3.2 | ||||||||||||||
| Banjos banjos (b) | 1 | −17.1 | 11.5 | 3.3 | ||||||||||||||
| Caragoides equula (b) | 3 | −19.5 | 0.3 | 11.7 | 0.3 | 3.3 | ||||||||||||
| Chelidoperca hirundinacea (b) | 1 | −18.0 | 10.4 | 2.9 | ||||||||||||||
| Clupea pallasii (p) * | 3 | −21.1 | 0.4 | 10.4 | 0.4 | 3.1 | ||||||||||||
| Coelorhynchus longissimus (bp) | 1 | −18.0 | 10.6 | 3.0 | ||||||||||||||
| Conger myriaster (b) * | 3 | −19.0 | 0.8 | 12.4 | 0.3 | 3.5 | ||||||||||||
| Dasycottus setiger (b) | 3 | −19.0 | 0.4 | 11.8 | 0.8 | 3.5 | ||||||||||||
| Foetorepus altivelis (b) | 1 | −18.0 | 8.4 | 2.4 | ||||||||||||||
| Gadus macrocephalus (b) | 2 | −19.7 | 0.4 | 12.4 | 0.3 | 3.7 | 4 | −19.7 | 0.7 | 12.5 | 0.6 | 3.7 | ||||||
| Glyptocephalus stelleri (b) | 3 | −19.2 | 0.5 | 11.1 | 0.4 | 3.3 | 3 | −18.9 | 0.5 | 11.3 | 0.2 | 3.3 | ||||||
| Halieutaea stellata (b) | 1 | −17.1 | 12.1 | 3.4 | ||||||||||||||
| Hemilepidotus gilberti (b) | 1 | −18.8 | 12.7 | 3.8 | 1 | −18.5 | 13.6 | 4.0 | ||||||||||
| Hemitripterus villosus (b) | 3 | −19.4 | 0.4 | 12.7 | 0.2 | 3.8 | ||||||||||||
| Hippoglossoides dubius (b) | 3 | −19.9 | 0.5 | 11.3 | 0.1 | 3.4 | 3 | −18.9 | 0.5 | 11.3 | 0.4 | 3.3 | ||||||
| Hoplichthys giberti (b) | 2 | −18.2 | 0.5 | 9.3 | 0.2 | 2.6 | ||||||||||||
| Icelus cataphractus (b) | 3 | −19.0 | 0.1 | 11.3 | 0.2 | 3.3 | ||||||||||||
| Laeops kitaharae (b) | 1 | −22.4 | 7.4 | 2.1 | ||||||||||||||
| Lepidotrigla microptera (b) | 1 | −18.0 | 11.2 | 3.2 | ||||||||||||||
| Limanda schrencki (b) | 1 | −18.0 | 10.5 | 3.0 | ||||||||||||||
| Malakichthys wakiyae (p) | 3 | −18.9 | 0.7 | 9.2 | 0.1 | 2.6 | ||||||||||||
| Pagrus major (b) | 2 | −18.1 | 0.1 | 12.0 | 0.4 | 3.4 | ||||||||||||
| Pampus argenteus (bp) | 1 | −17.3 | 11.5 | 3.3 | ||||||||||||||
| Plectranthias kelloggi azumanus (b) | 1 | −18.1 | 10.8 | 3.0 | ||||||||||||||
| Pleurogrammus azonus (b) * | 3 | −21.2 | 0.3 | 9.8 | 0.2 | 2.9 | ||||||||||||
| Podothecus thompsoni (b) | 1 | −18.3 | 12.8 | 3.8 | ||||||||||||||
| Psenopsis anomala (bp) | 2 | −17.9 | 1.0 | 11.6 | 0.8 | 3.3 | ||||||||||||
| Pseudorhombus cinnamoneus (b) | 3 | −18.6 | 0.3 | 10.2 | 0.5 | 2.9 | ||||||||||||
| Pseudorhombus pentophthalmus (b) | 2 | −17.6 | 0.1 | 11.5 | 0.2 | 3.3 | ||||||||||||
| Scorpaenodes littoralis (b) | 1 | −17.7 | 11.8 | 3.4 | ||||||||||||||
| Sebastes owstoni (b) * | 3 | −20.9 | 0.4 | 11.1 | 0.2 | 3.3 | 1 | −18.8 | 10.5 | 3.1 | ||||||||
| Sphyraena pinguis (p) | 4 | −17.0 | 0.2 | 13.2 | 0.3 | 3.8 | ||||||||||||
| Taurocottus bergi (b) | 3 | −19.3 | 0.5 | 12.9 | 0.4 | 3.9 | ||||||||||||
| Zenopsis nebulosa (bp) | 2 | −17.1 | 0.8 | 12.4 | 0.7 | 3.5 | ||||||||||||
| Zeus faber (bp) | 2 | −17.4 | 0.4 | 12.4 | 0.1 | 3.5 | ||||||||||||
| Cephalopod | ||||||||||||||||||
| Euprymna morsei (bp) | 1 | −18.9 | 11.9 | 3.5 | ||||||||||||||
| Watasenia scintillans (p) | 2 | −21.4 | 0.7 | 10.8 | 0.2 | 3.2 | ||||||||||||
| Species Name | St. A | St. B | St. C | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | δ13C | δ15N | TP | n | δ13C | δ15N | TP | n | δ13C | δ15N | TP | |||||||
| Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | |||||||
| Fish | ||||||||||||||||||
| Allolepis hollandi (b) | 2 | −18.8 | 0.1 | 12.6 | 0.0 | 3.6 | ||||||||||||
| Anoplagonus occidentalis (b) | 1 | −19.8 | 11.1 | 3.2 | ||||||||||||||
| Argentina kagoshimae (b) | 6 | −18.9 | 0.5 | 11.5 | 0.4 | 3.3 | ||||||||||||
| Ascoldia variegata (b) | 2 | −18.9 | 0.3 | 13.4 | 0.4 | 3.9 | ||||||||||||
| Aulopus japonicus (b) | 2 | −18.2 | 0.3 | 11.0 | 0.0 | 3.1 | ||||||||||||
| Beringraja pulchra (b) | 1 | −16.4 | 11.6 | 3.3 | ||||||||||||||
| Caragoides equula (b) | 4 | −18.8 | 0.7 | 12.0 | 1.0 | 3.4 | ||||||||||||
| Chelidonichthys spinosus (b) | 1 | −15.3 | 11.0 | 3.1 | ||||||||||||||
| Chelidoperca hirundinacea (b) | 2 | −18.1 | 0.0 | 11.1 | 0.4 | 3.2 | ||||||||||||
| Clupea pallasii (p) * | 1 | −20.0 | 11.4 | 3.3 | 4 | −21.6 | 0.5 | 10.8 | 0.3 | 3.0 | ||||||||
| Conger myriaster (b) * | 2 | −19.1 | 1.1 | 12.2 | 0.3 | 3.5 | ||||||||||||
| Cookeolus japonicus (b) | 1 | −19.2 | 10.5 | 3.0 | ||||||||||||||
| Cottiusculus gonez (b) | 1 | −19.4 | 10.8 | 3.1 | 2 | −19.3 | 0.6 | 11.4 | 0.0 | 3.2 | ||||||||
| Dentex tumifrons (b) | 6 | −17.9 | 0.4 | 12.3 | 0.3 | 3.5 | ||||||||||||
| Echelus uropterus (b) | 1 | −16.8 | 11.9 | 3.4 | ||||||||||||||
| Enophrys diceraus (b) | 1 | −18.0 | 12.7 | 3.7 | ||||||||||||||
| Eopsetta grigorjewi (b) | 2 | −17.5 | 0.4 | 11.0 | 0.2 | 3.1 | ||||||||||||
| Foetorepus altivelis (b) | 2 | −18.3 | 0.7 | 9.3 | 0.2 | 2.6 | ||||||||||||
| Gadus macrocephalus (b) | 5 | −19.7 | 0.5 | 11.6 | 1.4 | 3.4 | 5 | −19.1 | 0.8 | 12.0 | 1.1 | 3.4 | ||||||
| Gymnocanthus herzensteini (b) * | 2 | −18.1 | 0.1 | 12.0 | 0.4 | 3.5 | 2 | −18.1 | 12.7 | 0.5 | 3.6 | |||||||
| Hippoglossoides dubius (b) | 6 | −18.7 | 0.4 | 11.3 | 0.3 | 3.2 | ||||||||||||
| Hoplichthys giberti (b) | 2 | −17.9 | 0.3 | 10.1 | 0.0 | 2.8 | ||||||||||||
| Hoplobrotula armata (b) | 1 | −17.5 | 12.0 | 3.4 | ||||||||||||||
| Hypsagonus quadricornis (b) | 1 | −18.9 | 12.5 | 3.6 | ||||||||||||||
| Icelus cataphractus (b) | 6 | −18.8 | 0.3 | 11.9 | 0.4 | 3.4 | ||||||||||||
| Leiognathus nuchalis (b) | 2 | −19.9 | 0.3 | 13.4 | 0.2 | 3.8 | ||||||||||||
| Lepidotrigla microptera (b) | 4 | −18.2 | 0.5 | 11.1 | 0.2 | 3.2 | ||||||||||||
| Leptagonus leptorhynchus (b) | 3 | −18.5 | 0.4 | 12.4 | 0.5 | 3.6 | 2 | −18.0 | 0.1 | 12.9 | 0.0 | 3.7 | ||||||
| Liparis tessellatus (b) | 2 | −20.0 | 0.6 | 10.7 | 0.4 | 3.0 | ||||||||||||
| Lophius litulon (b) | 4 | −17.4 | 0.5 | 12.8 | 0.2 | 3.6 | ||||||||||||
| Lycodes nakamurae (b) | 2 | −19.3 | 0.1 | 12.7 | 0.1 | 3.6 | ||||||||||||
| Maurolicus muelleri (b) | 2 | −22.0 | 0.6 | 10.8 | 0.7 | 3.0 | ||||||||||||
| Monocentris japonica (b) | 1 | −17.7 | 13.4 | 3.8 | ||||||||||||||
| Petroschmidtia toyamensis (b) | 2 | −18.2 | 0.5 | 12.7 | 0.1 | 3.6 | ||||||||||||
| Plectranthias kelloggi azumanus (b) | 1 | −18.0 | 9.9 | 2.8 | ||||||||||||||
| Podothecus thompsoni (b) | 4 | −19.2 | 0.1 | 12.4 | 0.5 | 3.6 | 2 | −18.1 | 0.1 | 13.0 | 0.5 | 3.7 | ||||||
| Psenopsis anomala (b) | 1 | −19.1 | 11.8 | 3.4 | ||||||||||||||
| Repomucenus ornatipinnis (b) | 1 | −17.9 | 11.1 | 3.1 | ||||||||||||||
| Saurida undosquamis (b) | 1 | −18.9 | 13.8 | 3.9 | ||||||||||||||
| Sebastes owstoni (b) * | 2 | −20.7 | 0.3 | 10.9 | 0.1 | 3.1 | ||||||||||||
| Sebastiscus marmoratus (b) | 2 | −16.9 | 0.4 | 11.0 | 0.4 | 3.1 | ||||||||||||
| Stichaeus grigorjewi (b) | 2 | −19.2 | 0.3 | 13.0 | 0.2 | 3.7 | ||||||||||||
| Synagrops philippinensis (b) | 4 | −19.2 | 0.5 | 10.9 | 0.3 | 3.1 | ||||||||||||
| Synodus macrops (b) | 2 | −18.2 | 0.1 | 11.3 | 0.0 | 3.2 | ||||||||||||
| Taurocottus bergi (bp) | 1 | −19.2 | 13.1 | 3.8 | 1 | −18.3 | 13.3 | 3.8 | ||||||||||
| Thamnaconus modestus (b) | 2 | −17.4 | 0.3 | 13.1 | 0.5 | 3.7 | ||||||||||||
| Trachurus japonicus (p) * | 4 | −19.2 | 0.4 | 11.8 | 0.4 | 3.4 | ||||||||||||
| Triglops jordani (b) | 1 | −18.7 | 11.9 | 3.4 | 1 | −18.5 | 11.8 | 3.4 | ||||||||||
| Upeneus japonicus (b) | 3 | −18.4 | 0.9 | 11.2 | 0.6 | 3.2 | ||||||||||||
| Zenopsis nebulosa (bp) | 2 | −17.7 | 0.5 | 11.7 | 0.5 | 3.3 | ||||||||||||
| Zeus faber (bp) | 6 | −16.7 | 0.7 | 12.8 | 0.9 | 3.7 | ||||||||||||
| Cephalopod | ||||||||||||||||||
| Berryteuthis magister (bp) | 1 | −22.1 | 10.3 | 3.0 | ||||||||||||||
| Enteroctopus dofleini (b) * | 4 | −19.0 | 0.2 | 11.5 | 0.6 | 3.3 | ||||||||||||
| Euprymna morsei (bp) | 1 | −18.7 | 12.0 | 3.5 | ||||||||||||||
| Loligo bleekeri (p) | 3 | −19.2 | 0.2 | 10.8 | 0.1 | 3.1 | ||||||||||||
| Loligo beka (p) | 4 | −18.2 | 0.7 | 12.0 | 0.4 | 3.4 | ||||||||||||
| Loligo japonica (p) | 2 | −18.9 | 0.3 | 11.6 | 0.1 | 3.3 | ||||||||||||
| Octopus longispadiceus (b) | 1 | −18.8 | 11.6 | 3.4 | ||||||||||||||
| Sepia esculenta (b) | 2 | −18.0 | 0.0 | 10.1 | 0.1 | 2.9 | ||||||||||||
| Todarodes pacificus (p) | 4 | −19.9 | 0.6 | 9.9 | 0.6 | 2.8 | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, D.; Park, T.H.; Lee, C.-I.; Hwang, K.; Kim, D.N.; Lee, S.-J.; Kang, S.; Park, H.J. Characterization of Trophic Structure of Fish Assemblages in the East and South Seas of Korea Based on C and N Stable Isotope Ratios. Water 2022, 14, 58. https://doi.org/10.3390/w14010058
Shin D, Park TH, Lee C-I, Hwang K, Kim DN, Lee S-J, Kang S, Park HJ. Characterization of Trophic Structure of Fish Assemblages in the East and South Seas of Korea Based on C and N Stable Isotope Ratios. Water. 2022; 14(1):58. https://doi.org/10.3390/w14010058
Chicago/Turabian StyleShin, Donghoon, Tae Hee Park, Chung-Il Lee, Kangseok Hwang, Doo Nam Kim, Seung-Jong Lee, Sukyung Kang, and Hyun Je Park. 2022. "Characterization of Trophic Structure of Fish Assemblages in the East and South Seas of Korea Based on C and N Stable Isotope Ratios" Water 14, no. 1: 58. https://doi.org/10.3390/w14010058
APA StyleShin, D., Park, T. H., Lee, C.-I., Hwang, K., Kim, D. N., Lee, S.-J., Kang, S., & Park, H. J. (2022). Characterization of Trophic Structure of Fish Assemblages in the East and South Seas of Korea Based on C and N Stable Isotope Ratios. Water, 14(1), 58. https://doi.org/10.3390/w14010058









