Treatment of Actual Winery Wastewater by Fenton-like Process: Optimization to Improve Organic Removal, Reduce Inorganic Sludge Production and Enhance Co-Treatment at Municipal Wastewater Treatment Facilities
Abstract
1. Introduction
2. Materials and Methods
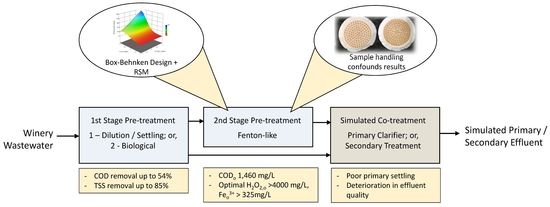
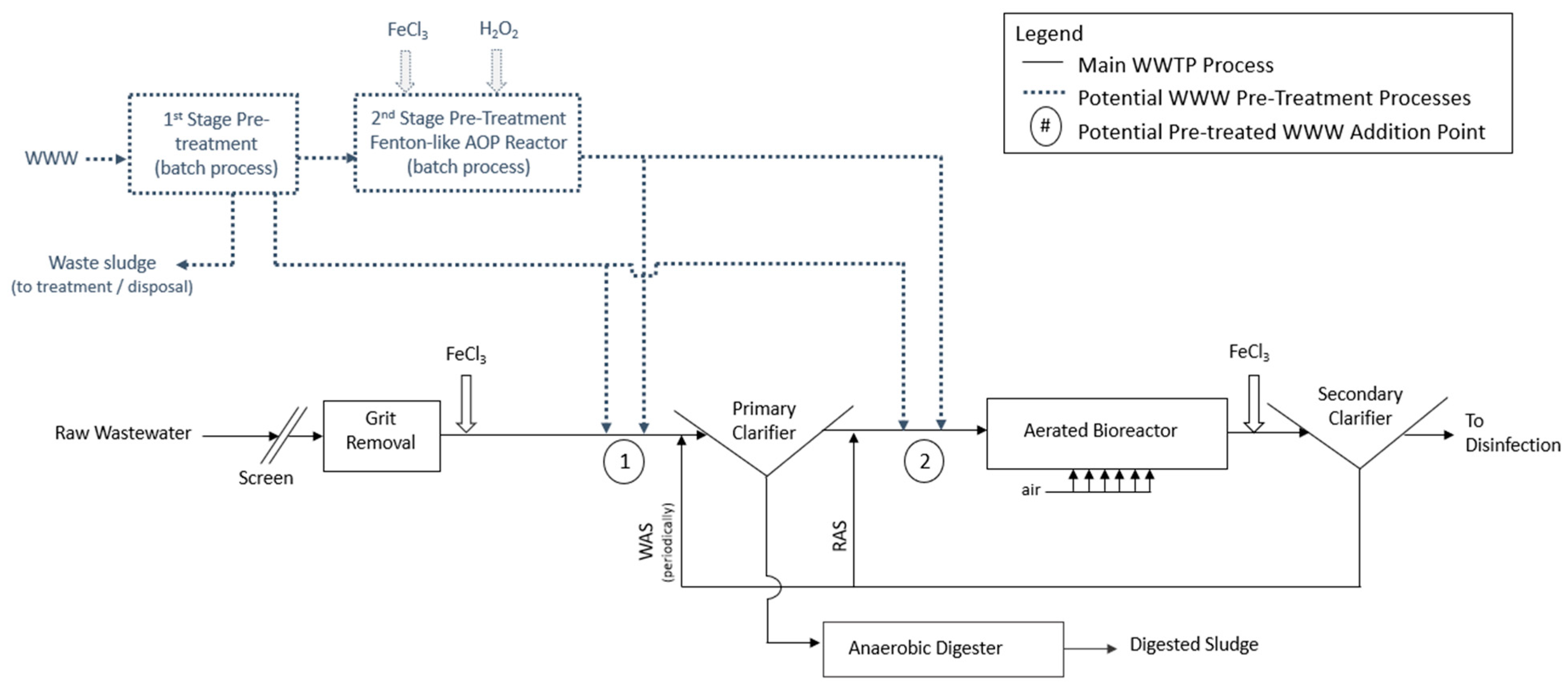
2.1. Overview of Fenton-like Pre-Treatment Concept
2.2. Chemicals
2.3. Winery Wastewater
2.4. Mixed Liquor
2.5. Experimental Methods
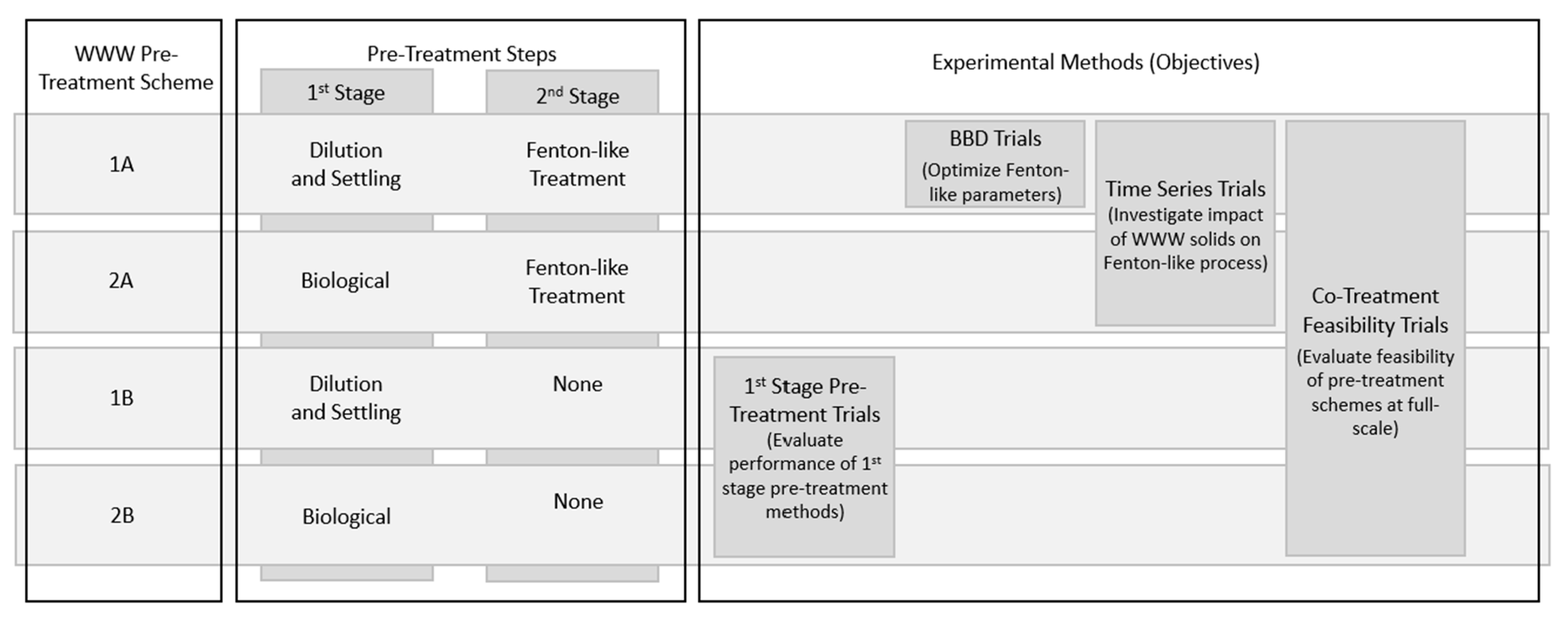
2.5.1. First Stage Pre-Treatment Trials
- Dilution and settling;
- Biological.
- Dilution and Settling
- Biological
2.5.2. BBD Fenton-like Trials
2.5.3. Fenton-like Time Series Trials
2.5.4. Co-Treatment Trials
- Simulated Primary Clarification
- Simulated Secondary Treatment
2.6. Analytical Methods
2.7. BBD Experimental Design and Optimization
3. Results and Discussion
3.1. Assessment of First Stage WWW Pre-Treatment Options
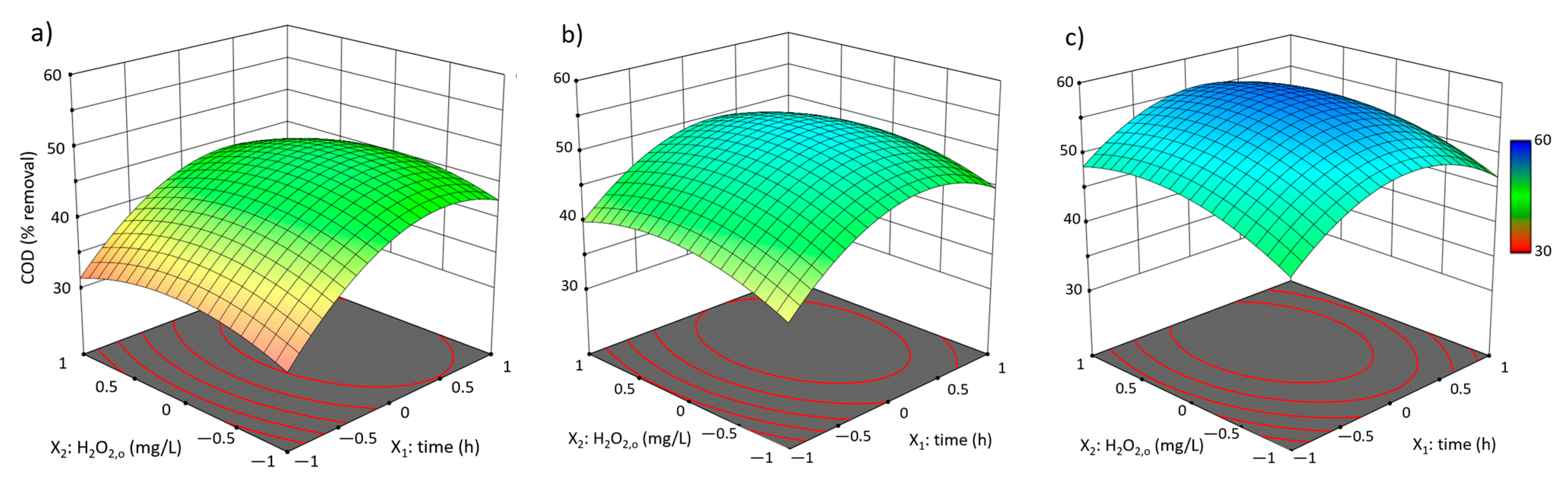
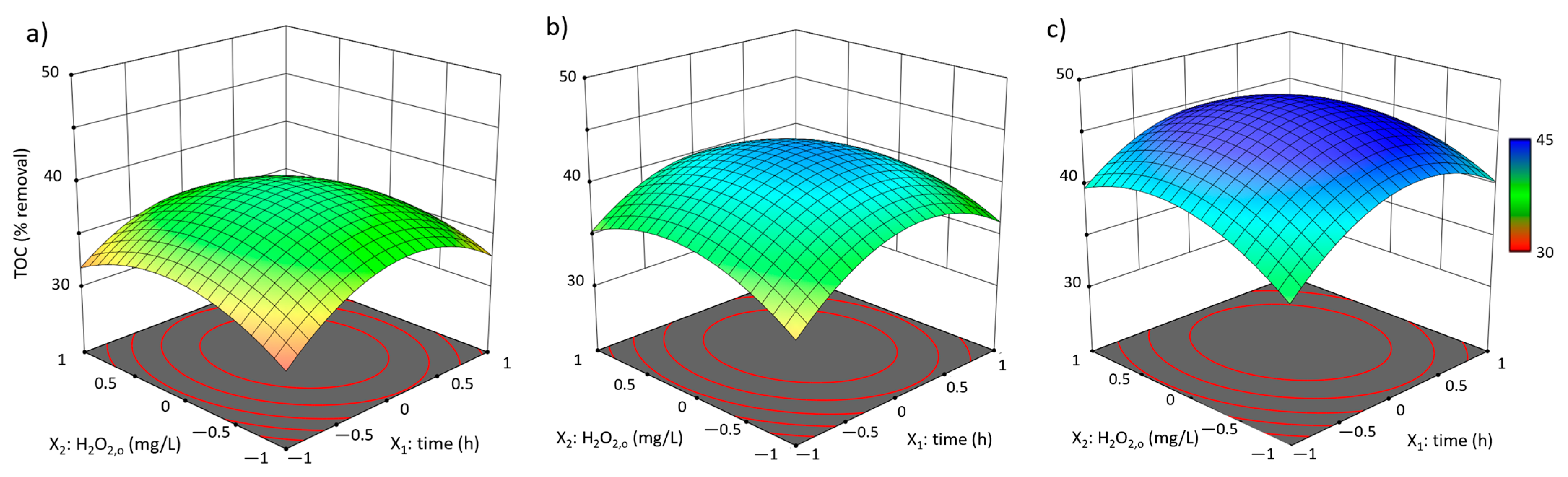
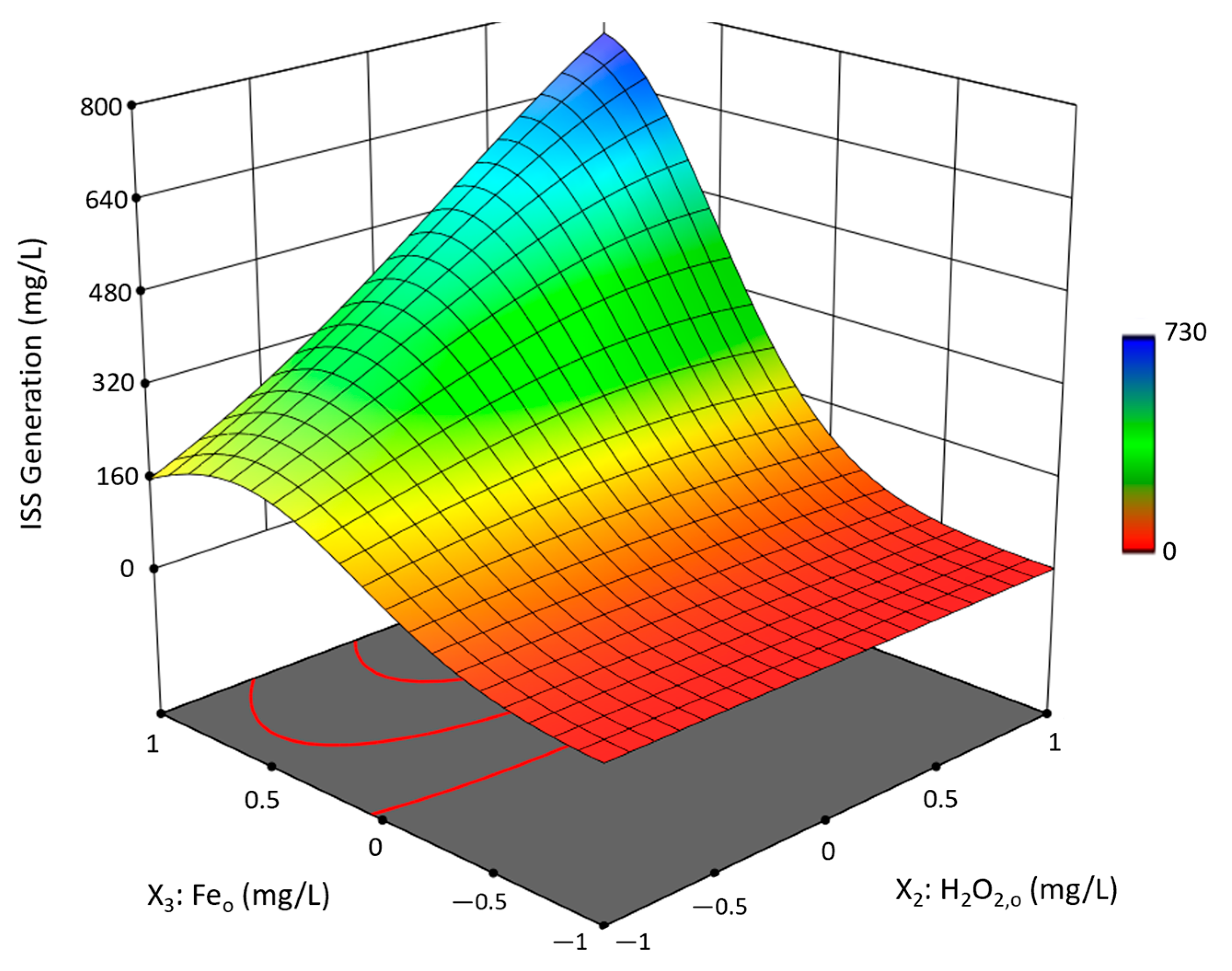
3.2. Box-Behnken Design Fenton-like Trials
3.2.1. Experimental Results and Model Development
3.2.2. Analysis and Discussion
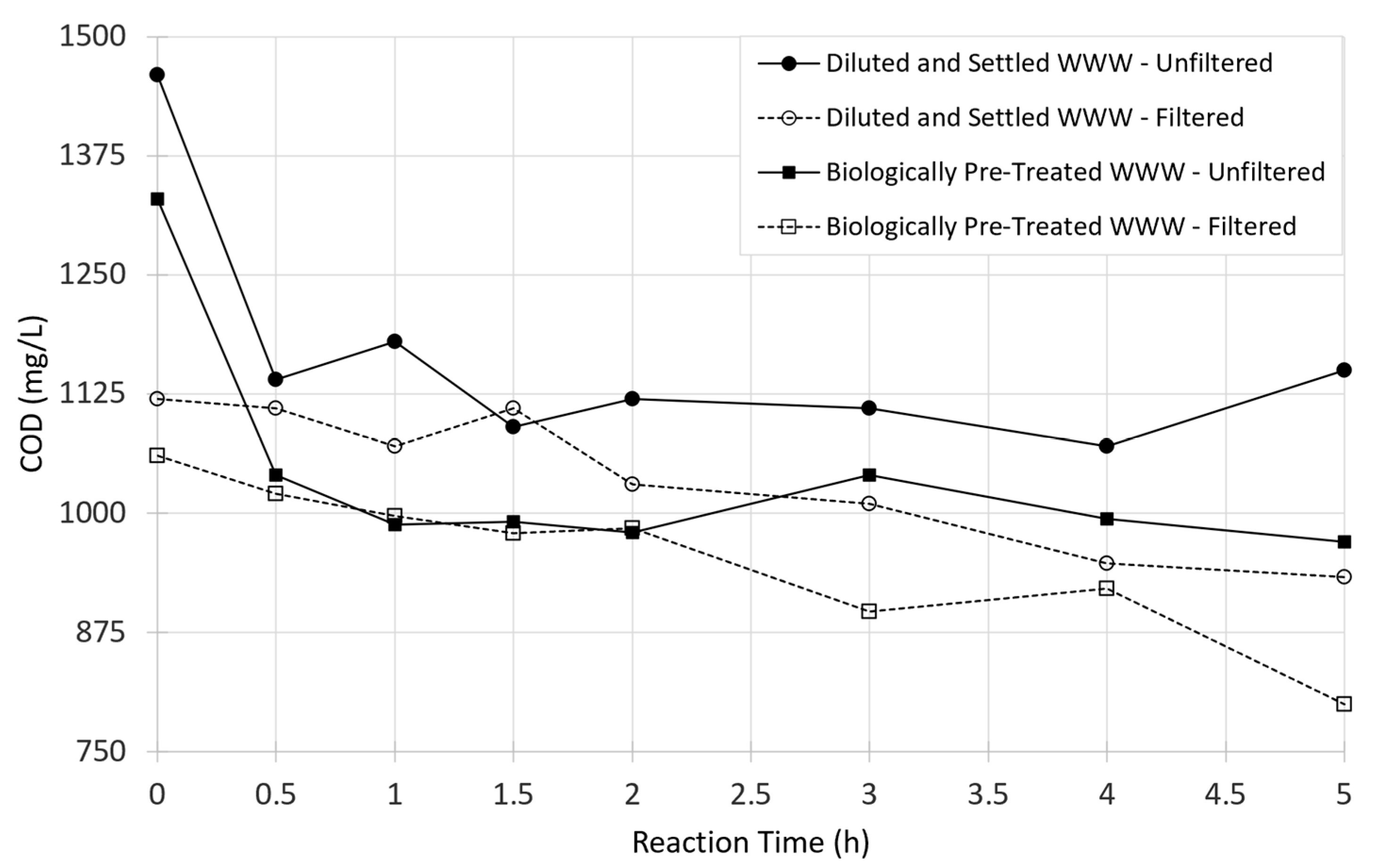
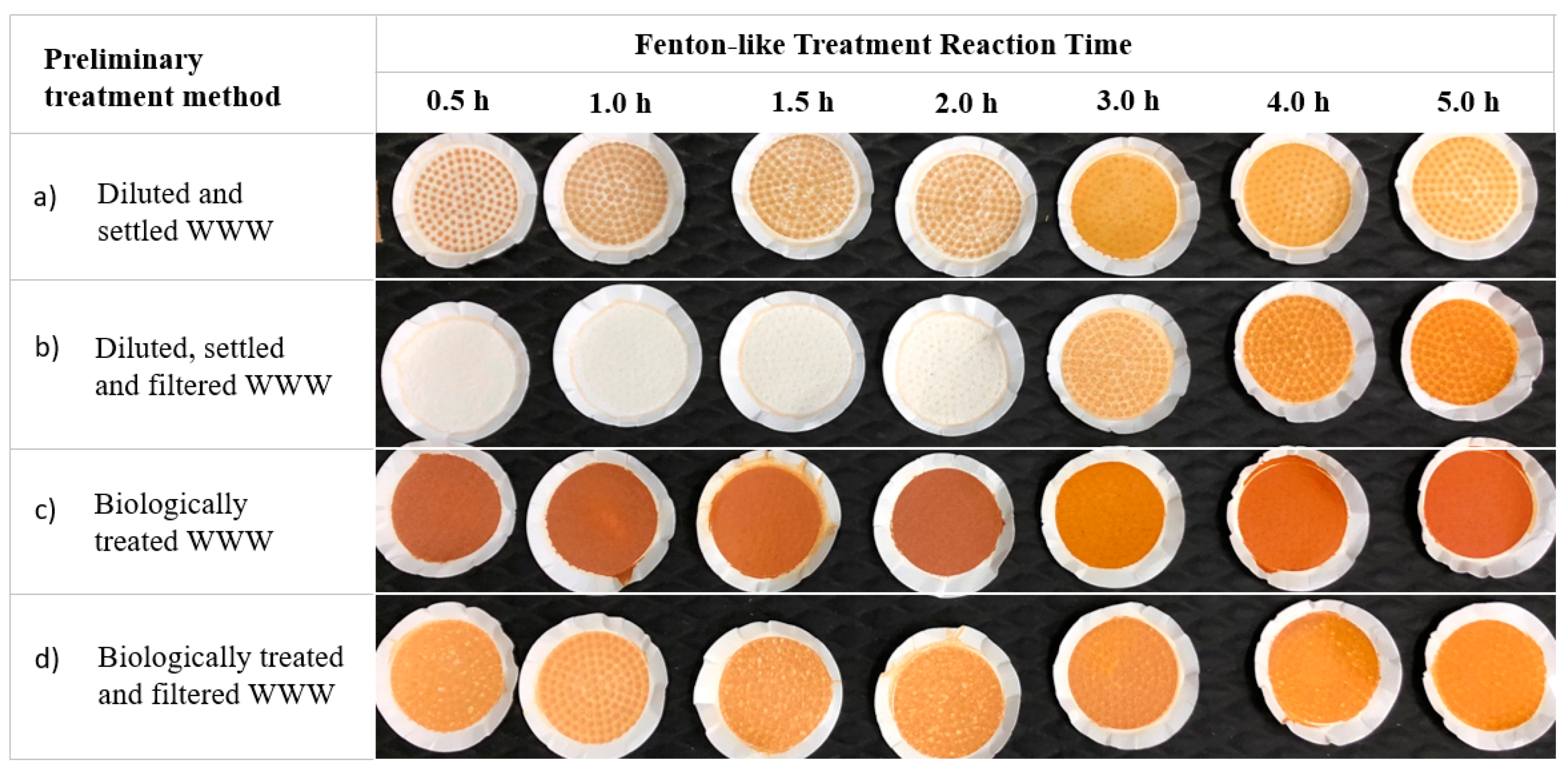
3.3. Time Series Treatment Trials
3.4. Co-Treatment Feasibility Trials
3.4.1. Impact on Primary Clarification Performance
3.4.2. Impact on Aerobic Biological Treatment Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, M.B.; Mehrvar, M. Characterising winery wastewater composition to optimise treatment and reuse. Aust. J. Grape Wine Res. 2020, 26, 410–416. [Google Scholar] [CrossRef]
- Johnson, M.B.; Mehrvar, M. From Field to Bottle: Water Footprint Estimation in the Winery Industry. In Water Footprint; Muthu, S., Ed.; Springer Nature: Singapore, 2021; pp. 103–136. [Google Scholar] [CrossRef]
- Bolzonella, D.; Papa, M.; Da Ros, C.; Muthukumar, L.A.; Rosso, D. Winery wastewater treatment: A critical overview of advanced biological processes. Crit. Rev. Biotechnol. 2019, 39, 489–507. [Google Scholar] [CrossRef]
- Johnson, M.B.; Mehrvar, M. Winery wastewater management and treatment in the Niagara Region of Ontario, Canada: A review and analysis of current regional practices and treatment performance. Can. J. Chem. Eng. 2020, 98, 5–24. [Google Scholar] [CrossRef]
- Lofrano, G.; Meric, S. A comprehensive approach to winery wastewater treatment: A review of the state-of-the-art. Desalin. Water Treat. 2016, 57, 3011–3028. [Google Scholar] [CrossRef]
- Bolzonella, D.; Zanette, M.; Battistoni, P.; Cecchi, F. Treatment of winery wastewater in a conventional municipal activated sludge process: Five years of experience. Water Sci. Technol. 2007, 56, 79–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Heredia, J.B.; Dominguez, J.; Partido, E. Physico-chemical treatment for the depuration of wine distillery wastewaters (vinasses). Water Sci. Technol. 2005, 51, 159–166. [Google Scholar] [CrossRef]
- Hadavifar, M.; Zinatizadeh, A.A.; Younesi, H.; Galehdar, M. Fenton and photo-Fenton treatment of distillery effluent and optimization of treatment conditions with response surface methodology. Asia-Pac. J. Chem. Eng. 2010, 5, 454–464. [Google Scholar] [CrossRef]
- Martins, R.C.; Rossi, A.F.; Quinta-Ferreira, R.M. Fenton’s oxidation process for phenolic wastewater remediation and biodegradability enhancement. J. Hazard. Mater. 2010, 180, 716–721. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Duran, A.; Corral, J.M.; Carnicer, A.; Frades, J.M.; Alonso, M.A. Ferrioxalate-induced solar photo-Fenton system for the treatment of winery wastewaters. Chem. Eng. J. 2012, 181–182, 281–288. [Google Scholar] [CrossRef]
- Gimeno, O.; Rivas, F.J.; Beltran, F.J.; Carbajo, M. Photo-catalytic ozonation of winery wastewaters. J. Agric. Food Chem. 2007, 55, 9944–9950. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.S.; Peres, J.A.; Li Puma, G. Treatment of winery wastewater by ozone-based advanced oxidation processes (O3, O3/UV and O3/UV/H2O2) in a pilot-scale bubble column reactor and process economics. Sep. Purif. Technol. 2010, 72, 235–241. [Google Scholar] [CrossRef]
- Lucas, M.S.; Peres, J.A.; Lan, B.Y.; Li Puma, G. Ozonation kinetics of winery wastewaters in a pilot-scale bubble column reactor. Water Res. 2009, 43, 1523–1532. [Google Scholar] [CrossRef]
- Augustina, T.E.; Ang, H.M.; Pareek, V.L. Treatment of winery wastewater using a photocatalytic/photolytic reactor. Chem. Eng. J. 2007, 135, 151–156. [Google Scholar] [CrossRef]
- Ioannou, L.A.; Puma, G.L.; Fatta-Kassinos, D. Treatment of winery wastewater by physicochemical, biological and advanced processes: A review. J. Hazard. Mater. 2015, 286, 343–368. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, N.; Monou, M.; Mantzavinos, D.; Kassinos, D. Monitoring of the quality of winery influents/effluents and polishing of partially treated winery flows by homogenous Fe (II) photo-oxidation. Desalination 2009, 248, 836–842. [Google Scholar] [CrossRef]
- Lucas, M.S.; Mouta, M.; Pirra, A.; Peres, J.A. Winery wastewater treatment by a combined process: Long term aerated storage and Fenton’s reagent. Water Sci. Technol. 2009, 60, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Ioannu, L.A.; Fatta-Kassinos, D. Solar photo-Fenton oxidation against the bioresistant fractions of winery wastewater. J. Environ. Chem. Eng. 2013, 1, 703–712. [Google Scholar] [CrossRef]
- Beltran, F.J.; Garcia-Araya, J.F.; Alvarez, P.M. pH sequential ozonation of domestic and wine-distillery wastewaters. Water Res. 2001, 35, 929–936. [Google Scholar] [CrossRef]
- Beck, C.; Prades, G.; Sadowski, A.-G. Activated sludge wastewater treatment plants optimisation to face pollution overloads during grape harvest periods. Water Sci. Technol. 2005, 51, 81–88. [Google Scholar] [CrossRef][Green Version]
- Amaral-Silva, N.; Martins, R.C.; Paiva, C.; Castro-Silva, S.; Quinta-Ferreira, R.M. A new winery wastewater treatment approach during vintage periods integrating ferric coagulation, Fenton reaction and activated sludge. J. Environ. Chem. Eng. 2016, 4, 2207–2215. [Google Scholar] [CrossRef]
- Johnson, M.B.; Mehrvar, M. An assessment of the grey water footprint of winery wastewater in the Niagara Region of Ontario, Canada. J. Clean. Prod. 2019, 214, 623–632. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Beltran, J.; Rivas, J.; Alvarez, P.M.; Alonso, M.A.; Acedo, B. A kinetic model for advanced oxidation processes of aromatic hydrocarbons in water: Application to phenanthrene and nitrobenzene. Ind. Eng. Chem. Res. 1999, 38, 4189–4199. [Google Scholar] [CrossRef]
- Gernjak, W. Solar Photo-Fenton Treatment of EU Priority Substances—Process Parameters and Control Strategies. Ph.D. Thesis, Universitat fur Bodenkultur Wein, Vienna, Austria, March 2006. [Google Scholar]
- Gernjak, W.; Maldonado, M.I.; Malato, S.; Caceres, J.; Krutzler, T.; Glaser, A.; Bauer, R. Pilot-plant treatment of olive mill wastewater (OMW) by solar TiO2 photocatalysis and solar photo-Fenton. Sol. Energy 2004, 77, 567–572. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012; ISBN 0875530133. [Google Scholar]
- Ahmadi, M.; Vahabzadeh, B.; Bonakdapur, B.; Mofarrah, E.; Mehranidn, M. Application of the central composite design and response surface methodology to the advanced treatment of olive oil processing wastewaters using Fenton’s peroxidation. J. Hazard. Mater. 2005, 123, 187–195. [Google Scholar] [CrossRef]
- Ramirez, J.H.; Costa, C.A.; Madeira, L.M. Experimental design to optimize the degradation of the synthetic dye orange II using Fenton’s reagent. Catal. Today 2009, 107–118, 68–76. [Google Scholar] [CrossRef]
- Yang, J.; Xing, M.; Zhou, X. Degaradation of recalcitrant organics from winery wastewater by Fenton’s reaction. Environ. Eng. Sci. 2008, 25, 1229–1234. [Google Scholar] [CrossRef]
- Ministry of the Environment. Design Guidelines for Sewage Works. 2008. Available online: https://www.ontario.ca/document/design-guidelines-sewage-works-0 (accessed on 1 October 2018).
- Ghafoori, S.; Mehrvar, M.; Chan, P.K. Free-Radical-Induced Degradation of Aqueous Polyethylene Oxide by UV/H2O2: Experimental Design, Reaction Mechanisms, and Kinetic Modeling. Ind. Eng. Chem. Res. 2012, 51, 14980–14993. [Google Scholar] [CrossRef]
- Bustillo-Lecompte, C.F.; Mehrvar, M. Treatment of actual slaughterhouse wastewater by combined anaerobic-aerobic processes for biogas generation and removal of organics and nutrients: An optimization study towards a cleaner production in the meat processing industry. J. Clean. Prod. 2016, 141, 278–289. [Google Scholar] [CrossRef]
- Johnson, M.B.; Mehrvar, M. Aqueous Metronidazole Degradation by UV/H2O2 Process in Single-and Multi-Lamp Tubular Photoreactors: Kinetics and Reactor Design. Ind. Eng. Chem. Res. 2008, 47, 6525–6537. [Google Scholar] [CrossRef]
- Kotta, E.; Kalogerakis, N.; Mantzavinos, D. The effect of solids on the electrochemical treatment of olive mill effluents. J. Chem. Technol. Biot. 2007, 85, 504–511. [Google Scholar] [CrossRef]
- Zorpas, A.A.; Saranti, A. Multi-criteria analysis of sustainable environmental clean technologies for the treatment of winery’s wastewater. Int. J. Glob. Environ. Issues 2016, 15, 151–168. [Google Scholar] [CrossRef]
- Charley, P.J.; Sarkar, B.; Stitt, C.F.; Saltman, P. Chelation of iron by sugars. Biochim. Biophys. Acta 1963, 69, 313–321. [Google Scholar] [CrossRef]
- Mehrvar, M.; Johnson, M.B. Method and System for Pre-Treating High Strength Wastewater. U.S. Patent Provisional Application No. 63/170,009, 2 April 2021. [Google Scholar]
| Independent Variable | Units | Symbol | Coded Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Reaction Time | h | X1 | 2 | 3 | 4 |
| H2O2,o | mg/L | X2 | 1000 | 2500 | 4000 |
| Feo3+ | mg/L | X3 | 75 | 200 | 325 |
| Parameter | Units | WWW | Pre-Treated WWW | ||
|---|---|---|---|---|---|
| Raw | Diluted (4% v/v) | Scheme 1B | Scheme 2B | ||
| COD | mg/L | 163,000 | 6520 | 4850 | 2970 |
| Filtered COD | mg/L | 103,200 | 4128 | 4550 | 2020 |
| TOC | mg/L | 57,300 | 2292 | 1300 | 678 |
| Filtered TOC | mg/L | 27,600 | 1104 | 994 | 595 |
| TSS | mg/L | 71,600 | 2864 | 575 | 440 |
| VSS | mg/L | 51,600 | 2064 | 560 | 419 |
| TP | mg/L | 30.2 | 1.21 | 7.34 | 6.40 |
| Filtered TP | mg/L | - | - | 2.61 | 1.37 |
| pH | - | 3.78 | - | - | - |
| COD Removal | % | - | - | 26 | 54 |
| Filtered COD Removal | % | - | - | −10 | 51 |
| TOC Removal | % | - | - | 43 | 70 |
| Filtered TOC Removal | % | - | - | 10 | 46 |
| TSS Removal | % | - | - | 80 | 85 |
| VSS Removal | % | - | - | 73 | 80 |
| TP Removal | % | - | - | −513 | −429 |
| Run | Independent Coded Variables | COD Removal (%) Y1 | TOC Removal (%) Y2 | ISS Generation (mg/L) Y3 | |||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Observed | Predicted | Observed | Predicted | Observed | Predicted | |
| 1 | −1 | −1 | 0 | 36.58 | 37.27 | 33.04 | 32.98 | 146 | 178 |
| 2 | 1 | −1 | 0 | 45.55 | 44.87 | 35.35 | 36.3 | 119 | 123 |
| 3 | −1 | 1 | 0 | 39.32 | 40.00 | 36.37 | 35.41 | 112 | 109 |
| 4 | 1 | 1 | 0 | 46.64 | 45.95 | 35.6 | 35.66 | 154 | 127 |
| 5 | −1 | 0 | −1 | 33.84 | 34.31 | 33.81 | 34.32 | 3 | 3 |
| 6 | 1 | 0 | −1 | 43.56 | 45.4 | 35.86 | 35.35 | 1 | 1 |
| 7 | −1 | 0 | 1 | 50.96 | 49.12 | 40.72 | 41.23 | 354 | 304 |
| 8 | 1 | 0 | 1 | 52.05 | 51.58 | 44.3 | 43.79 | 409 | 415 |
| 9 | 0 | −1 | −1 | 44.38 | 43.22 | 36.62 | 36.18 | 6 | 5 |
| 10 | 0 | 1 | −1 | 44.18 | 43.03 | 35.6 | 36.05 | 0 | 0 |
| 11 | 0 | −1 | 1 | 50.48 | 51.63 | 43.27 | 42.83 | 161 | 153 |
| 12 | 0 | 1 | 1 | 54.45 | 55.61 | 44.3 | 44.74 | 451 | 544 |
| 13 | 0 | 0 | 0 | 50.48 | 51.83 | 42.51 | 42.85 | 176 | 163 |
| 14 | 0 | 0 | 0 | 51.99 | 51.83 | 42.76 | 42.85 | 165 | 163 |
| 15 | 0 | 0 | 0 | 53.01 | 51.83 | 43.27 | 42.85 | 147 | 163 |
| Source | Sum of Squares | DF | Mean Square | F Value | p-Value | Remark |
|---|---|---|---|---|---|---|
| CODremoval model (Y1) | 530.1 | 9 | 58.90 | 16.67 | 0.0032 | Significant |
| X1 | 91.80 | 1 | 91.80 | 25.98 | 0.0038 | Significant |
| X2 | 7.220 | 1 | 7.220 | 2.043 | 0.2123 | Not significant |
| X3 | 220.3 | 1 | 220.3 | 62.35 | 0.0005 | Significant |
| X1X2 | 0.681 | 1 | 0.681 | 0.193 | 0.6791 | Not significant |
| X1X3 | 18.62 | 1 | 18.62 | 5.270 | 0.0702 | |
| X2X3 | 4.347 | 1 | 4.347 | 1.230 | 0.3178 | Not significant |
| X12 | 157.8 | 1 | 157.8 | 44.66 | 0.0011 | Significant |
| X22 | 39.41 | 1 | 39.41 | 11.15 | 0.0206 | Significant |
| X32 | 0.129 | 1 | 0.129 | 0.037 | 0.8559 | Not significant |
| Residual | 17.67 | 5 | 3.533 | |||
| Lack of fit | 14.43 | 3 | 4.809 | 2.968 | 0.2621 | Not significant |
| Pure error | 3.240 | 2 | 1.620 | |||
| Corrected total SS | 547.8 | 14 | ||||
| R2 | 0.968 | |||||
| Adjusted R2 | 0.910 | |||||
| Adequate precision | 13.88 | |||||
| TOCremoval model (Y2) | 239.2 | 9 | 26.58 | 33.21 | 0.0006 | Significant |
| X1 | 6.410 | 1 | 6.410 | 8.011 | 0.0367 | Significant |
| X2 | 1.603 | 1 | 1.603 | 2.003 | 0.2162 | Not significant |
| X3 | 117.7 | 1 | 117.7 | 147.1 | <0.0001 | Significant |
| X1X2 | 2.355 | 1 | 2.355 | 2.943 | 0.1469 | Not significant |
| X1X3 | 0.589 | 1 | 0.589 | 0.736 | 0.4302 | Not significant |
| X2X3 | 1.047 | 1 | 1.047 | 1.308 | 0.3046 | Not significant |
| X12 | 75.38 | 1 | 75.38 | 94.20 | 0.0002 | Significant |
| X22 | 38.75 | 1 | 38.75 | 48.43 | 0.0009 | Significant |
| X32 | 0.429 | 1 | 0.429 | 0.537 | 0.4967 | Not significant |
| Residual | 4.001 | 5 | 0.800 | |||
| Lack of fit | 3.696 | 3 | 1.232 | 8.071 | 0.1122 | Not significant |
| Pure error | 0.305 | 2 | 0.153 | |||
| Corrected total SS | 243.2 | 14 | ||||
| R2 | 0.984 | |||||
| Adjusted R2 | 0.954 | |||||
| Adequate precision | 16.11 | |||||
| ) | 57.57 | 9 | 6.397 | 149.7 | <0.0001 | Significant |
| X1 | 0.024 | 1 | 0.027 | 0.556 | 0.4894 | Not significant |
| X2 | 0.107 | 1 | 0.107 | 2.513 | 0.1738 | Not significant |
| X3 | 45.43 | 1 | 45.43 | 1063 | <0.0001 | Significant |
| X1X2 | 0.067 | 1 | 0.067 | 1.576 | 0.2649 | Not significant |
| X1X3 | 0.175 | 1 | 0.175 | 4.100 | 0.0988 | |
| X2X3 | 2.208 | 1 | 2.208 | 51.67 | 0.0008 | Significant |
| X12 | 7.239 × 10− 6 | 1 | 7.239 × 10−6 | 1.694 × 10−4 | 0.9901 | Not significant |
| X22 | 0.1575 | 1 | 0.1575 | 3.685 | 0.113 | Not significant |
| X32 | 9.473 | 1 | 9.473 | 221.68 | <0.0001 | Significant |
| Residual | 0.214 | 5 | 0.043 | |||
| Lack of fit | 0.197 | 3 | 0.066 | 8.000 | 0.1131 | |
| Pure error | 0.016 | 2 | 0.008 | |||
| Corrected total SS | 57.79 | 14 | ||||
| R2 | 0.996 | |||||
| Adjusted R2 | 0.990 | |||||
| Adequate precision | 37.04 |
| Parameter | Raw Wastewater 1 | Control—No WWW | Pre-Treatment Scheme | ||||
|---|---|---|---|---|---|---|---|
| No FeCl3 2 | Plus FeCl3 3 | 1A 4,5 | 2A 4,5 | 1B 3 | 2B 3 | ||
| COD (mg/L) | 537 | 221 | 94 | 327 | 306 | 323 | 311 |
| TOC (mg/L) | 110 | 60.0 | 22.4 | 81.5 | 79.0 | 77.8 | 79.4 |
| TSS (mg/L) | 338 | 69 | 17 | 63 | 56 | 132 | 139 |
| TP (mg/L) | 9.52 | 4.01 | 0.40 | 2.29 | 2.11 | 3.38 | 3.58 |
| Fe (mg/L) | 8.54 | 0.83 | 2.85 | 4.66 | 3.79 | 27.1 | 27.9 |
| COD Removal 6 (%) | - | 59 | 82 | 45 | 47 | 49 | 50 |
| TOC Removal 6 (%) | - | 45 | 80 | 37 | 38 | 44 | 39 |
| TSS Removal 6 (%) | - | 80 | 95 | 80 | 83 | 58 | 57 |
| TP Removal 6 (%) | - | 58 | 96 | 74 | 76 | 61 | 60 |
| Fe Removal 6 (%) | - | 90 | 93 | 88 | 91 | 33 | 31 |
| Parameter | Control—No WWW 1 | Pre-Treatment Scheme 2 | ||||
|---|---|---|---|---|---|---|
| No FeCl3 3 | Plus FeCl3 4 | 1A 5,6 | 2A 5,6 | 1B 4 | 2B 4 | |
| COD (mg/L) | 111 | 89 | 127 | 123 | 93 | 146 |
| Filtered COD (mg/L) | 43 | 46 | 66 | 77 | 59 | 62 |
| TOC (mg/L) | 16.7 | 12.5 | 23.6 | 24.5 | 14.6 | 23.6 |
| Filtered TOC (mg/L) | 9.7 | 7.7 | 12.6 | 13.0 | 8.7 | 12.1 |
| TSS (mg/L) | 35 | 24 | 46 | 50 | 44 | 52 |
| TP (mg/L) | 1.45 | 0.78 | 1.53 | 1.49 | 1.16 | 1.48 |
| Filtered TP (mg/L) | 0.28 | 0.08 | 0.26 | 0.25 | 0.13 | 0.24 |
| Fe (mg/L) | 1.68 | 2.89 | 6.27 | 5.69 | 7.42 | 10.5 |
| Filtered Fe (mg/L) | 0.08 | 0.08 | 0.15 | 0.21 | 1.29 | 2.8 |
| MLSSo (mg/L) | 1129 | 1129 | 1129 | 1129 | 1129 | 1129 |
| MLSSfinal (mg/L) | 991 | 1220 | 1169 | 1140 | 1199 | 1185 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, M.B.; Mehrvar, M. Treatment of Actual Winery Wastewater by Fenton-like Process: Optimization to Improve Organic Removal, Reduce Inorganic Sludge Production and Enhance Co-Treatment at Municipal Wastewater Treatment Facilities. Water 2022, 14, 39. https://doi.org/10.3390/w14010039
Johnson MB, Mehrvar M. Treatment of Actual Winery Wastewater by Fenton-like Process: Optimization to Improve Organic Removal, Reduce Inorganic Sludge Production and Enhance Co-Treatment at Municipal Wastewater Treatment Facilities. Water. 2022; 14(1):39. https://doi.org/10.3390/w14010039
Chicago/Turabian StyleJohnson, Melody Blythe, and Mehrab Mehrvar. 2022. "Treatment of Actual Winery Wastewater by Fenton-like Process: Optimization to Improve Organic Removal, Reduce Inorganic Sludge Production and Enhance Co-Treatment at Municipal Wastewater Treatment Facilities" Water 14, no. 1: 39. https://doi.org/10.3390/w14010039
APA StyleJohnson, M. B., & Mehrvar, M. (2022). Treatment of Actual Winery Wastewater by Fenton-like Process: Optimization to Improve Organic Removal, Reduce Inorganic Sludge Production and Enhance Co-Treatment at Municipal Wastewater Treatment Facilities. Water, 14(1), 39. https://doi.org/10.3390/w14010039