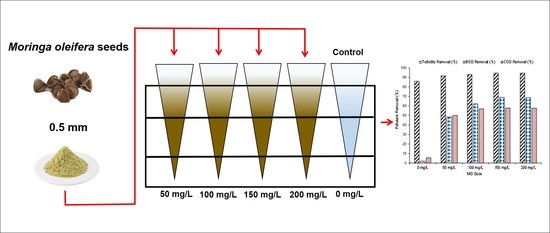
Kinetic and Prediction Modeling Studies of Organic Pollutants Removal from Municipal Wastewater using Moringa oleifera Biomass as a Coagulant
Abstract
1. Introduction
2. Materials and Methods
2.1. MO Biomass and Sample Preparation
2.2. Sampling and Characterization of Wastewater
2.3. Experimental Design and Calculation of Turbidity, BOD and COD Removal Efficiency
2.4. Kinetics of Turbidity, BOD and COD Removal
2.5. Modified Gompertz Model for Prediction of Turbidity, BOD and COD Removal
2.6. Statistics
3. Results and Discussion
3.1. Characteristics of Municipal Wastewater Used in This Study
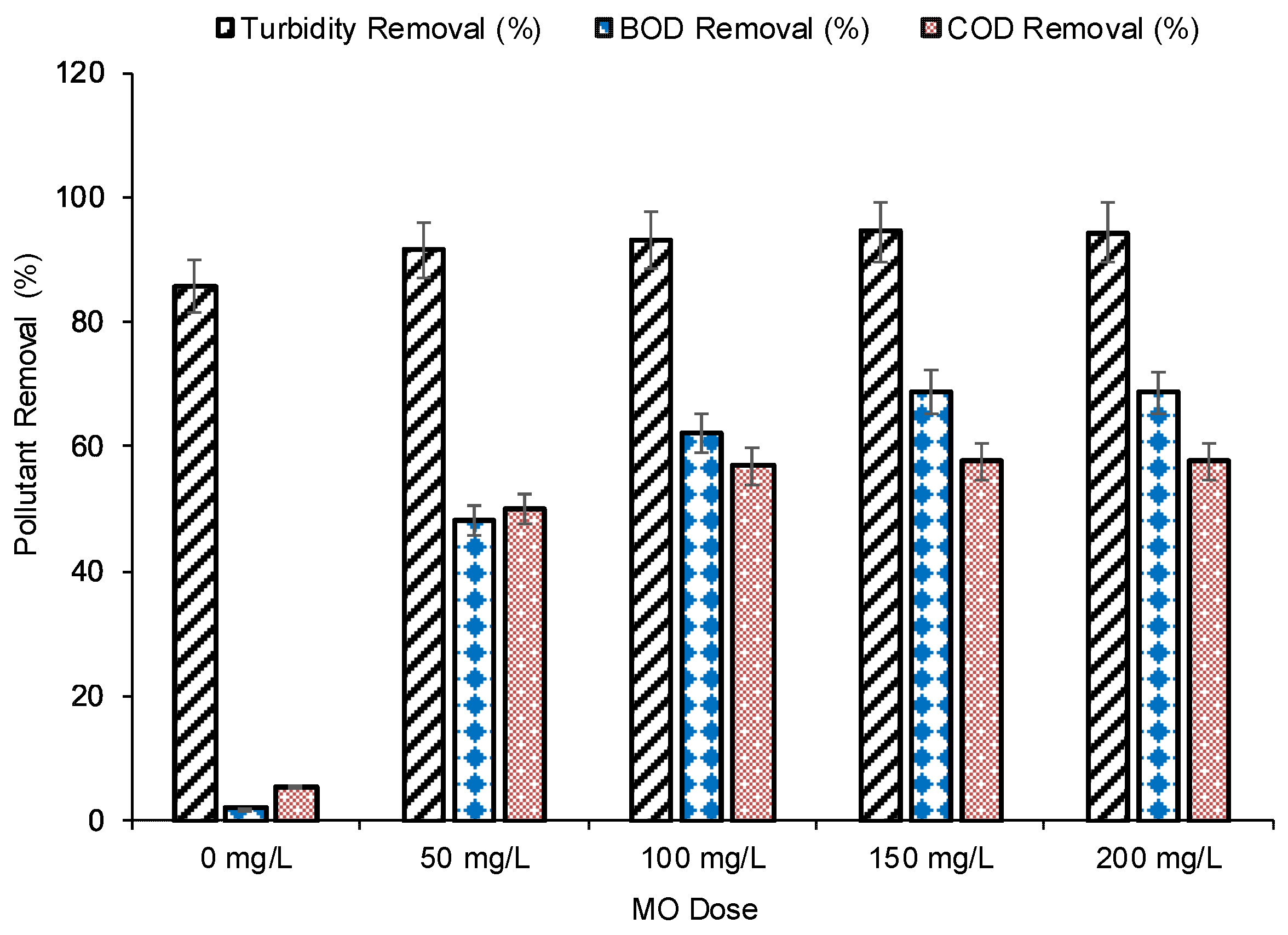
3.2. Effect of MO Dose on Turbidity, BOD and COD Removal
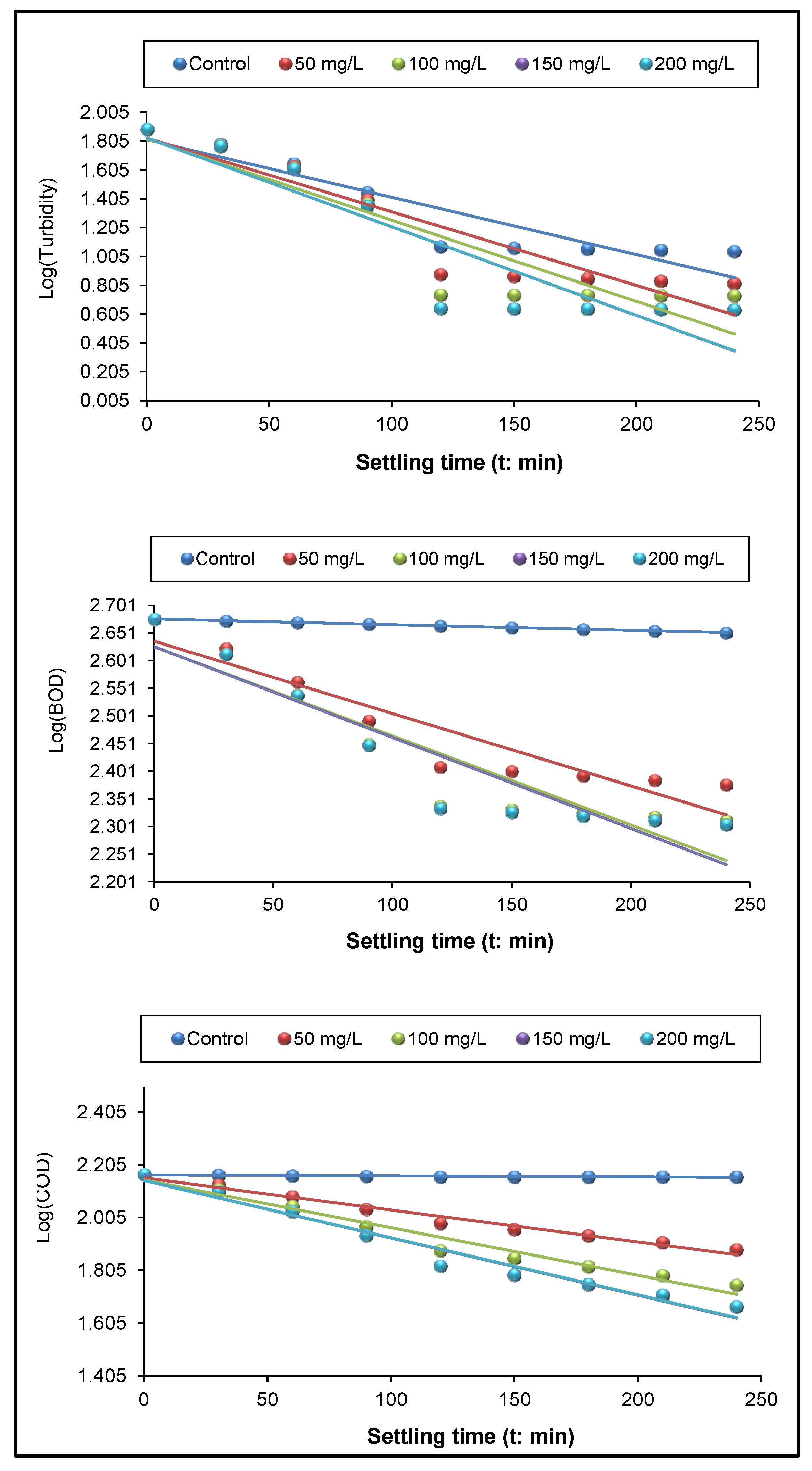
3.3. Kinetics of Turbidity, BOD, and COD Removal
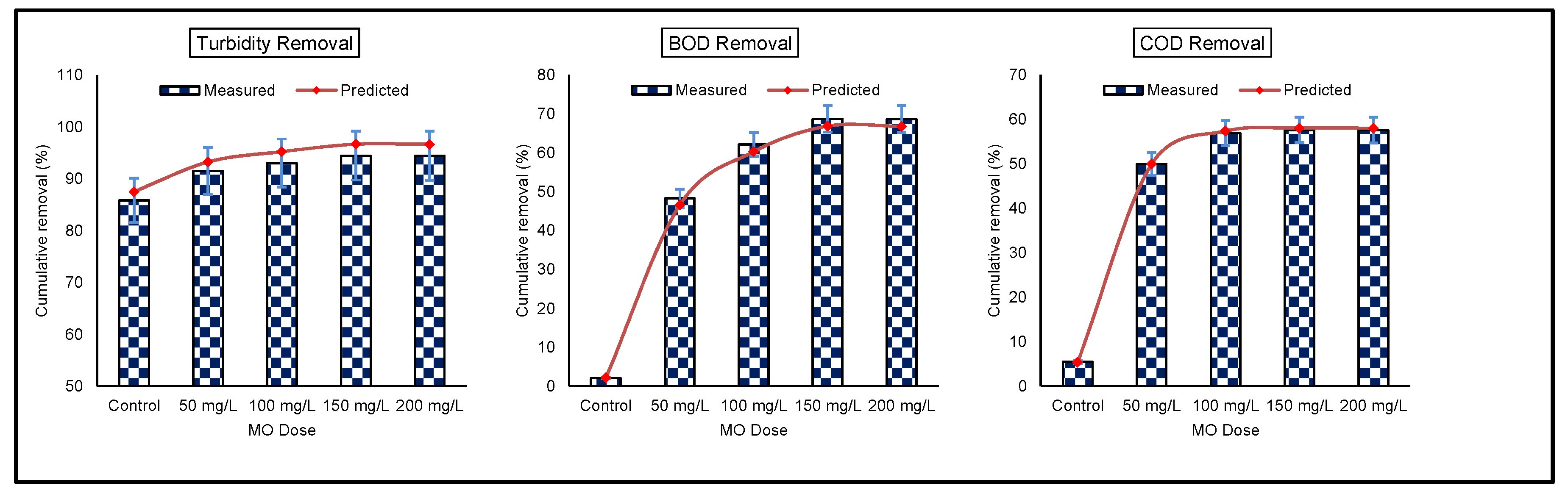
3.4. Prediction and Validation of Model Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Freitas, J.H.E.S.; de Santana, K.V.; do Nascimento, A.C.C.; de Paiva, S.C.; de Moura, M.C.; Coelho, L.C.B.B.; de Oliveira, M.B.M.; Paiva, P.M.G.; do Nascimento, A.E.; Napoleão, T.H. Evaluation of using aluminum sulfate and water-soluble Moringa oleifera seed lectin to reduce turbidity and toxicity of polluted stream water. Chemosphere 2016, 163, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Vunain, E.; Masoamphambe, E.F.; Mpeketula, P.M.G.; Monjerezi, M.; Etale, A. Evaluation of coagulating efficiency and water borne pathogens reduction capacity of Moringa oleifera seed powder for treatment of domestic wastewater from Zomba, Malawi. J. Environ. Chem. Eng. 2019, 7, 103118. [Google Scholar] [CrossRef]
- Abaliwano, J.K.; Ghebremichael, K.A.; Amy, G.L. Application of the Purified Moringa Oleifera Coagulant for Surface Water Treatment; United Nations Educational, Scientific and Cultural Organization, Institute of water Education (UNESCO-IHE): Delft, The Netherlands, 2008; Volume 5. [Google Scholar]
- Kansal, S.K.; Kumari, A. Potential of M. oleifera for the treatment of water and wastewater. Chem. Rev. 2014, 114, 4993–5010. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Xiong, B.; Piechowicz, B.; Wang, Z.; Marinaro, R.; Clement, E.; Carlin, T.; Uliana, A.; Kumar, M.; Velegol, S.B. Moringa oleifera f-sand Filters for Sustainable Water Purification. Environ. Sci. Technol. Lett. 2018, 5, 38–42. [Google Scholar] [CrossRef]
- Tripathi, A.; Mishra, A.K. Knowledge and passive adaptation to climate change: An example from Indian farmers. Clim. Risk Manag. 2017, 16, 195–207. [Google Scholar] [CrossRef]
- Valverde, K.C.; Coldebella, P.F.; Silva, M.F.; Nishi, L.; Bongiovani, M.C.; Bergamasco, R. Moringa oleifera Lam. and Its Potential Association with Aluminium Sulphate in the Process of Coagulation/Flocculation and Sedimentation of Surface Water. Int. J. Chem. Eng. 2018, 2018, 12–15. [Google Scholar] [CrossRef]
- Nwoba, E.G.; Vadiveloo, A.; Ogbonna, C.N.; Ubi, B.E.; Ogbonna, J.C.; Moheimani, N.R. Algal Cultivation for Treating Wastewater in African Developing Countries: A Review. Clean Soil Air Water 2020, 48, 1–14. [Google Scholar] [CrossRef]
- Hoa, N.T.; Hue, C.T. Enhanced water treatment by Moringa oleifera seeds extract as the bio-coagulant: Role of the extraction method. J. Water Supply Res. Technol. 2018, 67, 634–647. [Google Scholar] [CrossRef]
- Shirani, Z.; Santhosh, C.; Iqbal, J.; Bhatnagar, A. Waste Moringa oleifera seed pods as green sorbent for efficient removal of toxic aquatic pollutants. J. Environ. Manag. 2018, 227, 95–106. [Google Scholar] [CrossRef]
- Rocha, V.V.F.; dos Santos, I.F.S.; Silva, A.M.L.; Sant’Anna, D.O.; Junho, A.L.; Barros, R.M. Clarification of high-turbidity waters: A comparison of Moringa oleifera and virgin and recovered aluminum sulfate-based coagulants. Environ. Dev. Sustain. 2019, 1–12. [Google Scholar] [CrossRef]
- Adelodun, B.; Odedishemi, F.; Segun, M.; Choi, K.-S. Dosage and settling time course optimization of Moringa oleifera in municipal wastewater treatment using response surface methodology. Desalin. Water Treat. 2019, 167, 45–56. [Google Scholar] [CrossRef]
- Bello, O.S.; Lasisi, B.M.; Adigun, O.J.; Ephraim, V. Scavenging Rhodamine B dye using moringa oleifera seed pod. Chem. Speciat. Bioavailab. 2017, 29, 120–134. [Google Scholar] [CrossRef]
- Camacho, F.P.; Sousa, V.S.; Bergamasco, R.; Ribau Teixeira, M. The use of Moringa oleifera as a natural coagulant in surface water treatment. Chem. Eng. J. 2017, 313, 226–237. [Google Scholar] [CrossRef]
- Villaseñor-Basulto, D.L.; Astudillo-Sánchez, P.D.; del Real-Olvera, J.; Bandala, E.R. Wastewater treatment using Moringa oleifera Lam seeds: A review. J. Water Process Eng. 2018, 23, 151–164. [Google Scholar] [CrossRef]
- Ndabigengesere, A.; Narasiah, K.S.; Talbot, B.G. Active agents and mechanism of coagulation of turbid waters using Moringa oleifera. Water Res. 1995, 29, 703–710. [Google Scholar] [CrossRef]
- Muyibi, S.A.; Evison, L.M. Optimizing physical parameters affecting coagulation of turbid water with Morninga oleifera seeds. Water Res. 1995, 29, 2689–2695. [Google Scholar] [CrossRef]
- Okuda, T.; Baes, A.U.; Nishijima, W.; Okada, M. Coagulation mechanism of salt solution-extracted active component in Moringa oleifera seeds. Water Res. 2001, 35, 830–834. [Google Scholar] [CrossRef]
- Viotti, P.V.; Moreira, W.M.; dos Santos, O.A.A.; Bergamasco, R.; Vieira, A.M.S.; Vieira, M.F. Diclofenac removal from water by adsorption on Moringa oleifera pods and activated carbon: Mechanism, kinetic and equilibrium study. J. Clean. Prod. 2019, 219, 809–817. [Google Scholar] [CrossRef]
- Natarajan, R.; Manivasagan, R. Effect of operating parameters on dye wastewater treatment using Prosopis cineraria and kinetic modeling. Environ. Eng. Res. 2019, 25, 788–793. [Google Scholar] [CrossRef]
- Farzadkia, M.; Ehrampush, M.H.; Mehrizi, E.A.; Sadeghi, S.; Talebi, P.; Salehi, A.; Kermani, M. Investigating the efficiency and kinetic coefficients of nutrient removal in the subsurface artificial wetland of Yazd wastewater treatment plant. Environ. Health Eng. Manag. 2015, 2, 23–30. [Google Scholar]
- Adelodun, B.; Ajibade, F.O.; Abdulkadir, T.S.; Bakare, H.O.; Choi, K.S. SWOT analysis of agro-waste based adsorbents for persistent dye pollutants removal from wastewaters. In Environmental Degradation: Causes and Remediation Strategies; Kumar, V., Singh, J., Kumar, P., Eds.; Agro Environ Media—Agriculture and Ennvironmental Science Academy: Haridwar, India, 2020; pp. 88–103. [Google Scholar]
- Delelegn, A.; Sahile, S.; Husen, A. Water purification and antibacterial efficacy of Moringa oleifera Lam. Agric. Food Secur. 2018, 7, 1–10. [Google Scholar] [CrossRef]
- Muyibi, S.A.; Noor, M.J.M.M.; Leong, T.K.; Loon, L.H. Effects of oil extraction from Moringa oleifera seeds on coagulation of turbid water. Int. J. Environ. Stud. 2002, 59, 243–254. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. APHA Standard Methods for the Examination of Water and Wastewater, 22nd ed.; APHA, AWWA, WEF: Washington, DC, USA, 2012. [Google Scholar] [CrossRef]
- El Shahawy, A.; Heikal, G. Organic pollutants removal from oily wastewater using clean technology economically, friendly biosorbent (Phragmites australis). Ecol. Eng. 2018, 122, 207–218. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, J.; Kumar, P.; Kumar, P. Response surface methodology based electro-kinetic modeling of biological and chemical oxygen demand removal from sugar mill effluent by water hyacinth (Eichhornia crassipes) in a Continuous Stirred Tank Reactor (CSTR). Environ. Technol. Innov. 2019, 14, 100327. [Google Scholar] [CrossRef]
- Báez, L.C.; Castro Rosas, J.; Villagómez Ibarra, J.R.; Palma Quiroz, I.; Páez Lerma, J.B.; Gómez Aldapa, C.A. Production of benzyl carbonyl (rose aroma) from whey and its effect on pollutant load removal. Environ. Dev. Sustain. 2019, 21, 609–619. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Guidelines for Water Reuse; United States Environmental Protection Agency: Washington, DC, USA, 2012.
- NESREA. National Environmental Standards and Regulations Enforcement Agency (Establishment) Act; NESREA: Abuja, Nigeria, 2009; pp. 1–21.
- Kayode, O.; Luethi, C.; Rene, E. Management Recommendations for Improving Decentralized Wastewater Treatment by the Food and Beverage Industries in Nigeria. Environments 2018, 5, 41. [Google Scholar] [CrossRef]
- Kane, C.; Bâ, A.; Mahamat, S.A.M.; Ayessou, N.; Mbacké, M.K.; Mar Diop, C.G. Combination of alum and extracted Moringa oleifera bioactive molecules powder for municipal wastewater treatment. Int. J. Biol. Chem. Sci. 2016, 10, 1918–1929. [Google Scholar] [CrossRef]
- Boulaadjoul, S.; Zemmouri, H.; Bendjama, Z.; Drouiche, N. A novel use of Moringa oleifera seed powder in enhancing the primary treatment of paper mill effluent. Chemosphere 2018, 206, 142–149. [Google Scholar] [CrossRef]
- Dotto, J.; Fagundes-Klen, M.R.; Veit, M.T.; Palácio, S.M.; Bergamasco, R. Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J. Clean. Prod. 2019, 208, 656–665. [Google Scholar] [CrossRef]
- Al-gheethi, A.A.; Mohamed, R.M.S.R.; Wurochekke, A.A.; Nurulainee, N.R.; Mas Rahayu, J.; Amir Hashim, M.K. Efficiency of Moringa oleifera Seeds for Treatment of Laundry Wastewater. MATEC Web Conf. 2017, 103, 1–8. [Google Scholar] [CrossRef]
- Rosmawanie, M.; Mohamed, R.; Al-Gheethi, A.; Pahazri, F.; Amir-Hashim, M.K.; Nur-Shaylinda, M.Z. Sequestering of pollutants from public market wastewater using Moringa oleifera and Cicer arietinum flocculants. J. Environ. Chem. Eng. 2018, 6, 2417–2428. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, J.; Bu, X.H. Recent advances about metal–organic frameworks in the removal of pollutants from wastewater. Coord. Chem. Rev. 2019, 378, 17–31. [Google Scholar] [CrossRef]
- Samal, K.; Dash, R.R.; Bhunia, P. Effect of hydraulic loading rate and pollutants degradation kinetics in two stage hybrid macrophyte assisted vermifiltration system. Biochem. Eng. J. 2018, 132, 47–59. [Google Scholar] [CrossRef]
- Dai, Y.D.; Shah, K.J.; Huang, C.P.; Kim, H.; Chiang, P.C. Adsorption of nonylphenol to multi-walled carbon nanotubes: Kinetics and isotherm study. Appl. Sci. 2018, 8, 2295. [Google Scholar] [CrossRef]
- Sacher, F.; Gerstner, P.; Merklinger, M.; Thoma, A.; Kinani, A.; Roumiguières, A.; Bouchonnet, S.; Richard-Tanaka, B.; Layousse, S.; Ata, R.; et al. Determination of monochloramine dissipation kinetics in various surface water qualities under relevant environmental conditions-Consequences regarding environmental risk assessment. Sci. Total Environ. 2019, 685, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ren, P.; Li, T.; Trembly, J.P.; Liu, X. Zinc removal from model wastewater by electrocoagulation: Processing, kinetics and mechanism. Chem. Eng. J. 2018, 349, 358–367. [Google Scholar] [CrossRef]
- Carvajal, A.; Akmirza, I.; Navia, D.; Pérez, R.; Muñoz, R.; Lebrero, R. Anoxic denitrification of BTEX: Biodegradation kinetics and pollutant interactions. J. Environ. Manag. 2018, 214, 125–136. [Google Scholar] [CrossRef]
- Hernández-Martínez, R.; Valdivia-Rivera, S.; Betto-Sagahon, J.; Coreño-Alonso, A.; Tzintzun-Camacho, O.; Lizardi-Jiménez, M.A. Solubilization and removal of petroleum hydrocarbons by a native microbial biomass in a bubble column reactor. Rev. Mex. Ing. Quím. 2019, 18, 181–189. [Google Scholar] [CrossRef]


| Parameter | MO Treatment | Before Treatment | CV (%) | After Treatment | USEPA Permissible Limit for Wastewater Reuse in Agriculture | NESREA Permissible Limit for Wastewater Discharge | Unit |
|---|---|---|---|---|---|---|---|
| pH | Control | 7.26 ± 0.09 | 0.01 | 7.37 ± 0.03 ** | 6–8.4 | 6–9 | - |
| 50 mg/L | 6.93 ± 0.05 *** | ||||||
| 100 mg/L | 6.88 ± 0.05 *** | ||||||
| 150 mg/L | 7.02 ± 0.05 *** | ||||||
| 200 mg/L | 7.01 ± 0.05 *** | ||||||
| Total suspended solids (TSS) | Control | 111.93 ± 15.23 | 13.50 | 91.88 ± 4.25 *** | 5 | 25 | mg/L |
| 50 mg/L | 70.43 ± 2.05 *** | ||||||
| 100 mg/L | 62.13 ± 2.36 *** | ||||||
| 150 mg/L | 41.70 ± 2.15 *** | ||||||
| 200 mg/L | 41.71 ± 2.00 *** | ||||||
| Turbidity | Control | 76.74 ± 3.69 | 4.80 | 10.88 ± 2.00 *** | 2 | 5 | NTU |
| 50 mg/L | 6.52 ± 1.15 *** | ||||||
| 100 mg/L | 5.35 ± 1.15 *** | ||||||
| 150 mg/L | 4.27 ± 1.05 *** | ||||||
| 200 mg/L | 4.28 ± 1.25 *** | ||||||
| Biochemical oxygen demand (BOD) | Control | 147.45 ± 12.87 | 8.72 | 144.40 ± 4.28 ns | 30 | 30 | mg/L |
| 50 mg/L | 76.28 ± 2.75 *** | ||||||
| 100 mg/L | 55.83 ± 2.55 *** | ||||||
| 150 mg/L | 46.12 ± 2.05 *** | ||||||
| 200 mg/L | 46.22 ± 3.16 *** | ||||||
| Chemical oxygen demand (COD) | Control | 474.18 ± 10.09 | 2.12 | 448.18 ± 3.45 ** | 120 | 60 | mg/L |
| 50 mg/L | 237.28 ± 3.00 *** | ||||||
| 100 mg/L | 204.30 ± 1.50 *** | ||||||
| 150 mg/L | 200.88 ± 2.52 *** | ||||||
| 200 mg/L | 201.00 ± 2.50 *** | ||||||
| Electrical conductance (EC) | Control | 402.44 ± 1.27 | 0.31 | 401.01 ± 0.03 ns | 700 | 400 | µS/cm |
| 50 mg/L | 429.09 ± 0.05 *** | ||||||
| 100 mg/L | 438.41 ± 0.05 *** | ||||||
| 150 mg/L | 447.55 ± 0.01 *** | ||||||
| 200 mg/L | 447.76 ± 0.05 *** | ||||||
| Alkalinity | Control | 385.00 ± 1.85 | 0.48 | 383 ± 1.26 ns | 50–150 | - | mg/L |
| 50 mg/L | 311.85 ± 0.95 *** | ||||||
| 100 mg/L | 327.25 ± 0.09 *** | ||||||
| 150 mg/L | 329.95 ± 1.06 *** | ||||||
| 200 mg/L | 330.05 ± 0.28 *** | ||||||
| Total hardness (TH) | Control | 128.50 ± 4.36 | 3.38 | 125.25 ± 1.25 ns | - | - | mg/L |
| 50 mg/L | 111.36 ± 0.12 *** | ||||||
| 100 mg/L | 104.96 ± 1.00 *** | ||||||
| 150 mg/L | 101.12 ± 1.05 *** | ||||||
| 200 mg/L | 98.56 ± 1.28 *** |
| Parameter | Model Variable | MO Treatment Dose | ||||
|---|---|---|---|---|---|---|
| Control | 50 mg/L | 100 mg/L | 150 mg/L | 200 mg/L | ||
| Turbidity | R2 | 0.8432 | 0.8534 | 0.8386 | 0.8335 | 0.8399 |
| r | 2.2216 | 2.8276 | 3.1608 | 3.6531 | 3.6472 | |
| y | −0.0042 x + 1.8143 | −0.0051 x + 1.8256 | −0.0056 x + 1.82 | −0.0062 x + 1.8265 | −0.0062 x + 1.8266 | |
| BOD | R2 | 0.8064 | 0.9774 | 0.9623 | 0.9638 | 0.9697 |
| r | 1.0664 | 1.2234 | 1.3184 | 1.3841 | 1.3833 | |
| y | −0.0005 x + 2.1667 | −0.0012 x + 2.1557 | −0.0018 x + 2.1478 | −0.0022 x + 2.146 | −0.0022 x + 2.1462 | |
| COD | R2 | 0.9912 | 0.8755 | 0.8534 | 0.8675 | 0.8696 |
| r | 0.8686 | 0.9696 | 0.9969 | 0.9999 | 0.9999 | |
| y | −0.0001 x + 2.6760 | −0.0013 x + 2.6357 | −0.0016 x + 2.6262 | −0.0016 x + 2.6269 | −0.0016 x + 2.6261 | |
| Parameter | Variable | MO Treatment Dose | ||||
|---|---|---|---|---|---|---|
| Control | 50 mg/L | 100 mg/L | 150 mg/L | 200 mg/L | ||
| Turbidity | REm | 85.82 | 91.49 | 93.02 | 94.44 | 94.42 |
| REp | 87.50 | 93.24 | 95.22 | 96.97 | 96.95 | |
| SE | 2.24 | 2.37 | 2.51 | 2.55 | 2.53 | |
| Λm | 45.94 | 45.99 | 45.56 | 45.57 | 45.58 | |
| µ | 0.0291 | 0.0290 | 0.0293 | 0.0296 | 0.0294 | |
| R2 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | |
| BOD | REm | 2.07 | 48.26 | 62.14 | 68.72 | 68.65 |
| REp | 2.10 | 46.58 | 61.71 | 68.15 | 68.08 | |
| SE | 0.05 | 1.64 | 1.57 | 1.61 | 1.62 | |
| Λm | 45.99 | 63.32 | 56.82 | 55.16 | 55.24 | |
| µ | 0.0290 | 0.0177 | 0.0205 | 0.0214 | 0.0213 | |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | |
| COD | REm | 5.48 | 49.96 | 56.92 | 57.64 | 57.61 |
| REp | 5.37 | 50.24 | 57.38 | 58.05 | 58.02 | |
| SE | 0.74 | 1.10 | 1.34 | 1.35 | 1.34 | |
| Λm | 117.89 | 48.31 | 47.15 | 47.28 | 47.29 | |
| µ | 0.0092 | 0.0265 | 0.0277 | 0.0276 | 0.0276 | |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adelodun, B.; Ogunshina, M.S.; Ajibade, F.O.; Abdulkadir, T.S.; Bakare, H.O.; Choi, K.S. Kinetic and Prediction Modeling Studies of Organic Pollutants Removal from Municipal Wastewater using Moringa oleifera Biomass as a Coagulant. Water 2020, 12, 2052. https://doi.org/10.3390/w12072052
Adelodun B, Ogunshina MS, Ajibade FO, Abdulkadir TS, Bakare HO, Choi KS. Kinetic and Prediction Modeling Studies of Organic Pollutants Removal from Municipal Wastewater using Moringa oleifera Biomass as a Coagulant. Water. 2020; 12(7):2052. https://doi.org/10.3390/w12072052
Chicago/Turabian StyleAdelodun, Bashir, Matthew Segun Ogunshina, Fidelis Odedishemi Ajibade, Taofeeq Sholagberu Abdulkadir, Hashim Olalekan Bakare, and Kyung Sook Choi. 2020. "Kinetic and Prediction Modeling Studies of Organic Pollutants Removal from Municipal Wastewater using Moringa oleifera Biomass as a Coagulant" Water 12, no. 7: 2052. https://doi.org/10.3390/w12072052
APA StyleAdelodun, B., Ogunshina, M. S., Ajibade, F. O., Abdulkadir, T. S., Bakare, H. O., & Choi, K. S. (2020). Kinetic and Prediction Modeling Studies of Organic Pollutants Removal from Municipal Wastewater using Moringa oleifera Biomass as a Coagulant. Water, 12(7), 2052. https://doi.org/10.3390/w12072052