Adsorption Properties of Magnetic Magnetite Nanoparticle for Coexistent Cr(VI) and Cu(II) in Mixed Solution
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials
2.2. Experimental Analysis Methods
2.2.1. Analysis Methods
2.2.2. Batch Adsorption Experiments
2.2.3. Desorption and Regeneration Experiments
3. Results and Discussion
3.1. The Effect of Adsorption Parameters
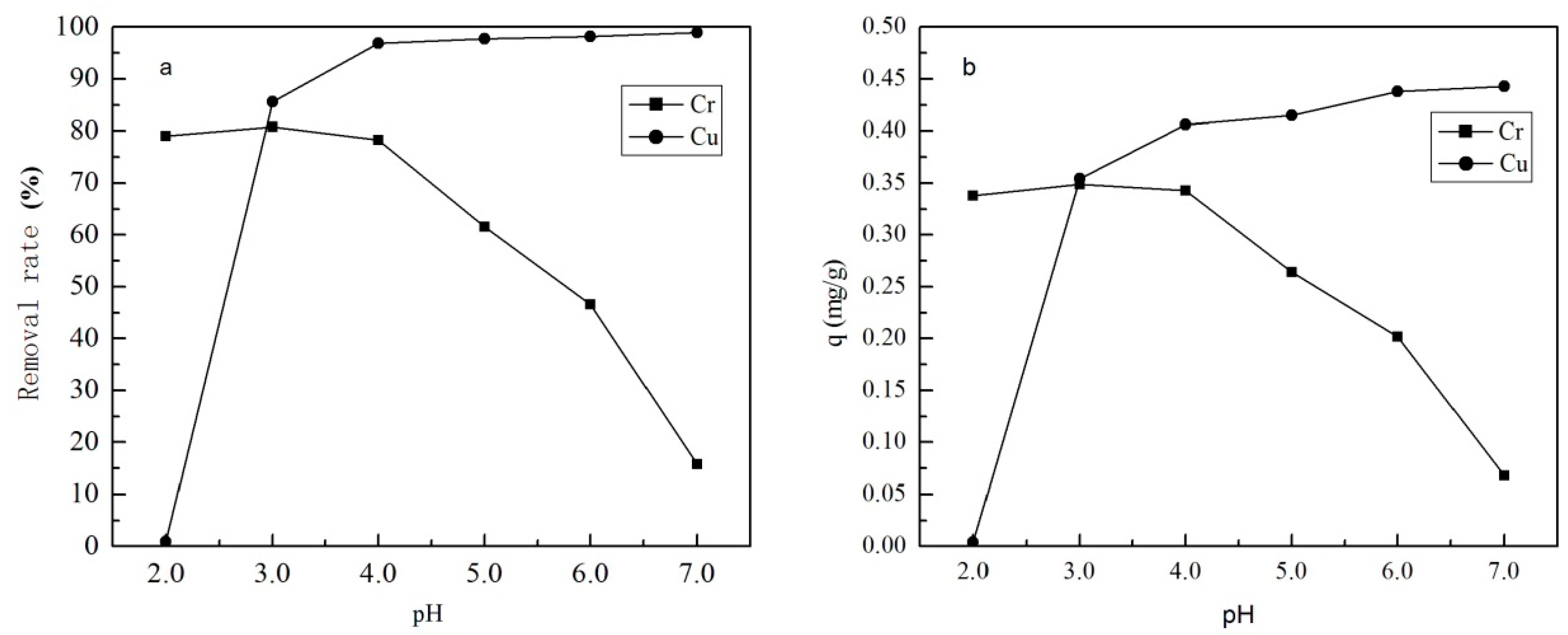
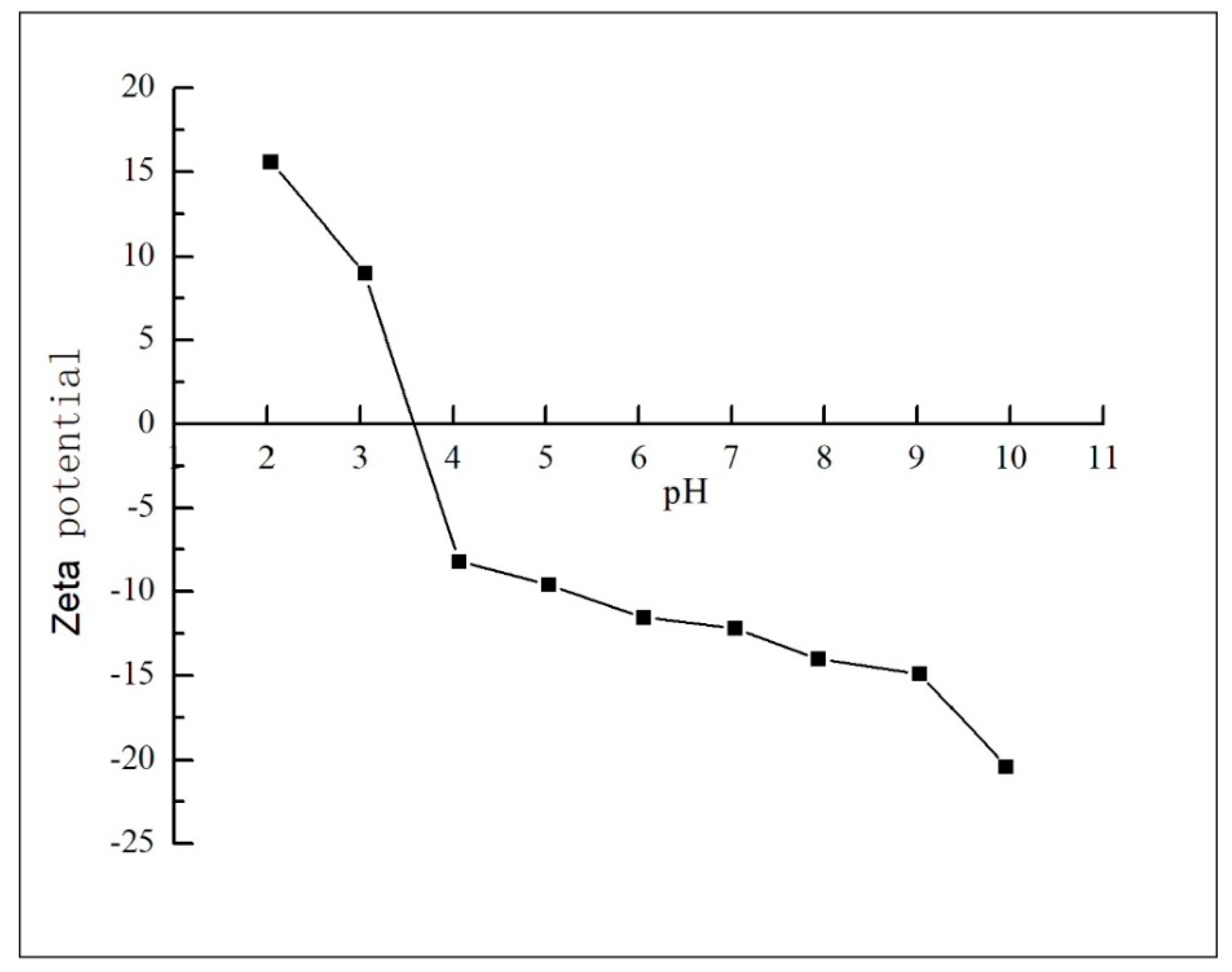
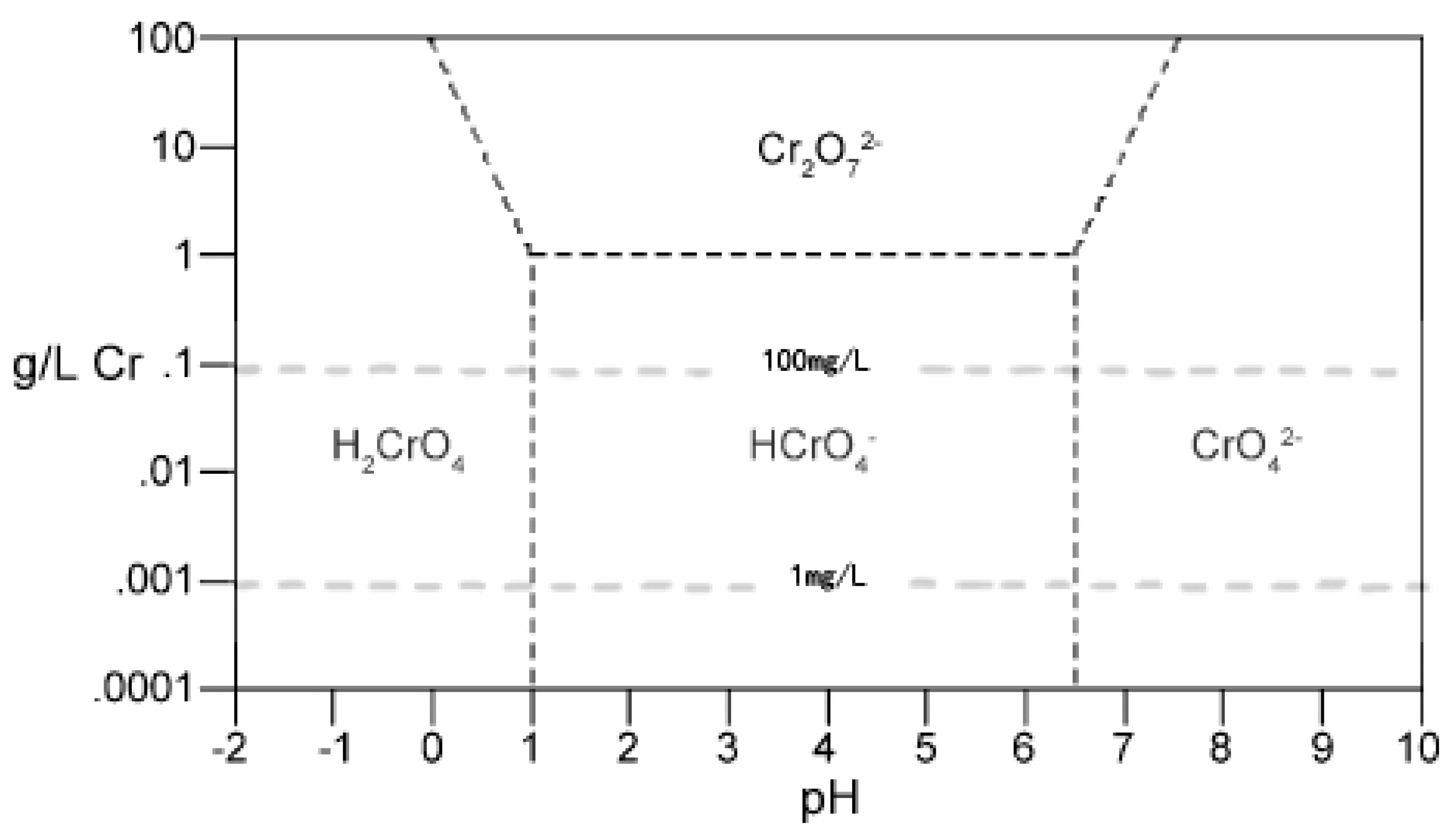
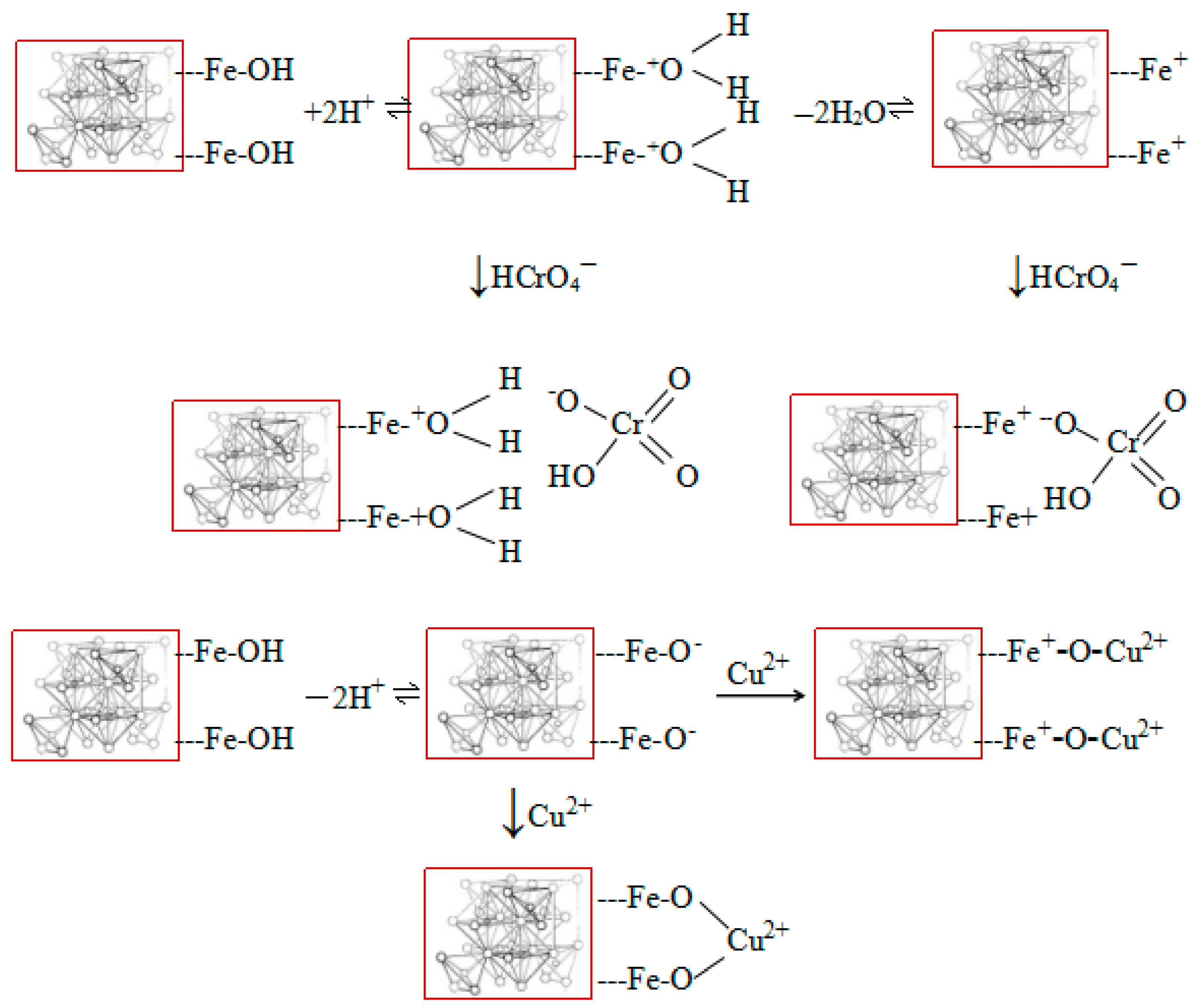
3.1.1. The Effect of pH and Zeta Potential
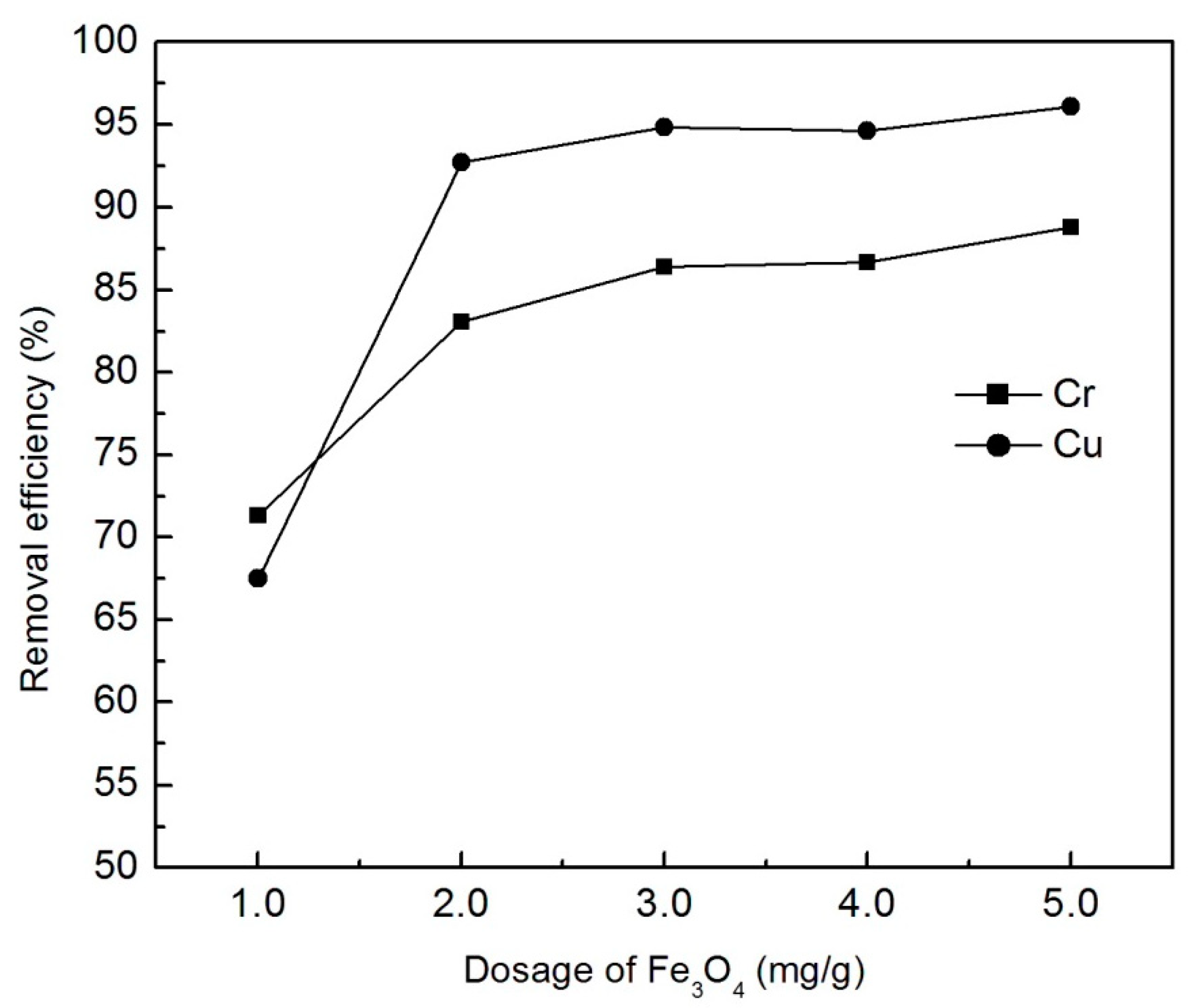
3.1.2. The Effect of Fe3O4 Dose
3.1.3. The Influence of Temperature
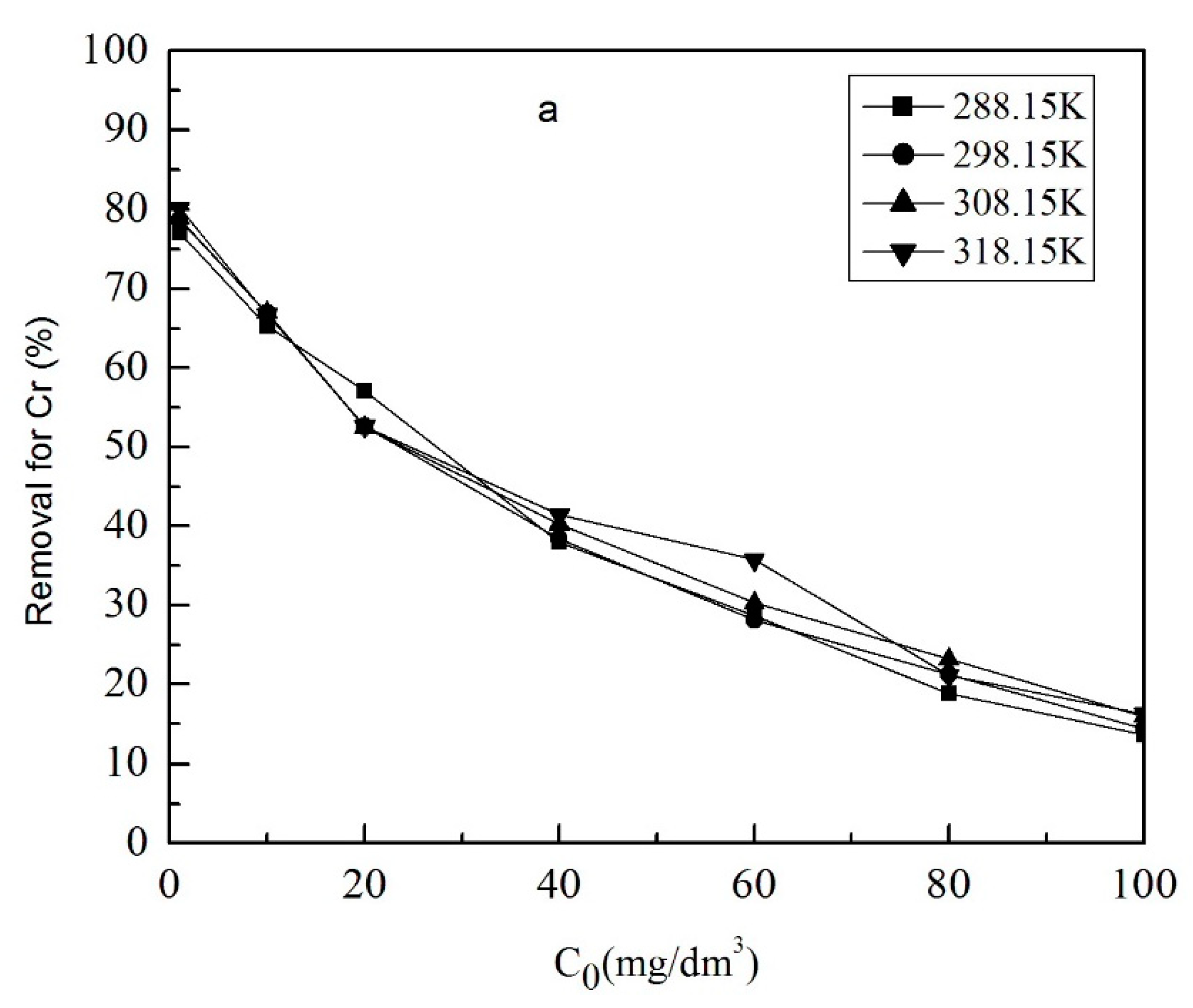
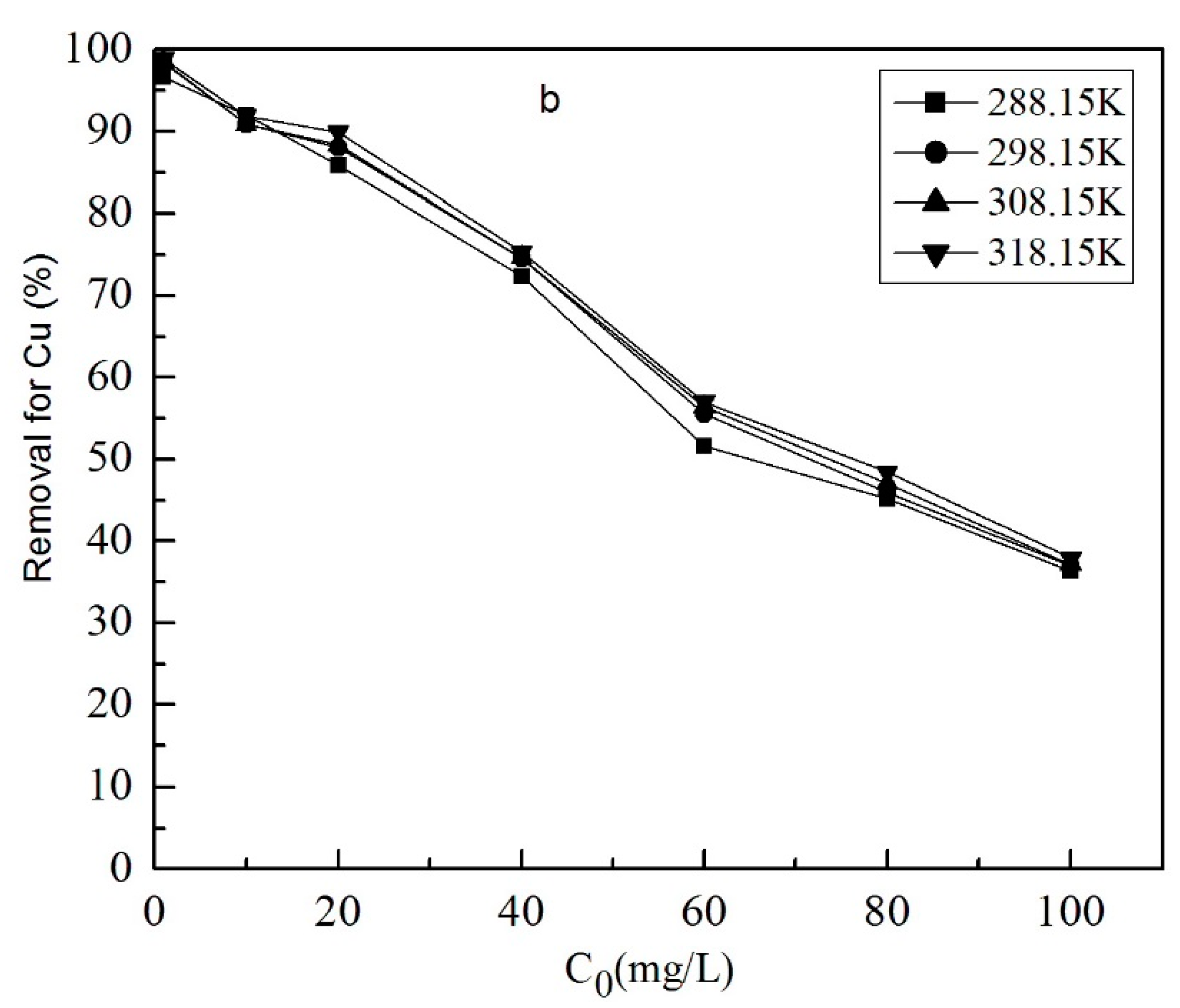
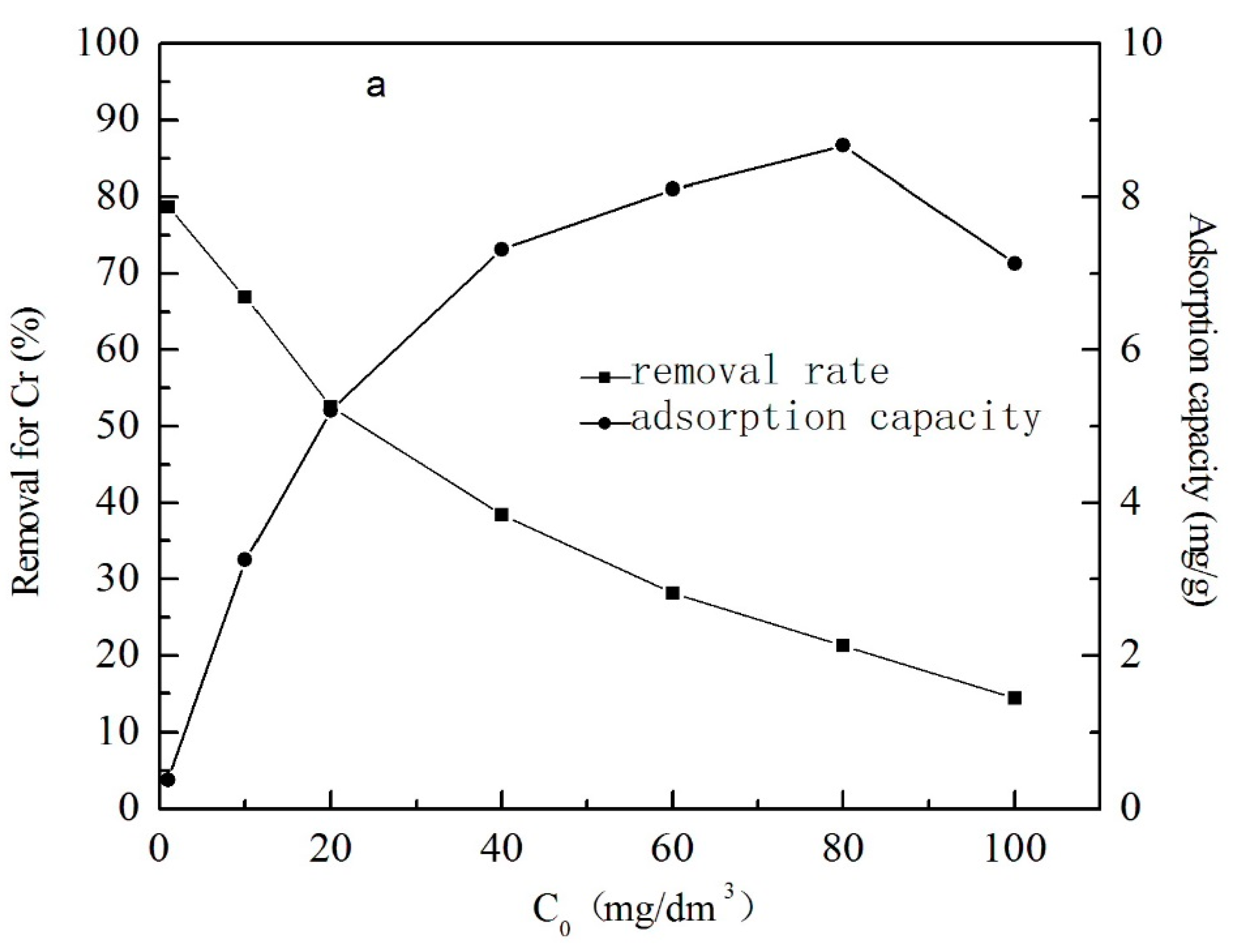
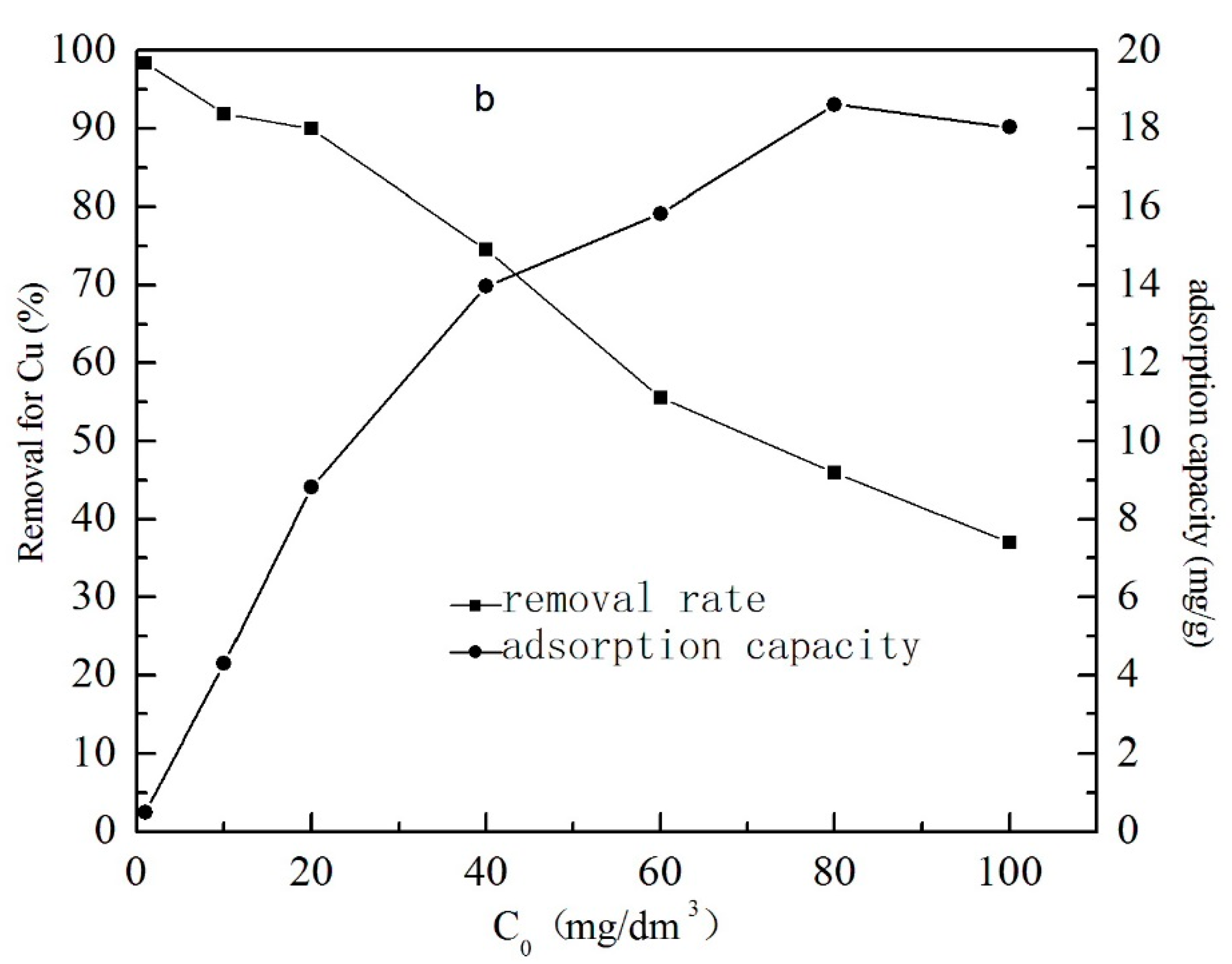
3.1.4. The Effect of Initial Concentration of Metal Ions
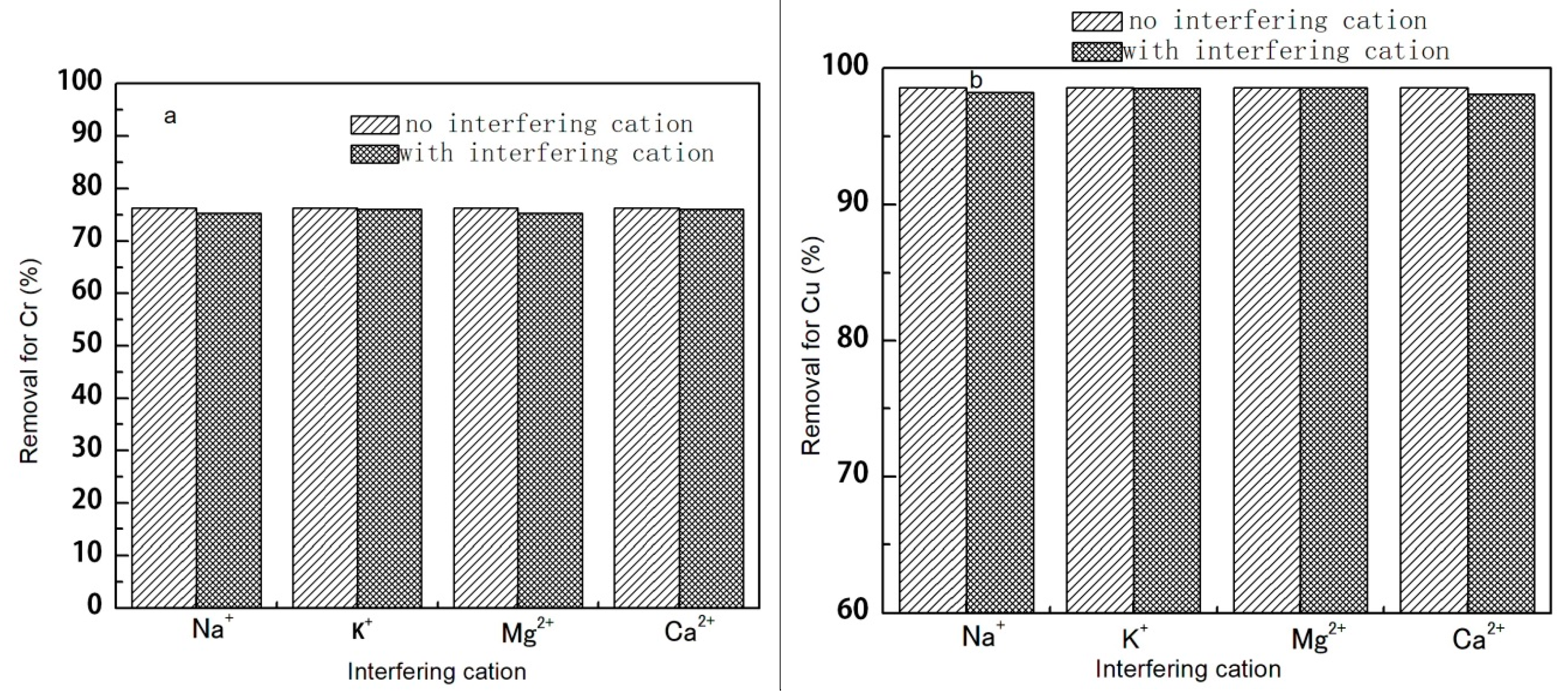
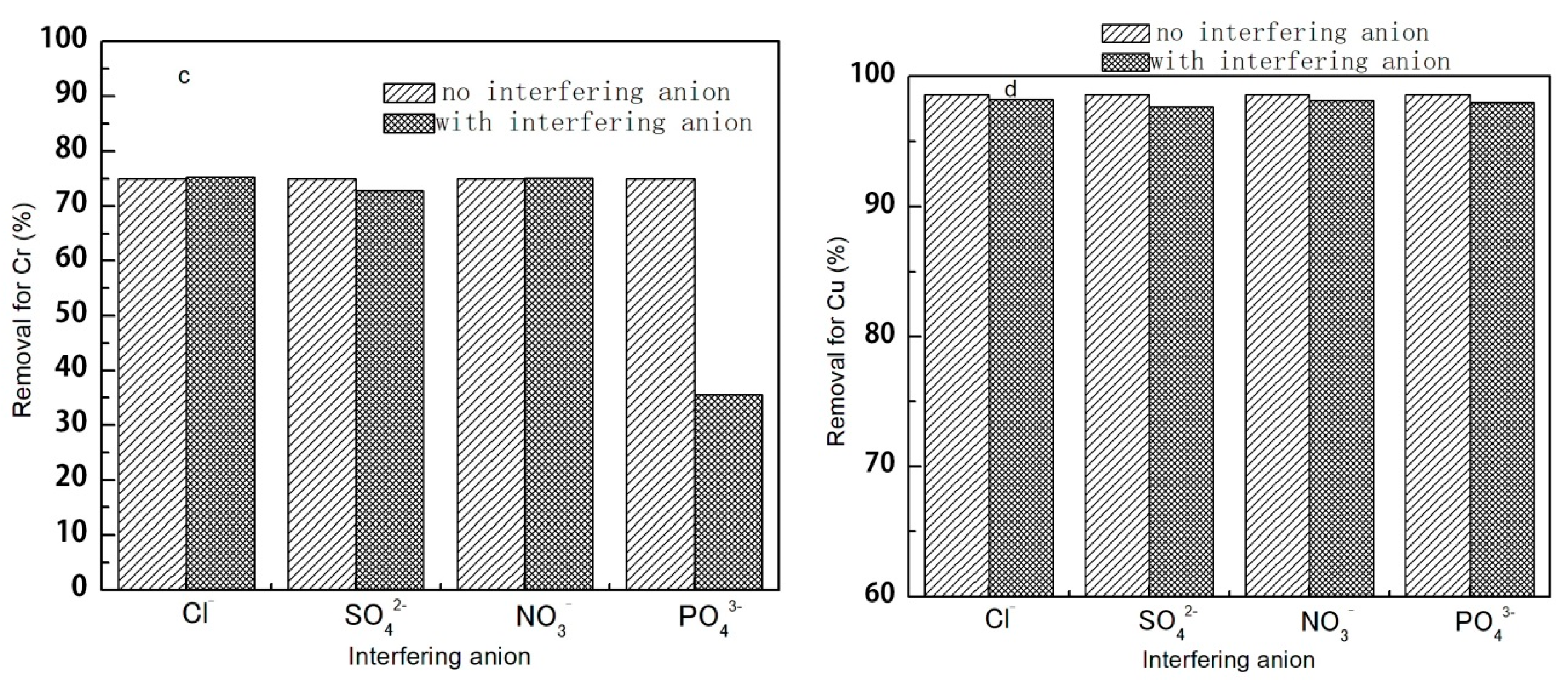
3.1.5. The Effect of Coexisting Common Ions
3.2. Study on Adsorption Isotherms
3.3. Adsorption Kinetics Study
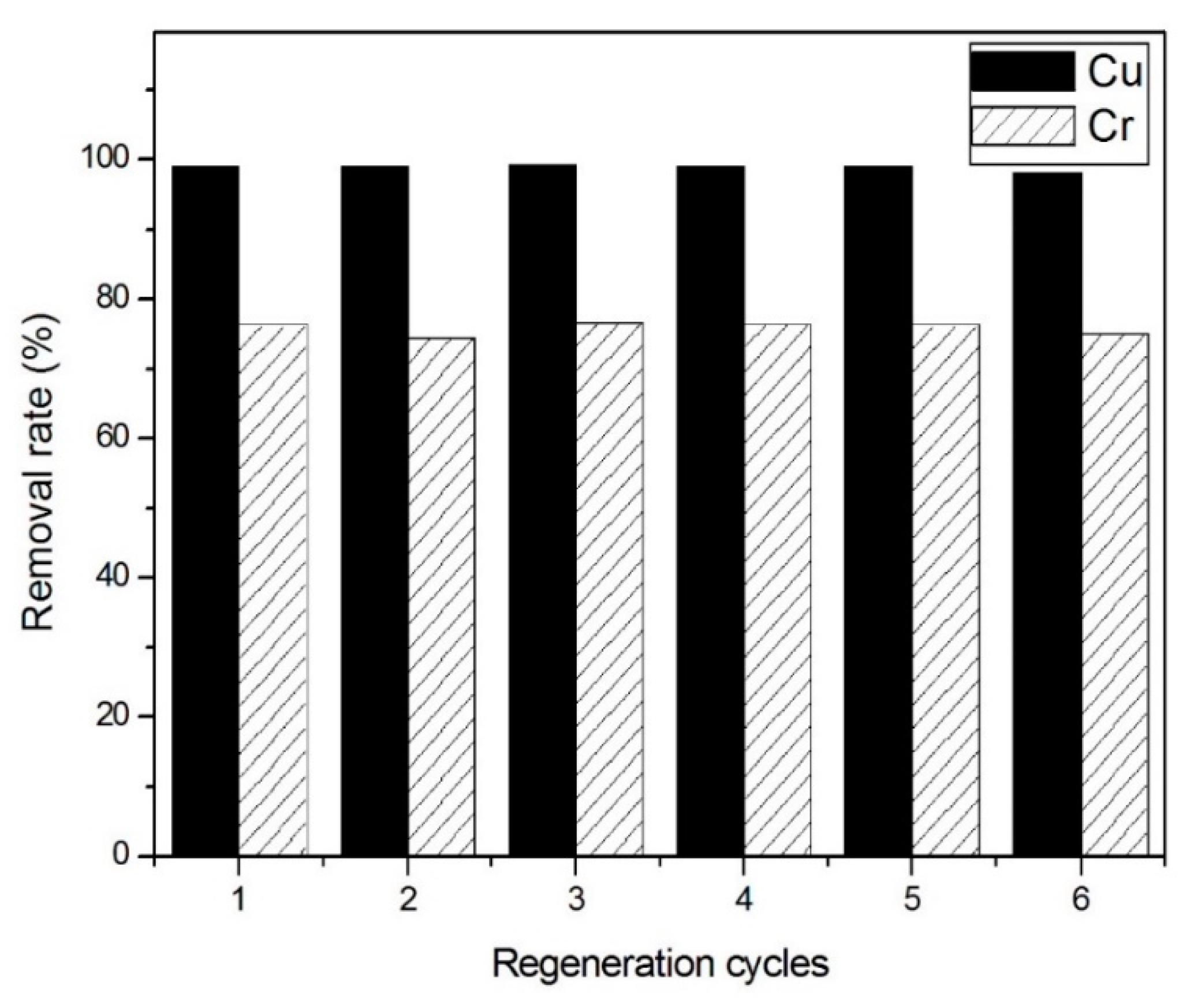
3.4. Desorption and Regeneration Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rozada, F.; Otero, M.; Morán, A.; Garcia, A.I. Adsorption of heavy metals onto sewage sludge-derived materials. Bioresour. Technol. 2008, 99, 6332–6338. [Google Scholar] [CrossRef] [PubMed]
- Mertz, W. Chromium in Human Nutrition: A Review. J. Nutr. 1993, 123, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Pelaez, M.; Dionysiou, D.D.; Entezari, M.H.; Tsoutsou, D.; O’Shea, K. Chromium(VI) removal by maghemite nanoparticles. Chem. Eng. J. 2013, 222, 527–533. [Google Scholar] [CrossRef]
- Brewer, G.J. Copper toxicity in the general population. Clin. Neurophysiol. 2010, 121, 459–460. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dasgupta, J.; Farooq, U.; Sikder, J.; Drioli, E.; Curcio, S. Experimental analysis, modeling and optimization of chromium (VI) removal from aqueous solutions by polymer-enhanced ultrafiltration. J. Membr. Sci. 2014, 456, 139–154. [Google Scholar] [CrossRef]
- Dowlatabadi, M.; Jahangiri, M.; Farhadian, N. Prediction of chlortetracycline adsorption on the Fe3O4 nanoparticle using molecular dynamics simulation. J. Biomol. Struct. Dyn. 2019, 37, 3616–3626. [Google Scholar] [CrossRef]
- Mohan, D.; Kumar, H.; Sarswat, A.; Alexandre-Franco, M.; Pittman, C.U. Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chem. Eng. J. 2014, 236, 513–528. [Google Scholar] [CrossRef]
- Moghaddam, A.Z.; Ghiamati, E.; Pourashuri, A.; Allahresani, A. Modified nickel ferrite nanocomposite/functionalized chitosan as a novel adsorbent for the removal of acidic dyes. Int. J. Biol. Macromol. 2018, 120, 1714–1725. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Ali, S.M.; El-Dek, S.I.; Galal, A. Magnetite-hematite nanoparticles prepared by green methods for heavy metal ions removal from water. Mater. Sci. Eng. B 2013, 178, 744–751. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Yanful, E.K.; Pratt, A.R. Chemical states in XPS and Raman analysis during removal of Cr(VI) from contaminated water by mixed maghemite-magnetite nanoparticles. J. Hazard. Mater. 2012, 235, 246–256. [Google Scholar] [CrossRef]
- Jung, C.; Heo, J.; Han, J.; Her, N.; Lee, S.J.; Oh, J.; Ryu, J.; Yoon, Y. Hexavalent chromium removal by various adsorbents: Powdered activated carbon, chitosan, and single/multi-walled carbon nanotubes. Sep. Purif. Technol. 2013, 106, 63–71. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurences and Uses, 2nd ed.; Wiley: Hoboken, NJ, USA, 2004; Available online: https://books.google.com.hk/books?hl=zh-TW&lr=&id=dlMuE3_klW4C&oi=fnd&pg=PA1&ots=l0oKXkS79M&sig=FfJYZEm_s8PIj_oRAszc1_y_zqA&redir_esc=y#v=onepage&q&f=false (accessed on 31 December 2019).
- Mahdavi, M.; Ahmad, M.B.; Haron, M.J.; Gharayebi, Y.; Shameli, K.; Nadi, B. Fabrication and Characterization of SiO2/(3-Aminopropyl) triethoxysilane-Coated Magnetite Nanoparticles for Lead(II) Removal from Aqueous Solution. J. Inorg. Organomet. Polym Mater. 2013, 23, 599–607. [Google Scholar] [CrossRef]
- Weng, X.; Huang, L.; Chen, Z.; Megharaj, M.; Naidu, R. Synthesis of iron-based nanoparticles by green tea extract and their degradation of malachite. Ind. Crop. Prod. 2013, 51, 342–347. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef]
- Demiral, H.; Demiral, I.; Tumsek, F.; Karabacakoglu, B. Adsorption of chromium(VI) from aqueous solution by activated carbon derived from olive bagasse and applicability of different adsorption models. Chem. Eng. J. 2008, 144, 188–196. [Google Scholar] [CrossRef]
- Tewari, N.; Vasudevan, P.; Guha, B.K. Study on biosorption of Cr(VI) by Mucor hiemalis. Biochem. Eng. J. 2005, 23, 185–192. [Google Scholar] [CrossRef]
- Ding, M.; De Jong, B.H.W.S.; Roosendaal, S.J.; Vredenberg, A. XPS studies on the electronic structure of bonding between solid and solutes: Adsorption of arsenate, chromate, phosphate, Pb2+, and Zn2+, ions on amorphous black ferric oxyhydroxide. Geochim. Cosmochim. Acta 2000, 64, 1209–1219. [Google Scholar] [CrossRef]
- Weng, C.H.; Wang, J.H.; Huang, C.P. Adsorption of Cr(VI) onto TiO2, from dilute aqueous solutions. Water Sci. Technol. 1997, 35, 55–62. [Google Scholar] [CrossRef]
- Ozmen, M.; Can, K.; Arslan, G.; Tor, A.; Cengeloglu, Y.; Ersoz, M. Adsorption of Cu(II) from aqueous solution by using modified Fe3O4 magnetic nanoparticles. Desalin 2010, 254, 162–169. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Preethi, J.; Meenakshi, S. Removal of chlorpyrifos, an insecticide using metal free heterogeneous graphitic carbon nitride (g-C3N4) incorporated chitosan as catalyst: Photocatalytic and adsorption studies. Int. J. Biol. Macromol. 2019, 132, 289–299. [Google Scholar] [CrossRef]
- Horst, M.F.; Alvarez, M.; Lassalle, V.L. Removal of heavy metals from wastewater using magnetic nanocomposites: Analysis of the experimental conditions. Sep. Sci. Technol. 2016, 51, 550–563. [Google Scholar] [CrossRef]
- Wasewar, K.L. Adsorption of metals onto tea factory waste: A review. Int. J. Res. Rev. Appl. Sci. 2010, 3, 303. [Google Scholar]
- Javadian, H.; Vahedian, P.; Toosi, M. Adsorption characteristics of Ni(II) from aqueous solution and industrial wastewater onto Polyaniline/HMS nanocomposite powder. Appl. Surf. Sci. 2013, 284, 13–22. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Lo, I.M. Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water Res. 2005, 39, 4528–4536. [Google Scholar] [CrossRef]
- Jiang, D.; Amano, Y.; Machida, M. Removal and recovery of phosphate from water by a magnetic Fe3O4 @ASC adsorbent. J. Envion. Chem. Eng. 2017, 5, 4229–4238. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef]
- Shin, K.Y.; Hong, J.Y.; Jang, J. Heavy metal ion adsorption behavior in nitrogen-doped magnetic carbon nanoparticles: Isotherms and kinetic study. J. Hazard. Mater 2011, 190, 36–44. [Google Scholar] [CrossRef]
- Jiang, M.-Q.; Wang, Q.P.; Jin, X.Y.; Chen, Z.L. Removal of Pb(II) from aqueous solution using modified and unmodified kaolinite clay. J. Hazard. Mater. 2009, 170, 332–339. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef]
- Poguberovic, S.S.; Krcmar, D.M.; Maletic, S.P.; Konya, Z.; Pilipovic, D.D.T.; Kerkez, D.V.; Roncevic, S.D. Removal of As(III) and Cr(VI) from aqueous solutions using“green”zero-valent iron nanoparticles produced by oak, mulberry and cherry leaf extracts. Ecol. Eng. 2016, 90, 42–49. [Google Scholar] [CrossRef]
- Rajput, S.; Pittman, C.U., Jr.; Mohan, D. Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb(2+)) and chromium (Cr(6+)) removal from water. J. Colloid Interface Sci. 2016, 468, 334–346. [Google Scholar] [CrossRef] [PubMed]

| Temperature (K) | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| qm–exp (mg/g) | qm (mg/g) | KL (dm3/mg) | R2 | KF (mg/g (dm3/mg)1/n) | n | R2 | ||
| Cr (VI) | 288.15 | 7.536 | 7.3475 | 0.5548 | 0.9801 | 1.5894 | 1.6170 | 0.9500 |
| 298.15 | 8.440 | 8.0270 | 0.3209 | 0.9740 | 1.6835 | 1.6487 | 0.9568 | |
| 308.15 | 8.500 | 8.7025 | 0.2568 | 0.9755 | 1.6616 | 1.6134 | 0.9599 | |
| 318.15 | 9.270 | 9.0196 | 0.2831 | 0.9641 | 1.7356 | 1.6216 | 0.9584 | |
| Cu (II) | 288.15 | 18.062 | 18.6498 | 0.3621 | 0.9928 | 3.6012 | 2.1267 | 0.9131 |
| 298.15 | 18.360 | 18.7935 | 0.4258 | 0.9954 | 4.1201 | 2.2847 | 0.9382 | |
| 308.15 | 18.815 | 18.9322 | 0.4733 | 0.9978 | 4.1636 | 2.2690 | 0.9400 | |
| 318.15 | 19.367 | 19.8373 | 0.4515 | 0.9827 | 4.4247 | 2.3173 | 0.9436 | |
| Pseudo-First-Order Kinetics | Pseudo-Second-Order Kinetics | ||||||
|---|---|---|---|---|---|---|---|
| qe (mg/g) | k1 | R2 | k2 | R2 | |||
| Cr (VI) | 0.4224 | 0.3939 | 0.9815 | 0.9804 | 0.4079 | 5.8069 | 0.9922 |
| Cu (II) | 0.4398 | 0.4362 | 0.9035 | 0.9961 | 0.4484 | 5.7268 | 0.9971 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Lin, S.; Han, M.; Su, Q.; Xia, L.; Hui, Z. Adsorption Properties of Magnetic Magnetite Nanoparticle for Coexistent Cr(VI) and Cu(II) in Mixed Solution. Water 2020, 12, 446. https://doi.org/10.3390/w12020446
Zhang J, Lin S, Han M, Su Q, Xia L, Hui Z. Adsorption Properties of Magnetic Magnetite Nanoparticle for Coexistent Cr(VI) and Cu(II) in Mixed Solution. Water. 2020; 12(2):446. https://doi.org/10.3390/w12020446
Chicago/Turabian StyleZhang, Jin, Shuang Lin, Meiling Han, Qing Su, Lianqiu Xia, and Zhaocong Hui. 2020. "Adsorption Properties of Magnetic Magnetite Nanoparticle for Coexistent Cr(VI) and Cu(II) in Mixed Solution" Water 12, no. 2: 446. https://doi.org/10.3390/w12020446
APA StyleZhang, J., Lin, S., Han, M., Su, Q., Xia, L., & Hui, Z. (2020). Adsorption Properties of Magnetic Magnetite Nanoparticle for Coexistent Cr(VI) and Cu(II) in Mixed Solution. Water, 12(2), 446. https://doi.org/10.3390/w12020446




