Broad Diet Composition and Seasonal Feeding Variation Facilitate Successful Invasion of the Shimofuri Goby (Tridentiger bifasciatus) in a Water Transfer System
Abstract
1. Introduction
2. Materials and Methods
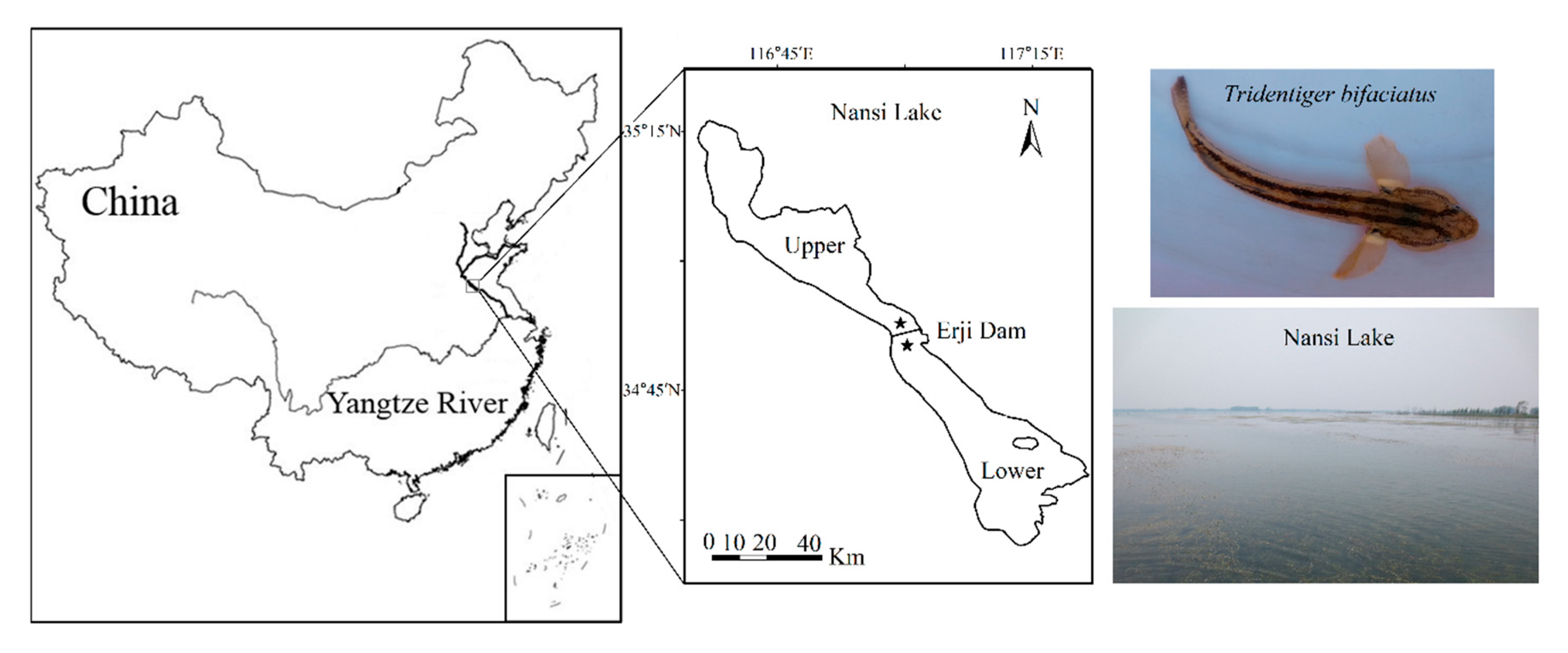
2.1. Study Area and Fish Sampling
2.2. Diet Analysis
3. Results
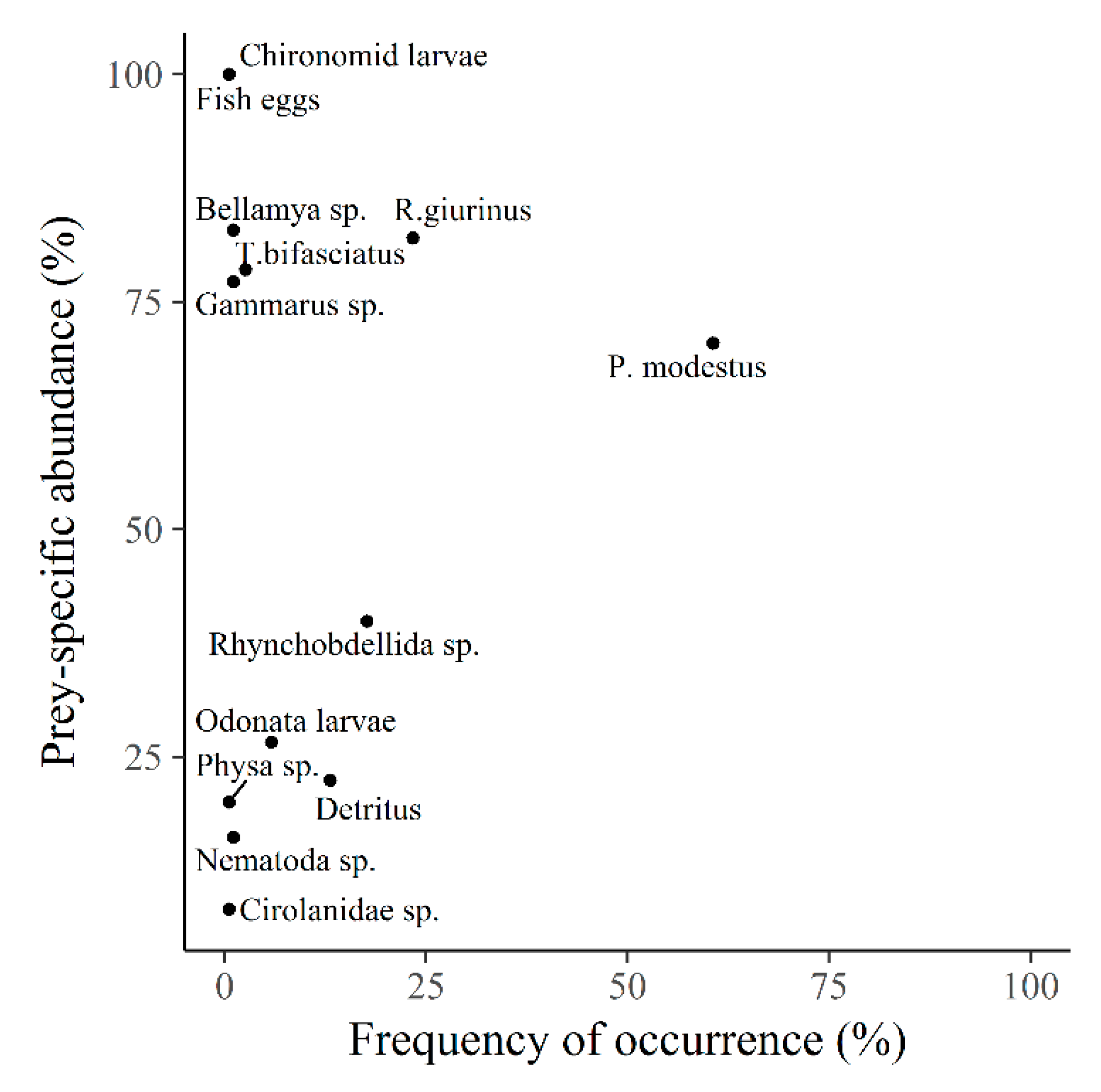
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cucherousset, J.; Olden, J.D. Ecological Impacts of Nonnative Freshwater Fishes. Fisheries 2011, 36, 215–230. [Google Scholar] [CrossRef]
- Dick, J.T.A.; Gallagher, K.; Avlijas, S.; Clarke, H.C.; Lewis, S.E.; Leung, S.; Minchin, D.; Caffrey, J.; Alexander, M.E.; Maguire, C.; et al. Ecological impacts of an invasive predator explained and predicted by comparative functional responses. Biol. Invasions 2013, 15, 837–846. [Google Scholar] [CrossRef]
- Brandner, J.; Auerswald, K.; Cerwenka, A.F.; Geist, J. Comparative feeding ecology of invasive Ponto-Caspian gobies. Hydrobiologia 2013, 703, 113–131. [Google Scholar] [CrossRef]
- Ricciardi, A.; Kipp, R. Predicting the number of ecologically harmful species in an aquatic system. Divers. Distrib. 2008, 14, 374–380. [Google Scholar] [CrossRef]
- Copp, G.H.; Russell, I.C.; Peeler, E.J.; Gherardi, F.; Tricarico, E.; Macleod, A.; Mumford, J. European Non-native Species in Aquaculture Risk Analysis Scheme–a summary of assessment protocols and decision support tools for use of alien species in aquaculture. Fish. Manag. Ecol. 2016, 23, 1–11. [Google Scholar] [CrossRef]
- Witter, F.; Goldschmidt, T.; Goudswaard, P.C.; Ligtvoet, W.; Van Oijen, M.J.P.; Wanink, J.H. Species extinction and concomitant ecological changes in Lake Victoria. Neth. J. Zool. 1992, 42, 214–232. [Google Scholar] [CrossRef]
- Copp, G.H.; Kováč, V.; Zweimüller, I.; Dias, A.; Nascimento, M.; Balážová, M. Preliminary study of dietary interactions between invading Ponto-Caspian gobies and some native fish species in the River Danube near Bratislava (Slovakia). Aquat. Invasions 2008, 3, 193–200. [Google Scholar] [CrossRef]
- Rahel, F.J. Biogeographic barriers, connectivity and homogenization of freshwater faunas: It’s a small world after all. Freshw. Biol. 2007, 52, 696–710. [Google Scholar] [CrossRef]
- Zhan, A.B.; Zhang, L.; Xia, Z.Q.; Ni, P.; Xiong, W.; Chen, Y.Y.; Haffner, G.D.; Maclsaac, H.J. Water diversions facilitate spread of non-native species. Biol. Invasions 2015, 17, 3073–3080. [Google Scholar] [CrossRef]
- Gallardo, B.; Aldridge, D.C. Inter-basin water transfers and expansion of aquatic invasive species. Water Res. 2018, 143, 282–291. [Google Scholar] [CrossRef]
- Baltz, D.M.; Moyle, P.B. Invasion resistance to introduced species by a native assemblage of California stream fishes. Ecol. Appl. 1993, 3, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Moyle, P.B.; Light, T. Fish invasions in California: Do abiotic factors determine success? Ecology 1996, 77, 1666–1670. [Google Scholar] [CrossRef]
- Kadye, W.T.; Booth, A.J. Integrating stomach content and stable isotope analyses to elucidate the feeding habits of non-native sharptooth catfish Clarias gariepinus. Biol. Invasions 2012, 14, 779–795. [Google Scholar] [CrossRef]
- Liu, C.M.; Zuo, D.K.; Xu, Y.X. Water transfer in China: The east route project. Int. J. Water Resour. D 1984, 2, 103–118. [Google Scholar] [CrossRef]
- Qin, J.; Cheng, F.; Zhang, L.; Schmidt, B.V.; Liu, J.; Xie, S.G. Invasions of two estuarine gobiid species interactively induced from water diversion and saltwater intrusion. Manag. Biol. Invasions 2019, 10, 139–150. [Google Scholar] [CrossRef]
- Matern, S.A. The invasion of the shimofuri goby (Tridentiger bifasciatus) into California: Establishment, potential for spread, and likely effects. Ph.D. Thesis, University of California, Davis, CA, USA, 1999. [Google Scholar]
- Wu, H.L.; Zhong, J.S. Chinese Animals: Osteichthyes, Gobioidei; Scientific Press: Beijing, China, 2008; p. 951. [Google Scholar]
- Qin, J.; Xiang, M.; Jia, M.X.; Cheng, F.; Zhang, L.; Schmidt, B.V.; Liu, J.; Xie, S.G. Combined opportunistic and equilibrium life-history traits facilitate successful invasions of the Shimofuri goby (Tridentiger bifasciatus). Aquat. Invasions 2020, 15, 514–528. [Google Scholar] [CrossRef]
- Matern, S.A.; Fleming, K.J. Invasion of a third Asian goby, Tridentiger bifasciatus, into California. Calif. Fish Game 1995, 81, 71–76. [Google Scholar]
- Matern, S.A.; Moyle, P.B.; Pierce, L.C. Native and alien fishes in a California estuarine marsh: Twenty-one years of changing assemblages. Tran. Am. Fish. Soc. 2002, 131, 797–816. [Google Scholar] [CrossRef]
- Matern, S.A.; Brown, L.R. Invaders eating invaders: Exploitation of novel alien prey by the Shimofuri goby in the San Francisco Estuary, California. Biol. Invasions 2005, 7, 497–507. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, S.L.; Hou, H.P.; Yang, Y.Y.; Gong, Y.L. (2019) Assessing the changes of ecosystem services in the Nansi Lake wetland, China. Water 2019, 11, 788. [Google Scholar] [CrossRef]
- Hyslop, E.J. Stomach contents analysis-a review of methods and their application. J. Fish. Biol. 1980, 7, 411–429. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecology Methodology; Addison Wesley Educational Publishers: Boston, CA, USA, 1999. [Google Scholar]
- Amundsen, P.A.; Gabler, H.M.; Staldvik, F.J. A new approach to graphical analysis of feeding strategy from stomach contents data–modification of the Costello (1990) method. J. Fish. Biol. 1996, 48, 607–614. [Google Scholar] [CrossRef]
- Grabowska, J.; Przybylski, M. Life-history traits of nonnative freshwater fish invaders differentiate them from natives in the Central European bioregion. Rev. Fish Biol. Fish. 2014, 25, 165–178. [Google Scholar] [CrossRef]
- Grabowska, J.; Grabowski, M. Diel-feeding activity in early summer of racer goby Neogobius gymnotrachelus (Gobiidae): A new invader in the Baltic basin. J. Appl. Ichthyol. 2005, 21, 282–286. [Google Scholar] [CrossRef]
- Kakareko, T.; Zbikowski, J.; Zytkowicz, J. Diel partitioning in summer of two syntopic neogobiids from two different habitats of the lower Vistula River, Poland. J. Appl. Ichthol. 2005, 21, 292–295. [Google Scholar] [CrossRef]
- Pothoven, S.A. Seasonal feeding ecology of co-existing native and invasive benthic fish along a nearshore to offshore gradient in Lake Michigan. Environ. Biol. Fishes 2018, 101, 1161–1174. [Google Scholar] [CrossRef]
- Neves, M.P.; Delariva, R.L.; Guimaraes, A.T.B.; Sanches, P.V. Carnivory during Ontogeny of the Plagioscion squamosissimus: A Successful Non-Native Fish in a Lentic Environment of the Upper Parana River Basin. PLoS ONE 2015, 10, e0141651. [Google Scholar] [CrossRef] [PubMed]
- Townsend, C.R.; Winfield, I.J. The application of optimal foraging theory to feeding behaviour in fish. In Fish Energetics: New Perspectives; Tytler, P., Calow, P., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 1985; pp. 67–98. [Google Scholar]
- Nurkse, K.; Kotta, J.; Orav-Kotta, H.; Ojaveer, H. A successful non-native predator, round goby, in the Baltic Sea: Generalist feeding strategy, diverse diet and high prey consumption. Hydrobiologia 2016, 777, 271–281. [Google Scholar] [CrossRef]
- Fox, L.R. Cannibalism in Natural Populations. Annu. Rev. Ecol. Syst. 1975, 6, 87–106. [Google Scholar] [CrossRef]
- Smith, C.; Reay, P. Cannibalism in teleost fish. Rev. Fish Biol. Fish. 1991, 1, 41–64. [Google Scholar] [CrossRef]
- Pereira, L.S.; Agostinho, A.A.; Winemiller, K.O. Revisiting cannibalism in fishes. Rev. Fish Biol. Fish. 2017, 27, 499–513. [Google Scholar] [CrossRef]
- Muñoz-Arroyo, S.; Martínez-Rincón, R.O.; Findley, L.T.; Hernández-Olalde, L.; Balart, E.F. Reproductive behaviors and sex roles during a diurnal cycle of the goby, Lythrypnus pulchellus (Teleostei: Gobiidae). J. Ethol. 2020, 38, 79–98. [Google Scholar] [CrossRef]
- Marconato, A.; Bisazza, A.; Fabris, M. The cost of parental care and egg cannibalism in the river bullhead, Cottus gobio L. (Pisces, Cottidae). Behav. Ecol. Sociobiol. 1993, 32, 229–237. [Google Scholar] [CrossRef]
- Xie, S.; Li, Z.; Cui, Y.; Murphy, B.R. Distribution, feeding and body condition of four small fish species in the near-shore and central areas of Liangzi Lake, China. Environ. Biol. Fishes 2005, 74, 379–387. [Google Scholar] [CrossRef]
- Janssen, J.; Jude, D.J. Recruitment failure of mottled sculpin Cottus bairdi in Calumet Harbor, southern Lake Michigan, induced by the newly introduced round goby Neogobius melanostomus. J. Great Lakes Res. 2001, 27, 319–328. [Google Scholar] [CrossRef]
- Matern, S.A. Using temperature and salinity tolerances to predict the success of the Shimofuri Goby, a recent invader into California. Tran. Am. Fish. Soc. 2001, 130, 592–599. [Google Scholar] [CrossRef]
| September | November | January | March | May | July | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Fish | 35 | 37 | 17 | 35 | 41 | 26 | |||||||||||||
| SL (mm) BW(g) | Mean ± SD | 52.9 ± 7.2 | 51.9 ± 10.7 | 60.2 ± 7.6 | 72.3 ± 6.7 | 50.4 ± 7.6 | 37.0 ± 4.6 | ||||||||||||
| Mean ± SD | 2.869 ± 1.128 | 3.202 ± 2.371 | 4.562 ± 1.729 | 8.869 ± 2.757 | 2.275 ± 1.239 | 0.925 ± 0.408 | |||||||||||||
| Prey items | %F | %W | %IRI | %F | %W | %IRI | %F | %W | %IRI | %F | %W | %IRI | %F | %W | %IRI | %F | %W | %IRI | |
| Mollusca | 2.70 | 0.19 | 0.01 | 4.88 | 4.40 | 0.50 | |||||||||||||
| Bellamya sp. | 2.70 | 0.19 | 0.01 | 2.44 | 4.25 | 0.24 | |||||||||||||
| Physa sp. | 2.44 | 0.15 | 0.01 | ||||||||||||||||
| Aquatic insects | 17.14 | 4.12 | 1.38 | 13.51 | 6.63 | 1.59 | 5.88 | 4.60 | 0.53 | 2.86 | 0.14 | 0.01 | |||||||
| Chironomid larvae | 2.86 | 0.11 | 0.01 | ||||||||||||||||
| Odonata larvae | 11.42 | 3.56 | 0.80 | 13.51 | 6.63 | 1.59 | 5.88 | 4.6 | 0.53 | 2.86 | 0.14 | 0.01 | |||||||
| Cirolanidae sp. | 2.86 | 0.45 | 0.03 | ||||||||||||||||
| Other Macroinvertebrate | 5.71 | 0.53 | 0.06 | 2.70 | 0.25 | 0.01 | 46.34 | 1.47 | 1.58 | 23.07 | 38.28 | 23.76 | |||||||
| Nematoda sp. | 5.71 | 0.53 | 0.06 | 2.70 | 0.25 | 0.01 | 7.69 | 3.91 | 0.63 | ||||||||||
| Rhynchobdellida sp. | 46.34 | 1.47 | 1.58 | 57.69 | 34.38 | 41.78 | |||||||||||||
| Shrimp | 62.86 | 73.24 | 90.23 | 72.97 | 68.67 | 88.70 | 76.47 | 57.4 | 86.73 | 74.29 | 37.43 | 48.04 | 68.29 | 38.23 | 60.57 | 11.54 | 18.75 | 5.82 | |
| Palaemon modestus | 62.86 | 73.24 | 90.74 | 72.97 | 68.67 | 88.70 | 76.47 | 57.4 | 86.73 | 74.29 | 37.43 | 59.32 | 68.29 | 38.23 | 60.73 | 3.85 | 7.29 | 0.59 | |
| Gammarus sp. | 7.69 | 11.46 | 1.86 | ||||||||||||||||
| Fish | 14.29 | 17.35 | 4.86 | 24.32 | 22.03 | 9.48 | 17.65 | 33.57 | 11.71 | 48.57 | 61.57 | 51.66 | 29.27 | 54.58 | 37.07 | 3.85 | 5.47 | 0.57 | |
| Rhinogobius giurinus | 14.29 | 17.35 | 4.89 | 24.32 | 22.03 | 9.48 | 17.65 | 33.57 | 11.71 | 42.86 | 35.34 | 32.31 | 29.27 | 54.58 | 37.16 | 3.85 | 5.47 | 0.44 | |
| Tridentiger bifasciatus | 14.29 | 26.23 | 8.00 | ||||||||||||||||
| Fish egg | 2.44 | 4.41 | 0.25 | ||||||||||||||||
| Detritus | 37.14 | 4.76 | 3.46 | 5.41 | 2.22 | 0.21 | 11.76 | 4.44 | 1.03 | 20.00 | 0.86 | 0.30 | 2.44 | 0.54 | 0.03 | ||||
| Unidentified digested food | 69.23 | 37.50 | 69.85 | ||||||||||||||||
| Unidentified digested food | 69.23 | 37.50 | 54.69 | ||||||||||||||||
| Niche breadth | 0.13 | 0.18 | 0.41 | 0.50 | 0.21 | 0.51 | |||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, J.; Xie, S.; Cheng, F. Broad Diet Composition and Seasonal Feeding Variation Facilitate Successful Invasion of the Shimofuri Goby (Tridentiger bifasciatus) in a Water Transfer System. Water 2020, 12, 3411. https://doi.org/10.3390/w12123411
Qin J, Xie S, Cheng F. Broad Diet Composition and Seasonal Feeding Variation Facilitate Successful Invasion of the Shimofuri Goby (Tridentiger bifasciatus) in a Water Transfer System. Water. 2020; 12(12):3411. https://doi.org/10.3390/w12123411
Chicago/Turabian StyleQin, Jiao, Songguang Xie, and Fei Cheng. 2020. "Broad Diet Composition and Seasonal Feeding Variation Facilitate Successful Invasion of the Shimofuri Goby (Tridentiger bifasciatus) in a Water Transfer System" Water 12, no. 12: 3411. https://doi.org/10.3390/w12123411
APA StyleQin, J., Xie, S., & Cheng, F. (2020). Broad Diet Composition and Seasonal Feeding Variation Facilitate Successful Invasion of the Shimofuri Goby (Tridentiger bifasciatus) in a Water Transfer System. Water, 12(12), 3411. https://doi.org/10.3390/w12123411





