Removing Nutrients from Crab-Breeding Wastewater by a Floating Plant–Effective Microorganism Bed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flume Test
2.1.1. Aquaculture Wastewater Preparation
2.1.2. Flume Equipment
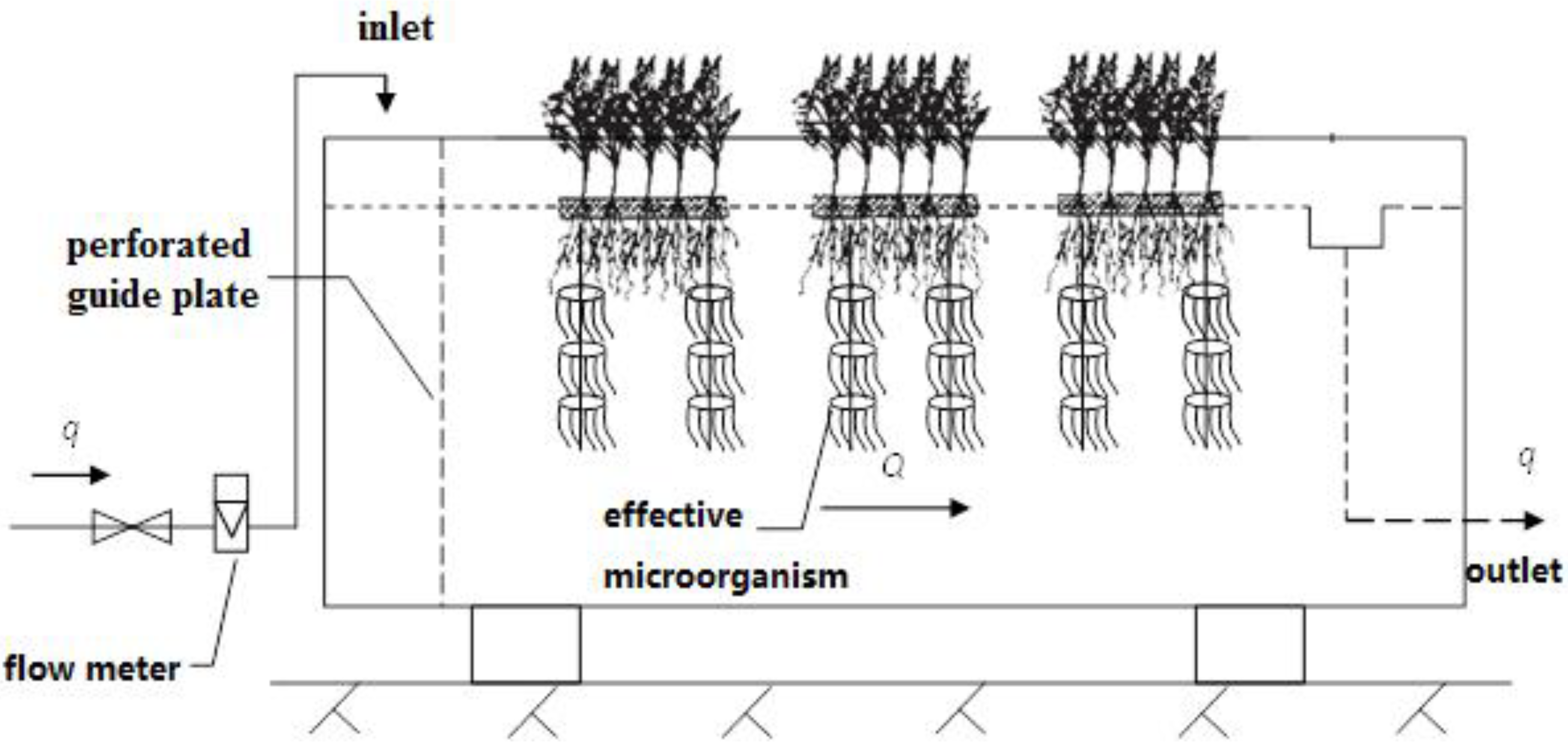
2.1.3. Floating Bed–Effective Microorganism Treatment System
2.1.4. Treatment under Various Hydraulic Loadings
2.1.5. Calculation of the Pollutant Removal Efficiency and Removal Load
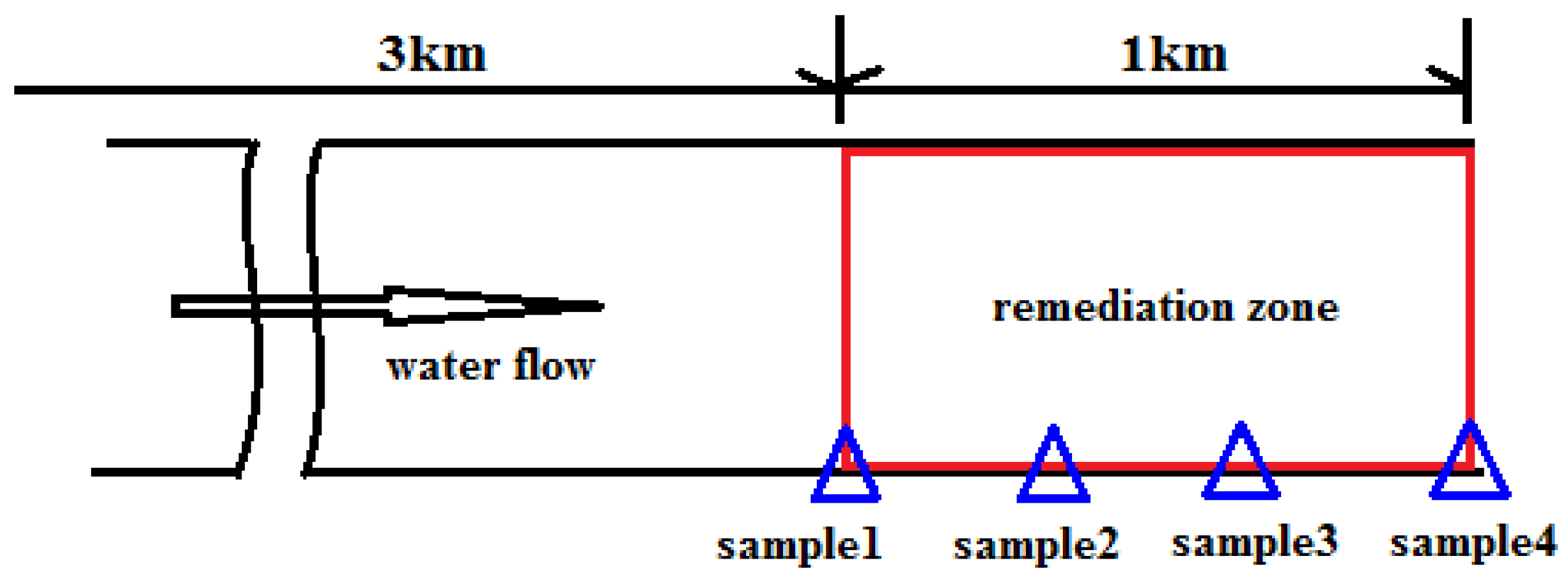
2.2. Field Test
2.3. Calculation of the Pollutant Comprehensive Degradation Coefficient
3. Results and Discussion
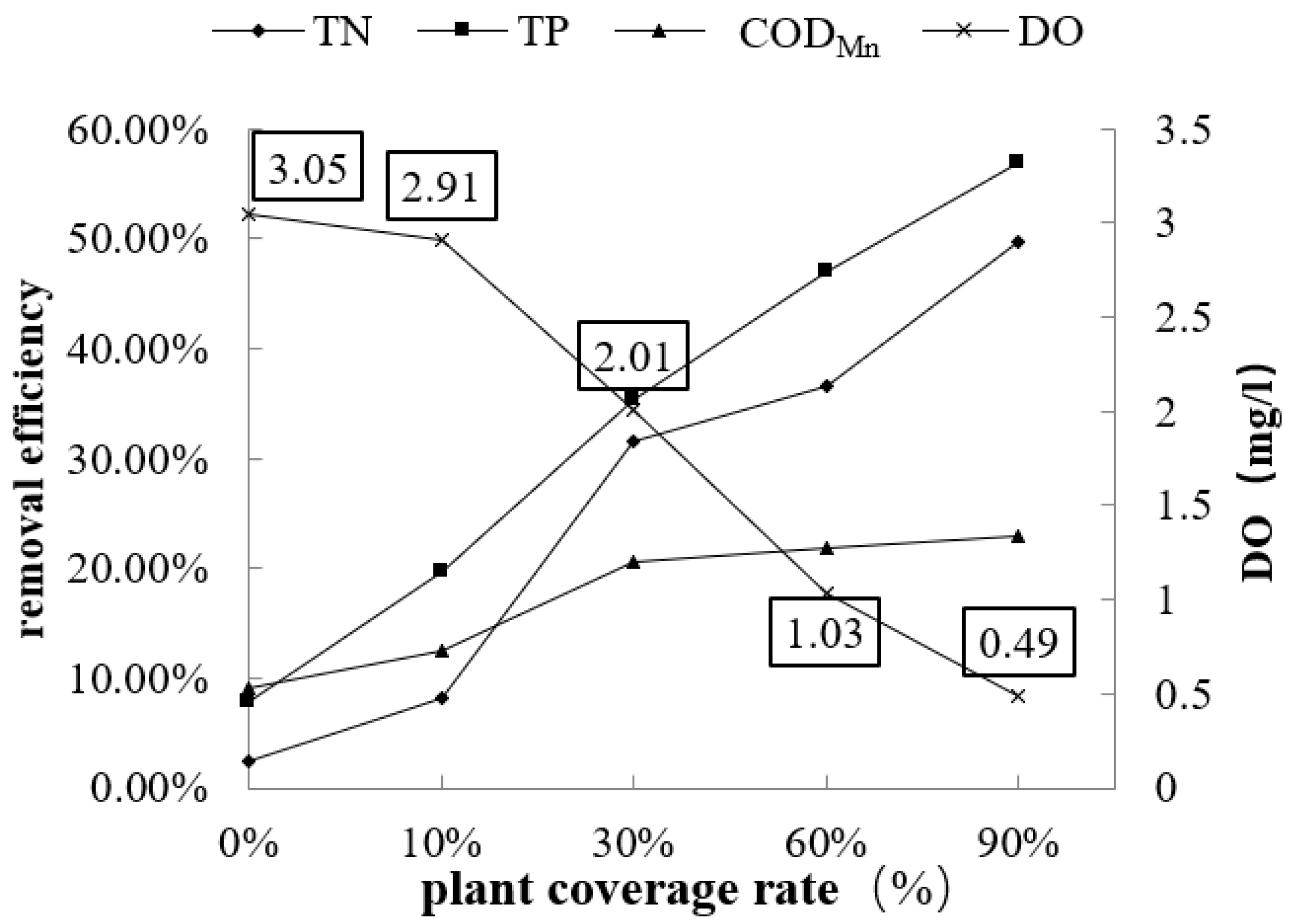
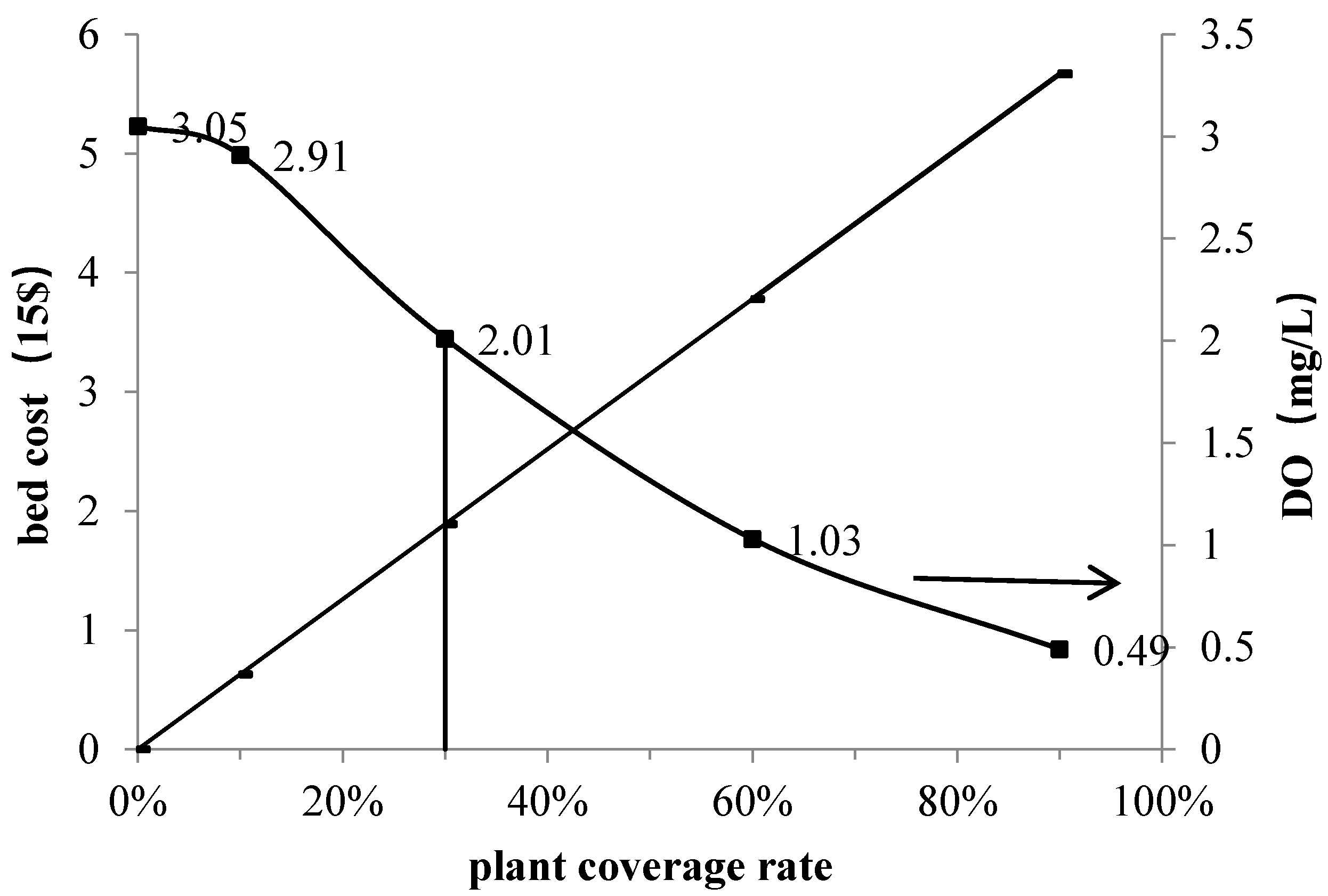
3.1. Effect of the Plant Coverage Rate on Purification Performance
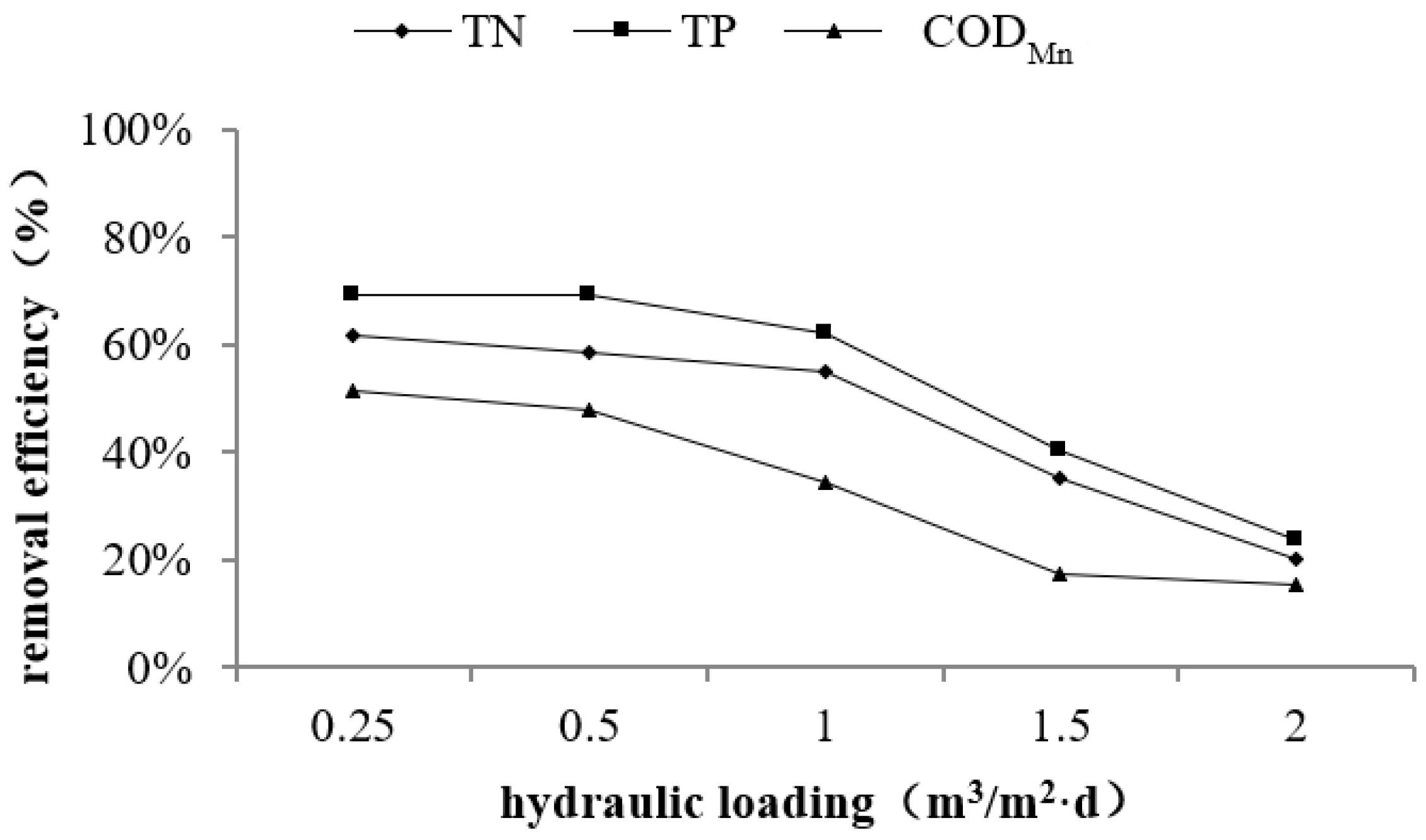
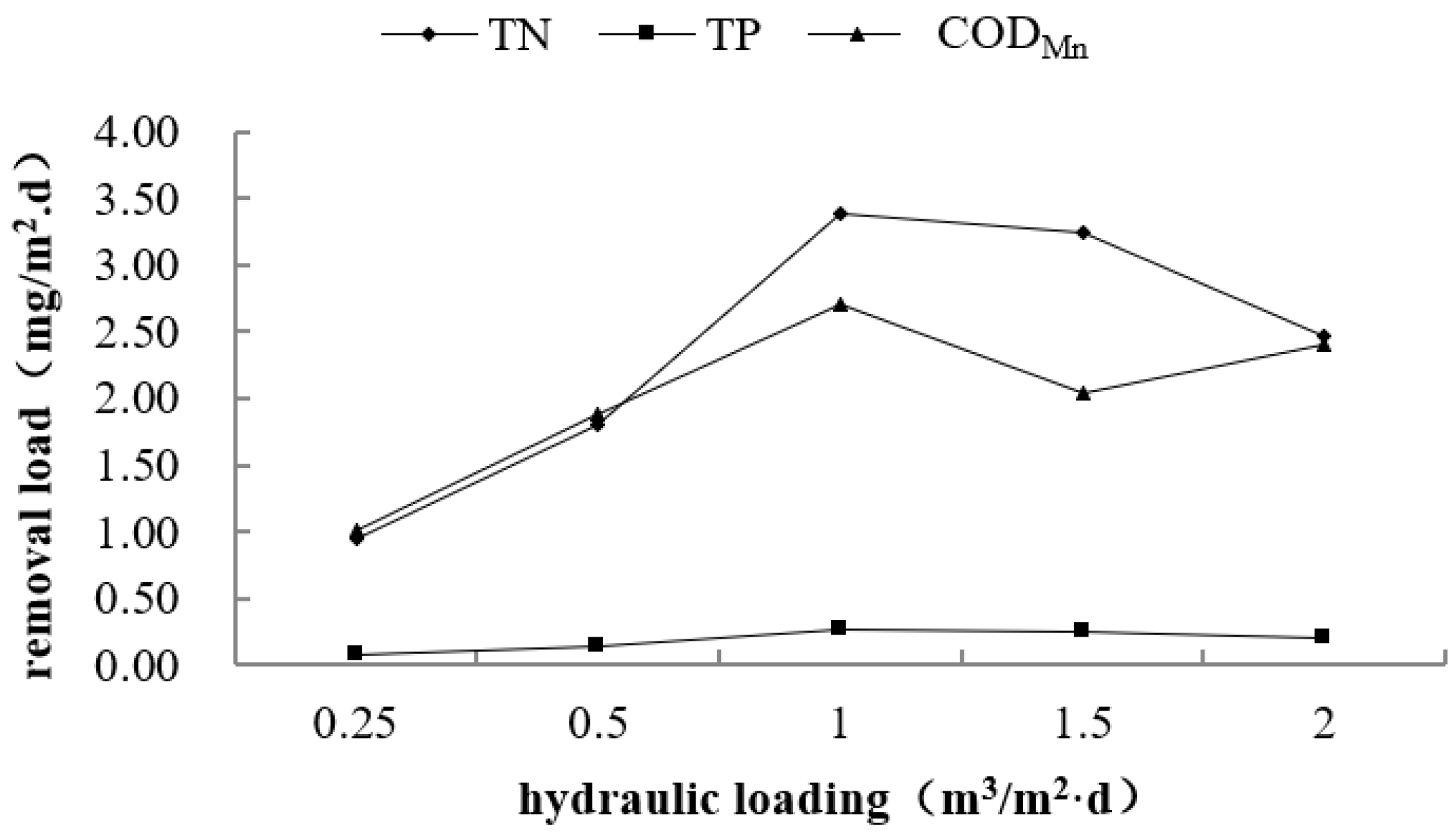
3.2. Effect of Hydraulic Loading on Purification Performance
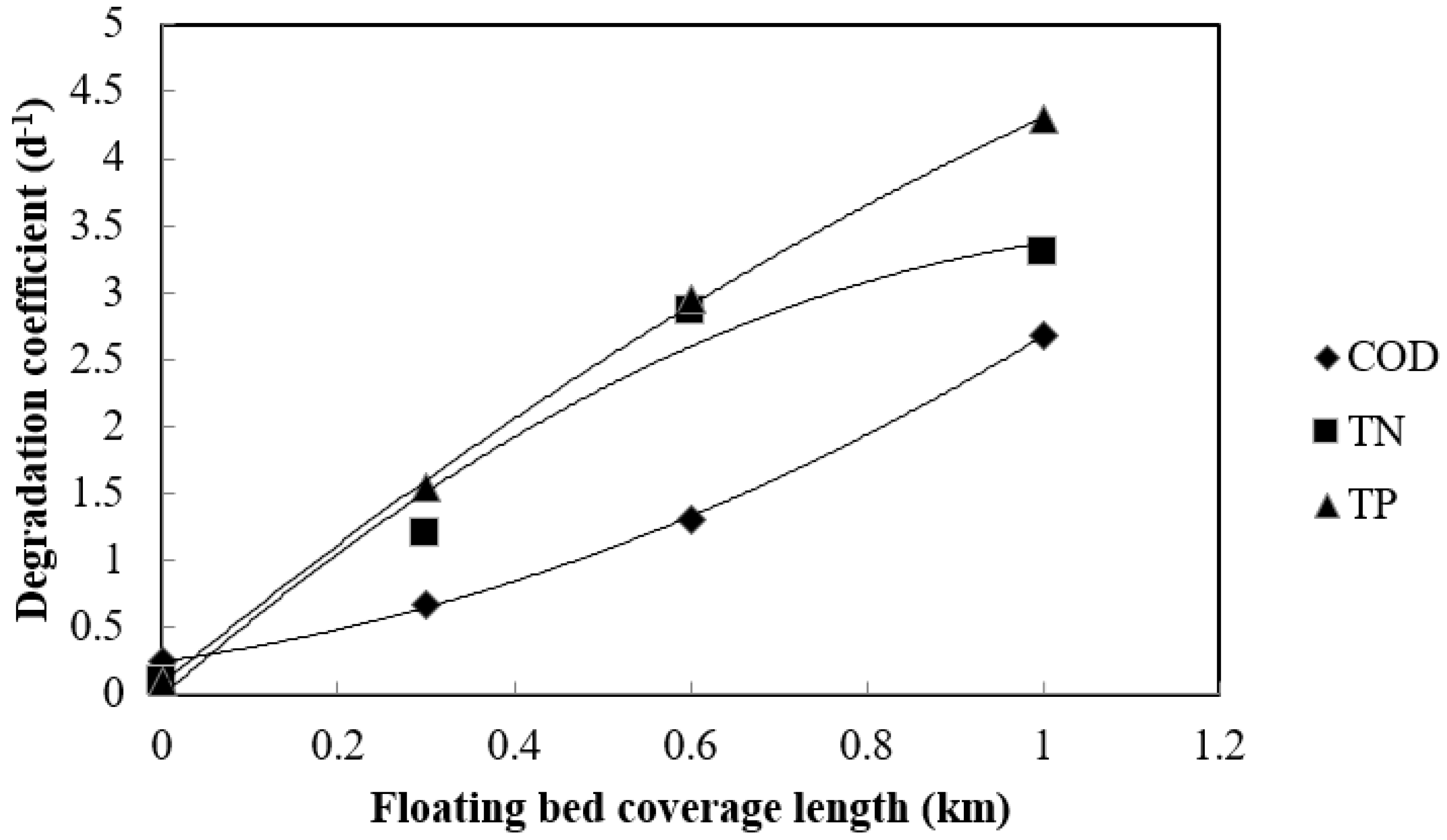
3.3. Floating Bed–Effective Microorganism Field Application
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nasir, N.M.; Bakar, N.S.A.; Lananan, F.; Abdul Hamid, S.H.; Lam, S.S.; Jusoh, A. Treatment of African catfish, Clarias gariepinus wastewater utilizing phytoremediation of microalgae, Chlorella sp. with Aspergillus niger bio-harvesting. Bioresour. Technol. 2015, 190, 492–498. [Google Scholar] [CrossRef]
- Gondwe, M.J.; Guildford, S.J.; Hecky, R.E. Tracing the flux of aquaculture-derived organic wastes in the southeast arm of Lake Malawi using carbon and nitrogen stable isotopes. Aquaculture 2012, 350–353, 8–18. [Google Scholar] [CrossRef]
- Ghasemi, Z.; Sourinejad, I.; Kazemian, H.; Rohani, S. Application of zeolites in aquaculture industry: A review. Rev. Aquac. 2018, 10, 75–95. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, S.; Chen, S.B.; Zhang, L.; Zhu, J.X. The effects of current density on the efficiency of aquaculture wastewater treatment by electrochemical technology. Fish. Mod. 2018, 2, 13–20. [Google Scholar]
- Rodziewicz, J.; Filipkowska, U.; Janczukowicz, W. Application of rotating biological contactor for treatment of wastewater from fish breeding. Rocz. Ochr. Srodowiska 2011, 13, 1233–1244. [Google Scholar]
- Li, Z.; Yu, E.; Zhang, K.; Gong, W.; Xie, J. Water treatment effect, microbial community structure, and metabolic characteristics in a field-scale aquaculture wastewater treatment system. Front. Microbiol. 2020, 11, 930. [Google Scholar] [CrossRef]
- Nizam, N.U.M.; Hanafiah, M.M.; Noor, I.M.; Karim, H.I.A. Efficiency of Five Selected Aquatic Plants in Phytoremediation of Aquaculture Wastewater. Appl. Sci. 2020, 10, 1–11. [Google Scholar]
- Lananan, F.; Hamid, S.H.A.; Din, W.N.S.; Ali, N.; Khatoon, H.; Jusoh, A.; Endut, A. Symbiotic bioremediation of aquaculture wastewater in reducing ammonia and phosphorus utilizing Effective Microorganism (EM-1) and microalgae (Chlorella sp.). Int. Biodeterior. Biodegrad. 2014, 95, 127–134. [Google Scholar] [CrossRef]
- Wu, H.; Lu, K.; Qian, W.; Zheng, Z.; Chen, H.; Wan, L. Effectiveness of remediating aquaculture wastewater by immobi-lized microorganisms and Myriophyllum aquaticum. J. Fish. Sci. China 2013, 20, 316–326. [Google Scholar] [CrossRef]
- Chen, T.; Yang, J.; Huang, B.; Liu, W.; Lu, C. Combined process of biofilm+roots for aquaculture wastewater remediation. IOP Conf. Ser. Earth Environ. Sci. 2020, 508, 012102. [Google Scholar] [CrossRef]
- Suresh, B.; Ravishankar, G.A. Phytoremediation-a novel and promising approach for environmental clean-up. Crit. Rev. Biotechnol. 2004, 24, 97–124. [Google Scholar] [CrossRef]
- Aken, V.B. Transgenic plants for phytoremediation: Helping nature to clean up environmental pollution. Trends Biotechnol. 2008, 26, 225–227. [Google Scholar] [CrossRef]
- Cheng, S.P.; Xiao, J.; Xiao, H.P.; Zhang, L.P.; Wu, Z.B. Phytoremediation of triazophos by Canna Indica Linn. In a hydroponic system. Int. J. Phytoremed. 2007, 9, 453–463. [Google Scholar] [CrossRef]
- Fraser, L.H.; Carty, S.M.; Steer, D. A test of four plant species to reduce total nitrogen and total phosphorus from soil leachate in subsurface wetland microcosms. Bioresour. Technol. 2004, 94, 185–192. [Google Scholar] [CrossRef]
- Wei, R.X.; Wu, H.Q.; Zhang, J.R.; Li, F.P. Remediation effects of plant floating bed-microorganism on contaminated water. Ecol. Environ. Sci. 2009, 18, 68–74. [Google Scholar]
- Rehman, K.; Imran, A.; Amin, I.; Afzal, M. Inoculation with bacteria in floating treatment wetlands positively modulates the phytoremediation of oil field wastewater. J. Hazard. Mater. 2018, 349, 242–251. [Google Scholar] [CrossRef]
- Grosse, W.; Wissing, F.W.; Perfler, R.; Wu, Z.; Chang, J.; Lei, Z. Waterqualityimprovement in tropical and subtropical areas for reuse and rehabilitation of aquatic ecosystem. In Freshwater Contamination in China, Chapter III; Gawlik, B.M., Platzer, B., Muntau, H., Eds.; Office for Official Publications of the European Communities, European Communities: Brussells, Belgium, 2001; pp. 19–32. [Google Scholar]
- Cheng, S.; Ren, F.; Grosse, W.; Wu, Z. Effects of cadmium on chlorophyll content, photochemical efficiency, and photosynthetic intensity of Canna indica Linn. Int. J. Phytoremed. 2002, 4, 239–246. [Google Scholar] [CrossRef]
- Huang, Z.T.; Jiang, Y.L.; Song, X.F. Research progress of aquaculture wastewater treatment with aerobic denitrification technology. Fish. Mod. 2018, 5, 7–13. [Google Scholar]
- Ansari, F.A.; Singh, P.; Guldhe, A.; Bux, F. Microalgal cultivation using aquaculture wastewater: Integrated biomass generation and nutrient remediation. Algal Res. 2017, 21, 169–177. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Lu, K.H.; Liang, J.J. Role of rhizosphere microorganisms of aquatic plants in phytoremediation of wastewater. Environ. Sci. Technol. 2010, 33, 75–80. [Google Scholar]

| Pollutant | CODMn | TN | TP |
|---|---|---|---|
| Concentration (mg/L) | 7.5–10.5 | 6.0–7.5 | 0.4–0.6 |
| Index | Inlet Concentration | Plant Coverage Rate | ||||
|---|---|---|---|---|---|---|
| 0% | 10% | 30% | 60% | 90% | ||
| DO (mg/L) | 1.98 | 3.05 | 2.91 | 2.01 | 1.03 | 0.49 |
| TN (mg/L) | 7.16 | 6.99 | 6.57 | 4.90 | 4.54 | 3.60 |
| TP (mg/L) | 0.51 | 0.47 | 0.41 | 0.33 | 0.27 | 0.22 |
| CODMn (mg/L) | 10.05 | 9.13 | 8.79 | 7.98 | 7.85 | 7.75 |
| cost(*×15$) | 0 | 0.63 | 1.89 | 3.78 | 5.67 | |
| Index | Inlet Concentration | Hydraulic Loading (m3/m2·day) | ||||
|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1.0 | 1.5 | 2.0 | ||
| TN (mg/L) | 6.13 | 2.36 | 2.54 | 2.75 | 3.97 | 4.90 |
| TP (mg/L) | 0.42 | 0.13 | 0.13 | 0.16 | 0.25 | 0.32 |
| CODMn (mg/L) | 7.86 | 3.83 | 4.11 | 5.16 | 6.50 | 6.66 |
| Pollutants | Floating Bed Coverage Length (km) | ||||
|---|---|---|---|---|---|
| 0 | 0.3 | 0.6 | 1 | ||
| CODMn | Concentration (mg/L) | 9.040 | 8.970 | 8.780 | 8.420 |
| Degradation coefficient (d−1) | 0.244 | 0.671 | 1.313 | 2.681 | |
| TN | Concentration (mg/L) | 1.944 | 1.938 | 1.865 | 1.702 |
| Degradation coefficient (d−1) | 0.097 | 1.204 | 2.868 | 3.300 | |
| TP | Concentration (mg/L) | 0.1231 | 0.1227 | 0.1168 | 0.1063 |
| Degradation coefficient (d−1) | 0.102 | 1.545 | 2.954 | 4.293 | |
| Pollutant | Regression Equation | R2 |
|---|---|---|
| CODMn | y = 1.5636x2 + 0.8615x + 0.2512 | 0.9998 |
| TN | y = −2.4222x2 + 5.8022x − 0.0108 | 0.9723 |
| TP | y = −1.2010x2 + 5.4245x + 0.0822 | 0.9994 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Ling, H.; Tan, J.; Shao, X. Removing Nutrients from Crab-Breeding Wastewater by a Floating Plant–Effective Microorganism Bed. Water 2020, 12, 3384. https://doi.org/10.3390/w12123384
Chen L, Ling H, Tan J, Shao X. Removing Nutrients from Crab-Breeding Wastewater by a Floating Plant–Effective Microorganism Bed. Water. 2020; 12(12):3384. https://doi.org/10.3390/w12123384
Chicago/Turabian StyleChen, Lina, Hong Ling, Junyi Tan, and Xiaohou Shao. 2020. "Removing Nutrients from Crab-Breeding Wastewater by a Floating Plant–Effective Microorganism Bed" Water 12, no. 12: 3384. https://doi.org/10.3390/w12123384
APA StyleChen, L., Ling, H., Tan, J., & Shao, X. (2020). Removing Nutrients from Crab-Breeding Wastewater by a Floating Plant–Effective Microorganism Bed. Water, 12(12), 3384. https://doi.org/10.3390/w12123384




