Impacts of Temperature and Solids Retention Time, and Possible Mechanisms of Biological Hydrolysis Pretreatment on Anaerobic Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Hydrolysis-Anaerobic Digestion (BH-AD) Process Flow Design
2.2. Biochemical Methane Potential (BMP) Tests
2.3. Continuous Stirred-Tank Reactor (CSTR) Tests
2.4. Sludge Analysis
3. Results
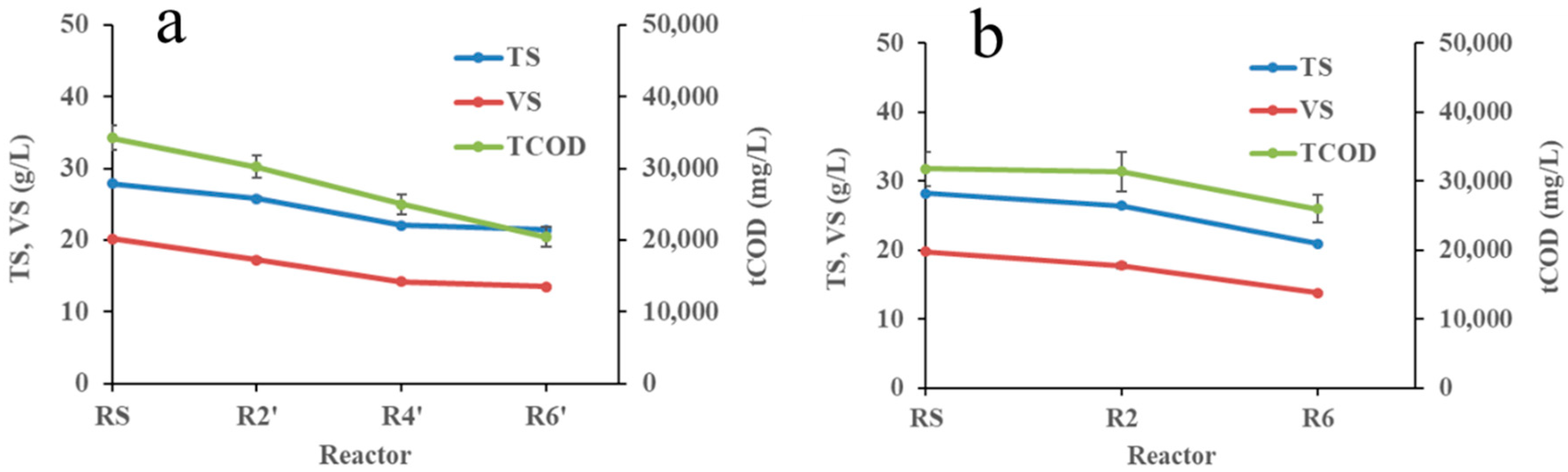
3.1. Characteristics of Substrate and Inoculum Sludge under Different Test Modes
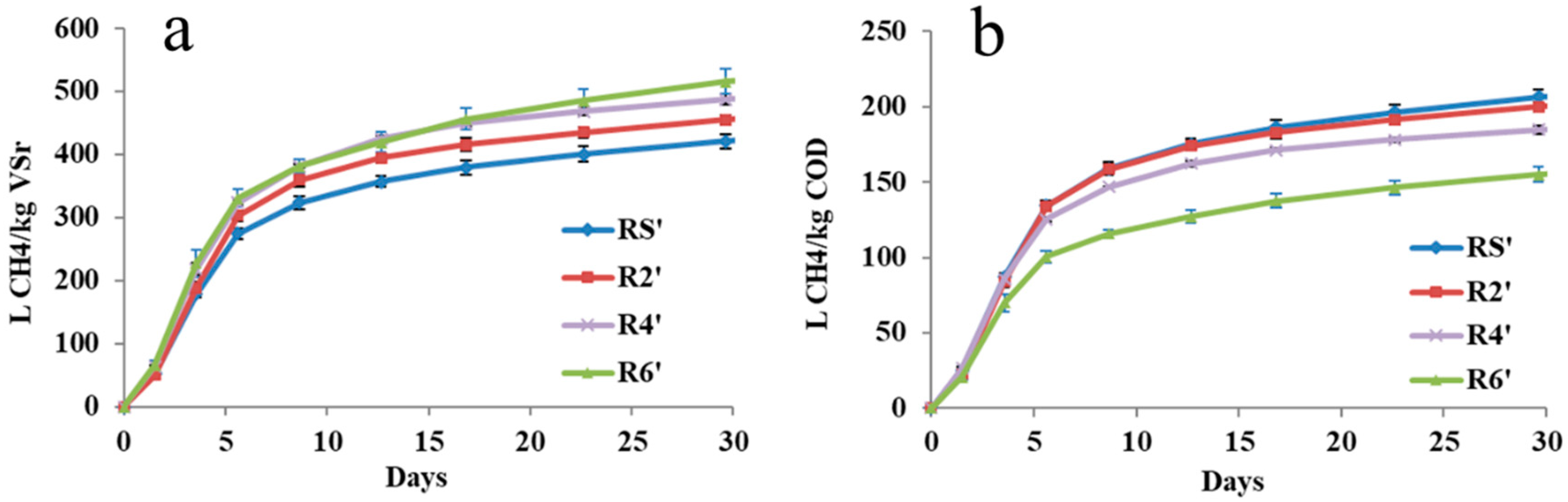
3.2. Evaluation of BH42 or BH61 Pretreatment by BMP Tests
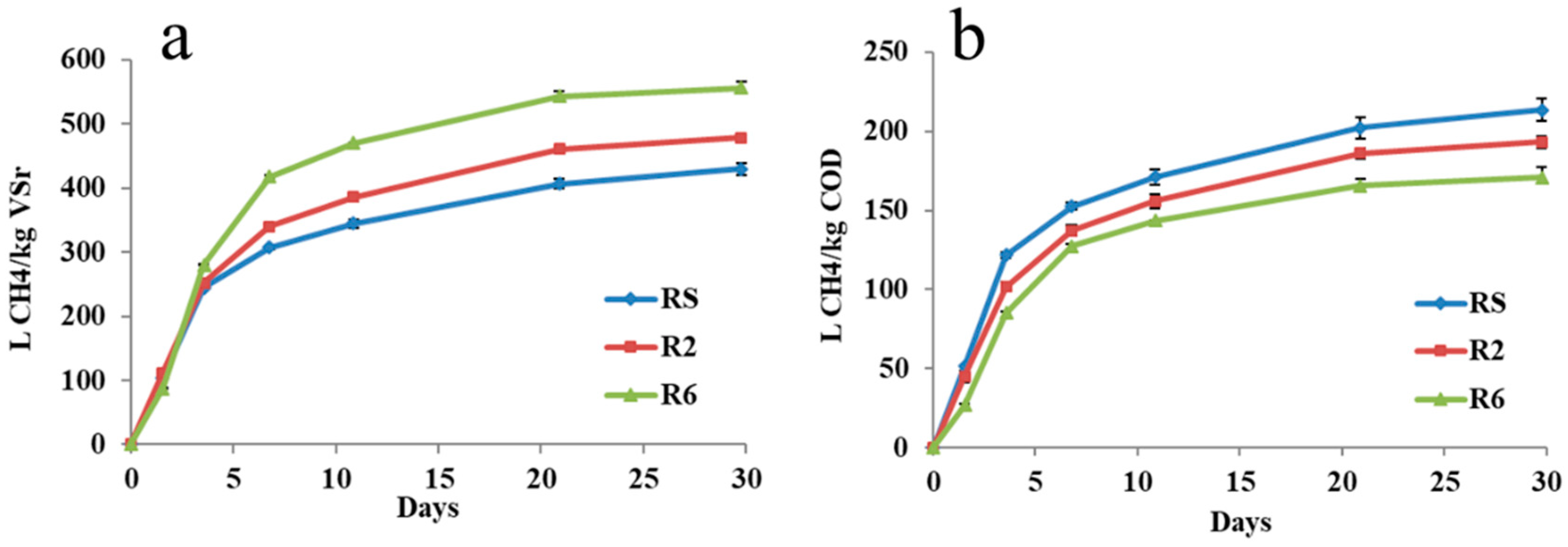
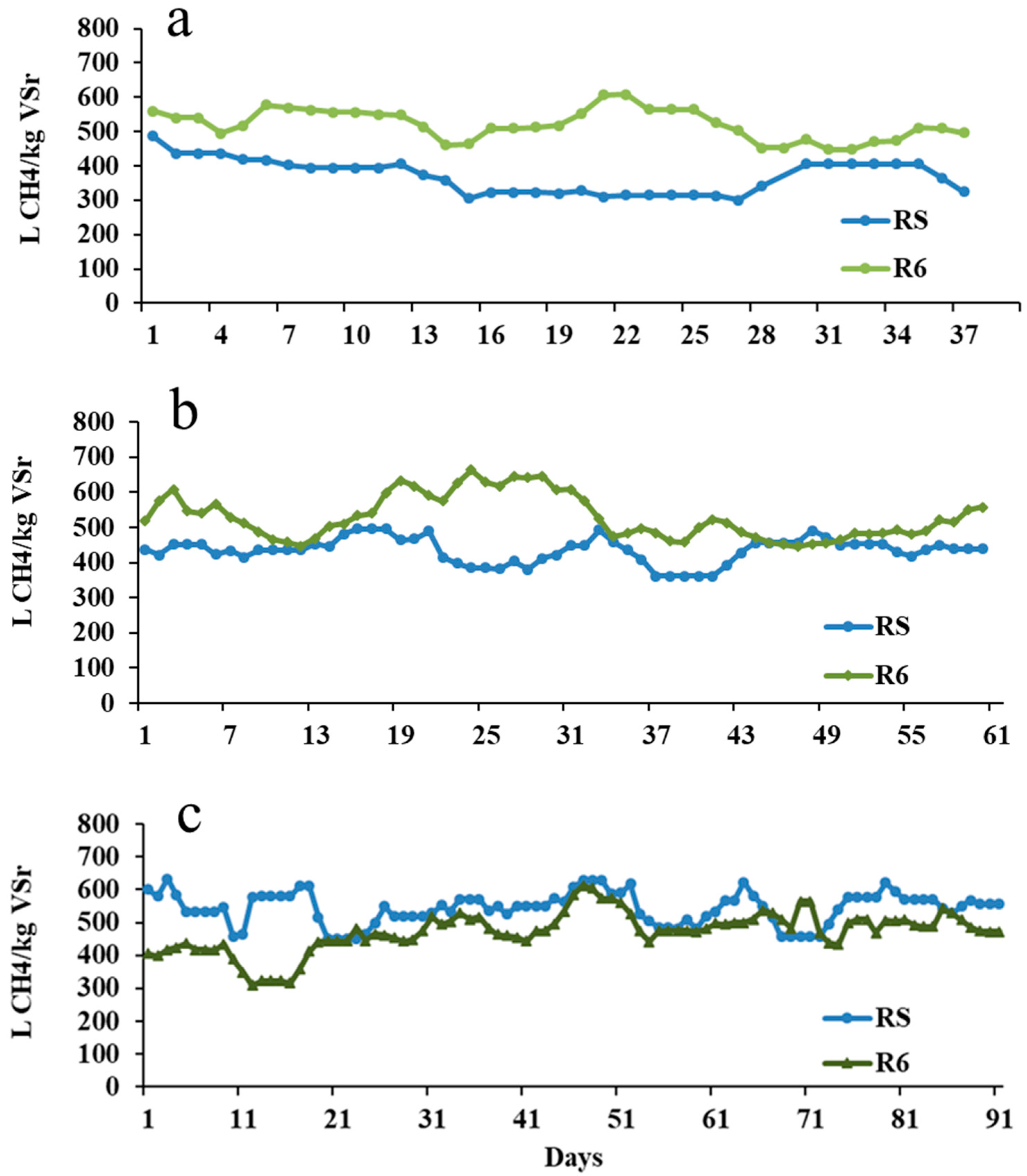
3.3. Evaluation of BH61 Pretreatment by CSTR Tests
4. Discussion
- (1)
- SRT and temperature played critical roles in the BH process; onsite pilot or full-scale tests are of practical importance to achieve the optimum condition in VS reduction, biogas generation, and/or pathogen reduction after AD;
- (2)
- Substrate characteristics, especially the ratio between primary sludge and waste active sludge (or other sources of biosolid/biowaste), were essential for the selection of SRT and temperature for BH pretreatment;
- (3)
- BH process involved stimulated growth of the microorganisms and production of extracellular hydrolytic enzymes; the increased apparent hydrolysis rate was reported to be more significant than overall degradability after AD, and higher VS removal might not result in more biogas generation in earlier stages;
- (4)
- In this study, SRT and temperature simultaneously contributed to enhanced AD performance; either longer SRT or higher temperature pretreatment had higher overall biogas generation and VS reduction than untreated sludge;
- (5)
- Compared with BH at 42 °C for 3 days (BH42), the combination of low–high temperature (from 42 to 61 °C, up to 2 days) had higher overall biogas generation and VS reduction after 30-day BMP tests;
- (6)
- The enhancement is less significant when increasing BH SRT longer than 48 h, while temperature (up to 61 °C) played more important roles starting from 36-h SRT of BH pretreatment.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Anaerobic Digestion |
| BH | Biological Hydrolysis |
| BMP | Biochemical Methane Potential |
| CH4 | Methane |
| COD | Chemical Oxygen Demand |
| CSTR | Continuous Stirred-tank Reactor |
| GC | Gas Chromatography |
| OLR | Organic Loading Rate |
| RS | Raw Sludge |
| SRT | Solid Retention Time |
| STP | Standard Temperature and Pressure |
| TCOD | Total Chemical Oxygen Demand |
| TS | Total Solids |
| TSS | Total Suspended Solids |
| VFA | Volatile Fatty Acid Concentration |
| VS | Volatile Solids |
| VSR | Volatile Solids Reduction |
| VSS | Suspended Volatile Solids |
| WAS | Waste Activated Sludge |
| WWTP | Wastewater Treatment Plant |
References
- Murphy, J.D.; Power, N.M. A technical, economic and environmental comparison of composting and anaerobic digestion of biodegradable municipal waste. J. Environ. Sci. Health Part A-Toxic/Hazard. Subst. Environ. Eng. 2006, 41, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Mata-Alvarez, J.; Mace, S.; Llabres, P. Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Mahapatra, K.; Ramteke, D.S.; Paliwal, L.J.; Naik, N.K. Agronomic application of food processing industrial sludge to improve soil quality and crop productivity. Geoderma 2013, 207, 205–211. [Google Scholar] [CrossRef]
- USEPA. A Plain English Guide to the Epa Part 503 Biosolids Rule; US Environmental Protection Agency Office of Wastewater Management (USEPA): Washington, DC, USA, 1994.
- Kepp, U.; Machenbach, I.; Weisz, N.; Solheim, O.E. Enhanced stabilisation of sewage sludge through thermal hydrolysis-three years of experience with full scale plant. Water Sci. Technol. 2000, 42, 89–96. [Google Scholar] [CrossRef]
- Morgan-Sagastume, F.; Pratt, S.; Karlsson, A.; Cirne, D.; Lant, P.; Werker, A. Production of volatile fatty acids by fermentation of waste activated sludge pre-treated in full-scale thermal hydrolysis plants. Bioresour. Technol. 2011, 102, 3089–3097. [Google Scholar] [CrossRef] [PubMed]
- Devlin, C.D.; Esteves, S.R.R.; Dinsdale, R.M.; Guwy, A.J. The effect of acid pretreatment on the anaerobic digestion and dewatering of waste activated sludge. Bioresour. Technol. 2011, 102, 4076–4082. [Google Scholar] [CrossRef]
- Ge, H.Q.; Jensen, P.D.; Batstone, D.J. Pre-treatment mechanisms during thermophilic-mesophilic temperature phased anaerobic digestion of primary sludge. Water Res. 2010, 44, 123–130. [Google Scholar] [CrossRef]
- Ge, H.Q.; Jensen, P.D.; Batstone, D.J. Increased temperature in the thermophilic stage in temperature phased anaerobic digestion (tpad) improves degradability of waste activated sludge. J. Hazard. Mater. 2011, 187, 355–361. [Google Scholar] [CrossRef]
- Biswal, K.B.; Huang, H.; Dai, J.; Chen, G.H.; Wu, D. Impact of low-thermal pretreatment on physicochemical properties of saline waste activated sludge, hydrolysis of organics and methane yield in anaerobic digestion. Bioresour. Technol. 2020, 297. [Google Scholar] [CrossRef]
- Werker, A.G.; Carlsson, M.; Morgan-Sagastume, F.; Le, M.S.; Harrison, D.; Warrington, W.A. Full scale demonstration and assessment of enzymic hydrolysis pre-treatment for mesophilic anaerobic digestion of municipal wastewater treatment sludge. In Proceedings of the WEFTEC Proceedings, California, CA, USA, 13–17 October 2007. [Google Scholar]
- Bungay, S.; Abdelwahab, M. Monsal enzymic hydrolysis–new developments and lessons learnt. In Proceedings of the 13th European Biosolids & Organic Resources Conference & Workshop, Manchester, UK, 13–14 November 2008. [Google Scholar]
- Agabo-Garcia, C.; Perez, M.; Rodriguez-Morgado, B.; Parrado, J.; Solera, R. Biomethane production improvement by enzymatic pre-treatments and enhancers of sewage sludge anaerobic digestion. Fuel 2019, 255, 115713. [Google Scholar] [CrossRef]
- Bolzonella, D.; Battista, F.; Mattioli, A.; Nicolato, C.; Frison, N.; Lampis, S. Biological thermophilic post hydrolysis of digestate enhances the biogas production in the anaerobic digestion of agro-waste. Renew. Sustain. Energy Rev. 2020, 134, 110174. [Google Scholar] [CrossRef]
- Mlaik, N.; Khoufi, S.; Hamza, M.; Masmoudi, M.A.; Sayadi, S. Enzymatic pre-hydrolysis of organic fraction of municipal solid waste to enhance anaerobic digestion. Biomass Bioenergy 2019, 127. [Google Scholar] [CrossRef]
- Ding, H.H.H.; Chang, S.; Liu, Y. Biological hydrolysis pretreatment on secondary sludge: Enhancement of anaerobic digestion and mechanism study. Bioresour. Technol. 2017, 244, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Filer, J.; Ding, H.H.H.; Chang, S. Biochemical methane potential (bmp) assay method for anaerobic digestion research. Water 2019, 11, 921. [Google Scholar] [CrossRef]
- Gorris, L.G.M.; Vandeursen, J.M.A.; Vanderdrift, C.; Vogels, G.D. Inhibition of propionate degradation by acetate in methanogenic fluidized-bed reactors. Biotechnol. Lett. 1989, 11, 61–66. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Ballesteros, M.; Gonzalez-Fernandez, C. Efficient anaerobic digestion of microalgae biomass: Proteins as a key macromolecule. Molecules 2018, 23, 1098. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, S.; Jayashree, C.; Kumar, S.A.; Yeom, I.T.; Banu, J.R. The enhancement of anaerobic biodegradability of waste activated sludge by surfactant mediated biological pretreatment. Bioresour. Technol. 2014, 168, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Eastman, J.A.; Ferguson, J.F. Solubilization of particulate organic-carbon during the acid phase of anaerobic-digestion. J. Water Pollut. Control Fed. 1981, 53, 352–366. [Google Scholar]
- Chang, T.C.; Wu, Y.C.; Ouyang, C.F.; Hao, O.J. Anaerobic sludge-digestion using mesophilic thermophilic phase-separation. J. Chem. Technol. Biotechnol. 1989, 45, 85–96. [Google Scholar] [CrossRef]
- Ghosh, S. Pilot-scale demonstration of two-phase anaerobic digestion of activated sludge. Water Sci. Technol. 1991, 23, 1179–1188. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Madura, R.L.; Walling, D.A.; Farrell, J.B. Volatile solids reduction in two-phase and conventional anaerobic sludge digestion. Water Res. 1996, 30, 1041–1048. [Google Scholar] [CrossRef]
- Wang, Q.; Noguchi, C.; Hara, Y.; Sharon, C.; Kakimoto, K.; Kato, Y. Studies on anaerobic digestion mechanism: Influence of pretreatment temperature on biodegradation of waste activated sludge. Environ. Technol. 1997, 18, 999–1008. [Google Scholar] [CrossRef]
- Gavala, H.N.; Yenal, U.; Skiadas, I.V.; Westermann, P.; Ahring, B.K. Mesophilic and thermophilic anaerobic digestion of primary and secondary sludge. Effect of pre-treatment at elevated temperature. Water Res. 2003, 37, 4561–4572. [Google Scholar] [CrossRef]
- Kim, H.W.; Han, S.K.; Shin, H.S. Anaerobic co-digestion of sewage sludge and food waste using temperature-phased anaerobic digestion process. Water Sci. Technol. 2004, 50, 107–114. [Google Scholar] [CrossRef]
- Skiadas, I.V.; Gavala, H.N.; Lu, J.; Ahring, B.K. Thermal pre-treatment of primary and secondary sludge at 70 c prior to anaerobic digestion. Water Sci. Technol. 2005, 52, 161–166. [Google Scholar] [CrossRef]
- Watts, S.; Hamilton, G.; Keller, J. Two-stage thermophilic-mesophilic anaerobic digestion of waste activated sludge-from a biological nutrient removal plant. Water Sci. Technol. 2006, 53, 149–157. [Google Scholar] [CrossRef]
- Santha, H.; Sandino, J.; Shimp, G.F.; Sung, S.W. Performance evaluation of a ‘sequential-batch’ temperature-phased anaerobic digestion (tpad) scheme for producing class a biosolids. Water Environ. Res. 2006, 78, 221–226. [Google Scholar] [CrossRef]
- Kim, H.W.; Nam, J.Y.; Shin, H.S. A comparison study on the high-rate co-digestion of sewage sludge and food waste using a temperature-phased anaerobic sequencing batch reactor system. Bioresour. Technol. 2011, 102, 7272–7279. [Google Scholar] [CrossRef]
- Coelho, N.M.G.; Droste, R.L.; Kennedy, K.J. Evaluation of continuous mesophilic, thermophilic and temperature phased anaerobic digestion of microwaved activated sludge. Water Res. 2011, 45, 2822–2834. [Google Scholar] [CrossRef]
- Yu, J.W.; Zheng, M.X.; Tao, T.; Zuo, J.; Wang, K.J. Waste activated sludge treatment based on temperature staged and biologically phased anaerobic digestion system. J. Environ. Sci. 2013, 25, 2056–2064. [Google Scholar] [CrossRef]
- Qin, Y.; Higashimori, A.; Wu, L.J.; Hojo, T.; Kubota, K.; Li, Y.Y. Phase separation and microbial distribution in the hyperthermophilic-mesophilic-type temperature-phased anaerobic digestion (tpad) of waste activated sludge (was). Bioresour. Technol. 2017, 245, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Buffiere, P.; Dooms, M.; Hattou, S.; Benbelkacem, H. The hydrolytic stage in high solids temperature phased anaerobic digestion improves the downstream methane production rate. Bioresour. Technol. 2018, 259, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Kim, J.; Hwang, K.; Lee, C. A comparative study of single- and two-phase anaerobic digestion of food waste under uncontrolled ph conditions. Waste Manag. 2018, 78, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Mehari, B.B.; Chang, S.; Hong, Y.; Chen, H. Temperature-phased biological hydrolysis and thermal hydrolysis pretreatment for anaerobic digestion performance enhancement. Water 2018, 10, 1812. [Google Scholar] [CrossRef]
- Ma, S.J.; Ma, H.J.; Hu, H.D.; Ren, H.Q. Effect of mixing intensity on hydrolysis and acidification of sewage sludge in two-stage anaerobic digestion: Characteristics of dissolved organic matter and the key microorganisms. Water Res. 2019, 148, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, A.; Wandera, S.M.; Bi, S.J.; Song, Y.L.; Qiao, W.; Dong, R.J. Response of the microbial community to the methanogenic performance of biologically hydrolyzed sewage sludge with variable hydraulic retention times. Bioresour. Technol. 2019, 288. [Google Scholar] [CrossRef]
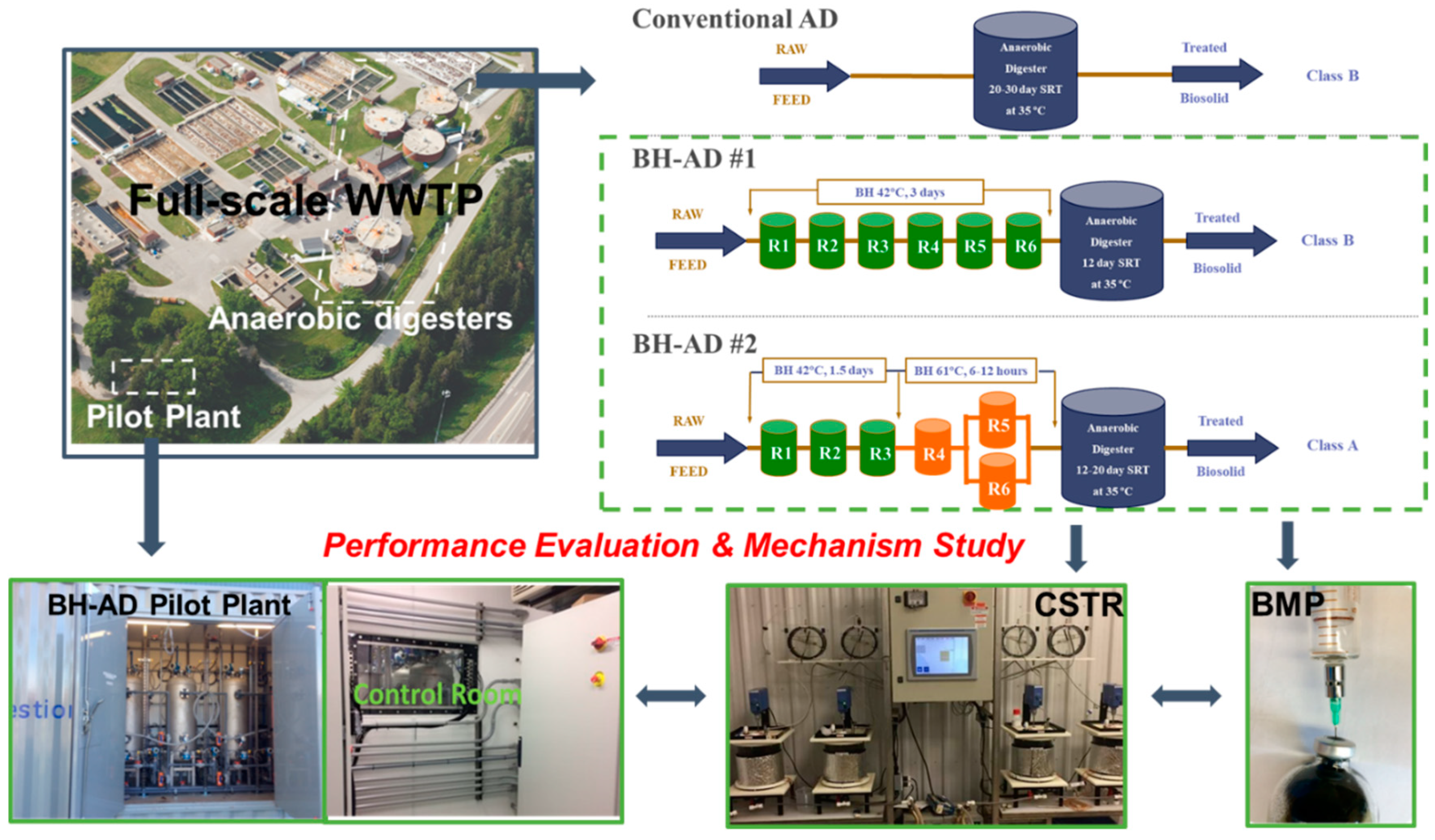
| BH Pretreatment | BH Stage 1 (Plug-Flow) | BH Stage 2 (Batch) | |
|---|---|---|---|
| BH Pretreated Sludge | Temperature/SRT | Temperature/SRT | |
| BH42 (Pilot plant was consistently operated for around 120 days) | RS’ | - | - |
| R2′ | 42 °C/24 h | - | |
| R4′ | 42 °C/48 h | - | |
| R6′ | 42 °C/72 h | - | |
| BH61 (Pilot plant was consistently operated for around 400 days) | RS | - | - |
| R2 | 42 °C/36 h | - | |
| R6 | 42 °C/36 h | 61 °C/6 h | |
| Parameter/Test Mode | pH | TS | TSS | VS | VSS | TCOD | SCOD | VFA | OLR | F/M | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| g/L | g/L | g/L | g/L | mg/L | mg/L | mg/L | g VS/L/d | TCOD/VSS | |||
| BMP *: BH42 test mode | Inoculum | 7.69 ± 0.05 | 18.35 ± 0.12 | 16.69 ± 0.07 | 10.78 ± 0.19 | 10.03 ± 0.08 | 15,610 ± 1513 | 687 ± 50 | 241 ± 12 | - | - |
| RS | 6.55 ± 0.01 | 27.85 ± 0.27 | 26.53 ± 0.25 | 20.22 ± 0.26 | 19.99 ± 0.93 | 34,275 ± 1722 | 2448 ± 211 | 1489 ± 349 | - | 1.65 | |
| R2′ | 7.13 ± 0.07 | 25.75 ± 0.24 | 24.39 ± 0.21 | 17.25 ± 0.30 | 16.5 ± 0.22 | 30,225 ± 1534 | 3688 ± 341 | 1974 ± 283 | - | 1.63 | |
| R4′ | 7.25 ± 0.08 | 22.04 ± 0.10 | 21.2 ± 0.13 | 14.22 ± 0.11 | 13.88 ± 0.13 | 24,975 ± 1428 | 3320 ± 237 | 1394 ± 154 | - | 1.35 | |
| R6′ | 7.49 ± 0.07 | 21.42 ± 0.10 | 20.5 ± 0.17 | 13.5 ± 0.12 | 13.02 ± 0.09 | 20,450 ± 1410 | 2148 ± 203 | 696 ± 76 | - | 1.10 | |
| BMP *: BH61 test mode | Inoculum | 7.95 ± 0.02 | 20.74 ± 0.26 | 20.11 ± 0.28 | 12.43 ± 0.24 | 12.02 ± 0.30 | 19,775 ± 1809 | 871 ± 30 | 686 ± 120 | - | - |
| RS | 7.01 ± 0.15 | 28.19 ± 0.11 | 27.55 ± 0.15 | 19.76 ± 0.21 | 18.07 ± 0.34 | 31,780 ± 2519 | 2348 ± 138 | 1232 ± 208 | - | 1.19 | |
| R2 | 7.31 ± 0.11 | 26.43 ± 0.25 | 25.75 ± 0.40 | 17.71 ± 0.34 | 17.39 ± 0.31 | 31,400 ± 2908 | 4116 ± 221 | 1986 ± 107 | - | 1.11 | |
| R6 | 7.82 ± 0.14 | 20.95 ± 0.16 | 19.73 ± 0.29 | 13.78 ± 0.14 | 12.91 ± 0.23 | 25,975 ± 1975 | 6420 ± 285 | 3068 ± 308 | - | 1.07 | |
| CSTR **: BH61–12 day | Inoculum | - | - | - | - | - | - | - | - | - | - |
| RS | 7.22 ± 0.12 | 27.8 ± 0.36 | - | 19.48 ± 0.21 | - | - | - | - | 1.62 | - | |
| R6 | 7.72 ± 0.15 | 19.5 ± 1.59 | - | 12.31 ± 0.9 | - | - | - | - | 1.02 | - | |
| CSTR **: BH61–16 day | Inoculum | - | - | - | - | - | - | - | - | - | - |
| RS | 6.96 ± 0.15 | 26.13 ± 1.8 | - | 17.5 ± 1.24 | - | - | - | - | 1.09 | - | |
| R6 | 7.72 ± 0.18 | 19.78 ± 0.38 | - | 12.38 ± 0.29 | - | - | - | - | 0.77 | - | |
| CSTR **: BH61–20 day | Inoculum | - | - | - | - | - | - | - | - | - | - |
| RS | 7.01 ± 0.15 | 23.92 ± 2.26 | - | 17.28 ± 1.78 | - | - | - | - | 0.86 | - | |
| R6 | 7.82 ± 0.24 | 17.8 ± 0.82 | - | 11.08 ± 0.53 | - | - | - | - | 0.55 | - |
| Year | Substrate Sludge | BH Pretreatment | Proposed Mechanism/Findings | Reference |
|---|---|---|---|---|
| 1981 | Primary sludge | 35 °C, 3 days | Separation of acid and methane phases, and the carbohydrates (mostly cellulose) were more degraded in the acid phase, while lipids were not degraded. The overall process was primarily determined by the rate of hydrolysis, not the bacterial growth kinetics. | [21] |
| 1989 | Combined sludge | 37 °C, 2 days | VS reduction was not affected by the feed sludge concentration, and reduction of SRT (from 2 days to 1 day) in the acid phase resulted in similar amount of VFA, slight decrease in VS reduction, and a significant decrease in CH4 content. | [22] |
| 1991 | Waste activated sludge (WAS) | 36.8 °C, 3.1 days | SRT between 3 and 4 days were optimum for acid-phase digestion of WAS; pre-hydrolysis, acidification, sulfate and nitrate reductions were the predominant reactions in the acid digester. | [23] |
| 1996 | Combined sludge | 35 °C, 2–2.7 days | There was 3.9–25.6% enhancement of VS reduction; and sludge origins largely affected VS reduction (such as industrial waste tested in the feed sludge contributed to lower overall VS reduction). | [24] |
| 1997 | WAS | 60–120 °C, 5–60 min | No significant difference of BH process between 60–120 °C for 5–60 min. | [25] |
| 2003 | Primary sludge & WAS | 70 °C, 1–7 days | The ratio of primary to secondary sludge was important for the selection of BH pretreatment SRT and temperature. | [26] |
| 2004 | Sewage sludge and food waste | 55 °C, 5 days | Longer SRT, fast hydrolysis, higher CH4 conversion rate, and balanced nutrient condition of co-substrate contributed the enhanced performance. | [27] |
| 2005 | Primary sludge | 70 °C, 2 days | VSS reduction was mainly took place in BH pretreatment. | [28] |
| 2006 | Combined sludge | 47–60 °C, 2 days | BH pretreatment of WAS up to 54 °C for 2 days did not show any benefits compared to the pretreatment at 60 °C, while the 6 °C increase resulted in a 43% and 31% increase in COD and VS removal, respectively. | [29] |
| 2006 | Combined sludge | 57–58 °C, 6–8 days | The thermophilic reactor accounted for nearly 80% of the overall VS reduction with the mesophilic stage contributing the remaining 20%. | [30] |
| 2008 | Combined sludge and WAS | 35~70 °C | The hydrolysis and acidogenesis stages are separated out of methanogenic stage during BH comparing with conventional mesophilic anaerobic digestion, and thus BH pretreatment could provide optimal conditions for hydrolysis and acidification. | [12] |
| 2010, 2011 | Primary sludge | 50–65 °C, 2 days | The improved performance was due to an increased apparent hydrolysis rate rather than overall degradability. Possible mechanisms involved stimulated growth of the microorganisms or production of extracellular hydrolytic enzymes. | [8,9] |
| 2011 | Thickened WAS | Four-stage anaerobic digestion (37–55 °C) | Higher VS removal did not result in more biogas generation in earlier stage; soluble organics generated were consumed in the subsequent reactors, resulting in more gas production. More biogas generation was observed from thermophilic systems. | [31] |
| 2011 | Combined sludge | 55 °C, 2 days | Microwave pretreatment had synergistic effects, and showed better performance compared with two-phase AD. | [32] |
| 2013 | WAS | Amylase and protease addition, 2–28 h | Amylase showed best enhancement compared with protease or mixed enzyme in terms of sludge solubilization and acidification. | [33] |
| 2014 | WAS | Bacterial inoculum (as enzyme), 40 °C, 42 h | Sodium dodecyl sulfate (SDS) acted as an enzyme modulator molecule, which increased the availability of substrates to bacteria. | [20] |
| 2017 | WAS | 55 °C, 70 °C, 6 days | BH process (up to 70 °C) did not substantiallyaccelerate degradation or solubilization in the BH stage, however, degradation was improved in anaerobic digester. | [34] |
| 2017 | WAS | 42 °C, 55 °C, 3–6 days | The methane yield of BH pretreated WAS at 15-day BMP test was comparable with untreated WAS after 30-day BMP. Extracellular polymeric substances (EPS) from untreated WAS contained three different molecular weight fractions, and high-MW fraction decreased from 134 kDa to 25 kDa during 6-day BH at 42 °C. | [16] |
| 2018 | Cattle slurry and maize silage | 37–72 °C, 2 days | Solubilization mostly took place during the first 24 h, but there was no correlation between COD solubilization and methane production rate. BH might affect the accessibility of particulate matter (not only its solubilization) in the high-solids temperature phased anaerobic digestion (TPAD) system. | [35] |
| 2018 | Food waste | 35 °C, 4 days | Single-phase configuration showed an advantage in food waste without pH control at high organic loading rate. Microbial community shifted with operational conditions. | [36] |
| 2018 | Combined sludge | 42 °C, and 55 °C, 3 days | High-low temperature combination during lab-scale BH (55 °C to 42 °C) showed higher methane enhancement. | [37] |
| 2019 | Combined sludge | Enzyme addition | The optimal pre-treatments were due to protein degradation using proteases. Enzyme addition increased the biogas generation up to 3.65 and 5.77 times, respectively, compared with control. | [13] |
| 2019 | WAS | 37 °C, 2 days | Mixing rate might have effects on biological hydrolysis or anaerobic digestion. Firmicutes and Actinobacteria increased with elevated mixing intensity, and Fusobacteria and Chloroflexi could contribute to hydrolysis and acidification. | [38] |
| 2019 | Combined sludge | 70 °C, 5 days | Methanogens (Sporosarcina and Methnosarcina) were positively correlated to VS removal and methane yield, and negatively correlated to volatile fatty acids’ accumulation. | [39] |
| 2019 | Municipal Solid Waste | Bacterial (Aspergillus niger) fermentation | Synergistic effect of varied hydrolytic enzymes (cellulases, hemicellulases, etc.) on carbohydrate compounds. | [15] |
| 2020 | Agro-waste digestate | 65 °C, 2–5 days | Post-treatment (65 °C) in digestate increased the biodegradability of complex organic compounds for anaerobic digestion. | [14] |
| 2020 | WAS (high salinity) | 60–120 °C, 12 h | Pretreatment of WAS at 80 °C was confirmed to be more economically viable for tested anaerobic digestion. Higher temperature (120 °C) and longer SRT were benefit for protein and carbohydrate solubilization, while lower temperature could help ammonia and phosphorus release. | [10] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.H.; Kotova, P.; Shaw, C.; Hong, Y.; Chang, S. Impacts of Temperature and Solids Retention Time, and Possible Mechanisms of Biological Hydrolysis Pretreatment on Anaerobic Digestion. Water 2020, 12, 3166. https://doi.org/10.3390/w12113166
Ding HH, Kotova P, Shaw C, Hong Y, Chang S. Impacts of Temperature and Solids Retention Time, and Possible Mechanisms of Biological Hydrolysis Pretreatment on Anaerobic Digestion. Water. 2020; 12(11):3166. https://doi.org/10.3390/w12113166
Chicago/Turabian StyleDing, Huihuang H., Polina Kotova, Christopher Shaw, Youngseck Hong, and Sheng Chang. 2020. "Impacts of Temperature and Solids Retention Time, and Possible Mechanisms of Biological Hydrolysis Pretreatment on Anaerobic Digestion" Water 12, no. 11: 3166. https://doi.org/10.3390/w12113166
APA StyleDing, H. H., Kotova, P., Shaw, C., Hong, Y., & Chang, S. (2020). Impacts of Temperature and Solids Retention Time, and Possible Mechanisms of Biological Hydrolysis Pretreatment on Anaerobic Digestion. Water, 12(11), 3166. https://doi.org/10.3390/w12113166





