Adsorptive Removal of Arsenic by Mesoporous Iron Oxide in Aquatic Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Mesoporous Iron Oxide (MI)
2.2. Materials Characterization
2.3. Batch Experiment
3. Results and Discussion
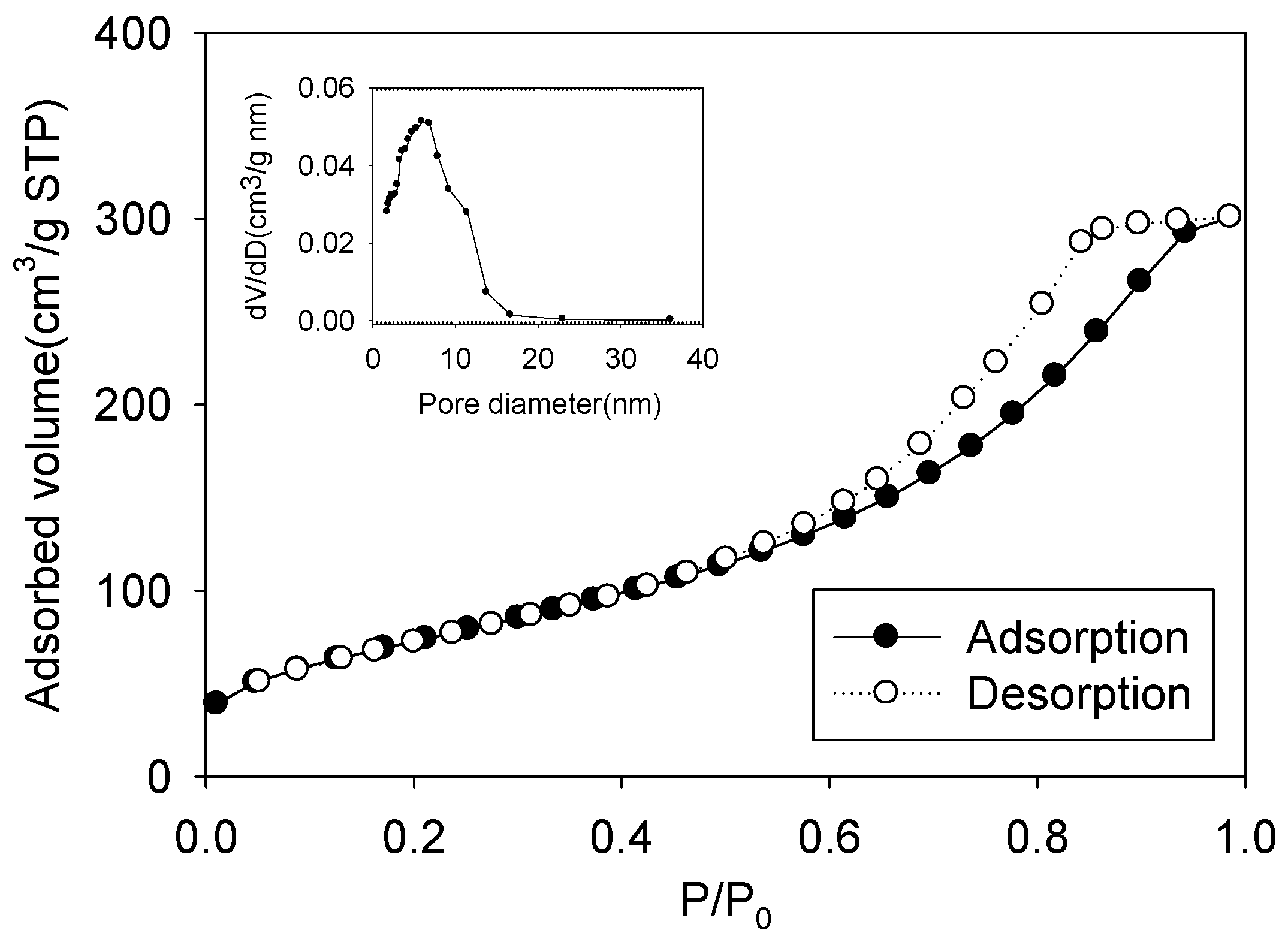
3.1. Characterization of MI
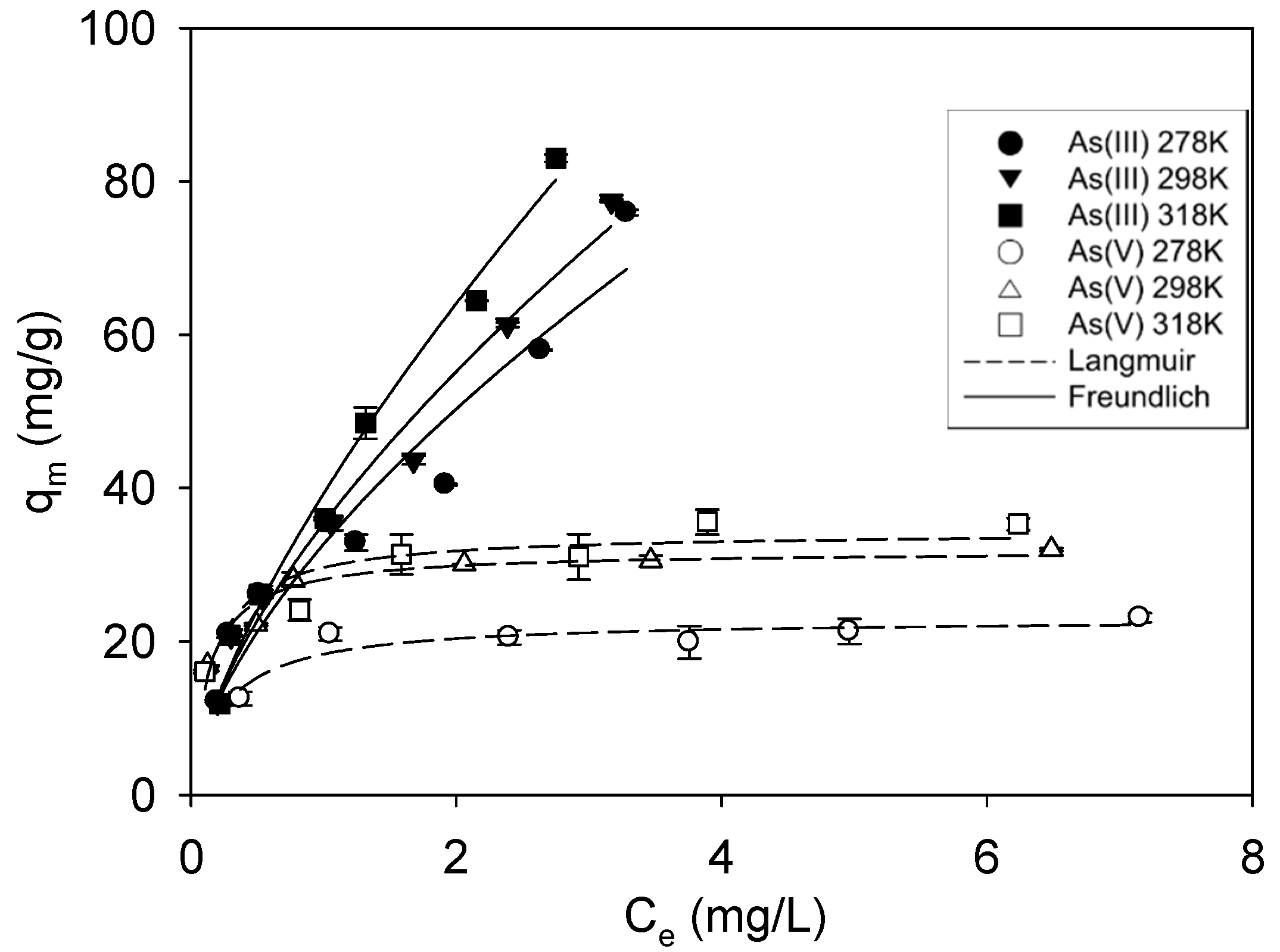
3.2. Adsorption Isotherms
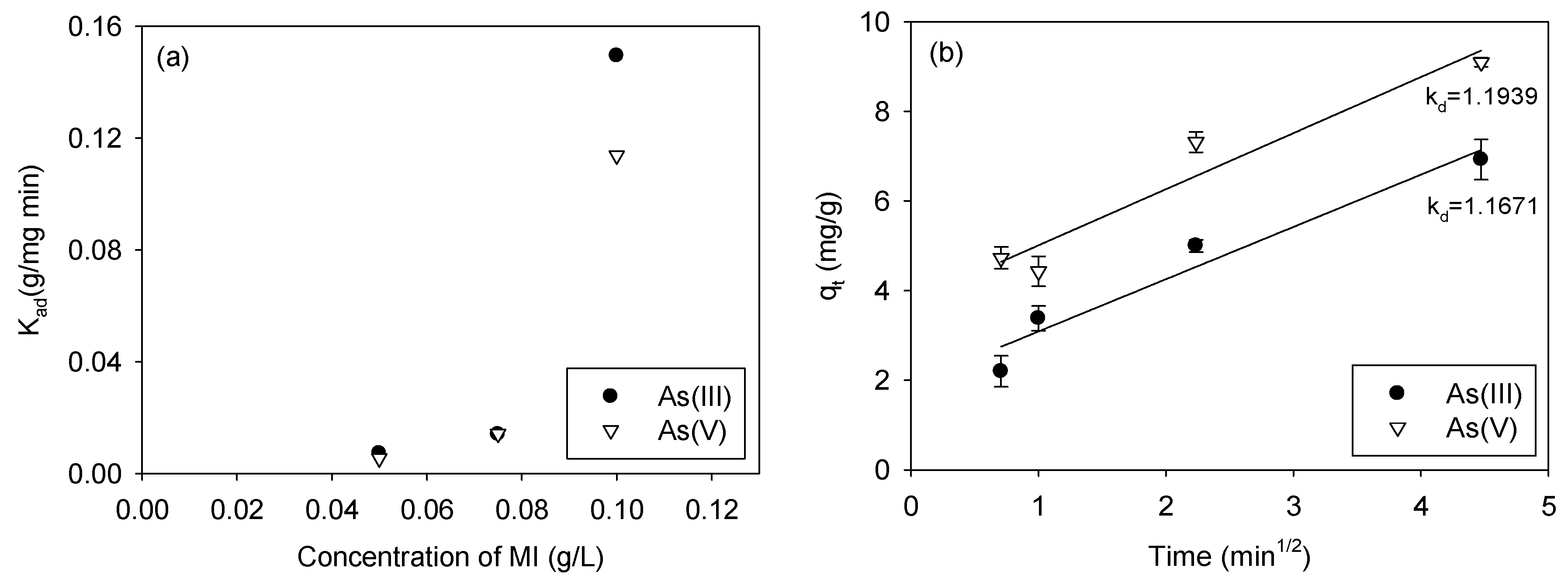
3.3. Kinetics of Arsenic Adsorption
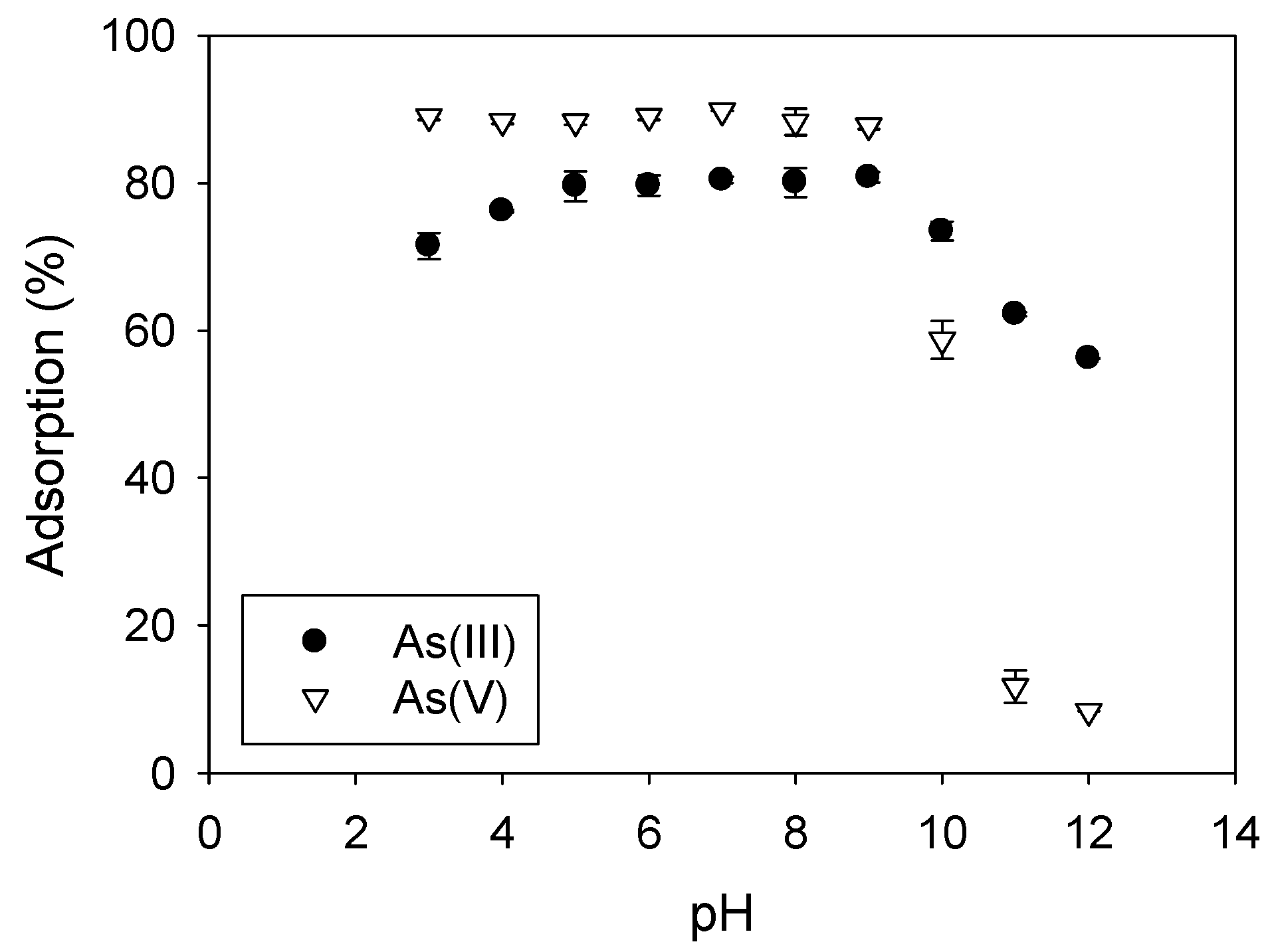
3.4. Effect of pH on Arsenic Adsorption
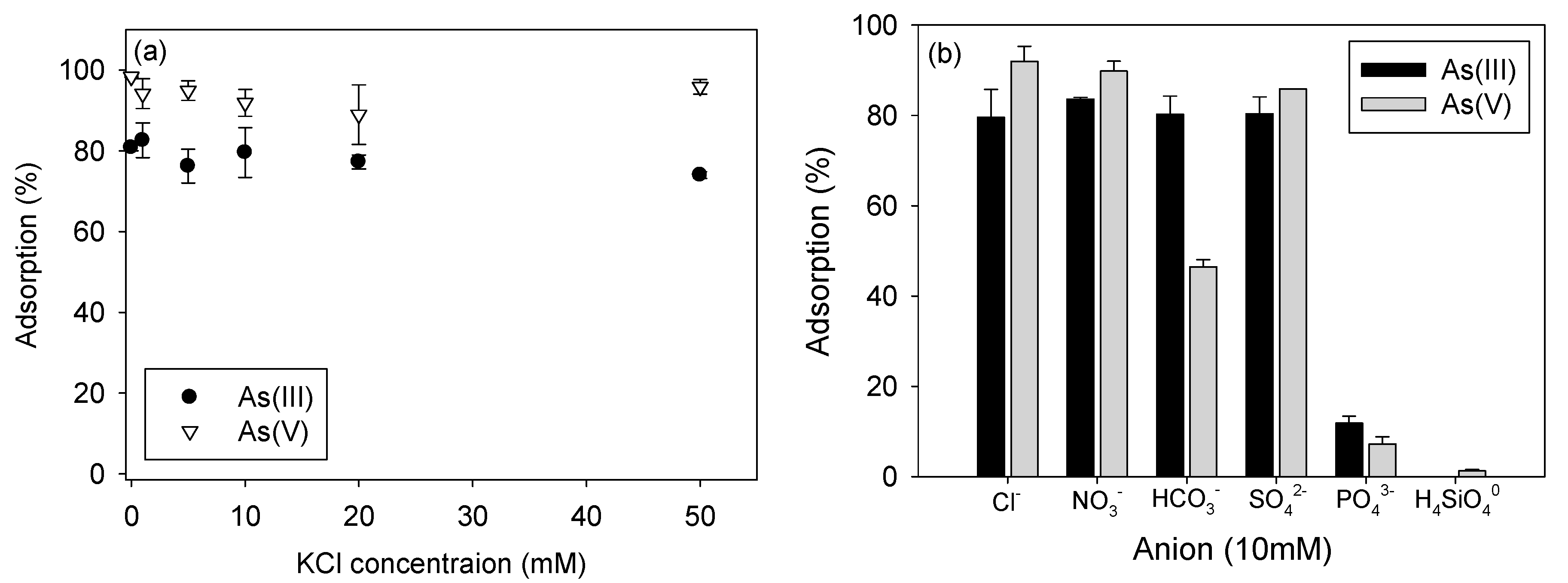
3.5. Effects of Ionic Strength and Anions on Arsenic Adsorption
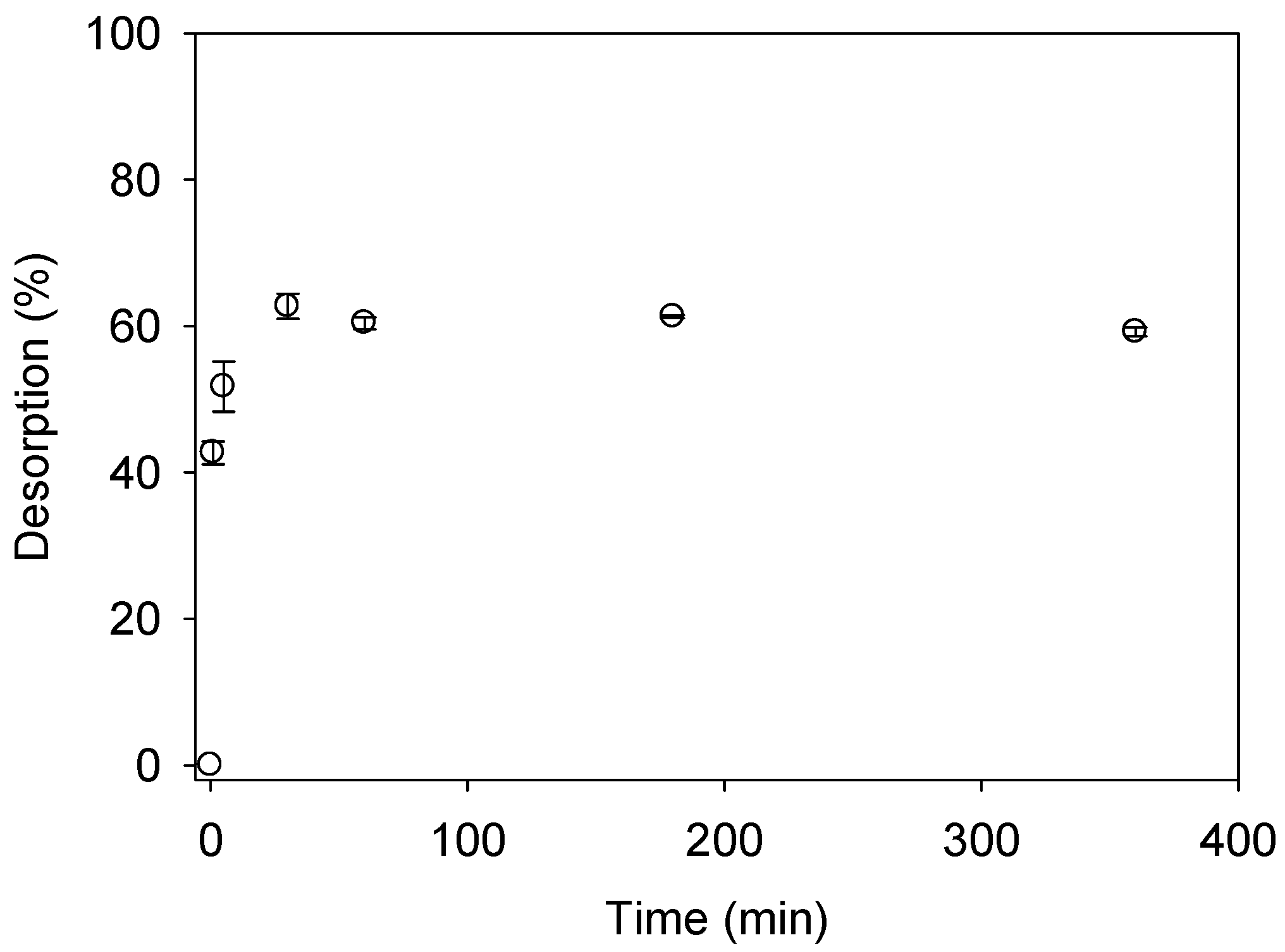
3.6. Regeneration of Arsenate Adsorbed MI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agency for Toxic Substances and Disease Registry. Top 20 Hazardous Substances: ATSDR/EPA Priority List for 1997; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 1997. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 10 November 2020).
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Schnoor, J. (Ed.) Environmental modeling. In Fate and Transport of Pollutants in Water, Air, and Soil; John Wiley Sons: New York, NY, USA, 1996. [Google Scholar]
- Xu, Y.H.; Nakajima, T.; Ohki, A. Adsorption and removal of arsenic(V) from drinking water by aluminum-loaded Shirasu-zeolite. J. Hazard. Mater. 2002, 92, 275–287. [Google Scholar] [CrossRef]
- Karim, M.M. Arsenic in groundwater and health problems in Bangladesh. Water Res. 2000, 34, 304–310. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking Water Quality, 2nd ed.; WHO: Geneva, Switzerland, 1993. [Google Scholar]
- USEPA. Proven Alternatives for Aboveground Treatment of Arsenic in Groundwater; Office of Solid Waste and Emergency Response: Washington, DC, USA, 2002. [Google Scholar]
- Lin, T.F.; Wu, J.K. Adsorption of arsenite and arsenate within activated alumina grains: Equilibrium and kinetics. Water Res. 2001, 35, 2049–2057. [Google Scholar] [CrossRef]
- Zuo, J.C.; Tong, S.R.; Yu, X.L.; Wu, L.Y.; Cao, C.Y.; Ge, M.F.; Song, W.G. Fe3+ and amino functioned mesoporous silica: Preparation, structural analysis and arsenic adsorption. J. Hazard. Mater. 2012, 235–236, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.D.; Jung, W.S.; Cho, J.M.; Ryu, B.G.; Yang, J.S.; Baek, K. Adsorption of Cr(VI) onto cationic surfactant-modified activated carbon. J. Hazard. Mater. 2009, 166, 642–646. [Google Scholar] [CrossRef]
- Min, S.Y.; Kim, B.L.; Park, S.J.; Chang, Y.Y.; Yang, J.K. Removal efficiency of arsenic by adsorbents having different type of metal oxides. Environ. Eng. Res. 2009, 14, 134–159. [Google Scholar] [CrossRef]
- Park, H.; Ayala, P.; Deshusses, M.A.; Mulchandani, A.; Choi, H.; Myung, N.V. Electrodeposition of maghemite (γ-Fe2O3) nanoparticles. Chem. Eng. J. 2008, 139, 208–212. [Google Scholar]
- Zouboulis, A.I.; Katsoyiannis, I.A. Arsenic removal using iron oxide loaded alginate beads. Ind. Eng. Chem. Res. 2002, 41, 6149–6155. [Google Scholar] [CrossRef]
- Park, Y.J.; Yang, J.K.; Choi, H.J.; Lee, S.M. Removal of arsenic from aqueous solution using hybrid metal oxide. Environ. Eng. Res. 2012, 17, S15–S19. [Google Scholar] [CrossRef]
- Sinha, S.; Amy, G.; Yoon, Y.; Her, N. Arsenic removal from water using various adsorbents: Magnetic ion exchange resins, hydrous ion oxideparticles, granular ferric hydroxide, activated alumina, sulfur modified iron, and iron oxide-coated microsand. Environ. Eng. Res. 2011, 16, 165–173. [Google Scholar] [CrossRef]
- Kanel, S.R.; Manning, B.; Charlet, L.; Choi, H. Removal of arsenic(III) from groundwater by nanoscale zero-valent iron. Environ. Sci. Technol. 2005, 39, 1291–1298. [Google Scholar] [CrossRef]
- Kanel, S.R.; Greneche, J.M.; Choi, H. Arsenic(V) removal kom groundwater using nano scale zero-valent iron as a colloidal reactive barrier material. Environ. Sci. Technol. 2006, 40, 2045–2050. [Google Scholar] [CrossRef]
- Ruiping, L.; Lihua, S.; Jiuhui, Q.; Guibai, L. Arsenic removal through adsorption, sand filtration and ultrafiltration: In situ precipitated ferric and manganese binary oxides as adsorbents. Desalination 2009, 249, 1233–1237. [Google Scholar] [CrossRef]
- Habuda-Stanić, M.; Kalajdžić, B.; Kuleš, M.; Velić, N. Arsenite and arsenate sorption by hydrous ferric oxide/polymeric material. Desalination 2008, 229, 1–9. [Google Scholar] [CrossRef]
- Banerjee, K.; Amy, G.L.; Prevost, M.; Nour, S.; Jekel, M.; Gallagher, P.M.; Blumenschein, C.D. Kinetic and thermodynamic aspects of adsorption of arsenic onto granular ferric hydroxide (GFH). Water Res. 2008, 42, 3371–3378. [Google Scholar] [CrossRef]
- Sabbatini, P.; Yrazu, F.; Rossi, F.; Thern, G.; Marajofsky, A.; de Cortalezzi, M.M.F. Fabrication and characterization of iron oxide ceramic membranes for arsenic removal. Water Res. 2010, 44, 5702–5712. [Google Scholar] [CrossRef]
- Qu, J. Research progress of novel adsorption processes in water purification: A review. J. Environ. Sci. 2008, 20, 1–13. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Ying, J.Y.; Mehnert, C.P.; Wong, M.S. Synthesis and applications of supramolecular-templated mesoporous materials. Angew. Chem. Int. Ed. 1999, 38, 56–77. [Google Scholar] [CrossRef]
- Zhao, X.S.; Lu, G.Q.; Millar, G.J. Advances in mesoporous molecular sieve MCM-41. Ind. Eng. Chem. Res. 1996, 35, 2075–2090. [Google Scholar] [CrossRef]
- Bui, T.X.; Choi, H. Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J. Hazard. Mater. 2009, 168, 602–608. [Google Scholar] [CrossRef]
- Sui, Q.; Huang, J.; Liu, Y.; Chang, X.; Ji, G.; Deng, S.; Xie, T.; Yu, G. Rapid removal of bisphenol A on highly ordered mesoporous carbon. J. Environ. Sci. 2011, 23, 177–182. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, C.M.; Choi, I.H.; Rengaraj, S.; Yi, J.H. Arsenic removal using mesoporous alumina prepared via a templating method. Environ. Sci. Technol. 2004, 38, 924–931. [Google Scholar] [CrossRef]
- Wang, P.; Lo, I.M.C. Synthesis of mesoporous magnetic γ-Fe2O3 and its application to Cr(VI) removal from contaminated water. Water Res. 2009, 43, 3727–3734. [Google Scholar] [CrossRef]
- Xin, X.; Wei, Q.; Yang, J.; Tan, L.; Feng, R.; Chen, G.; Du, B.; Li, H. Highly efficient removal of heavy metal ions by amine-functionalized mesoporous Fe3O4 nanoparticle. Chem. Eng. J. 2012, 184, 132–140. [Google Scholar] [CrossRef]
- Srivastava, D.N.; Perkas, N.; Gedanken, A.; Felner, I. Sonochemical synthesis of mesoporous iron oxide and accounts of its magnetic and catalytic properties. J. Phys. Chem. B 2002, 106, 1878–1883. [Google Scholar] [CrossRef]
- Sangwichien, C.; Aranovich, G.L.; Donohue, M.D. Density functional theory predictions of adsorption isotherms with hysteresis loops. Colloids Surf. Physicochem. Eng. Asp. 2002, 206, 313–320. [Google Scholar] [CrossRef]
- Vermeer, A.W.P.; McCulloch, J.K.; van Riemsdijk, W.H.; Koopal, L.K. Metal Ion Adsorption to Complexes of Humic Acid and Metal Oxides: Deviations from the Additivity Rule. Environ. Sci. Technol. 1999, 33, 3892–3897. [Google Scholar] [CrossRef]
- Gao, X.D.; Metge, D.W.; Ray, C.; Harvey, R.W.; Chorover, J. Surface Complexation of Carboxylate Adheres Cryptosporidium parvum Oocysts to the Hematite-Water Interface. Environ. Sci. Technol. 2009, 43, 7423–7429. [Google Scholar] [CrossRef]
- Ho, Y.S. Selection of optimum sorption isotherm. Carbon 2004, 42, 2115–2116. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents-A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Li, Q.; Gao, S.; Shang, J.K. Arsenic (III,V) removal from aqueous solution by ultrafine α-Fe2O3 nanoparticles synthesized from solvent thermal method. J. Hazard. Mater. 2011, 192, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S. Comment on "Arsenic removal using mesoporous alumina prepared via a templating method". Environ. Sci. Technol. 2004, 38, 3214–3215. [Google Scholar] [CrossRef]
- Mamindy-Pajany, Y.; Hurel, C.; Marmier, N.; Romeo, M. Arsenic adsorption onto hematite and goethite. C. R. Chim. 2009, 12, 876–881. [Google Scholar] [CrossRef]
- Southgate, B.A. Advances in Water Pollution Research: Proceedings of the International Conference; Symposium Publications Division; Pergamon Press: Oxford, UK, 1962. [Google Scholar]
- Su, C.M.; Puls, R.W. Arsenate and arsenite removal by zero-valent iron: Kinetics, redox transformation, and implications for in situ groundwater remediation. Environ. Sci. Technol. 2001, 35, 1487–1492. [Google Scholar] [CrossRef]
- Suzuki, N. Arsenic in Aquifers: Bicarbonate Ions and the Adsorption of Inorganic Arsenic by Iron Oxide Minerals. Senior Thesis Symposium, Environmental Sciences, Berkeley, University of California, 2011. Available online: https://nature.berkeley.edu/classes/es196/projects/2011final/SuzukiN_2011.pdf (accessed on 10 November 2020).
- Scerri, E.R.; Worrall, J. Prediction and the periodic table. Stud. Hist. Philos. Sci. 2001, 32A, 407–452. [Google Scholar] [CrossRef]
- Mamindy-Pajany, Y.; Hurel, C.; Marmier, N.; Roméo, M. Arsenic (V) adsorption from aqueous solution onto goethite, hematite, magnetite and zero-valent iron: Effects of pH, concentration and reversibility. Desalination 2011, 281, 93–99. [Google Scholar] [CrossRef]
- Fleming, B.A.; Crerar, D.A. Silicic acid ionization and calculation of silica solubility at elevated temperature and pH application to geothermal fluid processing and reinjection. Geothermics 1982, 11, 15–29. [Google Scholar] [CrossRef]
| MI (g/L) | As(III) | As(V) | ||||
|---|---|---|---|---|---|---|
| Kad (g/mg min) | Ksa (L/m2 hr) | R2 | Kad (g/mg min) | Ksa (L/m2 hr) | R2 | |
| 0.05 | 0.0073 | 0.0005 | 0.8532 | 0.0054 | 0.0004 | 0.9196 |
| 0.075 | 0.0140 | 0.0007 | 0.8715 | 0.0142 | 0.0007 | 0.8596 |
| 0.1 | 0.1443 | 0.0054 | 0.9105 | 0.1138 | 0.0042 | 0.9620 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, J.; Kim, S.; Kim, K.S.; Hwang, H.-K.; Choi, H. Adsorptive Removal of Arsenic by Mesoporous Iron Oxide in Aquatic Systems. Water 2020, 12, 3147. https://doi.org/10.3390/w12113147
Bae J, Kim S, Kim KS, Hwang H-K, Choi H. Adsorptive Removal of Arsenic by Mesoporous Iron Oxide in Aquatic Systems. Water. 2020; 12(11):3147. https://doi.org/10.3390/w12113147
Chicago/Turabian StyleBae, Jiyeol, Suho Kim, Kwang Soo Kim, Hwan-Kook Hwang, and Heechul Choi. 2020. "Adsorptive Removal of Arsenic by Mesoporous Iron Oxide in Aquatic Systems" Water 12, no. 11: 3147. https://doi.org/10.3390/w12113147
APA StyleBae, J., Kim, S., Kim, K. S., Hwang, H.-K., & Choi, H. (2020). Adsorptive Removal of Arsenic by Mesoporous Iron Oxide in Aquatic Systems. Water, 12(11), 3147. https://doi.org/10.3390/w12113147




