Abstract
The aquaculture industry requires solutions to several environmental challenges in order to become sustainable, including adequate wastewater management. Aquaculture wastewater (AWW) is rich in nitrogen, phosphorus, organic carbon, and other elements essential for microalgae. Due to the potential for AWW to be used as a microalgal growth medium and the potential of Chlorella sorokiniana to remediate wastewater, the growth of this species in AWW was evaluated. The microalgal growth in AWW was compared to the growth in a modified BG11 growth medium containing similar nutrient concentrations as the AWW. The effect of pH regulation and air-lifting the cell suspension at different airflow rates was also studied. As a result, it was found that C. sorokiniana can grow successfully in AWW; however, its cultivation required pH regulation. This microalga species can reach a biomass concentration of up to 476 mg/L and a biomass productivity of 140 mg/L/day. Furthermore, up to 78% of the nitrogen, 77% of the phosphorus, 70% of the magnesium, 90% of the zinc, and 99% of the nickel contained in the AWW were assimilated by the microalgae. The results of this study show that microalga cultivation in wastewater has great potential to reduce contamination while generating economic benefits.
1. Introduction
Phycoremediation refers to the use of microalgae or macroalgae to remove or transform pollutants from water and air, nutrients and harmful chemicals from wastewater, and CO2 from waste air [1]. Often, the pshycoremediation processes also involve the production of microalgal biomass. This biomass is a valuable stock in different industries, as several chemicals can be extracted or produced from it. These include biofuels such as biodiesel and bioethanol, which are produced from lipids and carbohydrates, respectively [2], and bioactive medicinal products, such as pigments and polyunsaturated fatty acids (PUFAs), which are commonly used in the health industry and play a vital role as precursors of vitamins, antioxidants, anti-inflammatory compounds, and wound healing additives [3,4]. In addition, the raw biomass is regularly used for human and animal consumption. It is highly digestible, contains large quantities of amino acids and proteins, and promotes animal growth and health [5].
The process of phycoremediation can be more efficient and less costly than conventional wastewater treatments. However, it might require more supervision and management, as the microalgal growth can alter the wastewater’s physicochemical factors, negatively affecting the growth. The pH, for instance, can change depending on the ions that the microalgae consume (e.g., ammonium assimilation decreases the pH, while nitrate assimilation increases the pH). Assimilation of nitrogen ions has the strongest influence on pH changes due to the fact that they are consumed in higher quantities than other elements [6]. Other ions that affect the pH include potassium, phosphate, sulfate, and magnesium [6]. The pH changes can be extreme, causing cell damage and even killing the cells [7].
Several challenges are present in pilot or large-scale phycoremediation systems. The challenges vary depending on the photobioreactor settings, wastewater used, and microalga species cultivated. The most common challenges are preventing microbial contamination, achieving proper mixing, and overcoming light and CO2 limitations. Microalgae, bacteria, and protozoans are often present in wastewater, and contamination by these microbes can affect the phycoremediation process negatively [8,9,10]. Thus, a method to sterilize the wastewater or reduce the concentration of microbes present is necessary. A suitable method to sterilize the wastewater in large-scale systems appears to be the use of UV-C radiation. Through this method, large volumes of water can be treated efficiently in a reasonable time and with low energy usage [11]. UV radiation also changes the quantity and quality of the dissolved organic matter, reducing light attenuation in the wastewater [12]. On the other hand, proper mixing and optimization of gas transfers can be achieved in column photobioreactors by air-lifting the cell suspension at an adequate airflow rate [13].
The aim of the study was to evaluate the potential of using the microalga Chlorella sorokiniana for phycoremediation of aquaculture wastewater (AWW) in a large scale photobioreactor. Although previous studies have been conducted to evaluate the feasibility of using the microalgae C. sorokiniana to remediate AWW, the studies have only been carried out using small scale systems [14,15]. Cultivation of plants and algae using aquaculture wastewater as a source of nutrients offers a solution to several environmental changes [16,17]. However, the literature about the use of AWW for microalgae cultivation is still limited and more research is needed to provide solutions for the industry. The importance of circular production methods is increasing as the natural resources are becoming scarce and the food demand increases [16]. The use of AWW provides the following benefits: (1) it is rich in chemicals required by microalgae: nitrogen, phosphorus, organic carbon, iron, molybdenum, zinc, sodium, nickel, magnesium, and potassium [14], (2) it does not contain detergents or other chemicals that could be harmful to green microalgae, and (3) it is considered a threat for aquaculture due to its potential to deteriorate freshwater ecosystems via the eutrophication [16]. On the other hand, the microalga C. sorokiniana was selected for this study due to efficiency it has shown to remediate wastewaters from different sources, for instance, urban and municipal wastewater [10,18,19], agro-industrial wastewater [20], industrial wastewater [21], and even aquaculture wastewater [14]. To evaluate the potential of C. sorokiniana to remediate AWW in a large-scale system, the experiments were performed in a 12 × 70 L column photobioreactor. Different cultivation conditions were tested: pH controlled and pH uncontrolled; air-lifting at 2, 4, 6, and 8 L/min; using two concentrations of CO2 in the air pumped into the culture. The microalgal growth in the AWW was compared to the growth in synthetic growth medium BG11. Additionally, the nutrient concentrations in the AWW were measured prior to and after microalga cultivation to evaluate the nutrient removal efficiency.
2. Materials and Methods
2.1. Photobioreactors
Two photobioreactors were used in this project. The first photobioreactor was the model HXDJB50-42 (Dalian Huixin Titanium Equipment Development Co., Ltd., Dalian, China). This system consists of a white-lighted shelf with air-bubbling connections and magnetic stirrers, which can fit 42 flasks. The stirrer system has stirring intervals of 10 s every 50 s. The pilot-scale photobioreactor was the HXDYKZ-12 (Dalian Huixin Titanium Equipment Development Co., Ltd., Dalian, China). This photobioreactor is divided into 12 columns, each with a capacity of approximately 70 L. Custom light-emitting diodes (LED) lamps were used in the lighting system, and each column used two lamps. Each lamp had 5 m of LED strips with LEDs of two colors, red and blue, in a ratio of 3:1. These LED stripes were designed for this project according to the light spectrum required for microalga growth [22]. The experiments were carried out in both photobioreactors under autotrophic conditions (24/0 h light/dark cycle) at a temperature of 28 ± 2 °C.
2.2. Microalga Species and Cultivation
The experiments were performed with the microalga Chlorella sorokiniana, Shihira and Krauss strain 211/8K (CCAP, Scotland, U.K.). The stock culture of C. sorokiniana was maintained in BG11 growth medium [23]. The experiments were performed using AWW as the growth medium, as well as a modified version of the BG11 growth medium (Table 1). The procedure of the phycoremediation process is simplified in Figure 1. The AWW was extracted directly from the fish tanks of a recirculating aquaculture system (RAS) wherein Oreochromis niloticus was the species farmed. After extraction, the AWW was passed through a UV-C lamp (Pond Friend 3600, by Pondteam) at a flow rate of 4.6 L/min and used immediately for microalga cultivation. Finally, the microalga was extracted from the wastewater. The aquaculture system consisted of the fish tanks, a sedimentation tank, and a moving bed biofilm reactor.

Table 1.
Specifications of the two groups and the control treatment evaluated in the experiment.
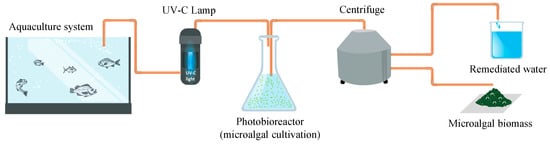
Figure 1.
Schematic design of a phycoremediation process for aquaculture wastewater. The wastewater is extracted from the aquaculture system, treated with a UV-C lamp and used for microalgae cultivation. Once the microalgae stop growing, the cell suspension is processed to harvest the microalgae.
2.3. Wastewater Characterization
The temperature and the pH (VisionPlus pH630, by Jenco), the electrical conductivity (EC meter, by Essentials), and dissolved oxygen (DO 300, by CyberScan) were measured in a fresh sample of AWW collected at the outlet of the UV-C lamp. The concentrations of total nitrogen, total phosphorus, iron ( Fe), magnesium ( Mg), potassium ( K), and quaternary ammonium compounds (QAC) were measured using the Hach Lange kits LCK 138, LCK 326, LCK 348, FerroVer Iron Reagent Set, Potassium Reagent Set, and Q. A. C. Reagent Set, respectively. The chemical oxygen demand (COD), ammonium ( NH4+), nitrate ( NO3−), nitrite ( NO2−), molybdenum ( Mo), zinc ( Zn), sodium ( Na), chloride ( Cl), and nickel ( Ni) were analyzed by the company Eurofins Umwelt Ost GmbH, Germany, using the methods DIN 38409-41 (H41):1980-12, DIN 38406-5 (E5):1983-10, DIN EN ISO 10304-1 (D20): 2009-07, DIN EN 26777 (D10):1993-04, DIN EN ISO 17294-2 (E29): 2017-01, DIN EN ISO 17294-2 (E29): 2017-01, DIN EN ISO 17294-2 (E29): 2017-01, DIN EN ISO 10304-1 (D20): 2009-07, and DIN EN ISO 17294-2 (E29): 2017-01, respectively. The bacterial density and total suspended solids were analyzed in fresh samples by the company Sýni, Iceland. All the samples were filtrated before the chemical analyses, except for the COD. All the analyses were performed in triplicate.
2.4. Microalgal Growth
The cell concentration was measured every 24 h via spectrophotometric methods at 750 nm using a DR 3900 spectrophotometer (Hach Lange, Dusseldorf, Germany). Furthermore, the biomass dry weight (DW) was measured via gravimetric methods at different growth stages to build a correlation between the optical density and dry weight biomass. The biomass dry weight was measured following the protocol designed by Zhu and Lee [24] with some modifications. The filters used were the Whatman GF/C (4.7 cm diameter); these were pre-washed in distillated water and then dried in an oven at 100 °C for 12 h and stored in a vacuum desiccator over KMnO4 crystals until use. The filtered biomass was also dried in an oven at 100 °C for 24 h and cooled down in a vacuum desiccator before weighing.
2.5. Controlling the pH of the Cell Suspension
The pH was measured every 24 h and maintained between 6 and 8, which is the ideal pH for the maximum growth of C. sorokiniana. To control the pH, a 0.5 M solution of potassium hydroxide (KOH) or a 0.5 M solution of hydrochloric acid (HCl) was added to the culture. The volume of solution added varied depending on the actual pH value of the culture. The experiment was performed on an HXDJB50-42 photobioreactor, using 2 L flasks filled with 1 L of the AWW. The airflow of the bubbling system was 1 L/min, approximately. As a control treatment, C. sorokiniana was cultivated under the same conditions; however, the pH was not controlled, only monitored. Both treatments were evaluated in triplicate.
2.6. Effect of the CO2 Content on Air and Airflow Rate in the Column Photobioreactor
2.6.1. Experiments
This experiment was divided into two groups and the control. In Group 1, AWW was used as the growth medium and pure air was used to air-lift the cell culture (Table 1). In Group 2, AWW was used as the growth medium and the air used to air-lift the cell culture was slightly enriched with pure CO2 (99% air and 1% CO2). Lastly, in the control group, modified BG11 growth medium was used (see below) and the air used to air-lift the cell culture was also slightly enriched with pure CO2 (99% air and 1% CO2). Four different airflow rates were evaluated in both groups and the control: 2, 4, 6, and 8 L per minute. Each flow rate was tested in triplicate under the same conditions. The pH was controlled every 12 h in Groups 1 and 2. The experiment was monitored until the microalgae reached its maximum biomass yield. Furthermore, the nutrient concentrations were measured at the beginning and end of the experiment to evaluate the efficiency of nutrient removal depending on the airflow.
2.6.2. Statistical Analysis
Permutational multivariate analysis of variance (PERMANOVA) was used to (1) detect significant differences between the average microalgal biomass productivity in the three treatments at the four airflow rates tested; (2) compare the maximum microalgal biomass obtained in the three treatments at the four different airflow rates; (3) evaluate the effect of the airflow rates on the growth of the microalgae throughout the entire cultivation period. The PERMANOVA analyses were performed using the Primer 6 with Permanova+ package (PRIMER-E Ltd., Plymouth, United Kingdom) [25]. The nutrient removal efficiencies were analyzed for each chemical independently. At first, Levene’s tests were performed to evaluate the homogeneity of the data. The data sets with normal distribution were analyzed via one-way ANOVA. The data sets with a non-normal distribution were analyzed via Kruskal–Wallis tests. Levene’s tests, one-way ANOVA, and Kruskal–Wallis tests were performed using R statistical software (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria).
2.6.3. Modified BG11 Growth Medium
AWW often contains microorganisms and chemical compounds that can affect microalgal growth. In order to evaluate the effect of these microorganisms and compounds on the growth efficiency of C. sorokiniana, microalga was also cultivated in a synthetic growth medium (BG11) that contained only the nutrients required by the microalgae (Table 2). Furthermore, as the concentration of nutrients also affects microalgal growth, the concentrations of nitrogen, phosphorus, magnesium, and chloride in the BG11 growth medium were changed to be the same as those in the AWW used. The rest of the chemical compounds included in the growth medium were diluted to 0.3, which is an estimated value of the concentration of those elements in the AWW used.

Table 2.
Concentrations of chemicals in the modified BG11 growth medium.
3. Results and Discussion
3.1. Wastewater Characterization
Microalgal growth is often limited by nutrient deficiency, toxic chemicals, acidic or basic environments, temperature, as well as other properties. Previous studies have shown that aquaculture wastewater contains essential nutrients required for microalgal growth [14]. To evaluate the potential of AWW to be used as a microalgal growth medium, the concentration of several chemicals, as well as some physicochemical properties of the AWW, was measured (Table 3). The ideal pH for cultivation of C. sorokiniana is between 6 and 8; however, the pH of the AWW was lower (5.22). A low pH affects the microalgae negatively, reducing the efficiency of the phycoremediation process. The AWW’s low pH is mainly caused by two factors: (1) carbon dioxide and carbonic acid dissolved in the water, produced during fish respiration, and (2) hydrogen ions ( H+) produced during the nitrification process [26]. On the other hand, the AWW contained several macronutrients and trace metals required by microalgae. For instance, organic carbon was detected in the wastewater with a concentration of COD = 64.3 ± 12.3 mg/L. Nitrogen was found in inorganic and organic forms in the AWW (Table 3). The most common inorganic form was NO3− (52.0 ± 4.0 mg/L), followed by NH4+ (12.8 ± 2.1 mg/L). The amount of toxic NO2− present was negligible (<0.01 mg/L). Although the concentration of NO3− is similar to that in the AWW used in other phycoremediation studies [14,27], the concentration of NH4+ is higher compared to that in the AWW used by Ansari, Singh, Guldhe and Bux [14] (5.3 mg/L). Furthermore, the bacterial density was found to be 2050 ± 50 CFU/mL and protozoans were observed under the microscope in samples of AWW pretreated with a UV-C lamp.

Table 3.
Aquaculture wastewater characterization.
3.2. Controlling the pH of the Cell Suspension
The effect of pH regulation during the cultivation of C. sorokiniana in AWW was studied. For comparison purposes, the microalgae were grown under two conditions: pH controlled and pH uncontrolled (Figure 2). Under both conditions, the pH behaved similarly over the first three days. During the first day, the pH increased as a result of CO2 assimilation by the microalgae, as well as the CO2 dissipation generated by the mixing and air bubbling of the cell culture. Then, the pH tended to decrease during the second and third days due to nitrogen assimilation. When the pH was not controlled, the pH dropped to its lowest (3.2) on the third day, causing the cells’ death. Differently, in the pH-controlled treatment, maintaining the pH closer to 7 promoted microalgal growth, and higher biomass concentrations were obtained. After the third day, the pH stopped decreasing and started to increase. This behavior has been reported in previous studies that attempted to cultivate C. sorokiniana in ammonium- and nitrate-rich growth media [6,7,28]. In this experiment, the pH was controlled successfully with the addition of KOH or HCl 0.5 M solution.
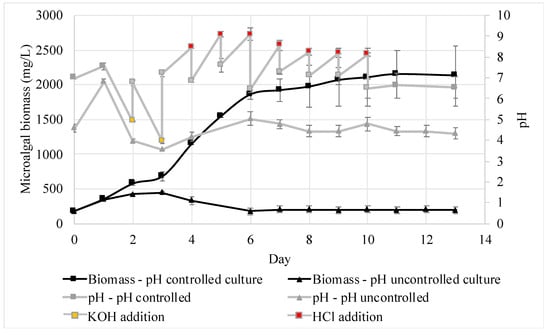
Figure 2.
pH measurements and biomass growth curves, with standard deviations, of two cultures of C. sorokiniana cultivated in AWW. In black, the biomass concentration, and in gray, the pH values. The boxes represent the pH-controlled culture, and the triangles, the pH-uncontrolled culture. Orange boxes indicate the pH values before the KOH addition. Red boxes indicate the pH value before HCl addition. Number of samples per treatment = 3.
The pH decreased during the second and third days due to the preference of C. sorokiniana to utilize ammonium when both ammonium and nitrate are available in the growth medium [28]. Scherholz and Curtis [7] explained this preference as a result of the inhibitory effect of ammonium over both the transport and reduction in nitrate. The assimilation of ammonium can be represented simplistically as
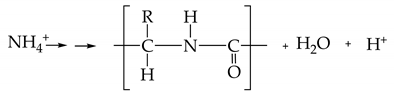 where the nitrogen contained in the ammonium becomes part of the peptide bond created by the condensation polymerization of amino acids. Along with the creation of the peptide bond, two other products are generated: H2O and H+ [28]. The proton exchange results in a pH drop, which affects the microalgae negatively. In this project, the lowest pH value measured during the cultivation of C. sorokiniana in the AWW was 3.2. However, this microalga species has an optimal growth rate when the pH is between 6 and 8, and it does not survive at pH 5 or lower [28].
where the nitrogen contained in the ammonium becomes part of the peptide bond created by the condensation polymerization of amino acids. Along with the creation of the peptide bond, two other products are generated: H2O and H+ [28]. The proton exchange results in a pH drop, which affects the microalgae negatively. In this project, the lowest pH value measured during the cultivation of C. sorokiniana in the AWW was 3.2. However, this microalga species has an optimal growth rate when the pH is between 6 and 8, and it does not survive at pH 5 or lower [28].

After the depletion of ammonium, the inhibition of the nitrate transporters stops, and the assimilation of nitrate takes place. The assimilation of nitrate can be represented simplistically as follows.

In these reactions, the nitrate is at first transformed into nitrite, then into ammonium, and finally, the nitrogen is used for protein building. As a result of the nitrate assimilation, chemical compounds such as O2 and OH− are produced. Thus, in contrast to ammonium assimilation, nitrate assimilation leads to a pH increment [28]. This explains the increase in the pH registered in the experiment.
Microalga cultivation in an ammonium-rich medium requires the addition of a basic chemical agent to regulate the pH. Some authors suggest the addition of NaOH to raise the pH [6], while other authors claim that KOH is the proper chemical agent to control the pH in microalga cultures, as K+ ions are essential for plant life [29]. These ions maintain the charge balance in the cytoplasm, are involved in enzyme reactions, and maintain the osmotic pressure of vacuoles. However, in contrast to K+ ions, Na+ ions are only essential for some C4 species of plants [29]. On the other hand, during the cultivation of microalgae in a nitrate-rich medium, the addition of an acidic chemical agent can maintain the pH within the optimal range [6]. Hydrochloric acid was the chemical agent used in this project and in the study carried out by Wang and Curtis [6]; the acid helped in both projects to obtain high-density cultures.
3.3. Effect of the CO2 Concentration on Air and Airflow in the Column Photobioreactor
3.3.1. Growth Rates and Maximum Biomass Concentrations
Two concentrations of CO2 in air and four different airflow rates were tested in order to optimize the phycoremediation process. The microalgal growth in the AWW was compared to the growth in the modified BG11 growth medium. Significant differences were found between the microalgal growth rates for the two CO2 concentrations tested (Pseudo F1,3 = 1133.29, p < 0.05). In addition, significant differences were found between the microalgal growth rates in the AWW and modified BG11 growth medium (Pseudo F2,3 = 528.04, p < 0.05). The highest growth rates of C. sorokiniana were obtained when the species was cultivated in AWW and CO2-enriched air, as shown in Figure 3. In these conditions, the microalgae produced more than 140 mg/L/day, on average. The second highest growth rates were obtained when the species was grown in the modified BG11 growth medium and air-lifted with CO2-enriched air. The difference in the growth rates between these treatments was mainly generated by the nitrogen source. In AWW, the nitrogen source is mainly ammonium, the assimilation of which requires less energy than the assimilation of nitrate. The nitrate must first be reduced to ammonium prior to its utilization [30]. On the other hand, the microalgae cultivated in AWW and air-lifted with pure air had the lowest growth rates registered in this project (below 25.7 mg/L/day). These low growth rates occurred as a result of CO2 limitation, since the air pumped into the cultures was not enriched with CO2.
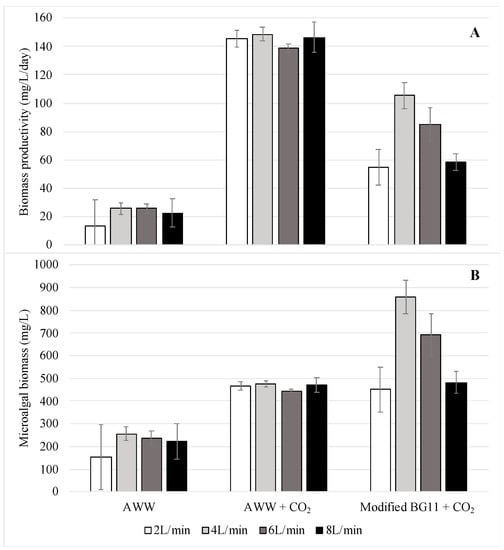
Figure 3.
Average biomass productivity (A) and maximum microalgal biomass (B), with standard deviations, of C. sorokiniana cultured in three different groups: AWW = AWW air-lifted with pure air; AWW + CO2 = AWW air-lifted with CO2-enriched air; BG11 + CO2 = modified BG11 growth medium air-lifted with CO2-enriched air. Four different airflow rates were tested in each group (2, 4, 6, and 8 L/min). Number of samples per treatment = 3.
The maximum biomass concentrations obtained were also significantly different among the groups tested (Pseudo F2,3 = 36.02, p < 0.05). Contrary to the biomass productivity (mg/L/day), the highest biomass concentrations (mg/L) were obtained by cultivation in the modified BG11 growth medium and CO2-enriched air, followed by cultivation in the AWW and CO2-enriched air, and, lastly, cultivation in AWW and pure air (Figure 3). In previous studies, it has been reported that C. sorokiniana reaches higher biomass concentrations when using nitrate as the nitrogen source instead of ammonium [31,32]. However, in these studies, the pH was not regulated when ammonium was used, which lowered the microalga growth and even caused cell lysis as the pH dropped. In this study, where the pH was regulated, the lower biomass concentrations obtained using AWW compared to those obtained using the modified BG11 growth medium could have been generated by the cell flocculation and precipitation phenomenon that occurred when AWW was used.
As the cultivation conditions were the same for all the treatments, the lower microalgal biomass yield obtained in AWW (476 mg/L) compared to the biomass yield obtained in the modified BG11 growth medium (858 mg/L) could have been caused by biological contaminants. Although the AWW was pretreated with a UV-C lamp, the bacterial concentration measured during the wastewater characterization was 2050 ± 50 CFU/mL. Additionally, different species of protozoans were observed in samples taken from the cell suspension during the cultivation period. Biological contamination affects microalgal growth negatively in different ways. It can reduce microalgal growth by outcompeting the species cultivated. The microalgal biomass yield can be reduced by predation of the cells. Some species of microalgae and bacteria can produce toxins that can be harmful to the species cultivated. Viruses can also infect microalgal cells, using them as host to replicate and finally causing cell lysis [33].
Another important difference between the groups cultivated in AWW and modified BG11 growth medium, both air-lifted with CO2-enriched air, is that they exhibited different growth patterns among the four airflow rates tested (Figure 4). Statistically, nonsignificant differences were found among the four different airflow rates used when C. sorokiniana was cultivated in AWW and CO2-enriched air (Pseudo F3,4 = 2.39, p > 0.05). Meanwhile, significant differences (Pseudo F3,9 = 50.90, p < 0.05) were found among the airflow rates for the microalgae cultivated in the modified BG11 growth medium and CO2-enriched air. In this last group, the highest biomass density was obtained at an airflow rate of 4 L/min, followed by 6 L/min, then 8 L/min, and, lastly, 2 L/min, with values of 858.3, 693.1, 481.8, and 451.2 mg/L, respectively. Possibly, the growth rate variations among the airflow rates were only generated in the modified BG11 growth medium due to differences between the physicochemical properties of the modified BG11 growth medium and those of the AWW.
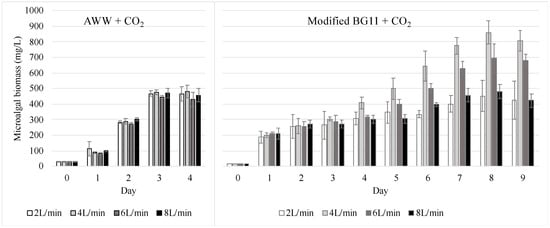
Figure 4.
Growth curves and standard deviations of C. sorokiniana cultured in two different conditions: AWW + CO2 = AWW and air-lifted with CO2-enriched air; BG11 + CO2 = modified BG11 growth medium and air-lifted with CO2-enriched air. Four different flowrates were tested in each condition (2, 4, 6, and 8 L/min). Number of samples per treatment = 3.
In this study, flocculation was observed when C. sorokiniana was cultivated in AWW. Although high growth rates were reached in the AWW, the flocculation could have negatively affected the microalgal growth. Ji et al. [34] asserted that auto-flocculation is enhanced in wastewater due to the high concentration and large number of ionic species, as this could decrease the double-layer repulsion between the colloidal particles on the cell wall. Flocculation was observed in this study at pH below 7; however, other authors have reported that flocculation occurs at pH higher than 8 [34,35,36]. These authors stated that a pH higher than 8 induces the production of insoluble precipitates such as struvite, calcium phosphate, and calcium carbonate, shifting the zeta potential of the cells and causing flocculation.
3.3.2. Nutrient Depletion
The concentrations of various nutrients present in the AWW were measured prior to and after the phycoremediation process. The microalga was harvested from the samples before the chemical analyses to ensure that only the chemicals that remained dissolved in the water would be measured. In a large-scale system, the microalgae can be harvested with different centrifuges that are available on the market. These concentrations were used to calculate the nutrient removal efficiency of C. sorokiniana. As shown in Figure 5, this species reduced the nutrient concentrations more efficiently when the culture was air-lifted with CO2-enriched air. Up to 78% of the nitrogen, up to 77% of the phosphorus, more than 70% of the magnesium, around 90% of the zinc, and over 99% of the nickel were assimilated by the microalgae. Within the nitrogen forms evaluated, ammonium was utilized in a higher percentage than nitrate: 99.9% and 75.2%, respectively. Ansari et al. [14] achieved similar nutrient removal percentages of ammonium and nitrate: 98.2% and 75.8%, respectively. Nonsignificant differences were found for the assimilation of nutrients among the airflow rates tested (p > 0.05). On the other hand, the percentages of nutrient assimilation were normally lower in the microalga cultures air-lifted with pure air. In addition, in this group, significant differences were found for the removal of nitrate at the different airflow rates used (p < 0.05). The lowest percentages of nutrient depletion occurred at 2 L/min, which correlate with the low growth obtained in this treatment (Figure 3).
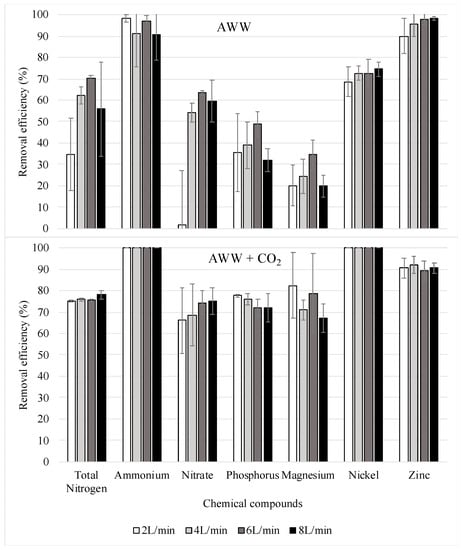
Figure 5.
Percentages and standard deviations of nutrients depleted by the C. sorokiniana cultivated in two different conditions: AWW = AWW air-lifted with pure air; AWW + CO2 = AWW air-lifted with CO2-enriched air. Four different airflow rates were tested in each group (2, 4, 6, and 8 L/min). Number of samples per treatment = 3.
4. Conclusions
This study demonstrates the potential and feasibility of the use of the microalga C. sorokiniana to remediate AWW in a large-scale photobioreactor. This species can utilize AWW as a growth medium. Its growth in AWW is at least 1.3 times faster than that in an artificial growth medium with similar concentrations of nutrients. High biomass concentrations can be obtained with the cultivation of C. sorokiniana in AWW, although higher biomass concentrations are reached in artificial growth medium. Aquaculture wastewater contains all the nutrients required by the microalga C. sorokiniana, and it is not necessary to add nutrients to the AWW for successful microalgal growth. However, as aquaculture wastewater contains ammonium, pH monitoring and regulation might be necessary for optimum microalgal growth. Such pH regulation can be executed effectively with the addition of HCl or KOH 0.5M solution. High nutrient removal percentages were achieved via phycoremediation: more than 78% of the nitrogen, 77% of the phosphorus, 70% of the magnesium, 90% of the zinc, and 99% of the nickel. Furthermore, using CO2-enriched air to air-lift the cell culture considerably promoted microalgal growth and biomass productivity. An airflow rate between 2 and 8 L/min did not generate significant differences in microalgal growth when CO2-enriched air was used. The high nutrient removal from AWW, high microalgal biomass production, and low chemical use make phycoremediation a favorable option for the treatment of AWW. Consequently, its application would be a step towards sustainability in the aquaculture industry.
Author Contributions
Conceptualization: L.A.L., R.I.T., S.B. (Sigfus Bjornsson), H.S., and S.J.; methodology: L.A.L., R.I.T., S.B. (Sigfus Bjornsson), S.B. (Sigurdur Brynjolfsson), H.S., and S.J.; term: L.A.L., R.I.T., and S.B. (Sigfus Bjornsson); validation: L.A.L. and S.B. (Sigurdur Brynjolfsson); formal analysis: L.A.L.; investigation: L.A.L.; statistical analysis: L.A.L., and O.P.P.; data curation: L.A.L.; resources: R.I.T., S.B. (Sigfus Bjornsson), S.B. (Sigurdur Brynjolfsson), O.P.P., H.S., and S.J.; writing—original draft preparation: L.A.L.; writing—review and editing: L.A.L., R.I.T., O.P.P., and S.B. (Sigurdur Brynjolfsson); visualization: L.A.L.; supervision: R.I.T., S.B. (Sigfus Bjornsson), and S.B. (Sigurdur Brynjolfsson); project administration: R.I.T., and H.S.; funding acquisition: R.I.T., S.B. (Sigfus Bjornsson), H.S., and S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Icelandic Technical Development Fund—Rannis (grant number: 175710-061).
Acknowledgments
The study was carried out by the company Samvist ehf. in collaboration with the University of Iceland and the Agricultural University of Iceland.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Olguín, E.J. Phycoremediation: Key issues for cost-effective nutrient removal processes. Biotechnol. Adv. 2003, 22, 81–91. [Google Scholar] [CrossRef]
- Suparmaniam, U.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T.; Shuit, S.H. Insights into the microalgae cultivation technology and harvesting process for biofuel production: A review. Renew. Sustain. Energy Rev. 2019, 115, 109361. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed]
- Sonani, R.R.; Rastogi, R.P.; Patel, R.; Madamwar, D. Recent advances in production, purification and applications of phycobiliproteins. World J. Biol. Chem. 2016, 7, 100–109. [Google Scholar] [CrossRef]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Kabir Chowdhury, M.A.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Wang, J.; Curtis, W.R. Proton stoichiometric imbalance during algae photosynthetic growth on various nitrogen sources: Toward metabolic pH control. J. Appl. Phycol. 2016, 28, 43–52. [Google Scholar] [CrossRef]
- Scherholz, M.L.; Curtis, W.R. Achieving pH control in microalgal cultures through fed-batch addition of stoichiometrically-balanced growth media. BMC Biotechnol. 2013, 13, 39. [Google Scholar] [CrossRef]
- Day, J.G.; Gong, Y.; Hu, Q. Microzooplanktonic grazers—A potentially devastating threat to the commercial success of microalgal mass culture. Algal Res. 2017, 27, 356–365. [Google Scholar] [CrossRef]
- Guldhe, A.; Kumari, S.; Ramanna, L.; Ramsundar, P.; Singh, P.; Rawat, I.; Bux, F. Prospects, recent advancements and challenges of different wastewater streams for microalgal cultivation. J. Environ. Manag. 2017, 203, 299–315. [Google Scholar] [CrossRef]
- Mennaa, F.Z.; Arbib, Z.; Perales, J.A. Urban wastewater treatment by seven species of microalgae and an algal bloom: Biomass production, N and P removal kinetics and harvestability. Water Res. 2015, 83, 42–51. [Google Scholar] [CrossRef]
- Qin, L.; Shu, Q.; Wang, Z.; Shang, C.; Zhu, S.; Xu, J.; Li, R.; Zhu, L.; Yuan, Z. Cultivation of Chlorella vulgaris in Dairy Wastewater Pretreated by UV Irradiation and Sodium Hypochlorite. Appl. Biochem. Biotechnol. 2014, 172, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Passero, M.L.; Cragin, B.; Hall, A.R.; Staley, N.; Coats, E.R.; McDonald, A.G.; Feris, K. Ultraviolet radiation pre-treatment modifies dairy wastewater, improving its utility as a medium for algal cultivation. Algal Res. 2014, 6, 98–110. [Google Scholar] [CrossRef]
- Wang, C.; Lan, C.Q. Effects of shear stress on microalgae—A review. Biotechnol. Adv. 2018, 36, 986–1002. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Singh, P.; Guldhe, A.; Bux, F. Microalgal cultivation using aquaculture wastewater: Integrated biomass generation and nutrient remediation. Algal Res. 2017, 21, 169–177. [Google Scholar] [CrossRef]
- Chen, J.-H.; Kato, Y.; Matsuda, M.; Chen, C.-Y.; Nagarajan, D.; Hasunuma, T.; Kondo, A.; Dong, C.-D.; Lee, D.-J.; Chang, J.-S. A novel process for the mixotrophic production of lutein with Chlorella sorokiniana MB-1-M12 using aquaculture wastewater. Bioresour. Technol. 2019, 290, 121786. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020; Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Yep, B.; Zheng, Y. Aquaponic trends and challenges—A review. J. Clean. Prod. 2019, 228, 1586–1599. [Google Scholar] [CrossRef]
- Bohutskyi, P. Effects of inoculum size, light intensity, and dose of anaerobic digestion centrate on growth and productivity of Chlorella and Scenedesmus microalgae and their poly-culture in primary and secondary wastewater. Algal Res. 2016, 19, 278–290. [Google Scholar] [CrossRef]
- Eladel, H.; Abomohra, A.E.-F.; Battah, M.; Mohmmed, S.; Radwan, A.; Abdelrahim, H. Evaluation of Chlorella sorokiniana isolated from local municipal wastewater for dual application in nutrient removal and biodiesel production. Bioprocess Biosyst. Eng. 2019, 42, 425–433. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Kuo, E.-W.; Nagarajan, D.; Ho, S.-H.; Dong, C.-D.; Lee, D.-J.; Chang, J.-S. Cultivating Chlorella sorokiniana AK-1 with swine wastewater for simultaneous wastewater treatment and algal biomass production. Bioresour. Technol. 2020, 302, 122814. [Google Scholar] [CrossRef]
- Asadi, P.; Rad, H.A.; Qaderi, F. Comparison of Chlorella vulgaris and Chlorella sorokiniana pa.91 in post treatment of dairy wastewater treatment plant effluents. Environ. Sci. Pollut. Res. 2019, 26, 29473–29489. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light requirements in microalgal photobioreactors: An overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.J.; Lee, Y.K. Determination of biomass dry weight of marine microalgae. J. Appl. Phycol. 1997, 9, 189–194. [Google Scholar] [CrossRef]
- Gorley, A.M.; Clarke, K. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Lekang, O.-I. Aquaculture Engineering; Blackwell Publishing Ltd.: Oxford, UK, 2007. [Google Scholar]
- Guo, Z.; Liu, Y.; Guo, H.; Yan, S.; Mu, J. Microalgae cultivation using an aquaculture wastewater as growth medium for biomass and biofuel production. J. Environ. Sci. 2013, 25, S85–S88. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, T.; Yu, X.; Bates, P.D.; Dong, T.; Chen, S. High-density fed-batch culture of a thermotolerant microalga Chlorella sorokiniana for biofuel production. Appl. Energy 2013, 108, 281–287. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Joun, J.M.; Lee, J.; Hong, M.E.; Pham, H.-M.; Chang, W.S.; Sim, S.J. Development of large-scale and economic pH control system for outdoor cultivation of microalgae Haematococcus pluvialis using industrial flue gas. Bioresour. Technol. 2017, 244, 1235–1244. [Google Scholar] [CrossRef]
- Podevin, M.; De Francisci, D.; Holdt, S.L.; Angelidaki, I. Effect of nitrogen source and acclimatization on specific growth rates of microalgae determined by a high-throughput in vivo microplate autofluorescence method. J. Appl. Phycol. 2015, 27, 1415–1423. [Google Scholar] [CrossRef]
- Li, T.; Zheng, Y.; Yu, L.; Chen, S. High productivity cultivation of a heat-resistant microalga Chlorella sorokiniana for biofuel production. Bioresour. Technol. 2013, 131, 60–67. [Google Scholar] [CrossRef]
- Wan, M.-X.; Wang, R.-M.; Xia, J.-L.; Rosenberg, J.N.; Nie, Z.-Y.; Kobayashi, N.; Oyler, G.A.; Betenbaugh, M.J. Physiological evaluation of a new Chlorella sorokiniana isolate for its biomass production and lipid accumulation in photoautotrophic and heterotrophic cultures. Biotechnol. Bioeng. 2012, 109, 1958–1964. [Google Scholar] [CrossRef]
- Lam, T.P.; Lee, T.-M.; Chen, C.-Y.; Chang, J.-S. Strategies to control biological contaminants during microalgal cultivation in open ponds. Bioresour. Technol. 2018, 252, 180–187. [Google Scholar] [CrossRef]
- Ji, M.-K.; Yun, H.-S.; Park, S.; Lee, H.; Park, Y.-T.; Bae, S.; Ham, J.; Choi, J. Effect of food wastewater on biomass production by a green microalga Scenedesmus obliquus for bioenergy generation. Bioresour. Technol. 2015, 179, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Beuckels, A.; Depraetere, O.; Vandamme, D.; Foubert, I.; Smolders, E.; Muylaert, K. Influence of organic matter on flocculation of Chlorella vulgaris by calcium phosphate precipitation. Biomass Bioenergy 2013, 54, 107–114. [Google Scholar] [CrossRef]
- Vandamme, D.; Beuckels, A.; Markou, G.; Foubert, I.; Muylaert, K. Reversible Flocculation of Microalgae using Magnesium Hydroxide. Bioenergy Res. 2015, 8, 716–725. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).