Small Patches of Riparian Woody Vegetation Enhance Biodiversity of Invertebrates
Abstract
1. Introduction
2. Materials and Methods
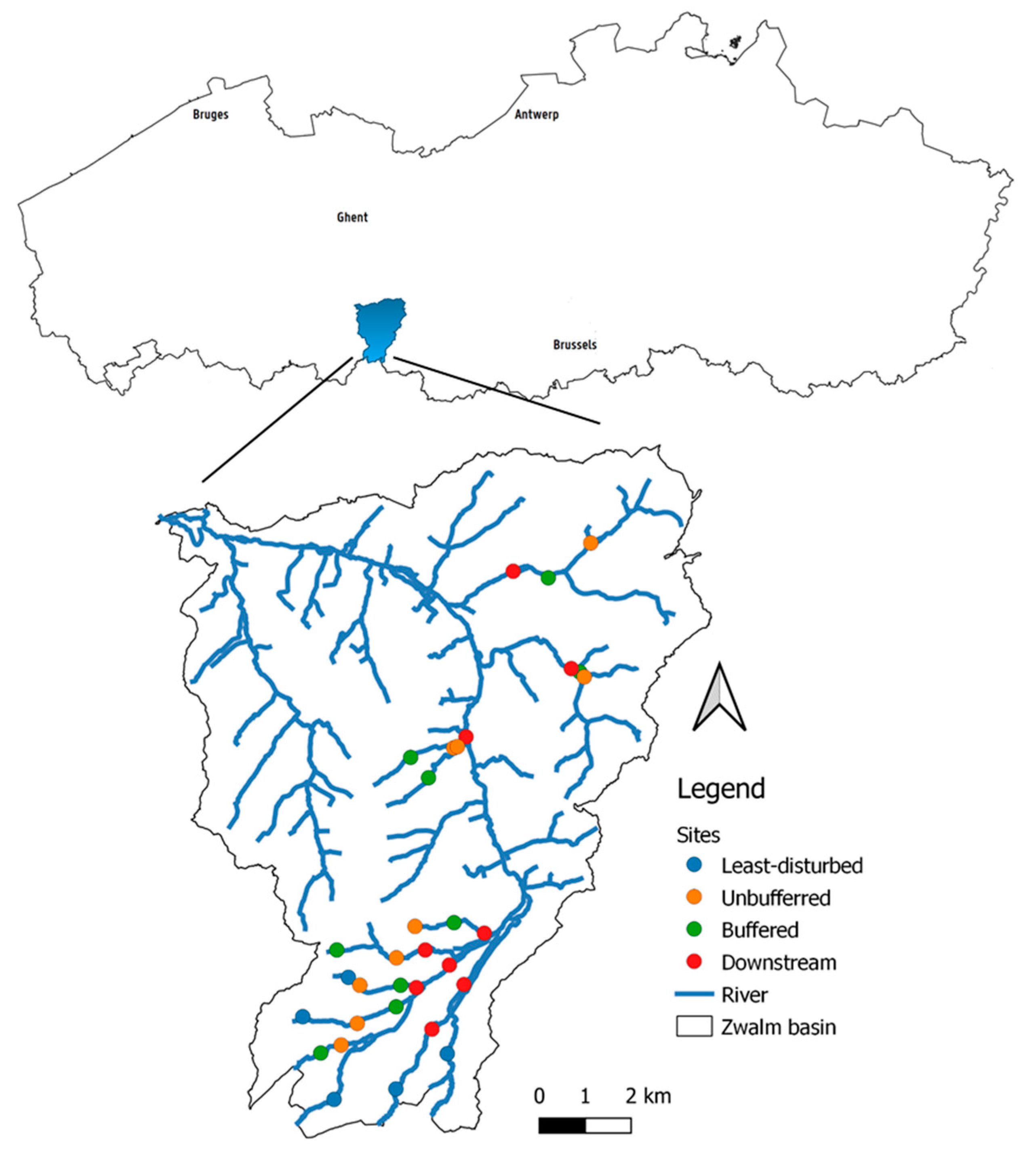
2.1. Study Area
2.2. Data Collection
2.2.1. Site Selection
2.2.2. Riparian Characterisation
2.2.3. Other Environmental Variables
2.3. Invertebrate Collection
2.4. Diversity Indices
2.5. Data Analysis
2.5.1. Riparian Attributes and Diversity Metrics: Differences between Site Types
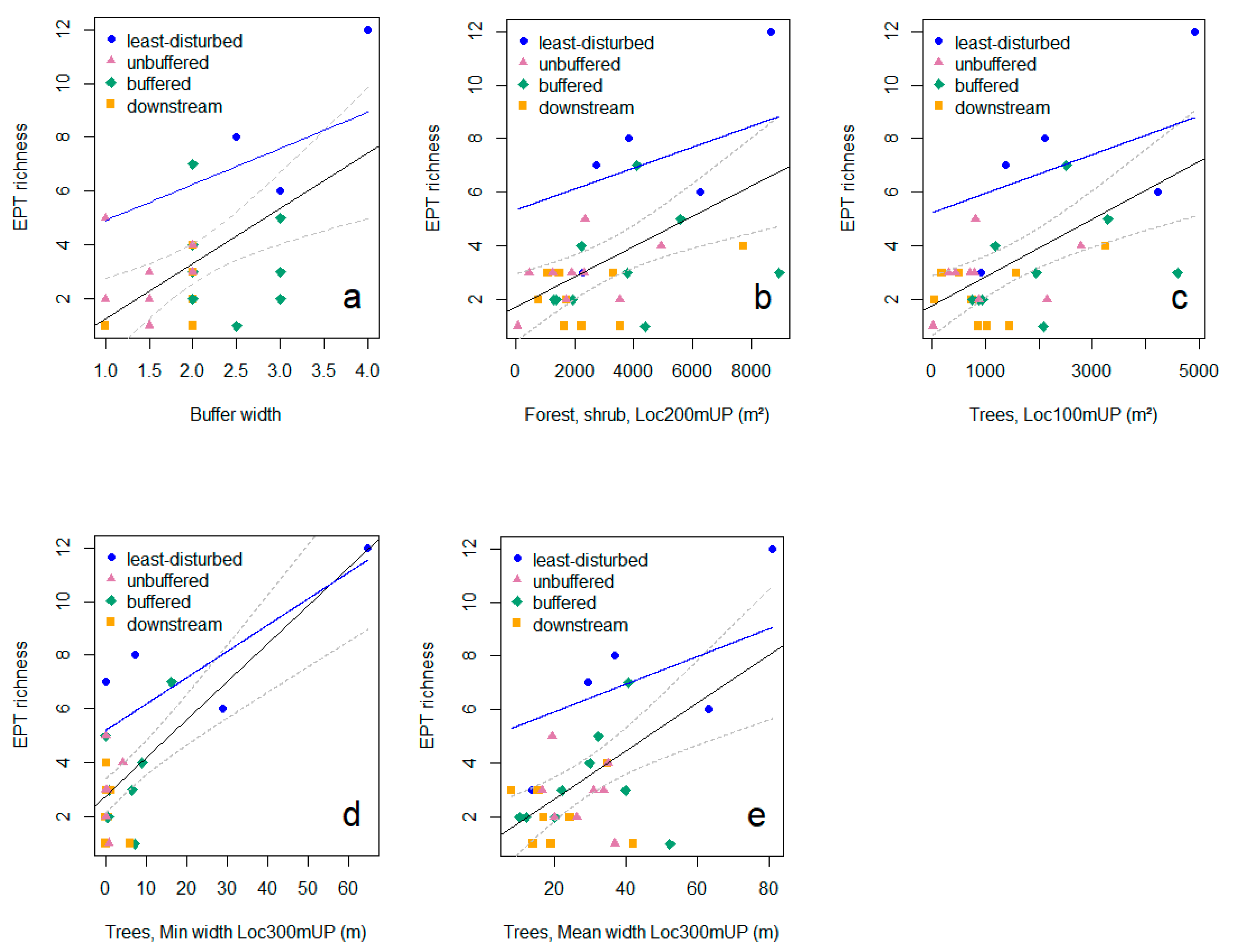
2.5.2. Relationships between Riparian Attributes and Diversity: Linear Regression Models
2.5.3. Redundancy Analysis
3. Results
3.1. Riparian Characteristics
3.1.1. Implications of Spatial Resolution and Data Collection Methods
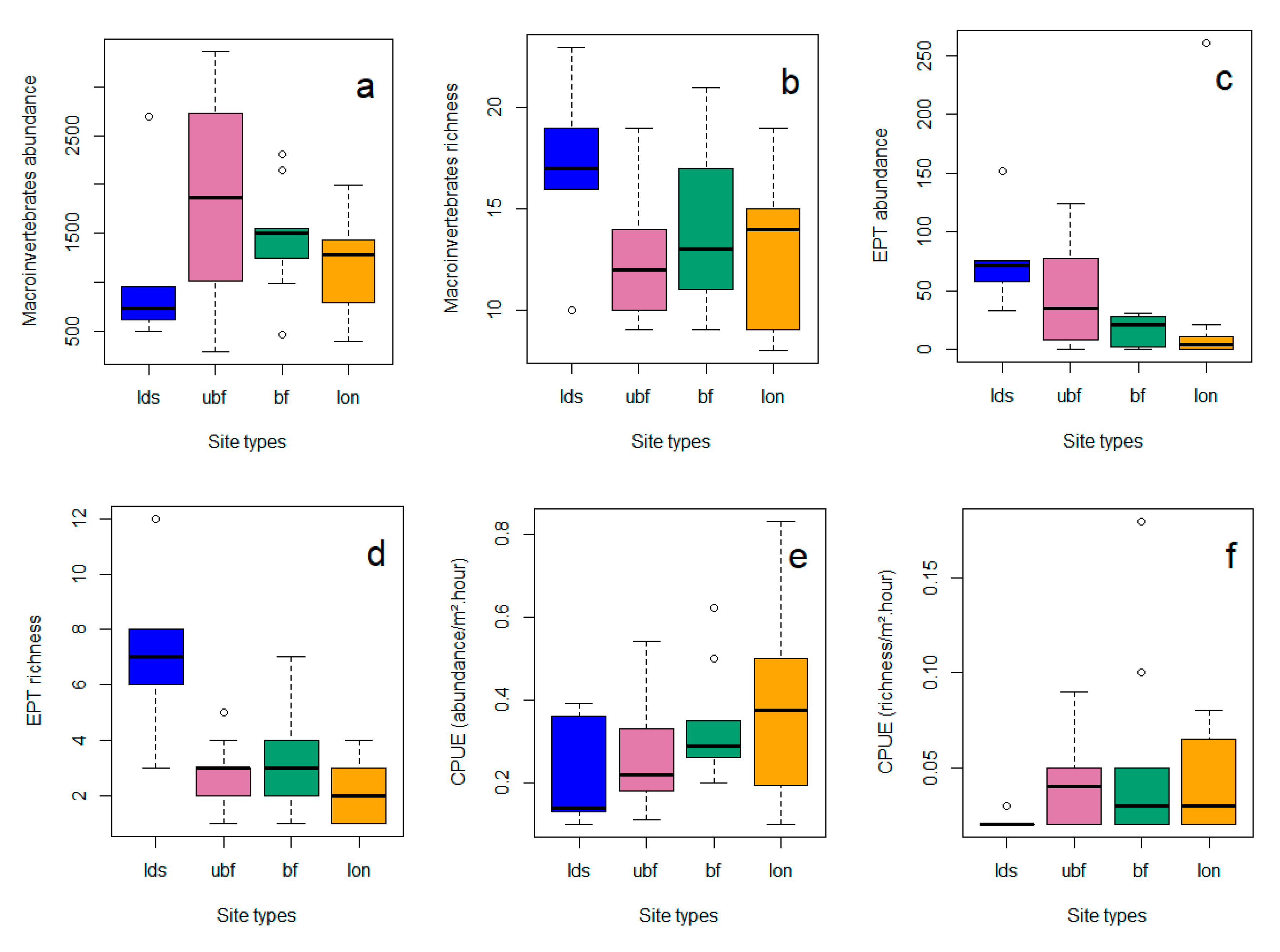
3.1.2. Differences among Site Types
3.2. Invertebrates
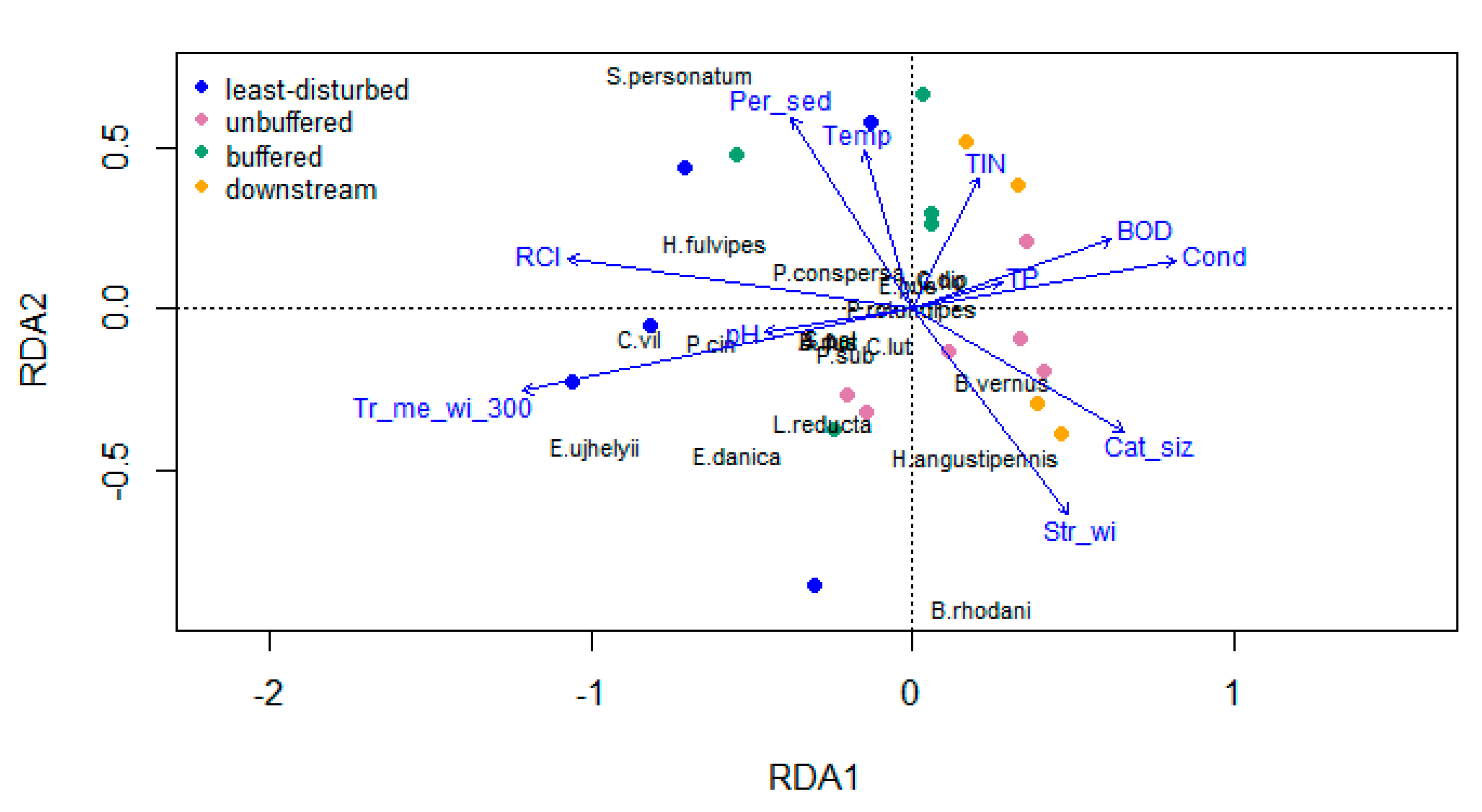
3.3. Relationships between Riparian Attributes and Invertebrate Diversity Metrics
4. Discussion
4.1. Environmental Variables and Invertebrate Diversity
4.2. Quantification of Riparian Attributes
4.3. Implications in Management and Future Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grzybowski, M.; Glińska-Lewczuk, K. Principal threats to the conservation of freshwater habitats in the continental biogeographical region of central Europe. Biodivers. Conserv. 2019, 28, 4065–4097. [Google Scholar] [CrossRef]
- Rodrigues, C.; Alves, P.; Bio, A.; Vieira, C.; Guimaraes, L.; Pinheiro, C.; Vieira, N. Assessing the ecological status of small mediterranean rivers using benthic macroinvertebrates and macrophytes as indicators. Environ. Monit. Assess. 2019, 191, 596. [Google Scholar] [CrossRef] [PubMed]
- Aarts, B.G.W.; Van den Brink, F.W.B.; Nienhuis, P.H. Habitat loss as the main cause of the slow recovery of fish faunas of regulated large rivers in Europe: The transversal floodplain gradient. River Res. Appl. 2004, 20, 3–23. [Google Scholar] [CrossRef]
- Grizzetti, B.; Pistocchi, A.; Liquete, C.; Udias, A.; Bouraoui, F.; van de Bund, W. Human pressures and ecological status of European rivers. Sci. Rep. 2017, 7, 205. [Google Scholar] [CrossRef]
- Lytle, D.A.; Poff, N.L. Adaptation to natural flow regimes. Trends Ecol. Evol. 2004, 19, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Welcomme, R.L.; Winemiller, K.O.; Cowx, I.G. Fish environmental guilds as a tool for assessment of ecological condition of rivers. River Res. Appl. 2006, 22, 377–396. [Google Scholar] [CrossRef]
- Forio, M.A.E.; Lock, K.; Radam, E.D.; Bande, M.; Asio, V.; Goethals, P. Assessment and analysis of ecological quality, macroinvertebrate communities and diversity in rivers of a multifunctional tropical island. Ecol. Indic. 2017, 77, 228–238. [Google Scholar] [CrossRef]
- Poff, N.L.; Allan, J.D. Functional-organization of stream fish assemblages in relation to hydrological variability. Ecology 1995, 76, 606–627. [Google Scholar] [CrossRef]
- Nguyen, T.; Forio, M.; Boets, P.; Lock, K.; Damanik Ambarita, M.; Suhareva, N.; Everaert, G.; Van der Heyden, C.; Dominguez-Granda, L.; Hoang, T.; et al. Threshold responses of macroinvertebrate communities to stream velocity in relation to hydropower dam: A case study from the Guayas river basin (Ecuador). Water 2018, 10, 1195. [Google Scholar] [CrossRef]
- Bayramoglu, B.; Chakir, R.; Lungarska, A. Impacts of land use and climate change on freshwater ecosystems in france. Environ. Model. Assess. 2020, 25, 147–172. [Google Scholar] [CrossRef]
- Deknock, A.; De Troyer, N.; Houbraken, M.; Dominguez-Granda, L.; Nolivos, I.; Van Echelpoel, W.; Forio, M.A.E.; Spanoghe, P.; Goethals, P. Distribution of agricultural pesticides in the freshwater environment of the Guayas river basin (Ecuador). Sci. Total Environ. 2019, 646, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Cambien, N.; Gobeyn, S.; Nolivos, I.; Forio, M.A.E.; Arias-Hidalgo, M.; Dominguez-Granda, L.; Witing, F.; Volk, M.; Goethals, P.L.M. Using the soil and water assessment tool to simulate the pesticide dynamics in the data scarce Guayas river basin, Ecuador. Water 2020, 12, 696. [Google Scholar] [CrossRef]
- Skoulikidis, N.T. The environmental state of rivers in the balkans-a review within the DPSIR framework. Sci. Total Environ. 2009, 407, 2501–2516. [Google Scholar] [CrossRef] [PubMed]
- Haygarth, P.M.; Wood, F.L.; Heathwaite, A.L.; Butler, P.J. Phosphorus dynamics observed through increasing scales in a nested headwater-to-river channel study. Sci. Total Environ. 2005, 344, 83–106. [Google Scholar] [CrossRef]
- Jiang, Y. China’s water scarcity. J. Environ. Manag. 2009, 90, 3185–3196. [Google Scholar] [CrossRef]
- Hering, D.; Johnson, R.K.; Kramm, S.; Schmutz, S.; Szoszkiewicz, K.; Verdonschot, P.F.M. Assessment of european streams with diatoms, macrophytes, macroinvertebrates and fish: A comparative metric-based analysis of organism response to stress. Freshw. Biol. 2006, 51, 1757–1785. [Google Scholar] [CrossRef]
- Mercado-Garcia, D.; Wyseure, G.; Goethals, P. Freshwater ecosystem services in mining regions: Modelling options for policy development support. Water 2018, 10, 531. [Google Scholar] [CrossRef]
- Wang, Q.R.; Kim, D.; Dionysiou, D.D.; Sorial, G.A.; Timberlake, D. Sources and remediation for mercury contamination in aquatic systems - a literature review. Environ. Pollut. 2004, 131, 323–336. [Google Scholar] [CrossRef]
- Clements, W.H.; Carlisle, D.M.; Lazorchak, J.M.; Johnson, P.C. Heavy metals structure benthic communities in colorado mountain streams. Ecol. Appl. 2000, 10, 626–638. [Google Scholar] [CrossRef]
- Forio, M.A.E.; Villa-Cox, G.; Van Echelpoel, W.; Ryckebusch, H.; Lock, K.; Spanoghe, P.; Deknock, A.; De Troyer, N.; Nolivos-Alvarez, I.; Dominguez-Granda, L.; et al. Bayesian belief network models as trade-off tools of ecosystem services in the Guayas river basin in Ecuador. Ecosyst. Serv. 2020, 44, 101124. [Google Scholar] [CrossRef]
- Wohl, E.; Angermeier, P.L.; Bledsoe, B.; Kondolf, G.M.; MacDonnell, L.; Merritt, D.M.; Palmer, M.A.; Poff, N.L.; Tarboton, D. River restoration. Water Resour. Res. 2005, 41, W10301. [Google Scholar] [CrossRef]
- Edwards, A.M.C.; Freestone, R.J.; Crockett, C.P. River management in the humber catchment. Sci. Total Environ. 1997, 194, 235–246. [Google Scholar] [CrossRef]
- Lock, K.; Asenova, M.; Goethals, P.L.M. Benthic macroinvertebrates as indicators of the water quality in Bulgaria: A case-study in the Iskar river basin. Limnol. Ecol. Manag. Inland Waters 2011, 41, 334–338. [Google Scholar] [CrossRef]
- Okumah, M.; Chapman, J.P.; Martin-Ortega, J.; Novo, P. Mitigating agricultural diffuse pollution: Uncovering the evidence base of the awareness–behaviour–water quality pathway. Water 2019, 11, 29. [Google Scholar] [CrossRef]
- Cole, L.J.; Stockan, J.; Helliwell, R. Managing riparian buffer strips to optimise ecosystem services: A review. Agric. Ecosyst. Environ. 2020, 296, 106891. [Google Scholar] [CrossRef]
- Dosskey, M.G.; Vidon, P.; Gurwick, N.P.; Allan, C.J.; Duval, T.P.; Lowrance, R. The role of riparian vegetation in protecting and improving chemical water quality in streams1. Jawra J. Am. Water Resour. Assoc. 2010, 46, 261–277. [Google Scholar] [CrossRef]
- Knight, K.W.; Schultz, R.C.; Mabry, C.M.; Isenhart, T.M. Ability of remnant riparian forests, with and without grass filters, to buffer concentrated surface runoff1. Jawra J. Am. Water Resour. Assoc. 2010, 46, 311–322. [Google Scholar] [CrossRef]
- Dosskey, M.G. Toward quantifying water pollution abatement in response to installing buffers on crop land. Environ. Manag. 2001, 28, 577–598. [Google Scholar] [CrossRef]
- Lowrance, R.; Altier, L.S.; Newbold, J.D.; Schnabel, R.R.; Groffman, P.M.; Denver, J.M.; Correll, D.L.; Gilliam, J.W.; Robinson, J.L.; Brinsfield, R.B.; et al. Water quality functions of riparian forest buffers in Chesapeake bay watersheds. Environ. Manag. 1997, 21, 687–712. [Google Scholar] [CrossRef]
- Osborne, L.L.; Kovacic, D.A. Riparian vegetated buffer strips in water-quality restoration and stream management. Freshw. Biol. 1993, 29, 243–258. [Google Scholar] [CrossRef]
- Raitif, J.; Plantegenest, M.; Roussel, J.-M. From stream to land: Ecosystem services provided by stream insects to agriculture. Agric. Ecosyst. Environ. 2019, 270–271, 32–40. [Google Scholar] [CrossRef]
- Spooner, P.; Lunt, I.; Robinson, W. Is fencing enough? The short-term effects of stock exclusion in remnant grassy woodlands in southern NSW. Ecol. Manag. Restor. 2002, 3, 117–126. [Google Scholar] [CrossRef]
- Maritz, B.; Alexander, G.J. Herpetofaunal utilisation of riparian buffer zones in an agricultural landscape near Mtunzini, South Africa. Afr. J. Herpetol. 2007, 56, 163–169. [Google Scholar] [CrossRef]
- McCracken, D.I.; Cole, L.J.; Harrison, W.; Robertson, D. Improving the farmland biodiversity value of riparian buffer strips: Conflicts and compromises. J. Env. Qual. 2012, 41, 355–363. [Google Scholar] [CrossRef]
- Gilbert, S.; Norrdahl, K.; Tuomisto, H.; Söderman, G.; Rinne, V.; Huusela-Veistola, E. Reverse influence of riparian buffer width on herbivorous and predatory Hemiptera. J. Appl. Entomol. 2015, 139, 539–552. [Google Scholar] [CrossRef]
- Cole, L.J.; Brocklehurst, S.; Elston, D.A.; McCracken, D.I. Riparian field margins: Can they enhance the functional structure of ground beetle (Coleoptera: Carabidae) assemblages in intensively managed grassland landscapes? J. Appl. Ecol. 2012, 49, 1384–1395. [Google Scholar] [CrossRef]
- Cole, L.J.; Brocklehurst, S.; Robertson, D.; Harrison, W.; McCracken, D.I. Riparian buffer strips: Their role in the conservation of insect pollinators in intensive grassland systems. Agric. Ecosyst. Environ. 2015, 211, 207–220. [Google Scholar] [CrossRef]
- Gericke, A.; Nguyen, H.H.; Fischer, P.; Kail, J.; Venohr, M. Deriving a bayesian network to assess the retention efficacy of riparian buffer zones. Water 2020, 12, 617. [Google Scholar] [CrossRef]
- Cole, L.J.; Brocklehurst, S.; McCracken, D.I.; Harrison, W.; Robertson, D. Riparian field margins: Their potential to enhance biodiversity in intensively managed grasslands. Insect Conserv. Divers. 2012, 5, 86–94. [Google Scholar] [CrossRef]
- Parkyn, S.M.; Davies-Colley, R.J.; Halliday, N.J.; Costley, K.J.; Croker, G.F. Planted riparian buffer zones in New Zealand: Do they live up to expectations? Restor. Ecol. 2003, 11, 436–447. [Google Scholar] [CrossRef]
- Grunblatt, J.; Meyer, B.E.; Wipfli, M.S. Invertebrate prey contributions to juvenile coho salmon diet from riparian habitats along three alaska streams: Implications for environmental change. J. Freshw. Ecol. 2019, 34, 617–631. [Google Scholar] [CrossRef]
- Braun, B.M.; Pires, M.M.; Stenert, C.; Maltchik, L.; Kotzian, C.B. Effects of riparian vegetation width and substrate type on riffle beetle community structure. Entomol. Sci. 2018, 21, 66–75. [Google Scholar] [CrossRef]
- Juen, L.; Cunha, E.J.; Carvalho, F.G.; Ferreira, M.C.; Begot, T.O.; Andrade, A.L.; Shimano, Y.; Leao, H.; Pompeu, P.S.; Montag, L.F.A. Effects of oil palm plantations on the habitat structure and biota of streams in eastern Amazon. River Res. Appl. 2016, 32, 2081–2094. [Google Scholar] [CrossRef]
- Castelle, A.J.; Johnson, A.W.; Conolly, C. Wetland and stream buffer size requirements—A review. J. Environ. Qual. 1994, 23, 878–882. [Google Scholar] [CrossRef]
- Urban, M.C.; Skelly, D.K.; Burchsted, D.; Price, W.; Lowry, S. Stream communities across a rural-urban landscape gradient. Divers. Distrib. 2006, 12, 337–350. [Google Scholar] [CrossRef]
- Burdon, J.F.; Ramberg, E.; Sargac, J.; Forio, M.A.E.; de Saeyer, N.; Mutinova, T.P.; Moe, F.T.; Pavelescu, O.M.; Dinu, V.; Cazacu, C.; et al. Assessing the benefits of forested riparian zones: A qualitative index of riparian integrity is positively associated with ecological status in European streams. Water 2020, 12, 1178. [Google Scholar] [CrossRef]
- Oldén, A.; Peura, M.; Saine, S.; Kotiaho, J.S.; Halme, P. The effect of buffer strip width and selective logging on riparian forest microclimate. For. Ecol. Manag. 2019, 453, 117623. [Google Scholar] [CrossRef]
- Pissarra, T.C.T.; Valera, C.A.; Costa, R.C.A.; Siqueira, H.E.; Martins, M.V.; do Valle, R.F.; Fernandes, L.F.S.; Pacheco, F.A.L. A regression model of stream water quality based on interactions between landscape composition and riparian buffer width in small catchments. Water 2019, 11, 1757. [Google Scholar] [CrossRef]
- Lock, K.; Goethals, P.L.M. Predicting the occurrence of stoneflies (Plecoptera) on the basis of water characteristics, river morphology and land use. J. Hydroinform. 2014, 16, 812–821. [Google Scholar] [CrossRef]
- Lock, K.; Goethals, P.L.M. Habitat suitability modelling for mayflies (Ephemeroptera) in Flanders (Belgium). Ecol. Inform. 2013, 17, 30–35. [Google Scholar] [CrossRef]
- Lock, K.; Goethals, P.L.M. Distribution and ecology of the caddisflies (Trichoptera) of Flanders (Belgium). Ann. Limnol. Int. J. Limnol. 2012, 48, 31–37. [Google Scholar] [CrossRef]
- Jacobus, L.M.; Macadam, C.R.; Sartori, M. Mayflies (Ephemeroptera) and their contributions to ecosystem services. Insects 2019, 10, 170. [Google Scholar] [CrossRef]
- Morse, J.C.; Frandsen, P.B.; Graf, W.; Thomas, J.A. Diversity and ecosystem services of Trichoptera. Insects 2019, 10, 125. [Google Scholar] [CrossRef]
- DeWalt, R.E.; Ower, G.D. Ecosystem services, global diversity, and rate of stonefly species descriptions (insecta: Plecoptera). Insects 2019, 10, 99. [Google Scholar] [CrossRef]
- Dedecker, A.P.; Goethals, P.L.; De Pauw, N. Comparison of artificial neural network (ANN) model development methods for prediction of macroinvertebrate communities in the Zwalm river basin in Flanders, Belgium. TheScientificWorldJournal 2002, 2, 96–104. [Google Scholar] [CrossRef][Green Version]
- Pauwels, V.R.N.; Verhoest, N.E.C.; De Troch, F.P. A metahillslope model based on an analytical solution to a linearized boussinesq equation for temporally variable recharge rates. Water Resour. Res. 2002, 38, 31–33. [Google Scholar] [CrossRef]
- Huygens, M.; Verhoeven, R.; De Sutter, R. Integrated river management of a small Flemish river catchment. Role Eros. Sediment. Transp. Nutr. Contam. Transf. Proc. 2000, 263, 191–199. [Google Scholar]
- Troch, P.A.; De Troch, F.P.; Brutsaert, W. Effective water table depth to describe initial conditions prior to storm rainfall in humid regions. Water Resour. Res. 1993, 29, 427–434. [Google Scholar] [CrossRef]
- Goethals, P.; Dedecker, A.; Gabriels, W.; de Pauw, N. Development and application of predictive river ecosystem models based on classification trees and artificial neural networks. In Ecological Informatics: Understanding Ecology By Biologically-Inspired Computation; Recknagel, F., Ed.; Springer: New York, NY, USA, 2003; pp. 91–107. [Google Scholar]
- Dedecker, A.P.; Goethals, P.L.M.; D’Heygere, T.; Gevrey, M.; Lek, S.; De Pauw, N. Application of artificial neural network models to analyse the relationships between gammarus pulex l. (Crustacea, Amphipoda) and river characteristics. Environ. Monit. Assess. 2005, 111, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.S.; Clapcott, J.; Quinn, J.; Hayes, J.; Joy, M.; Storey, R.; Greig, H.; Hay, J.; James, T.; Beech, M.; et al. Stream Habitat Assessment Protocols For Wadeable Rivers And Streams Of New Zealand; School of Biological Sciences, University of Canterbury: Christchurch, New Zealand, 2009. [Google Scholar]
- De Langhe, J.E.; Delvosalle, L.; Duvigneaud, J.; Lambinon, J.; Vanden Berghen, C. Flora Van België, Het Groot-Hertogdom Luxemburg, Noord-Frankrijk En De Aangrenzende Gebieden (Pteridofyten En Spermatofyten); Patrimonium van de Nationale Plantentuin van België, Meise: Meise, Belgium, 1988; p. 972. [Google Scholar]
- Agentschap Informatie Vlaanderen. Bodembedekkingskaart (BBK), 1 m Resolutie, Opname 2015; Vlaamse Overheid: Brussels, Belgium, 2019; Available online: http://www.geopunt.be/catalogus/datasetfolder/0230a22f-51c0-4aa5-bb5d-0d7eeeaf0ce8 (accessed on 1 October 2019).
- European Union; Copernicus Land Monitoring Service 2018; European Economic Area (EEA). Copernicus Tree Cover Density 2015. European Commission Joint Research Centre (JRC). 2020. Available online: https://land.copernicus.eu/pan-european/high-resolution-layers/forests/tree-cover-density/status-maps/2015?tab=mapview (accessed on 2 October 2019).
- Agentschap Informatie Vlaanderen. Digitaal Hoogtemodel Vlaanderen II, DTM, Raster, 5 m; Vlaamse Overheid: Brussels, Belgium, 2014; Available online: http://www.geopunt.be/catalogus/datasetfolder/9b0f82c7-57c4-463a-8918-432e41a66355 (accessed on 15 January 2019).
- Forio, M.A.E.; Goethals, P.L.M. An integrated approach of multi-community monitoring and assessment of aquatic ecosystems to support sustainable development. Sustainability 2020, 12, 5603. [Google Scholar] [CrossRef]
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Burdon, F.J.; Harding, J.S. The linkage between riparian predators and aquatic insects across a stream-resource spectrum. Freshw. Biol. 2008, 53, 330–346. [Google Scholar] [CrossRef]
- Nilsson, A. Aquatic Insects Of North Europe. A Taxonomic Handbook. Vol. 1: Ephemeroptera, Plecoptera, Heteroptera, Neuroptera, Megaloptera, Coleoptera, Trichoptera, Lepidoptera; Apollo Books: Stenstrup, Denmark, 1996; p. 274. [Google Scholar]
- Nilsson, A. Aquatic Insects Of North Europe. A Taxonomic Handbook. Vol. 2: Odonata, Diptera; Apollo Books: Stenstrup, Denmark, 1997; p. 440. [Google Scholar]
- de Pauw, N.; Vannevel, R. Macro-Invertebraten En Waterkwaliteit. Determineersleutels Voor Zoetwatermacro-Invertebraten En Methoden Ter Bepaling Van De Waterkwaliteit; Stichting Leefmilieu: Antwerp, Belgium, 1991; p. 316. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory Of Communication; University of Illinois Press: Urbana, IL, USA, 1949; p. 117. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Margalef, R. Information theory in ecology. Int. J. Gen. Syst. 1958, 3, 36–71. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models And Extensions Ion Ecology With R; Springer Science & Business Media, LLC.: New York, NY, USA, 2009. [Google Scholar]
- Dickman, P.W.; Sloggett, A.; Hills, M.; Hakulinen, T. Regression models for relative survival. Stat. Med. 2004, 23, 51–64. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 7 January 2019).
- Ter Braak, C.J.F.; Prentice, C. A theory of gradient analysis. In Advances in Ecological Research Vol. 34; Caswell, H., Ed.; Elsiever Academic Press: Cambridge, MA, USA, 2004; pp. 266–282. [Google Scholar]
- Buttigieg, P.L.; Ramette, A. A guide to statistical analysis in microbial ecology: A community-focused, living review of multivariate data analyses. FEMS Microbiol. Ecol. 2014, 90, 543–550. [Google Scholar] [CrossRef]
- Hair, J.F.; Anderson, R.E.; Babin, B.J.; Black, W.C. Multivariate Data Analysis: A Global Perspective; Pearson Education, Inc.: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Jari Oksanen, F.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-2. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 June 2020).
- Rios, S.L.; Bailey, R.C. Relationship between riparian vegetation and stream benthic communities at three spatial scales. Hydrobiologia 2006, 553, 153–160. [Google Scholar] [CrossRef]
- Death, R.G.; Collier, K.J. Measuring stream macroinvertebrate responses to gradients of vegetation cover: When is enough enough? Freshw. Biol. 2010, 55, 1447–1464. [Google Scholar] [CrossRef]
- Vought, L.B.M.; Pinay, G.; Fuglsang, A.; Ruffinoni, C. Structure and function of buffer strips from a water-quality perspective in agricultural landscapes. Landsc. Urban. Plan. 1995, 31, 323–331. [Google Scholar] [CrossRef]
- Clinnick, P.F. Buffer strip management in forest operations: A review. Aust. For. 1985, 48, 34–45. [Google Scholar] [CrossRef]
- Quinn, J.M.; Boothroyd, I.K.G.; Smith, B.J. Riparian buffers mitigate effects of pine plantation logging on New Zealand streams: 2. Invertebrate communities. For. Ecol. Manag. 2004, 191, 129–146. [Google Scholar] [CrossRef]
- Collier, K.J.; Smith, B.J. Dispersal of adult caddisflies (Trichoptera) into forests alongside three New Zealand streams. Hydrobiologia 1997, 361, 53–65. [Google Scholar] [CrossRef]
- Carlson, P.E.; McKie, B.G.; Sandin, L.; Johnson, R.K. Strong land-use effects on the dispersal patterns of adult stream insects: Implications for transfers of aquatic subsidies to terrestrial consumers. Freshw. Biol. 2016, 61, 848–861. [Google Scholar] [CrossRef]
- Hering, D.; Plachter, H. Riparian ground beetles (Coeloptera, Carabidae) preying on aquatic invertebrates: A feeding strategy in alpine floodplains. Oecologia 1997, 111, 261–270. [Google Scholar] [CrossRef]
- Samways, M.J.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Solutions for humanity on how to conserve insects. Biol. Conserv. 2020, 242, 108427. [Google Scholar] [CrossRef]
- Collier, K.J.; Smith, B.J. Interactions of adult stoneflies (Plecoptera) with riparian zones I. Effects of air temperature and humidity on longevity. Aquat. Insects 2000, 22, 275–284. [Google Scholar] [CrossRef]
- Ivkovic, M.; Milisa, M.; Baranov, V.; Mihaljevic, Z. Environmental drivers of biotic traits and phenology patterns of Diptera assemblages in karst springs: The role of canopy uncovered. Limnologica 2015, 54, 44–57. [Google Scholar] [CrossRef]
- Meleason, M.A.; Quinn, J.M. Influence of riparian buffer width on air temperature at whangapoua forest, coromandel peninsula, New Zealand. For. Ecol. Manag. 2004, 191, 365–371. [Google Scholar] [CrossRef]
- Jerves-Cobo, R.; Everaert, G.; Iñiguez-Vela, X.; Córdova-Vela, G.; Díaz-Granda, C.; Cisneros, F.; Nopens, I.; Goethals, P. A methodology to model environmental preferences of EPT taxa in the Machangara river basin (Ecuador). Water 2017, 9, 195. [Google Scholar] [CrossRef]
- Ab Hamid, S.; Md Rawi, C.S. Application of aquatic insects (ephemeroptera, plecoptera and trichoptera) in water quality assessment of Malaysian headwater. Trop. Life Sci. Res. 2017, 28, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Djoudi, E.; Marie, A.; Mangenot, A.; Puech, C.; Aviron, S.; Plantegenest, M.; Petillon, J. Farming system and landscape characteristics differentially affect two dominant taxa of predatory arthropods. Agric. Ecosyst. Environ. 2018, 259, 98–110. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.H.; Duan, M.C.; Yu, Z.R.; Axmacher, J.C. Different response patterns of epigaeic spiders and carabid beetles to varying environmental conditions in fields and semi-natural habitats of an intensively cultivated agricultural landscape. Agric. Ecosyst. Environ. 2018, 264, 54–62. [Google Scholar] [CrossRef]
- Schirmel, J.; Thiele, J.; Entling, M.H.; Buchholz, S. Trait composition and functional diversity of spiders and carabids in linear landscape elements. Agric. Ecosyst. Environ. 2016, 235, 318–328. [Google Scholar] [CrossRef]
- Malumbres-Olarte, J.; Vink, C.J.; Ross, J.G.; Cruickshank, R.H.; Paterson, A.M. The role of habitat complexity on spider communities in native alpine grasslands of New Zealand. Insect Conserv. Divers. 2013, 6, 124–134. [Google Scholar] [CrossRef]
- Magura, T.; Tóthmérész, B.; Molnár, T. Forest edge and diversity: Carabids along forest-grassland transects. Biodivers. Conserv. 2001, 10, 287–300. [Google Scholar] [CrossRef]
- Van Echelpoel, W.; Forio, A.M.; Van der Heyden, C.; Bermúdez, R.; Ho, L.; Rosado Moncayo, M.A.; Parra Narea, N.R.; Dominguez Granda, E.L.; Sanchez, D.; Goethals, L.P. Spatial characteristics and temporal evolution of chemical and biological freshwater status as baseline assessment on the tropical island San Cristóbal (Galapagos, Ecuador). Water 2019, 11, 880. [Google Scholar] [CrossRef]
- Jerves-Cobo, R.; Forio, M.A.E.; Lock, K.; Van Butsel, J.; Pauta, G.; Cisneros, F.; Nopens, I.; Goethals, P.L.M. Biological water quality in tropical rivers during dry and rainy seasons: A model-based analysis. Ecol. Indic. 2020, 108, 105769. [Google Scholar] [CrossRef]
- Sponseller, R.A.; Benfield, E.F.; Valett, H.M. Relationships between land use, spatial scale and stream macroinvertebrate communities. Freshw. Biol. 2001, 46, 1409–1424. [Google Scholar] [CrossRef]
- Roy, A.H.; Rosemond, A.D.; Paul, M.J.; Leigh, D.S.; Wallace, J.B. Stream macroinvertebrate response to catchment urbanisation (Georgia, USA). Freshw. Biol. 2003, 48, 329–346. [Google Scholar] [CrossRef]
- European Commission. Directive 2000/60/ec of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32000L0060 (accessed on 22 October 2000).
- Mouton, A.M.; Van der Most, H.; Jeuken, A.; Goethals, P.L.M.; De Pauw, N. Evaluation of river basin restoration options by the application of the water framework directive explorer in the Zwalm river basin (Flanders, Belgium). River Res. Appl. 2009, 25, 82–97. [Google Scholar] [CrossRef]
- Stutter, M.; Kronvang, B.; Ó hUallacháin, D.; Rozemeijer, J. Current insights into the effectiveness of riparian management, attainment of multiple benefits, and potential technical enhancements. J. Environ. Qual. 2019, 48, 236–247. [Google Scholar] [CrossRef]
| Spatial Units | Loc100mUP, Units | Loc200mUP, Units | Loc300mUP, Units | RipCatch100, Units | GIS Source |
|---|---|---|---|---|---|
| Land use | |||||
| Agricultural, forest and shrub Pasture and grassland Urban and industrial | x, m2 | x, m2 | x, % | x, % | BKK |
| Wetland and waterbodies | x, % | x, % | BKK | ||
| Tree cover | |||||
| Tree cover area | x, % | x, % | Copernicus | ||
| Tree cover density | x, % | x, % | Copernicus | ||
| Width of each riparian land use (agricultural, forest, pasture and urban) | |||||
| Min. width | x, m | x, m | x, m | BKK | |
| Mean width | x, m | x, m | x, m | BKK | |
| Distance between all riparian forest blocks upstream of a sampling site | |||||
| Distance between 100 m forest blocks | x, m | BKK | |||
| Distance between 50 m forest blocks | x, m | BKK | |||
| Distance between 25 m forest blocks | x, m | BKK | |||
| Indices | Equation | No. |
|---|---|---|
| % Insect | 1 | |
| Shannon–Wiener index (H′) | 2 | |
| Simpson’s index (D) | 3 | |
| Pielou′s evenness index (J′) | 4 | |
| Margalef diversity index (d) | 5 |
| Riparian Attributes | Abundance Inv 1 | Richness Inv 1 | Abundance EPT 2 | Richness EPT 2 | Richness Insects | % Insects | Shannon–Wiener | Margalef | Pielou’s Evenness Index | CPUE (Richness) |
|---|---|---|---|---|---|---|---|---|---|---|
| Quick Assessment | ||||||||||
| Adjacent groundcover | ↑ | ↑ | ||||||||
| Buffer groundcover | ↑ | ↑ | ||||||||
| Buffer width | ↑↑ | |||||||||
| Soil drainage | ↑ | ↑ | ↑ | ↑ | ||||||
| Riparian Condition Index (RCI) | ↑↑ | |||||||||
| Quantitative assessment | ||||||||||
| Unmanaged grass (%) | ↑ | ↑ | ↑ | |||||||
| Managed grass (%) | ↑↓ | |||||||||
| Quantification based on GIS (spatial units) | ||||||||||
| Local riparian attributes | ||||||||||
| Forest 3, shrub (Loc100mUP) (m2) | ↑↑ | ↑↑ | ||||||||
| Forest, shrub (Loc300mUP) (%) | ↑ | ↑↑ | ↑ | ↑ | ||||||
| Local riparian width attributes | ||||||||||
| Forest, shrub mean width (Loc100mUP) | ↑ | ↑↑ | ||||||||
| Forest, shrub mean width (Loc300mUP) | ↑↑ | ↑ | ↑ | |||||||
| Forest, shrub min. width (Loc100mUP) | ↑↑ | |||||||||
| Forest, shrub min. width (Loc300mUP) | ↑↑ | ↑ | ↑ | |||||||
| Full riparian corridor attributes | ||||||||||
| Forest, shrub (RipCatch100m) | ↑ | ↑↑ | ↑ | ↑ | ||||||
| Near distance to 25 m ForestBlocks (RipCatch100m) | ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forio, M.A.E.; De Troyer, N.; Lock, K.; Witing, F.; Baert, L.; Saeyer, N.D.; Rîșnoveanu, G.; Popescu, C.; Burdon, F.J.; Kupilas, B.; et al. Small Patches of Riparian Woody Vegetation Enhance Biodiversity of Invertebrates. Water 2020, 12, 3070. https://doi.org/10.3390/w12113070
Forio MAE, De Troyer N, Lock K, Witing F, Baert L, Saeyer ND, Rîșnoveanu G, Popescu C, Burdon FJ, Kupilas B, et al. Small Patches of Riparian Woody Vegetation Enhance Biodiversity of Invertebrates. Water. 2020; 12(11):3070. https://doi.org/10.3390/w12113070
Chicago/Turabian StyleForio, Marie Anne Eurie, Niels De Troyer, Koen Lock, Felix Witing, Lotte Baert, Nancy De Saeyer, Geta Rîșnoveanu, Cristina Popescu, Francis J. Burdon, Benjamin Kupilas, and et al. 2020. "Small Patches of Riparian Woody Vegetation Enhance Biodiversity of Invertebrates" Water 12, no. 11: 3070. https://doi.org/10.3390/w12113070
APA StyleForio, M. A. E., De Troyer, N., Lock, K., Witing, F., Baert, L., Saeyer, N. D., Rîșnoveanu, G., Popescu, C., Burdon, F. J., Kupilas, B., Friberg, N., Boets, P., Volk, M., McKie, B. G., & Goethals, P. (2020). Small Patches of Riparian Woody Vegetation Enhance Biodiversity of Invertebrates. Water, 12(11), 3070. https://doi.org/10.3390/w12113070











