High-Frequency Stable-Isotope Measurements of Evapotranspiration Partitioning in a Maize Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Stable-Isotope Method
2.2. Experimental Site
2.3. Isotope Measurements
2.4. Micro-Meteorological Instrumentation
2.5. Sap Flow Measurements
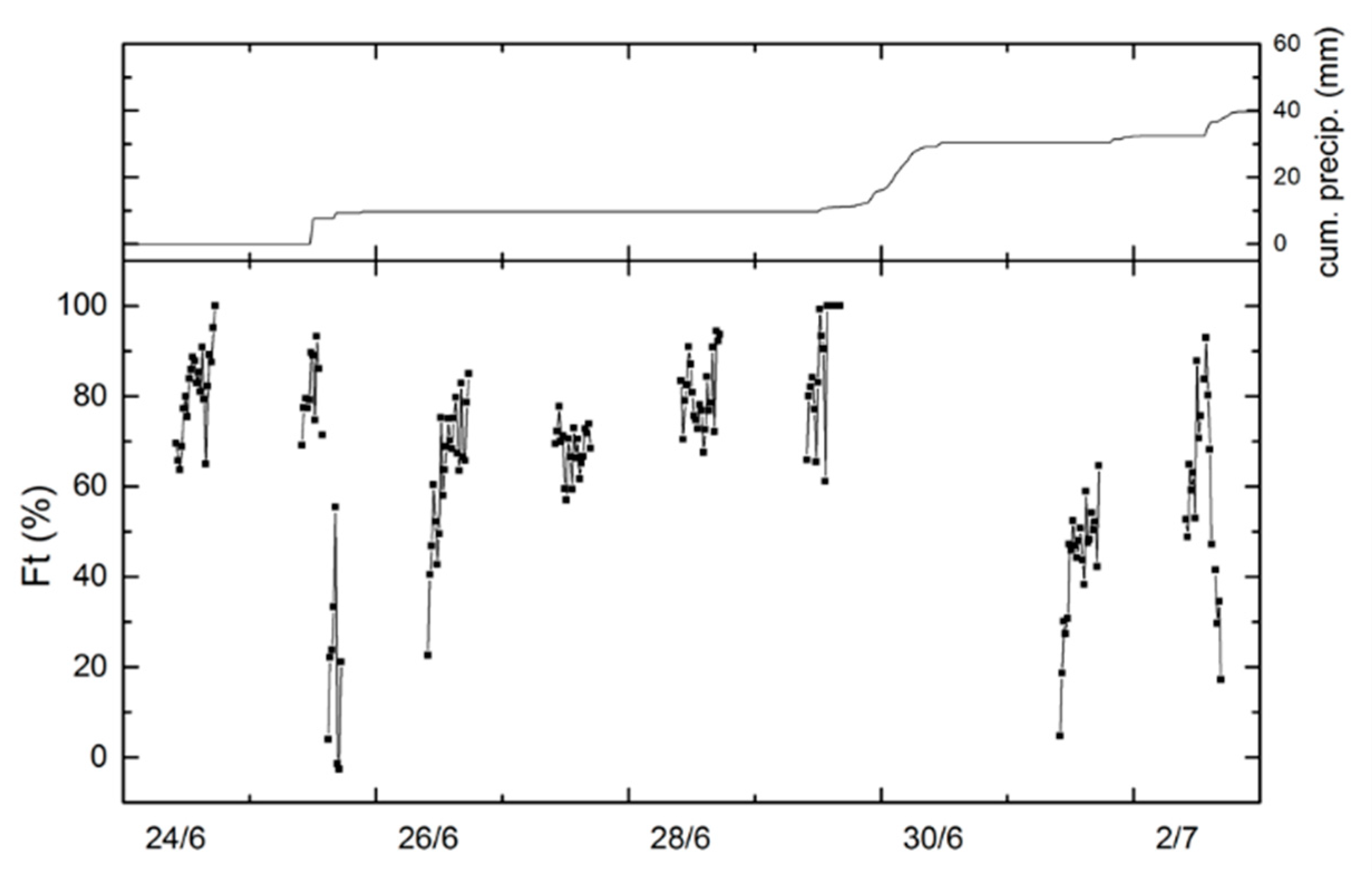
3. Results
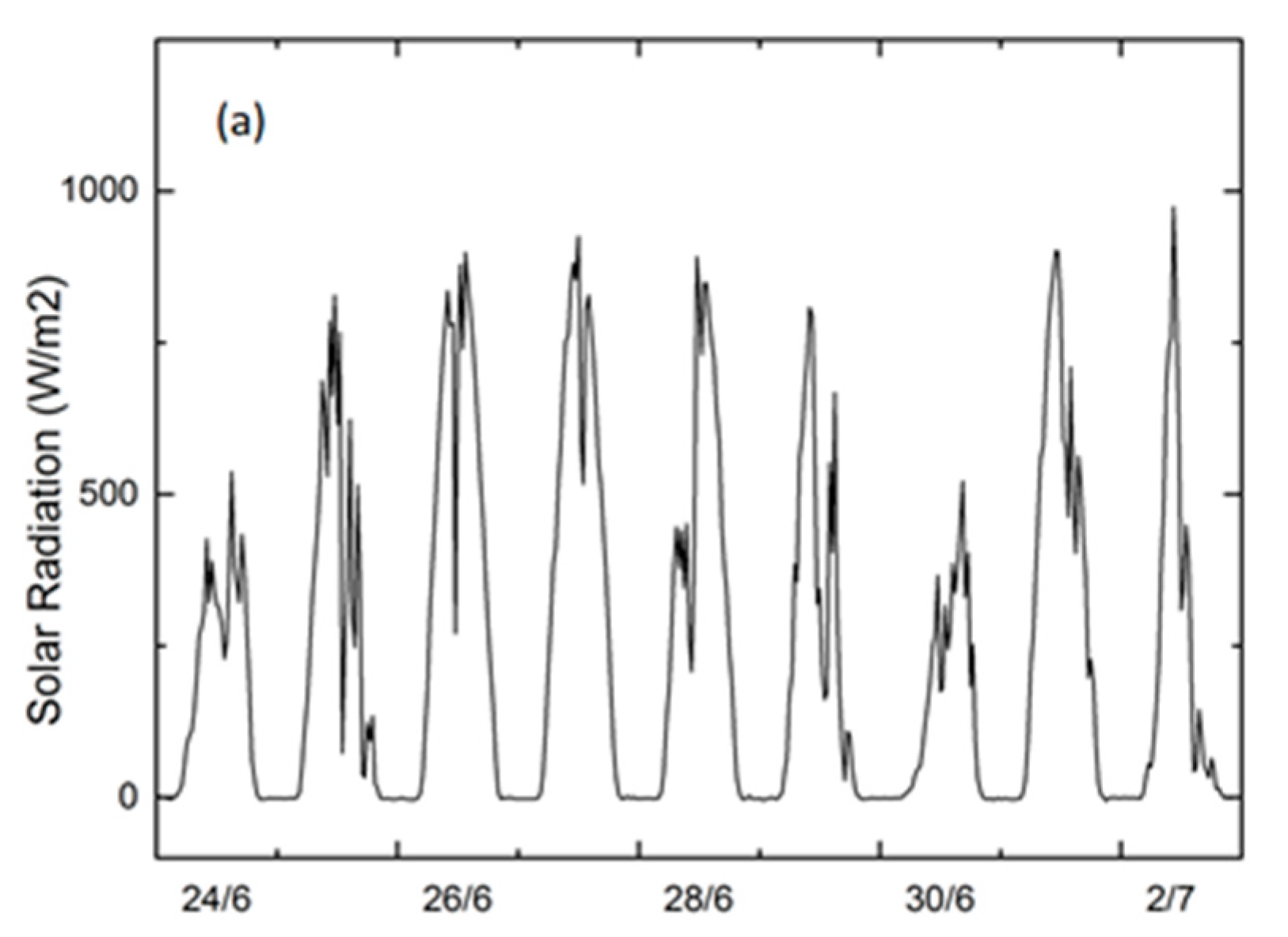
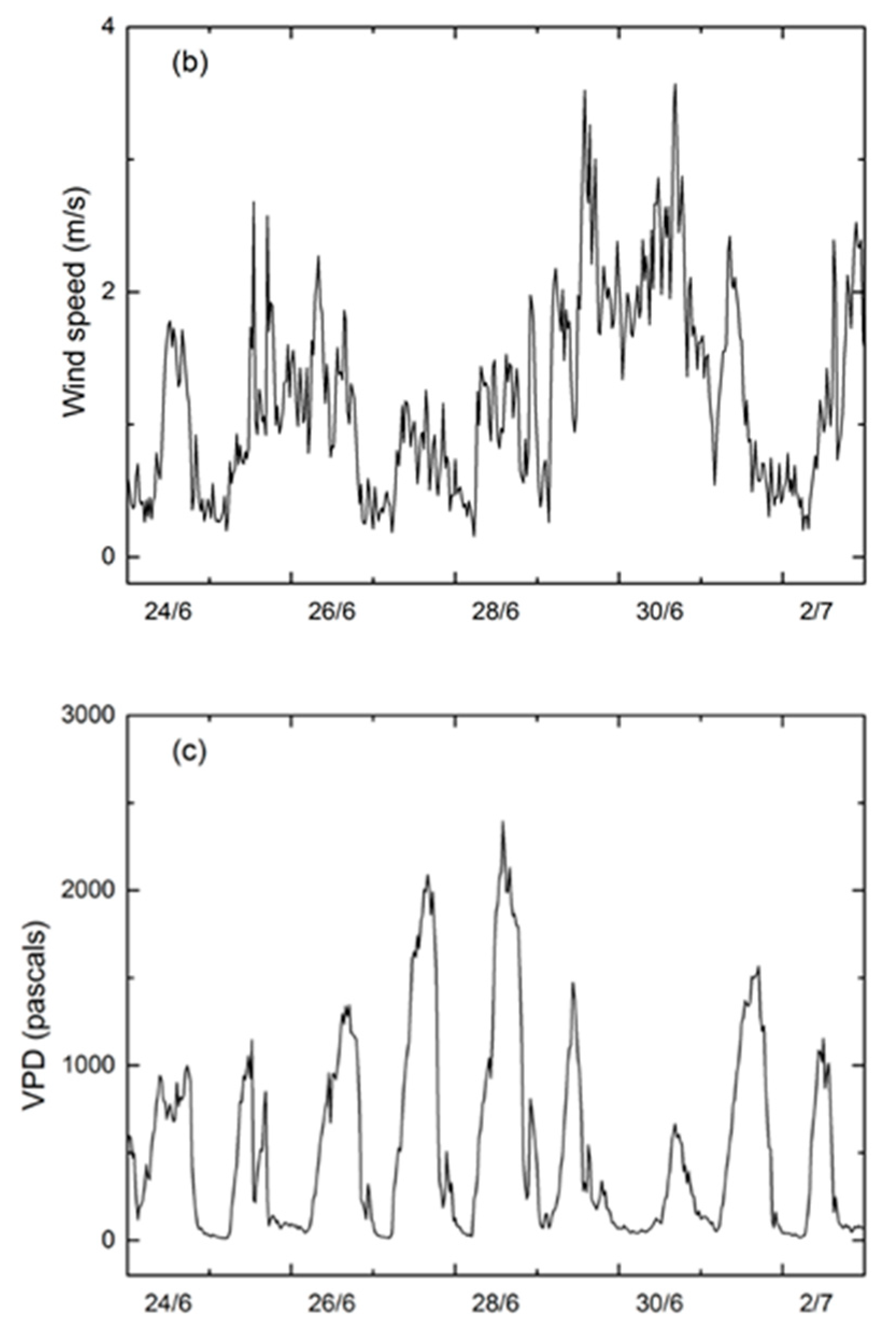
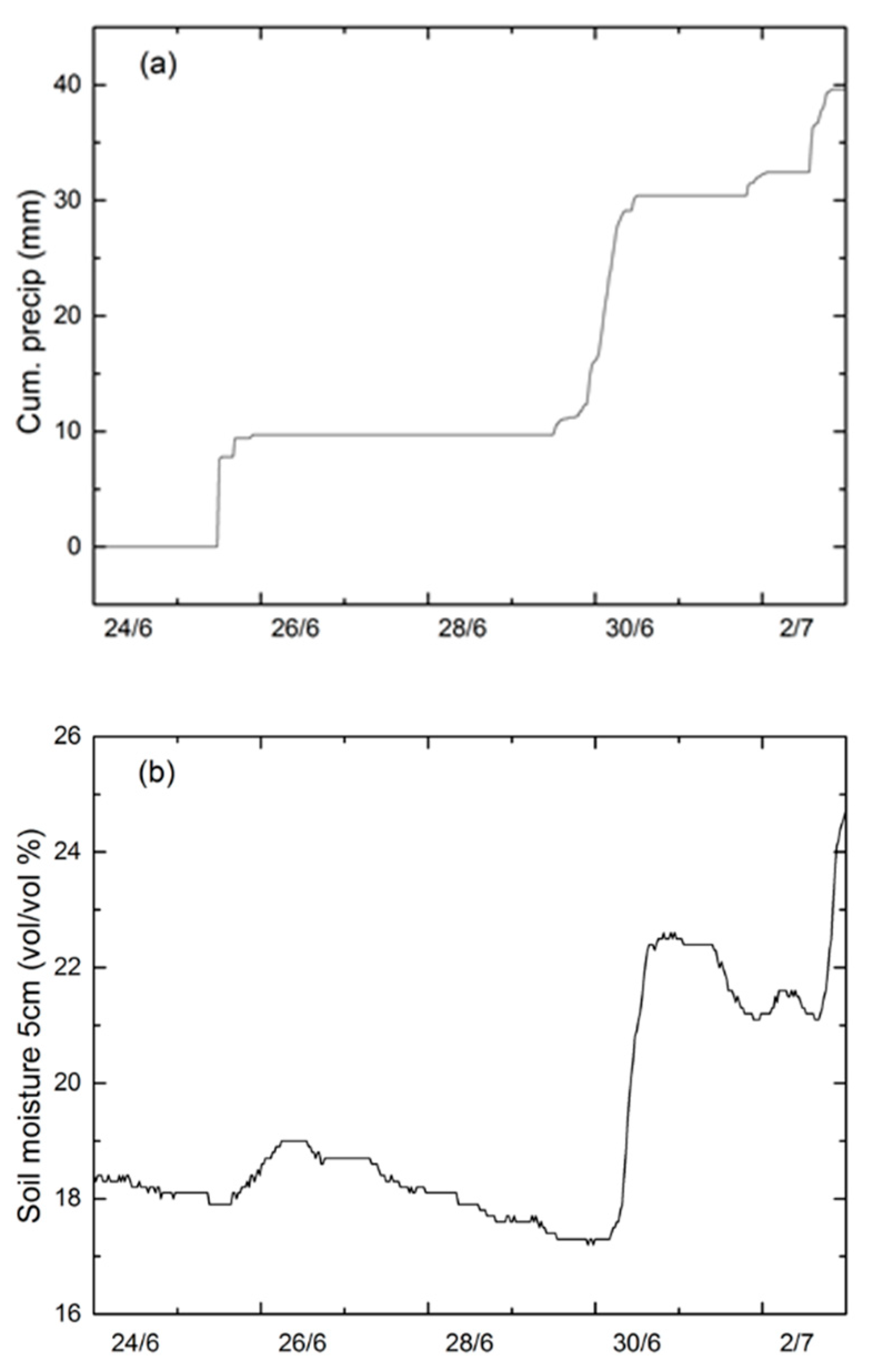
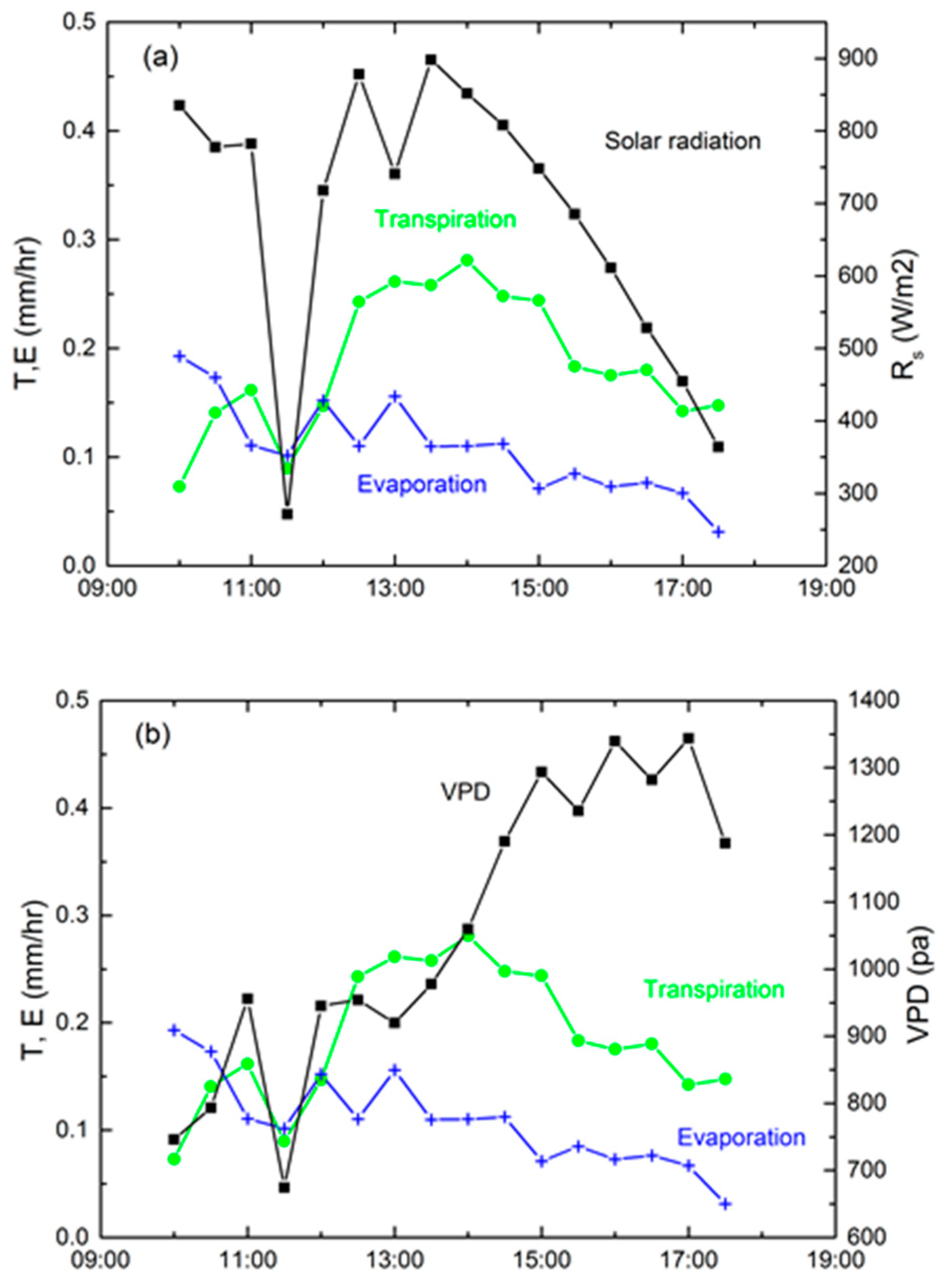
3.1. Environmental Conditions
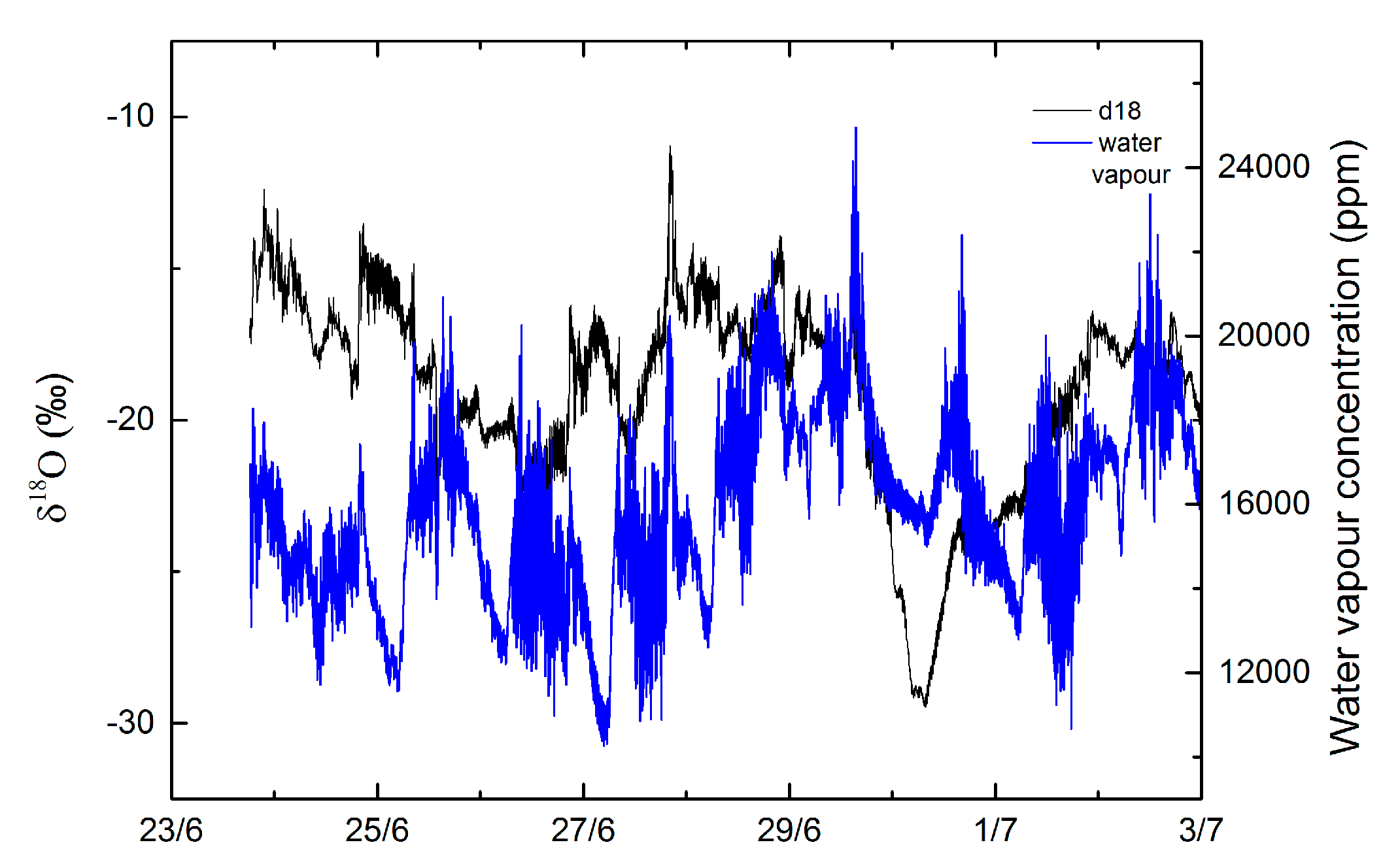
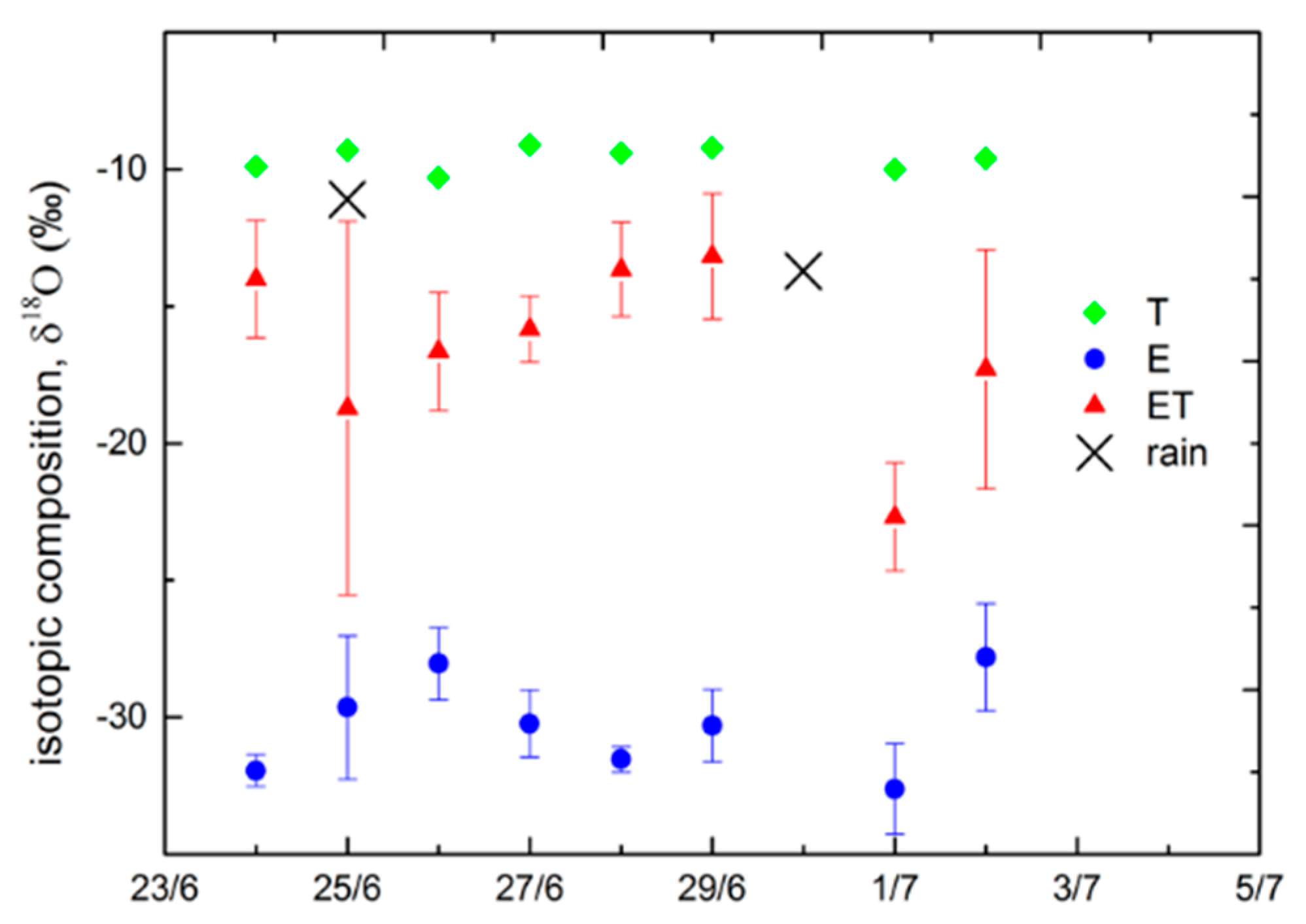
3.2. Isotope Results
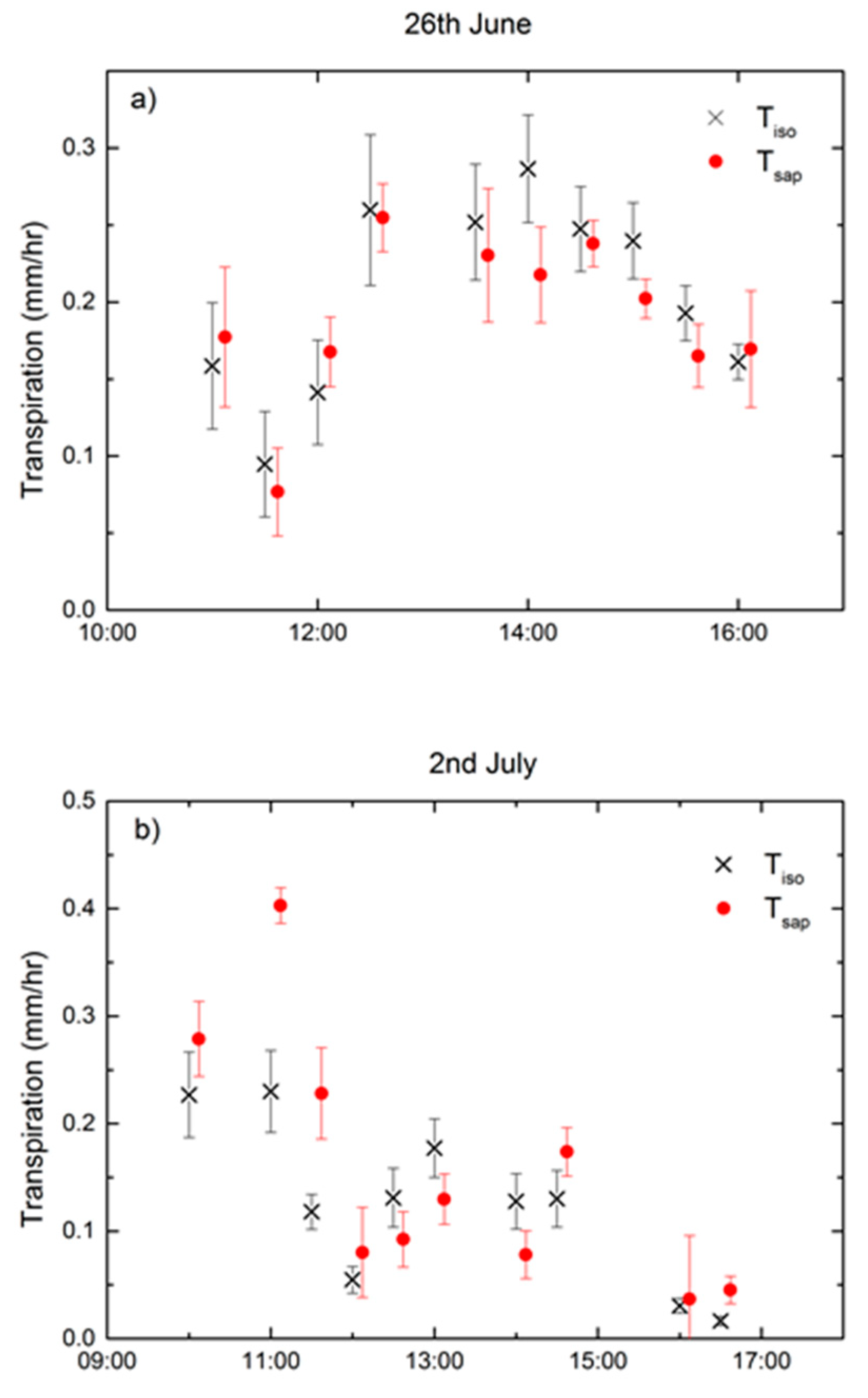
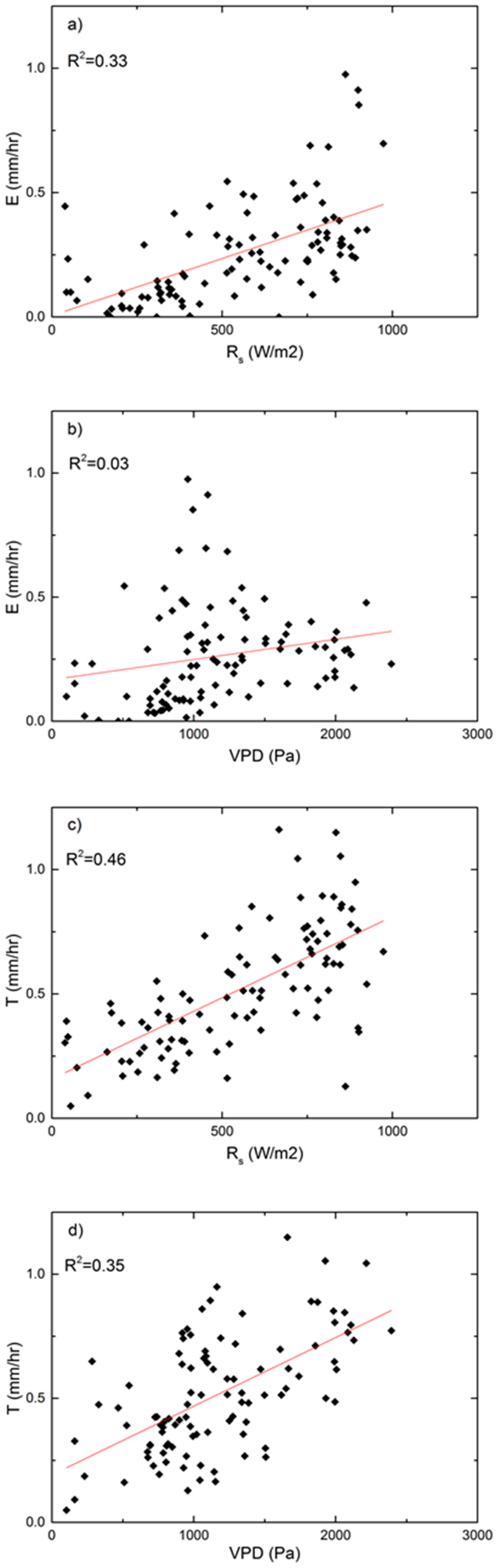
3.3. Technique Comparison and Environmental Controls
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bastiaanssen, W.G.M.; Pelgrum, H.; Wang, J.; Ma, Y.; Moreno, J.F.; Roerink, G.J.; van der, W.T. A Surface Energy Balance Algorithm for Land (SEBAL): Part 2 validation. J. Hydrol. 1998, 212, 213–229. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Y.; Sun, H.; Gates, J. Evapotranspiration and its partitioning in an irrigated winter wheat field: A combined isotopic and micrometeorologic approach. J. Hydrol. 2011, 408, 203–211. [Google Scholar] [CrossRef]
- Kelliher, F.M.; Köstner, B.M.M.; Hollinger, D.Y.; Byers, J.N.; Hunt, J.E.; McSeveny, T.M.; Meserth, R.; Weir, P.L.; Schulze, E.D. Evaporation, xylem sap flow, and tree transpiration in a New Zealand broad-leaved forest. Agric. For. Meteorol. 1992, 62, 53–73. [Google Scholar] [CrossRef]
- Klimešová, J.; Stŕedová, H.; Stŕeda, T. Maize transpiration in response to meteorological conditions. Contrib. Geophys. Geod. 2014, 43, 225–236. [Google Scholar] [CrossRef][Green Version]
- Zhou, S.; Liu, W.; Lin, W. The ratio of transpiration to evapotranspiration in a rainfed maize field on the Loess Plateau of China. Water Sci. Technol. Water Supply 2017, 17, 221–228. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Zhang, Y. Determination of daily evaporation and evapotranspiration of winter wheat and maize by large-scale weighing lysimeter and micro-lysimeter. Agric. For. Meteorol. 2002, 111, 109–120. [Google Scholar] [CrossRef]
- Kang, S.; Gu, B.; Du, T.; Zhang, J. Crop coefficient and ratio of transpiration to evapotranspiration of winter wheat and maize in a semi-humid region. Agric. Water Manag. 2003, 59, 239–254. [Google Scholar] [CrossRef]
- Ehleringer, J.; Field, C. Scaling Physiological Processes: Leaf to Globe; Physiological Ecology Academic Press: San Diego, CA, USA, 1993; p. 388. [Google Scholar]
- Moore, C.J. Frequency response corrections for eddy correlation systems. Bound. Layer Meteorol. 1986, 37, 17–35. [Google Scholar] [CrossRef]
- Raupach, M.R. Applying lagrangian fluid mechanics to infer scalar source distributions from concentration profiles in plant canopies. Agric. For. Meteorol. 1989, 47, 85–108. [Google Scholar] [CrossRef]
- Good, S.P.; Soderberg, K.; Guan, K.G.; King, E.; Scanlon, T.M.; Caylor, K.K. δ2H isotopic flux partitioning of evapotranspiration over a grass field following a water pulse and subsequent dry down. Water Resour. Res. 2014, 50, 1410–1432. [Google Scholar] [CrossRef]
- Yepez, E.A.; Williams, D.G.; Scott, R.L.; Lin, G. Partitioning overstory and understory evapotranspiration in a semiarid savanna woodland from the isotopic composition of water vapor. Agric. For. Meteorol. 2003, 119, 53–68. [Google Scholar] [CrossRef]
- Williams, D.; Cable, W.; Hultine, K.; Hoedjes, J.; Yepez, E.; Simonneaux, V.; Er-Raki, S.; Boulet, G.; de Bruin, H.; Chehbouni, A.; et al. Evapotranspiration components determined by stable isotope, sap flow and eddy covariance techniques. Agric. For. Meteorol. 2004, 125, 241–258. [Google Scholar] [CrossRef]
- Wang, L.; Niu, S.; Good, S.P.; Soderberg, K.; McCabe, M.F.; Sherry, R.A.; Luo, Y.; Zhou, X.; Xia, J.; Caylor, K.K.; et al. The effect of warming on grassland evapotranspiration partitioning using laser-based isotope monitoring techniques. Geochim. Cosmochim. Acta 2013, 111, 28–38. [Google Scholar] [CrossRef]
- Sutanto, S.J.; Wenninger, J.; Coenders-Gerrits, A.M.J.; Uhlenbrook, S. Partitioning of evaporation into transpiration, soil evaporation and interception: A comparison between isotope measurements and a HYDRUS-1D model. Hydrol. Earth Syst. Sci. 2012, 16, 2605–2616. [Google Scholar] [CrossRef]
- Flanagan, L.B.; Comstock, J.P.; Ehleringer, J.R. Comparison of modeled and observed environmental influences on the stable oxygen and hydrogen isotope composition of leaf water in Phaseolus vulgaris L. Plant Physiol. 1991, 96, 588–596. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Dawson, T.E. Water uptake by plants: Perspectives from stable isotope composition. Plant Cell Environ. 1992, 15, 1073–1082. [Google Scholar] [CrossRef]
- Roden, J.; Ehleringer, J. Observations of hydrogen and oxygen isotopes in leaf water confirm the craig-gordon model under wide-ranging environmental conditions. Plant Physiol. 1999, 120, 1165–1174. [Google Scholar] [CrossRef]
- Yakir, D.; Sternberg, L. The use of stable isotopes to study ecosystem gas exchange. Oecologia 2000, 123, 297–311. [Google Scholar] [CrossRef]
- Moreira, M.; Martinelli, L.; Victoria, R.; Barbosa, E.; Bonates, L.; Nepstads, D. Contribution of transpiration to forest ambient vapour based on isotopic measurements. Glob. Chang. Biol. 1997, 3, 439–450. [Google Scholar] [CrossRef]
- Yepez, E.A.; Huxman, T.E.; Ignace, D.D.; English, N.B.; Weltzin, J.F.; Castellanos, A.E.; Williams, D.G. Dynamics of transpiration and evaporation following a moisture pulse in semiarid grassland: A chamber-based isotope method for partitioning flux components. Agric. For. Meteorol. 2005, 132, 359–376. [Google Scholar] [CrossRef]
- Lai, C.T.; Ehleringer, J.R.; Bond, B.J.; Paw, U.K.T. Contributions of evaporation, isotopic non-steady state transpiration and atmospheric mixing on the δ18O of water vapour in Pacific Northwest coniferous forests. Plant Cell Environ. 2006, 29, 77–94. [Google Scholar] [CrossRef]
- Zhang, S.; Wen, X.; Wang, J.; Yu, G.; Sun, X. The use of stable isotopes to partition evapotranspiration fluxes into evaporation and transpiration. Acta Ecol. Sin. 2010, 30, 201–209. [Google Scholar] [CrossRef]
- Hu, Z.; Wen, X.; Sun, X.; Li, L.; Yu, G.; Lee, X.; Li, S. Partitioning of evapotranspiration through oxygen isotopic measurements of water pools and fluxes in a temperate grassland. J. Geophys. Res. Biogeosci. 2014, 119, 358–372. [Google Scholar] [CrossRef]
- Rothfuss, Y.; Biron, P.; Braud, I.; Canale, L.; Durand, J.L.; Gaudet, J.P.; Richard, P.; Vauclin, M.; Bariac, T. Partitioning evapotranspiration fluxes into soil evaporation and plant transpiration using water stable isotopes under controlled conditions. Hydrol. Process. 2010, 24, 3177–3194. [Google Scholar] [CrossRef]
- Wei, Z.; Yoshimura, K.; Okazaki, A.; Kim, W.; Liu, Z.; Yokoi, M. Partitioning of evapotranspiration using high-frequency water vapour isotopic measurement over a rice paddy field: Partitioning of evapotranspiration. Water Resour. Res. 2015, 51, 3716–3729. [Google Scholar] [CrossRef]
- Welp, L.R.; Lee, X.; Kim, K.; Gris, T.J.; Billmark, K.A.; Baker, J.M. δ18O of water vapor, evapotranspiration and the sites of leaf water evaporation in a soybean canopy. Plant Cell Environ. 2008, 31, 1214–1228. [Google Scholar] [CrossRef]
- Dubbert, M.; Cuntz, M.; Piayda, A.; Maguas, C.; Werner, C. Partitioning evapotranspiration: Testing the Craig and Gordon model with field measurements of oxygen isotope ratios of evaporative fluxes. J. Hydrol. 2013, 496, 142–153. [Google Scholar] [CrossRef]
- Hupet, F.; Vanclooster, M. Micro-variability of hydrological processes at the maize row scale: Implications for soil water content measurements and evapotranspiration estimates. J. Hydrol. 2005, 303, 247–270. [Google Scholar] [CrossRef]
- Craig, H.; Gordon, L. Deuterium and Oxygen-18 variations in the Ocean and Marine Atmosphere, Proceedings of the Conference on Stable Isotopes in Oceanographic Studies and Paleotemperatures; Tongiorgi, E., Ed.; Laboratory of Geology and Nuclear Science: Pisa, Italy, 1965; pp. 9–130. [Google Scholar]
- Majoube, M. Fractionnement en oxygene-18 et en deuterium entre leau et sa vapaeur. J. Chim. Phys. 1971, 68, 1423–1436. [Google Scholar] [CrossRef]
- Blöschl, G.; Blaschke, A.P.; Broer, M.; Bucher, C.; Carr, G.; Chen, X.; Eder, A.; Exner-Kittridge, M.; Farnleitner, A.; Flores-Orozco, A.; et al. The hydrological open air laboratory (HOAL) in Petzenkirchen: A hypothesis-driven observatory. Hydrol. Earth Syst. Sci. 2016, 20, 227–255. [Google Scholar] [CrossRef]
- Aubinet, M.; Vesala, T.; Papale, D. Eddy Covariance. A Practical Guide to Measurements and Data Analysis; Springer: Dorndrecht, Germany, 2012; p. 325. [Google Scholar]
- Mauder, M.; Foken, T. Eddy-Covariance Software TK3. Documentation and Instruction Manual of the Eddy-covariance Software Package TK3 (Update); University of Bayreuth: Bayreuth, Germany, 2015. [Google Scholar] [CrossRef]
- Webb, E.; Pearman, G.; Leuning, R. Correction of flux measurements for density effects due to heat and watervapourtransfer. Q. J. R. Meteorol. Soc. 1980, 106, 85–100. [Google Scholar] [CrossRef]
- Lindroth, A.; Cermák, J.; Kučera, J.; Cienciala, E.; Eckersten, H. Sap flow by heat balance method applied to small size Salix trees in a short-rotation forest. In Biomass and Bioenergy; Elsevier: Amsterdam, The Netherlands, 1995; Volume 8, pp. 7–15. [Google Scholar]
- Čermák, J.; Kučera, J.; Nadezhdina, N. Sap flow measurements with some thermodynamic methods, flow integration within trees and scaling up from sample trees to entire forest stands. Trees 2004, 18, 529–546. [Google Scholar]
- Phililips, D.L.; Gregg, J.W. Uncertainty in source partitioning using stable isotopes. Oecologia 2001, 127, 171–179. [Google Scholar] [CrossRef]
- Hollinger, D.; Richardson, A. Uncertainty in eddy covariance measurements and its application to physiological models. Tree Physiol. 2005, 25, 873–885. [Google Scholar] [CrossRef]
- Huang, S.; Gao, Y.; Li, Y.; Xu, L.; Tao, H.; Wang, P. Influence of plant architecture on maize physiology and yield in the Heilonggang River valley. Crop J. 2017, 5, 52–62. [Google Scholar] [CrossRef]
- Jara, J.; Stockle, C.O.; Kjelgaard, J. Measurement of evapotranspiration and its components in a corn (Zea Mays, L.) field. Agric. For. Meteorol. 1998, 92, 131–145. [Google Scholar] [CrossRef]
- Zhao, L.; He, Z.; Zhao, W.; Yang, Q. Extensive investigation of the sap flow of maize plants in an oasis farmland in the middle reach of the Heihe River, Northwest China. J. Plant Res. 2016, 129, 841–851. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, A.W.; Qiu, X.; Zhang, J.; Sun, J.; Wang, H. Plant transpiration in a maize/soybean intercropping system measured with heat balance method. Chin. J. Appl. Ecol. 2010, 21, 1283. [Google Scholar]
- Herbst, M.; Kappen, L.; Thamm, F.; Vanselow, R. Simultaneous measurements of transpiration, soil evaporation and total evaporation in a maize field in northern Germany. J. Exp. Bot. 1996, 47, 1957–1962. [Google Scholar] [CrossRef]
- Hossain, M.; Rumi, M.; Nahar, B.M.A. Radiation use efficiency in different row orientation of maize (Zea mays L.). J. Environ. Sci. Nat. Res. 2014, 7, 41–46. [Google Scholar] [CrossRef][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hogan, P.; Parajka, J.; Oismüller, M.; Heng, L.; Strauss, P.; Blöschl, G. High-Frequency Stable-Isotope Measurements of Evapotranspiration Partitioning in a Maize Field. Water 2020, 12, 3048. https://doi.org/10.3390/w12113048
Hogan P, Parajka J, Oismüller M, Heng L, Strauss P, Blöschl G. High-Frequency Stable-Isotope Measurements of Evapotranspiration Partitioning in a Maize Field. Water. 2020; 12(11):3048. https://doi.org/10.3390/w12113048
Chicago/Turabian StyleHogan, Patrick, Juraj Parajka, Markus Oismüller, Lee Heng, Peter Strauss, and Günter Blöschl. 2020. "High-Frequency Stable-Isotope Measurements of Evapotranspiration Partitioning in a Maize Field" Water 12, no. 11: 3048. https://doi.org/10.3390/w12113048
APA StyleHogan, P., Parajka, J., Oismüller, M., Heng, L., Strauss, P., & Blöschl, G. (2020). High-Frequency Stable-Isotope Measurements of Evapotranspiration Partitioning in a Maize Field. Water, 12(11), 3048. https://doi.org/10.3390/w12113048








