Stable Isotope Analysis Reveals the Importance of Riparian Resources as Carbon Subsidies for Fish Species in the Daning River, a Tributary of the Three Gorges Reservoir, China
Abstract
1. Introduction
2. Materials and Methods
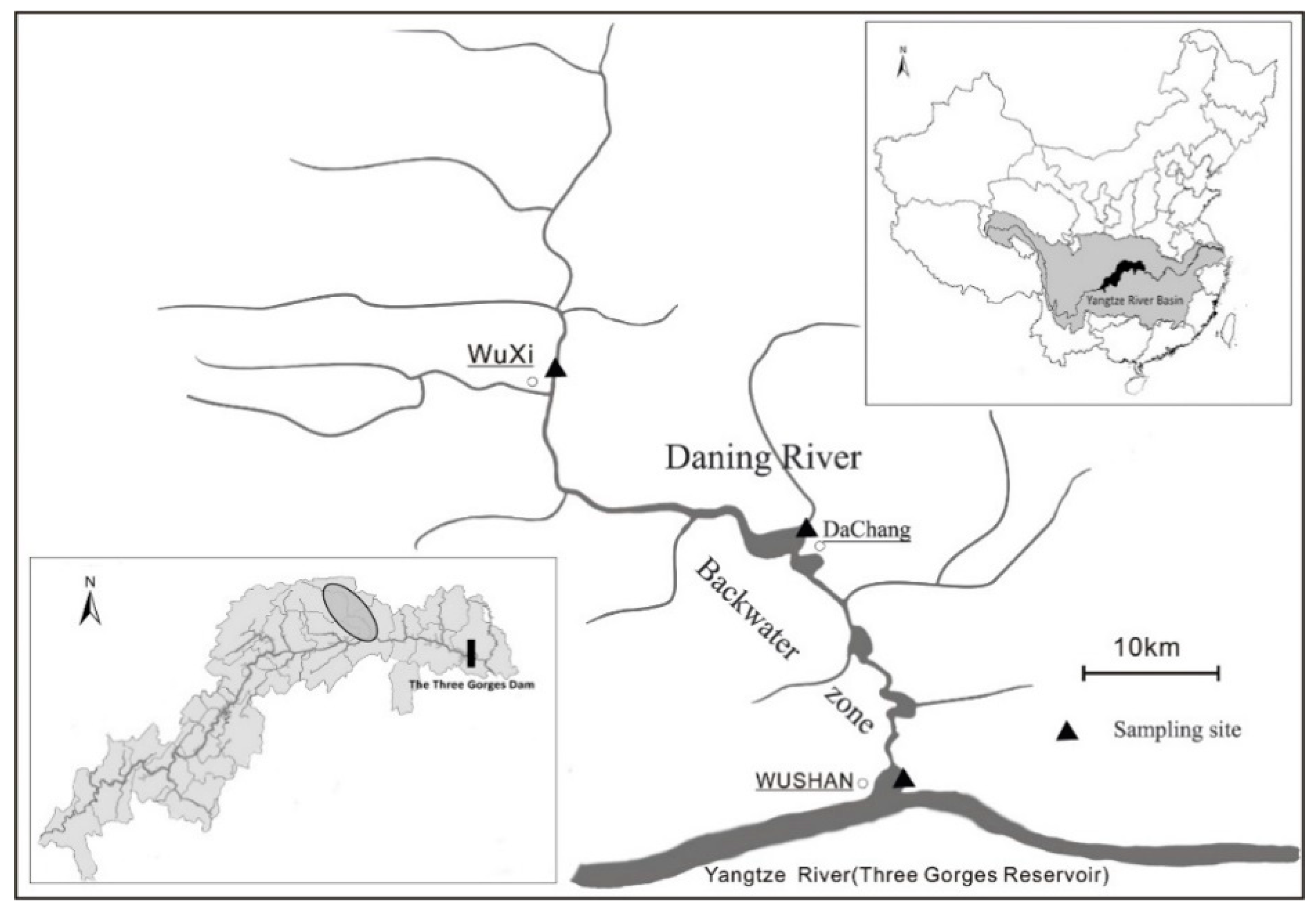
2.1. Study Area and Site Descriptions
2.2. Sample Collection and Analysis
2.3. Bayesian Mixing Model
2.4. Statistical Analyses
3. Results
3.1. Isotopic Signatures
3.2. Contributions of Basal Carbon Sources
3.3. Spatial Variability in Contribution
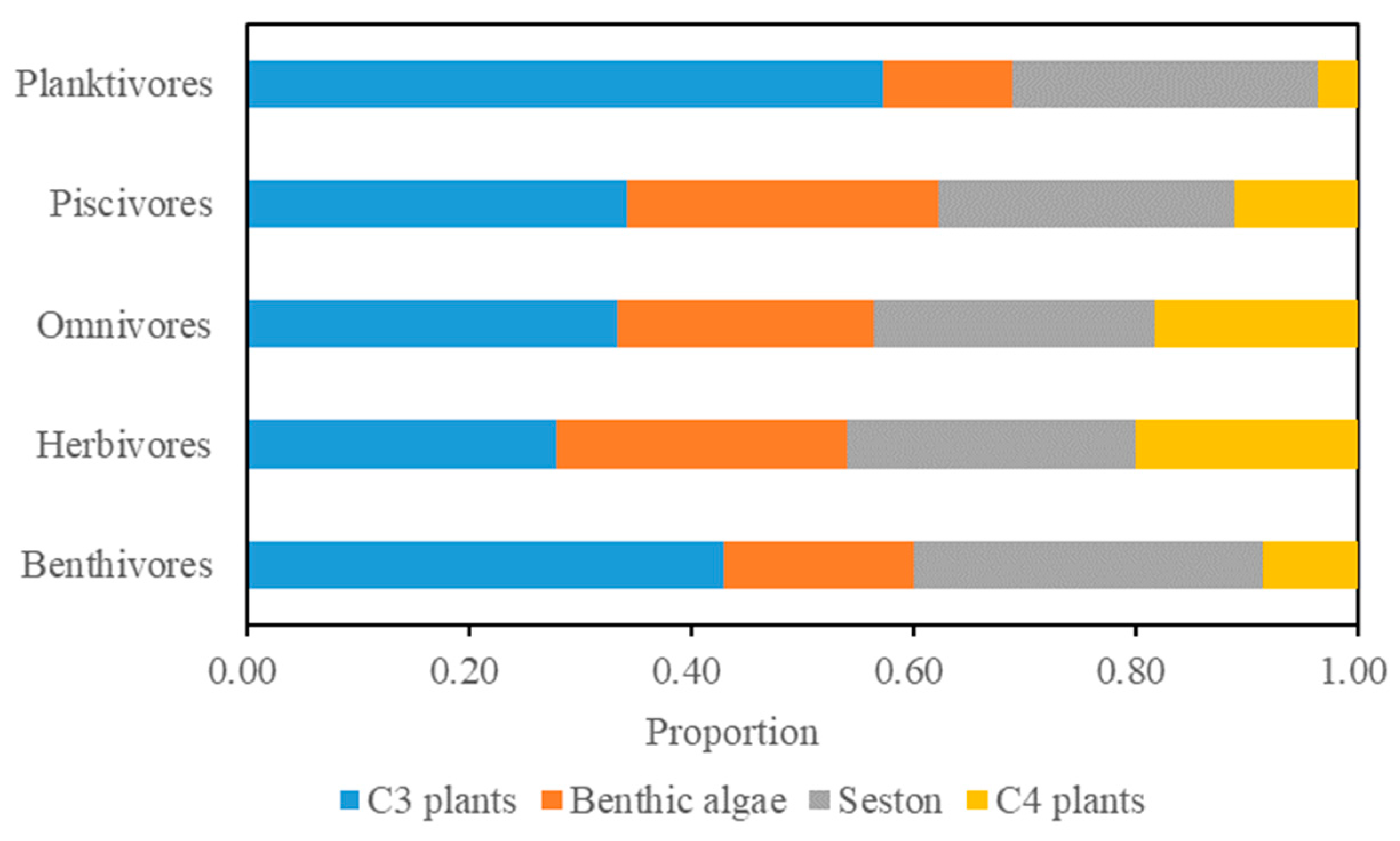
3.4. Differences in Contribution Among Feeding Groups
4. Discussion
4.1. The Relative Importance of Four Basic Carbon Sources
4.2. Spatial Variation of Carbon Source Contribution
4.3. The Carbon Pathway in the Influenced Backwater Zone
4.4. The Influence of Diet Strategies on the Importance of Major Carbon Sources
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Junk, W.J.; Bayley, P.B.; Sparks, R.E. The flood pulse concept in river-floodplain systems. Can. Spec. Publ. Fish. Aquat. Sci. 1989, 106, 110–127. [Google Scholar]
- Thorp, J.H.; Delong, M.D. The riverine productivity model: An heuristic view of carbon sources and organic processing in large river ecosystems. Oikos 1994, 70, 305–308. [Google Scholar] [CrossRef]
- Pingram, M.A.; Collier, K.J.; Hamilton, D.P.; David, B.O.; Hicks, B.J. Carbon sources supporting large river food webs: A review of ecological theories and evidence from stable isotopes. Freshw. Rev. 2012, 5, 85–103. [Google Scholar] [CrossRef]
- Thorp, J.H.; Delong, M.D.; Greenwood, K.S.; Casper, A.F. Isotopic analysis of three food web theories in constricted and floodplain regions of a large river. Oecologia 1998, 117, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Thorp, J.H.; Delong, M.D. Dominance of autochthonous autotrophic carbon in food webs of heterotrophic rivers. Oikos 2002, 96, 543–550. [Google Scholar] [CrossRef]
- Delong, M.D.; Thorp, J.H. Significance of instream autotrophs in trophic dynamics of the Upper Mississippi River. Oecologia 2006, 147, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Hoeinghaus, D.J.; Winemiller, K.O.; Agostinho, A.A. Landscape-scale hydrologic characteristics differentiate patterns of carbon flow in large-river food webs. Ecosystems 2007, 10, 1019–1033. [Google Scholar] [CrossRef]
- Zeug, S.C.; Winemiller, K.O. Evidence supporting the importance of terrestrial carbon in a large-river food web. Ecology 2008, 89, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Thorp, J.H.; Thoms, M.C.; Delong, M.D. The riverine ecosystem synthesis: Biocomplexity in river networks across space and time. River Res. Appl. 2006, 22, 123–147. [Google Scholar] [CrossRef]
- Thorp, J.H.; Thoms, M.C.; Delong, M.D. The Riverine Ecosystem Synthesis, 1st ed.; Elsevier: London, UK, 2008. [Google Scholar]
- Humphries, P.; Keckeis, H.; Finlayson, B. The river wave concept: Integrating river ecosystem models. BioScience 2014, 64, 870–882. [Google Scholar] [CrossRef]
- Mercado-Silva, N.; Helmus, M.R.; Zanden, M.J.V. The effects of impoundment and non-native species on a river food web in Mexico’s central plateau. River Res. Appl. 2009, 25, 1090–1108. [Google Scholar] [CrossRef]
- Freedman, J.A.; Lorson, B.D.; Taylor, R.B.; Carline, R.F.; Stauffer, J.R. River of the dammed: Longitudinal changes in fish assemblages in response to dams. Hydrobiologia 2014, 727, 19–33. [Google Scholar] [CrossRef]
- Carvalho, D.R.; Castro, D.; Callisto, M.; Moreira, M.Z.; Pompeu, P.S. Isotopic variation in five species of stream fishes under the influence of different land uses. J. Fish Biol. 2015, 87, 559–578. [Google Scholar] [CrossRef] [PubMed]
- Kaymak, N.; Winemiller, K.O.; Akin, S.; Altuner, Z.; Polat, F.; Dal, T. Stable isotope analysis reveals relative influences of seasonal hydrologic variation and impoundment on assimilation of primary production sources by fish in the Upper Yesilırmak River, Turkey. Hydrobiologia 2015, 753, 131–147. [Google Scholar] [CrossRef]
- Delong, M.D.; Thoms, M.C. Changes in the trophic status of fish feeding guilds in response to flow modification. J. Geophys. Res. Biogeosci. 2016, 121, 949–964. [Google Scholar] [CrossRef]
- Herwig, B.R.; Wahl, D.H.; Detters, J.M.; Soluk, D.A. Spatial and temporal patterns in the food web structure of a large floodplain river assessed using stable isotopes. Can. J. Fish. Aquat. Sci. 2007, 64, 495–508. [Google Scholar] [CrossRef]
- Poole, G.C. Stream hydrogeomorphology as a physical science basis for advances in stream ecology. J. N. Am. Benthol. Soc. 2010, 29, 12–25. [Google Scholar] [CrossRef]
- Power, M.E.; Dietrich, W.E.; Finlay, J.C. Dams and downstream aquatic biodiversity: Potential food web consequences of hydrologic and geomorphic change. Environ. Manag. 1996, 20, 887–895. [Google Scholar] [CrossRef]
- Doi, H.; Chang, K.H.; Ando, T.; Imai, H.; Nakano, S.I.; Kajimoto, A.; Katano, I. Drifting plankton from a reservoir subsidize downstream food webs and alter community structure. Oecologia 2008, 156, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Wellard Kelly, H.A.; Rosi-Marshall, E.J.; Kennedy, T.A.; Hall, R.O., Jr.; Cross, W.F.; Baxter, C.V. Macroinvertebrate diets reflect tributary inputs and turbidity-driven changes in food availability in the Colorado River downstream of Glen Canyon Dam. Freshw. Sci. 2013, 32, 397–410. [Google Scholar] [CrossRef]
- Fernandes, C.C.; Podos, J.; Lundberg, J.G. Amazonian ecology: Tributaries enhance the diversity of electric fishes. Science 2004, 305, 1960–1962. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, B.; Huang, J.; Han, X.; Lin, G.; Zheng, F.; Li, Y. Terrestrial contributions to the aquatic food web in the middle Yangtze River. PLoS ONE 2014, 9, e102473. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Z.J.; Yue, X.J.; Wang, Y.M.; Jin, L.; Zhang, Y.G. Influence of 175-m-impoundment in Three Gorges Reservoir Area on the food web energy sources of main commercial fishes in backwater area of Xiaojiang River. Chin. J. Appl. Ecol. 2013, 24, 1715–1721. [Google Scholar]
- Deng, C. Studies on Entrophication in the Three Gorgers Reservoir, 1st ed.; China Environmental Science Press: Beijing, China, 2007. [Google Scholar]
- Hang, Y. Study on Pollution Character and Water Environment Impact of the Wet-Dry Zones in the Three Gorges Reservoir. Ph.D. Thesis, Chongqing University, Chongqi, China, 2007. [Google Scholar]
- Shen, Z.; Hong, Q.; Yu, H.; Liu, R. Parameter uncertainty analysis of the non-point source pollution in the Daning River watershed of the Three Gorges Reservoir Region, China. Sci. Total Environ. 2008, 405, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yao, W.Z.; Deng, H.T.; Chen, D.Q.; Liu, S.P.; Duan, X.B. The current situation of fish resources in the Daning River after the impoundment of the Three Gorges Reservoir. Freshw. Fish. 2013, 43, 51–57. [Google Scholar]
- The Fish Laboratory of Institute Hydrobiology of Hubei Province. Fishes in the Yangtze River, 1st ed.; Science Press: Beijing, China, 1976. [Google Scholar]
- Zeng, X.Z. Fishery Resources of the Yangtze River Basin, 1st ed.; Marine Press: Beijing, China, 1990. [Google Scholar]
- Ding, R.H. The Fishes of Sichuan, 1st ed.; Sichuan Publishing House of Science and Technology: Chengdu, China, 1994. [Google Scholar]
- Zhang, T.L. Life-History Strategies, Trophic Patterns and Community Structure in the Fishes of Lake Biandantang. Ph.D. Thesis, Graduate University of the Chinese Academy of Sciences, Wuhan, China, 2005; pp. 61–118. [Google Scholar]
- Parnell, A.C.; Inger, R.; Bearhop, S.; Jackson, A.L. Source partitioning using stable isotopes: Coping with too much variation. PLoS ONE 2010, 5, e9672. [Google Scholar] [CrossRef] [PubMed]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Vander Zanden, M.J.; Rasmussen, J.B. Variation in δ15N and δ13C trophic fractionation: Implications for aquatic food web studies. Limnol. Oceanogr. 2001, 46, 2061–2066. [Google Scholar] [CrossRef]
- Marchese, M.R.; Saigo, M.; Zilli, F.L.; Capello, S.; Devercelli, M.; Montalto, L.; Paporello, G.; Wantzen, K.M. Food webs of the Paraná River floodplain: Assessing basal sources using stable carbon and nitrogen isotopes. Limnologica 2014, 46, 22–30. [Google Scholar] [CrossRef]
- Zeng, Y.; Lai, Z.; Yang, W.; Li, H. Stable isotopes reveal food web reliance on different carbon sources in a subtropical watershed in South China. Limnologica 2018, 69, 39–45. [Google Scholar] [CrossRef]
- Leigh, C.; Burford, M.A.; Sheldon, F.; Bunn, S.E. Dynamic stability in dry season food webs within tropical floodplain rivers. Mar. Freshw. Res. 2010, 61, 357–368. [Google Scholar] [CrossRef]
- Pingram, M.A.; Collier, K.J.; Hamilton, D.P.; Hicks, B.J.; David, B.O. Spatial and temporal patterns of carbon flow in a temperate, large river food web. Hydrobiologia 2014, 729, 107–131. [Google Scholar] [CrossRef]
- Neres-Lima, V.; Brito, E.F.; Krsulović, F.A.; Detweiler, A.M.; Hershey, A.E.; Moulton, T.P. High importance of autochthonous basal food source for the food web of a Brazilian tropical stream regardless of shading. Int. Rev. Hydrobiol. 2016, 101, 132–142. [Google Scholar] [CrossRef]
- Forsberg, B.R.; Araujo-Lima, C.A.R.M.; Martinelli, L.A.; Victoria, R.L.; Bonassi, J.A. Autotrophic carbon sources for fish of the central Amazon. Ecology 1993, 74, 643–652. [Google Scholar] [CrossRef]
- Marcarelli, A.M.; Baxter, C.V.; Mineau, M.M.; Hall, R.O. Quantity and quality: Unifying food web and ecosystem perspectives on the role of resource subsidies in freshwaters. Ecology 2011, 92, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Carroll, T.M.; Thorp, J.H.; Roach, K.A. Autochthony in karst spring food webs. Hydrobiologia 2016, 776, 173–191. [Google Scholar] [CrossRef]
- Brett, M.T.; Bunn, S.E.; Chandra, S.; Galloway, A.W.; Guo, F.; Kainz, M.J.; Kankaala, P.; Lau, D.C.; Moulton, T.P.; Power, M.E.; et al. How important are terrestrial organic carbon inputs for secondary production in freshwater ecosystems? Freshw. Biol. 2017, 62, 833–853. [Google Scholar]
- Oliveira, A.C.B.; Soares, M.G.M.; Martinelli, L.A.; Moreira, M.Z. Carbon sources of fish in an Amazonian floodplain lake. Aquat. Sci. 2006, 68, 229–238. [Google Scholar] [CrossRef]
- Mortillaro, J.M.; Pouilly, M.; Wach, M.; Freitas, C.E.C.; Abril, G.; Meziane, T. Trophic opportunism of central Amazon floodplain fish. Freshw. Biol. 2015, 60, 1659–1670. [Google Scholar] [CrossRef]
- Roach, K.A.; Winemiller, K.O.; Davis, S.E., III. Autochthonous production in shallow littoral zones of five floodplain rivers: Effects of flow, turbidity and nutrients. Freshw. Biol. 2014, 59, 1278–1293. [Google Scholar] [CrossRef]
- Finlay, J.C. Stable-carbon-isotope ratios of river biota: Implications for energy flow in lotic food webs. Ecology 2001, 82, 1052–1064. [Google Scholar] [CrossRef]
- Zhu, K.; Bi, Y.; Hu, Z. Responses of phytoplankton functional groups to the hydrologic regime in the Daning River, a tributary of Three Gorges Reservoir, China. Sci. Total Environ. 2013, 450, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zheng, B.; Wang, L.; Qin, Y.; Li, H.; Cao, W. Characterization of mixing processes in the confluence zone between the Three Gorges Reservoir mainstream and the Daning River using stable isotope analysis. Environ. Sci. Technol. 2015, 50, 9907–9914. [Google Scholar] [CrossRef] [PubMed]
- Holbach, A.; Wang, L.; Chen, H.; Hu, W.; Schleicher, N.; Zheng, B.; Norra, S. Water mass interaction in the confluence zone of the Daning River and the Yangtze River—A driving force for algal growth in the Three Gorges Reservoir. Environ. Sci. Pollut. Res. 2013, 20, 7027–7037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Zheng, B.H.; Liu, L.S.; Wang, L.P.; Huang, M.S.; Wu, G.Y. Seasonal variation of phytoplankton in the DaNing River and its relationships with environmental factors after impounding of the Three Gorges Reservoir: A four-year study. Procedia Environ. Sci. 2010, 2, 1479–1490. [Google Scholar] [CrossRef]
- Becker, V.; Huszar, V.L.M.; Naselli-Flores, L.U.I.G.I.; Padisak, J. Phytoplankton equilibrium phases during thermal stratification in a deep subtropical reservoir. Freshw. Biol. 2008, 53, 952–963. [Google Scholar] [CrossRef]
- Jepsen, D.B.; Winemiller, K.O. Basin geochemistry and isotopic ratios of fishes and basal production sources in four neotropical rivers. Ecol. Freshw. Fish. 2007, 16, 267–281. [Google Scholar] [CrossRef]
- Mao, Z.G.; Gu, X.H.; Zeng, Q.F.; Chen, H.H. Carbon sources and trophic structure in a macrophyte-dominated polyculture pond assessed by stable-isotope analysis. Freshw. Biol. 2016, 61, 1862–1873. [Google Scholar] [CrossRef]
- Webb, P.W. Body and fin form and strike tactics of four teleost predators attacking fathead minnow (Pimephales promelas) prey. Can. J. Fish. Aquat. Sci. 1984, 41, 157–165. [Google Scholar] [CrossRef]
- Drewe, K.E.; Horn, M.H.; Dickson, K.A.; Gawlicka, A. Insectivore to frugivore: Ontogenetic changes in gut morphology and digestive enzyme activity in the characid fish Brycon guatemalensis from Costa Rican rain forest streams. J. Fish. Biol. 2004, 64, 890–902. [Google Scholar] [CrossRef]
- Beaudoin, C.P.; Tonn, W.M.; Prepas, E.E.; Wassenaar, L.I. Individual specialization and trophic adaptability of northern pike (Esox lucius): An isotope and dietary analysis. Oecologia 1999, 120, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Correa, S.B.; Winemiller, K.O. Niche partitioning among frugivorous fishes in response to fluctuating resources in the Amazonian floodplain forest. Ecology 2014, 95, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.A. Life history and the relationship between food availability and foraging effort. Ecology 1991, 72, 1242–1252. [Google Scholar] [CrossRef]
- Peterson, C.C.; Winemiller, K.O. Ontogenic diet shifts and scale-eating in Roeboides dayi, a Neotropical characid. Environ. Biol. Fish. 1997, 49, 111–118. [Google Scholar] [CrossRef]
- Croxall, J.P.; Reid, K.; Prince, P.A. Diet, provisioning and productivity responses of marine predators to differences in availability of Antarctic krill. Mar. Ecol. Prog. Ser. 1999, 177, 115–131. [Google Scholar] [CrossRef]
- Vaudo, J.J.; Heithaus, M.R. Dietary niche overlap in a nearshore elasmobranch mesopredator community. Mar. Ecol. Prog. Ser. 2011, 425, 247–260. [Google Scholar] [CrossRef]
- Winemiller, K.O. Ontogenetic diet shifts and resource partitioning among piscivorous fishes in the Venezuelan ilanos. Environ. Biol. Fish. 1989, 26, 177–199. [Google Scholar] [CrossRef]
- Quevedo, M.; Svanbäck, R.; Eklöv, P. Intrapopulation niche partitioning in a generalist predator limits food web connectivity. Ecology 2009, 90, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Spataru, P.; Gophen, M. Feeding behaviour of silver carp Hypophthalmichthys molitrix Val. and its impact on the food web in Lake Kinneret, Israel. Hydrobiologia 1985, 120, 53–61. [Google Scholar] [CrossRef]
- Ke, Z.; Xie, P.; Guo, L.; Liu, Y.; Yang, H. In situ study on the control of toxic Microcystis blooms using phytoplanktivorous fish in the subtropical Lake Taihu of China: A large fish pen experiment. Aquaculture 2007, 265, 127–138. [Google Scholar] [CrossRef]
- Phillips, D.L.; Inger, R.; Bearhop, S.; Jackson, A.L.; Moore, J.W.; Parnell, A.C.; Semmens, B.X.; Ward, E.J. Best practices for use of stable isotope mixing models in food-web studies. Can. J. Zool. 2014, 92, 823–835. [Google Scholar] [CrossRef]
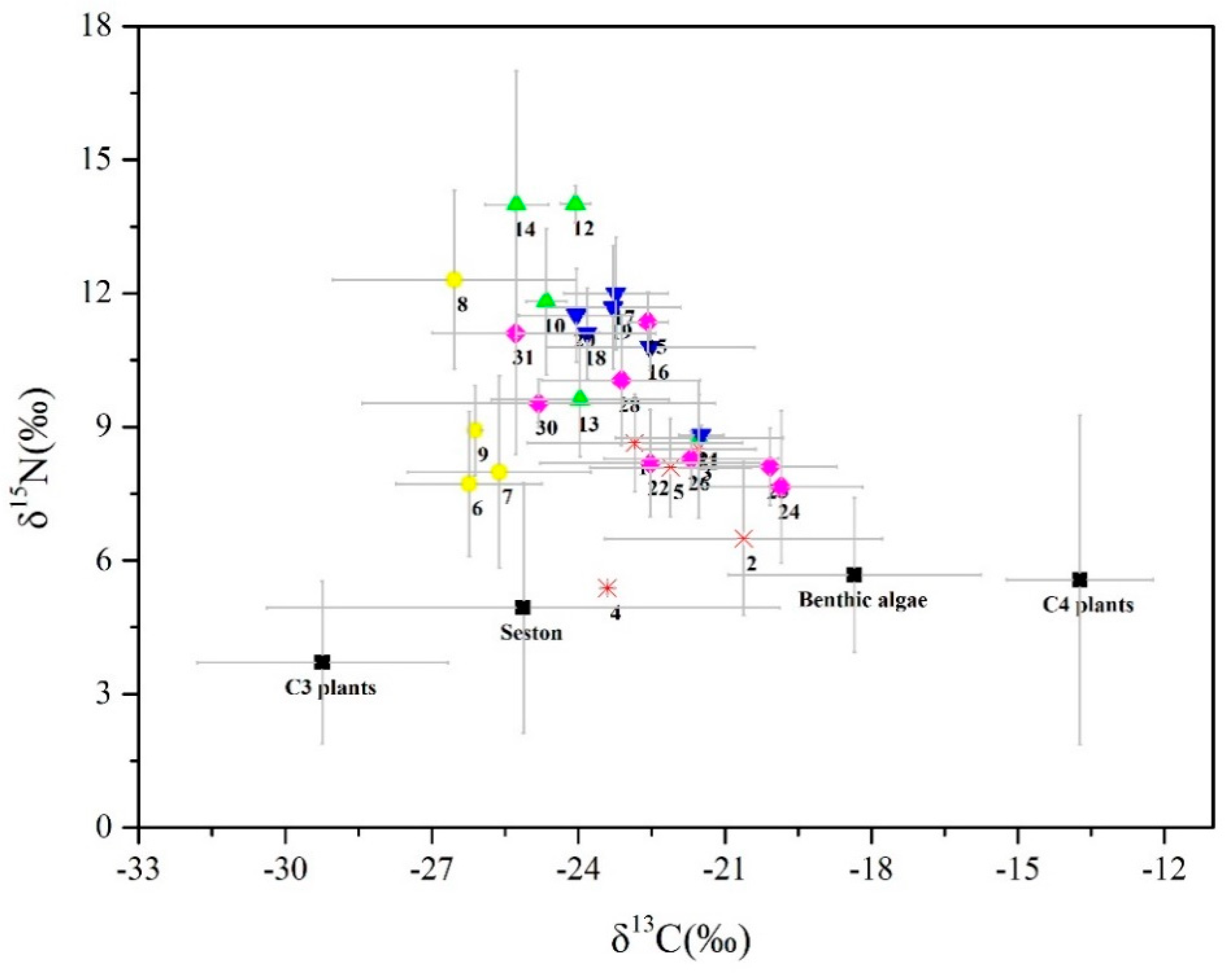
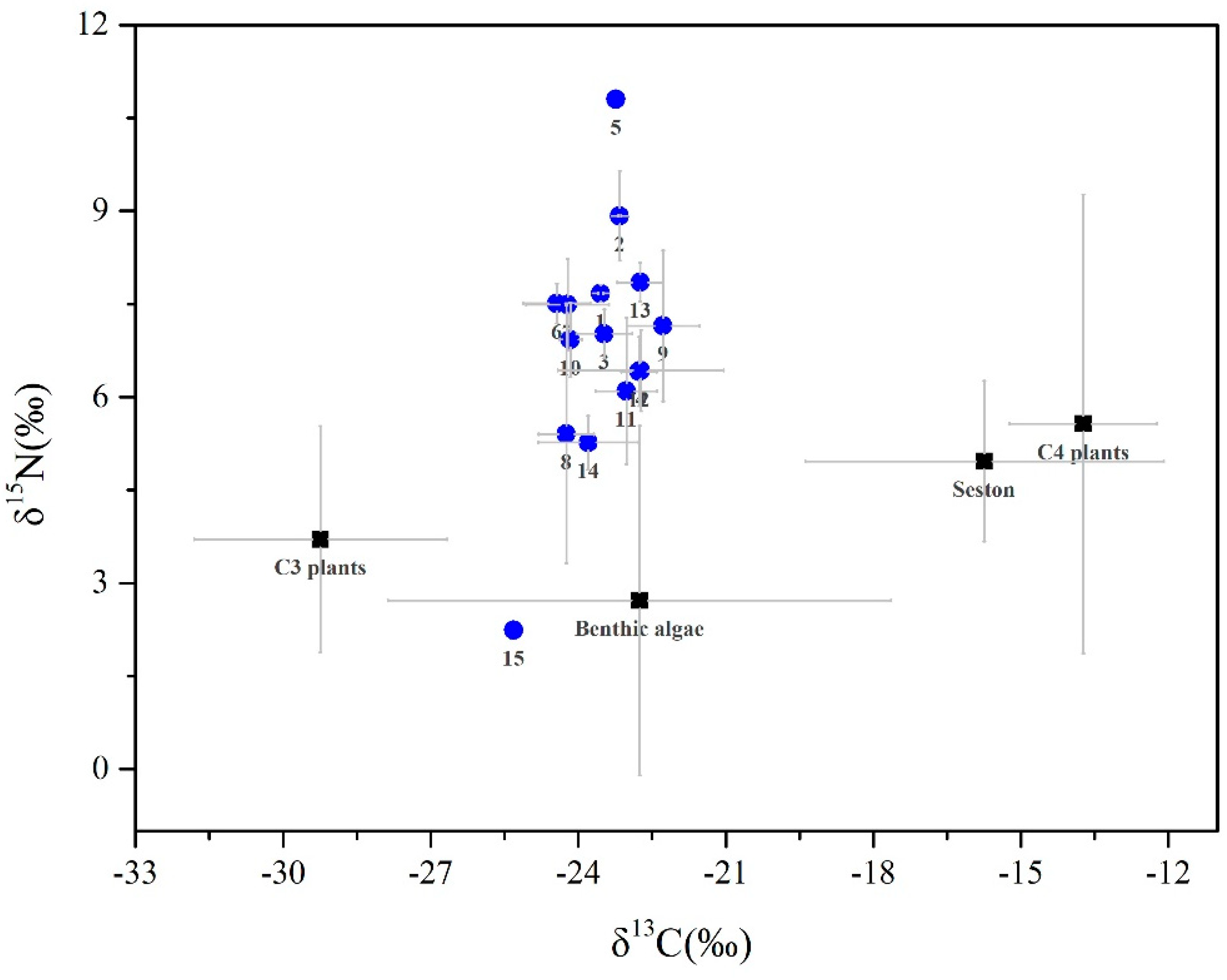
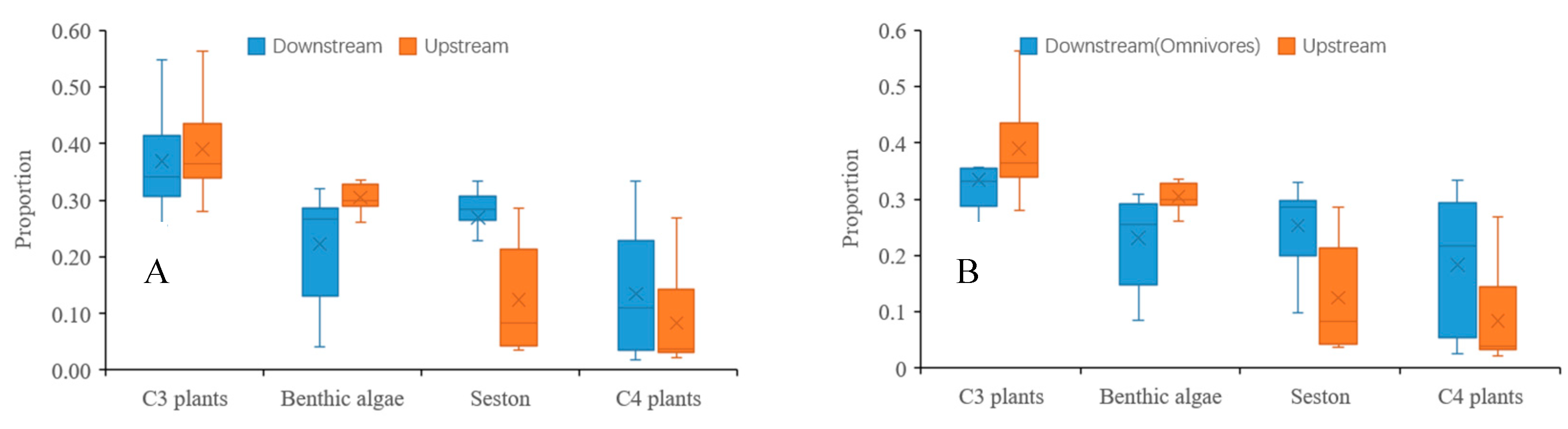
| Code | Taxa | C3 Plants | Benthic Algae | Seston | C4 Plants |
|---|---|---|---|---|---|
| Herbivores | |||||
| 1 | Parabramis pekinensis | 0.31 (0.10–0.49) | 0.31 (0.02–0.50) | 0.31 (0.02–0.48) | 0.13 (0.00–0.31) |
| 2 | Ctenopharyngodon idella | 0.27 (0.03–0.46) | 0.27 (0.00–0.45) | 0.27 (0.00–0.46) | 0.30 (0.06–0.48) |
| 3 | Squaliobarbus curriculus | 0.30 (0.03–0.46) | 0.29 (0.00–0.49) | 0.27 (0.00–0.44) | 0.25 (0.02–0.43) |
| 4 | Megalobrama amblycephala | 0.33 (0.03–0.56) | 0.26 (0.00–0.45) | 0.29 (0.00–0.48) | 0.21 (0.00–0.40) |
| 5 | Spinibarbus sinensis | 0.31 (0.08–0.50) | 0.30 (0.01–0.49) | 0.28 (0.01–0.43) | 0.19 (0.01–0.37) |
| Planktivores | |||||
| 6 | Hypophthalmichthys molitrix | 0.71 (0.46–0.85) | 0.04 (0.00–0.21) | 0.06 (0.00 –0.40) | 0.02 (0.00–0.15) |
| 7 | Aristichthys nobilis | 0.55 (0.33–0.80) | 0.04 (0.00–0.26) | 0.26 (0.00–0.47) | 0.03 (0.00–0.18) |
| 8 | Neosalanx taihuensis | 0.38 (0.06–0.75) | 0.05 (0.00–0.39) | 0.33 (0.00–0.59) | 0.03 (0.00–0.31) |
| 9 | Hyporhamphus intermedius | 0.31 (0.02–0.62) | 0.26 (0.00–0.45) | 0.28 (0.00–0.51) | 0.05 (0.00–0.40) |
| Benthivores | |||||
| 10 | Silurus asotus | 0.43 (0.20–0.70) | 0.19 (0.00–0.38) | 0.31 (0.01–0.49) | 0.03 (0.00–0.25) |
| 11 | Myxocyprinus asiaticus | 0.32 (0.11–0.53) | 0.27 (0.00–0.45) | 0.27 (0.00–0.43) | 0.25 (0.02–0.41) |
| 12 | Pelteobagrus nitidus | 0.36 (0.10–0.64) | 0.24 (0.00–0.42) | 0.31 (0.01–0.51) | 0.03 (0.00–0.31) |
| 13 | Pelteobagrus vachelli | 0.52 (0.29–0.72) | 0.05 (0.00–0.34) | 0.30 (0.00–0.45) | 0.07 (0.00–0.25) |
| 14 | Coreius heterodon | 0.42 (0.13–0.73) | 0.08 (0.00–0.38) | 0.32 (0.01–0.52) | 0.03 (0.00–0.28) |
| Piscivores | |||||
| 15 | Culter dabryi dabryi | 0.28 (0.04–0.45) | 0.32 (0.02–0.51) | 0.32 (0.03–0.52) | 0.13 (0.00–0.32) |
| 16 | Siniperca kneri | 0.40 (0.22–0.58) | 0.28 (0.01–0.45) | 0.24 (0.01–0.40) | 0.10 (0.00–0.29) |
| 17 | Elopichthys bambusa | 0.36 (0.15–0.61) | 0.29 (0.00–0.43) | 0.30 (0.01–0.48) | 0.05 (0.00–0.28) |
| 18 | Culter mongolicus | 0.34 (0.10–0.57) | 0.27 (0.00–0.45) | 0.29 (0.01–0.48) | 0.12 (0.00–0.32) |
| 19 | Culter alburnus | 0.43 (0.23–0.63) | 0.28 (0.01–0.42) | 0.27 (0.01–0.44) | 0.04 (0.00–0.25) |
| 20 | Leiocassis longirostris | 0.30 (0.09–0.50) | 0.30 (0.01–0.49) | 0.23 (0.00–0.41) | 0.24 (0.02–0.40) |
| Omnivores | |||||
| 21 | Hemiculter leucisculus | 0.35 (0.18–0.58) | 0.08 (0.00–0.41) | 0.30 (0.00–0.44) | 0.22 (0.01–0.35) |
| 22 | Carassius auratus | 0.28 (0.12–0.43) | 0.25 (0.01–0.44) | 0.10 (0.00–0.33) | 0.33 (0.16–0.50) |
| 23 | Cyprinus carpio | 0.25 (0.06–0.42) | 0.26 (0.00–0.46) | 0.18 (0.00–0.38) | 0.31 (0.14–0.53) |
| 24 | Opsariichthys bidens | 0.31 (0.04–0.51) | 0.28 (0.00–0.47) | 0.29 (0.00–0.46) | 0.21 (0.00–0.39) |
| 25 | Pseudobrama simoni | 0.32 (0.14–0.52) | 0.25 (0.00–0.45) | 0.25 (0.00–0.41) | 0.23 (0.02–0.39) |
| 26 | Squalidus argentatus | 0.36 (0.18–0.55) | 0.31 (0.01–0.46) | 0.30 (0.01–0.44) | 0.09 (0.00–0.29) |
| 27 | Hemiculter bleekeri | 0.34 (0.08–0.60) | 0.30 (0.00–0.46) | 0.28 (0.01–0.49) | 0.04 (0.00–0.33) |
| 28 | Saurogobio dabryi | 0.46 (0.25–0.73) | 0.11 (0.00–0.32) | 0.33 (0.01–0.52) | 0.02 (0.00–0.20) |
| Code | Taxa | C3 Plants | Benthicalgae | Seston | C4 Plants |
|---|---|---|---|---|---|
| 1 | Trilophysa bleekeri | 0.33 (0.01–0.60) | 0.29 (0.00–0.54) | 0.25 (0.00–0.43) | 0.09 (0.00–0.41) |
| 2 | Rhinogobius cliffordpopei | 0.41 (0.13–0.66) | 0.33 (0.01–0.52) | 0.04 (0.00–0.37) | 0.03 (0.00–0.33) |
| 3 | Hemibarbus labeo | 0.42 (0.20–0.68) | 0.32 (0.00–0.52) | 0.06 (0.00–0.34) | 0.03 (0.00–0.29) |
| 4 | Onychostoma macrolepis | 0.34 (0.07–0.61) | 0.31 (0.00–0.52) | 0.16 (0.00–0.41) | 0.04 (0.00–0.37) |
| 5 | Siniperca kneri | 0.36 (0.09–0.63) | 0.30 (0.00–0.50) | 0.25 (0.00–0.44) | 0.14 (0.00–0.34) |
| 6 | Glyptothorax fukiensis | 0.43 (0.11–0.72) | 0.34 (0.00–0.56) | 0.04 (0.00–0.36) | 0.04 (0.00–0.33) |
| 7 | Paracobitis variegatus | 0.46 (0.26–0.73) | 0.32 (0.01–0.55) | 0.04 (0.00–0.28) | 0.02 (0.00–0.24) |
| 8 | Carassius auratus | 0.32 (0.02–0.62) | 0.30 (0.00–0.52) | 0.04 (0.00–0.44) | 0.06 (0.00–0.43) |
| 9 | Acrossocheilus monticolus | 0.36 (0.11–0.60) | 0.29 (0.00–0.51) | 0.12 (0.00–0.41) | 0.14 (0.00–0.36) |
| 10 | Zacco platypus | 0.56 (0.30–0.73) | 0.26 (0.00–0.51) | 0.04 (0.00–0.26) | 0.02 (0.00–0.23) |
| 11 | Cyprinus carpio | 0.34 (0.03–0.58) | 0.30 (0.00–0.52) | 0.21 (0.00–0.43) | 0.11 (0.00–0.41) |
| 12 | Schizothorax prenanti | 0.34 (0.02–0.57) | 0.30 (0.00–0.52) | 0.21 (0.00–0.43) | 0.18 (0.00–0.41) |
| 13 | Saurogobio dabryi | 0.39 (0.18–0.63) | 0.33 (0.02–0.54) | 0.08 (0.00–0.36) | 0.04 (0.00–0.32) |
| 14 | Discogobio yunnanensis | 0.49 (0.26–0.71) | 0.33 (0.00–0.50) | 0.05 (0.00–0.31) | 0.04 (0.00–0.26) |
| 15 | Spinibarbus sinensis | 0.28 (0.00–0.49) | 0.27 (0.00–0.49) | 0.29 (0.00–0.48) | 0.27 (0.00–0.48) |
| Intergroup Dissimilarity (%) | Benthivores | Herbivores | Omnivores | Piscivores |
|---|---|---|---|---|
| Herbivores | 20.36 | |||
| Omnivores | 20.87 | 13.36 | ||
| Piscivores | 16.86 | 10.77 | 15.56 | |
| Planktivores | 22.42 | 33.03 | 31.38 | 27.84 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, H.; Li, Y.; Liu, M.; Duan, X.; Liu, S.; Chen, D. Stable Isotope Analysis Reveals the Importance of Riparian Resources as Carbon Subsidies for Fish Species in the Daning River, a Tributary of the Three Gorges Reservoir, China. Water 2018, 10, 1233. https://doi.org/10.3390/w10091233
Deng H, Li Y, Liu M, Duan X, Liu S, Chen D. Stable Isotope Analysis Reveals the Importance of Riparian Resources as Carbon Subsidies for Fish Species in the Daning River, a Tributary of the Three Gorges Reservoir, China. Water. 2018; 10(9):1233. https://doi.org/10.3390/w10091233
Chicago/Turabian StyleDeng, Huatang, Yun Li, Mingdian Liu, Xinbin Duan, Shaoping Liu, and Daqing Chen. 2018. "Stable Isotope Analysis Reveals the Importance of Riparian Resources as Carbon Subsidies for Fish Species in the Daning River, a Tributary of the Three Gorges Reservoir, China" Water 10, no. 9: 1233. https://doi.org/10.3390/w10091233
APA StyleDeng, H., Li, Y., Liu, M., Duan, X., Liu, S., & Chen, D. (2018). Stable Isotope Analysis Reveals the Importance of Riparian Resources as Carbon Subsidies for Fish Species in the Daning River, a Tributary of the Three Gorges Reservoir, China. Water, 10(9), 1233. https://doi.org/10.3390/w10091233






