Hydrogeochemical Evolution and Heavy Metal Contamination in Groundwater of a Reclaimed Land on Zhoushan Island
Abstract
:1. Introduction
2. Materials and Methods
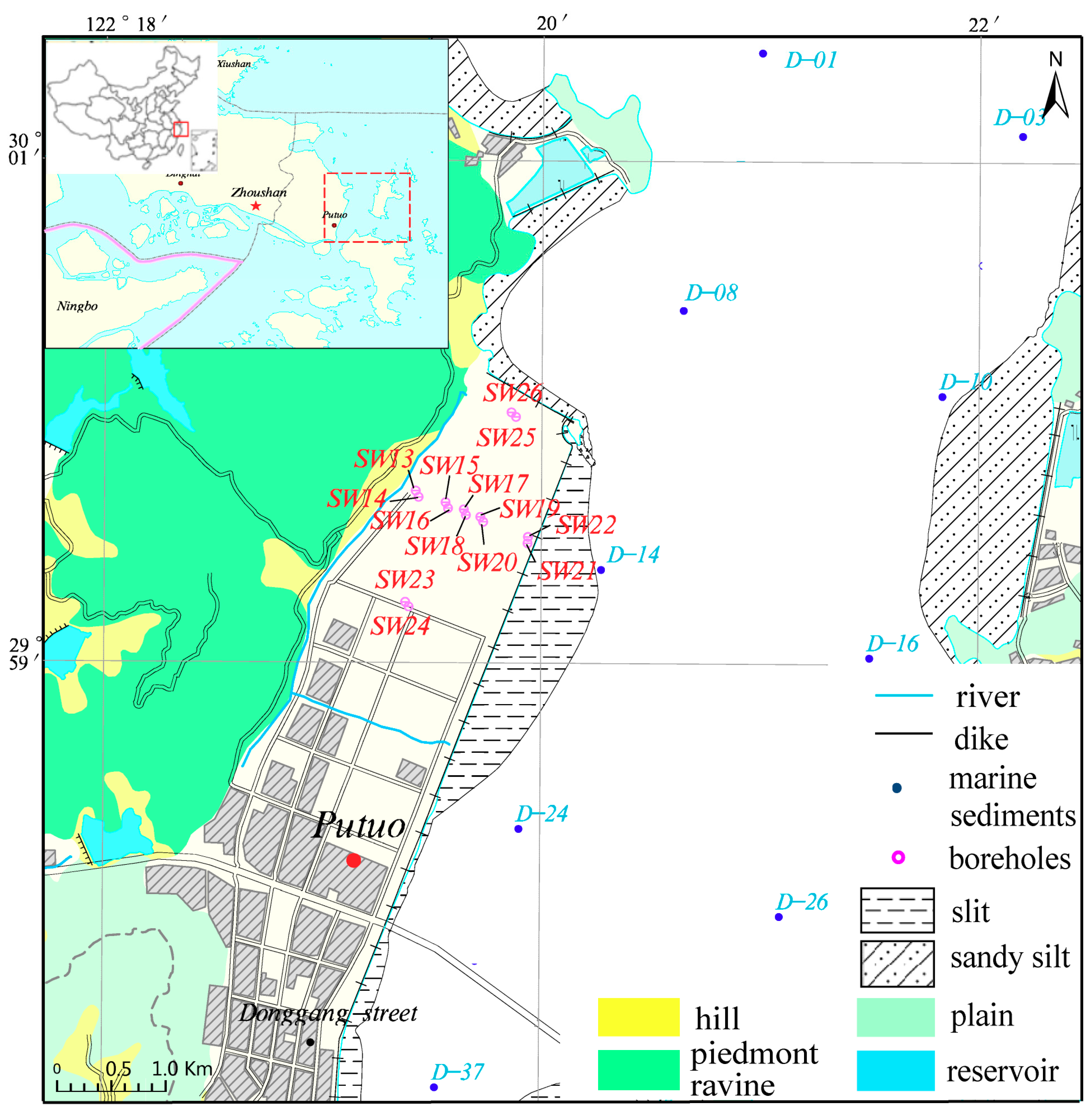
2.1. Study Area
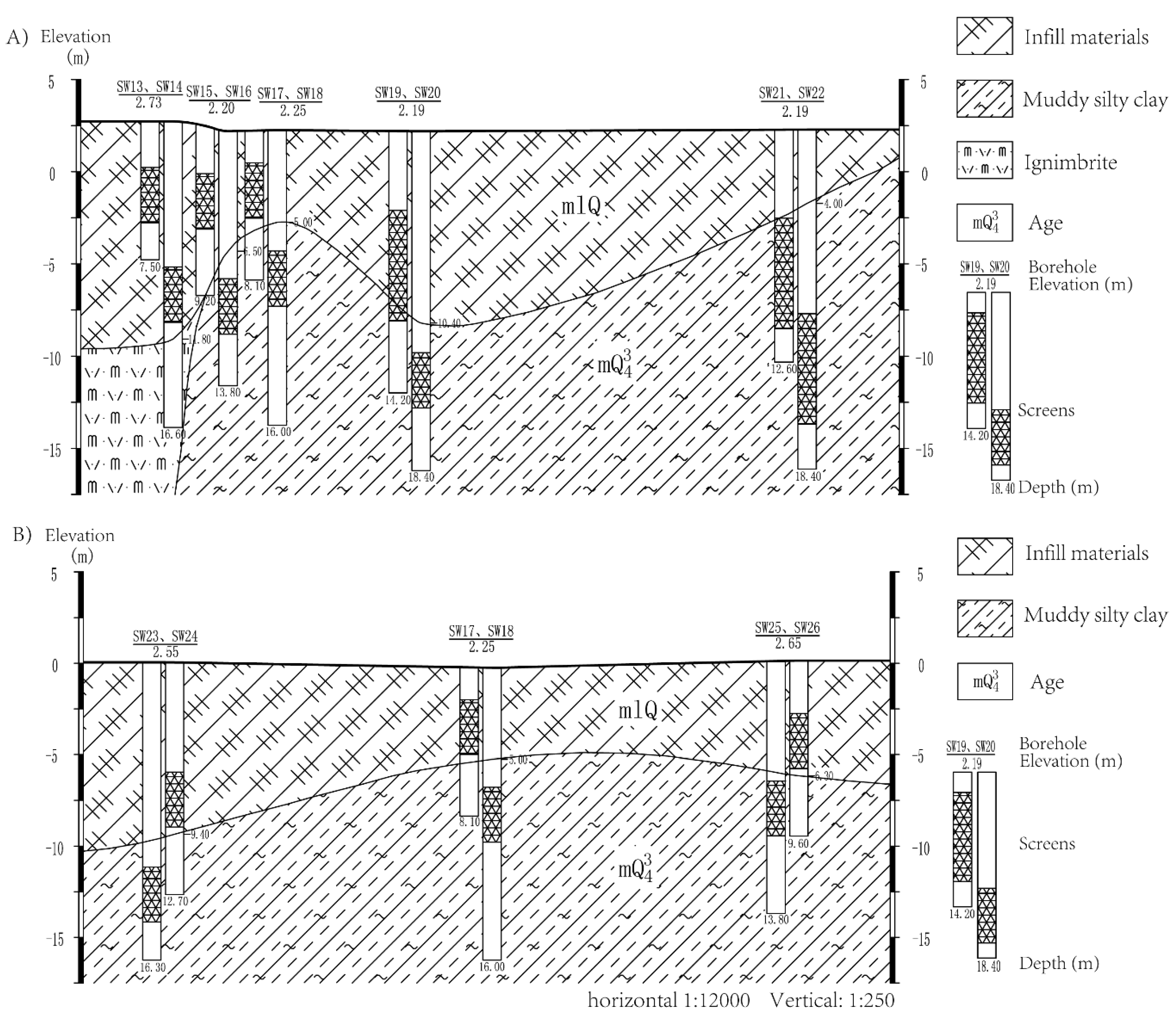
2.2. Geological Condition
2.3. Borehole Design and Sampling
2.4. Column Experiments
2.5. Kinetic Modeling
3. Results and Discussions
3.1. Physicochemical Characterization of Groundwater
3.2. Groundwater Hydrogeochemistry
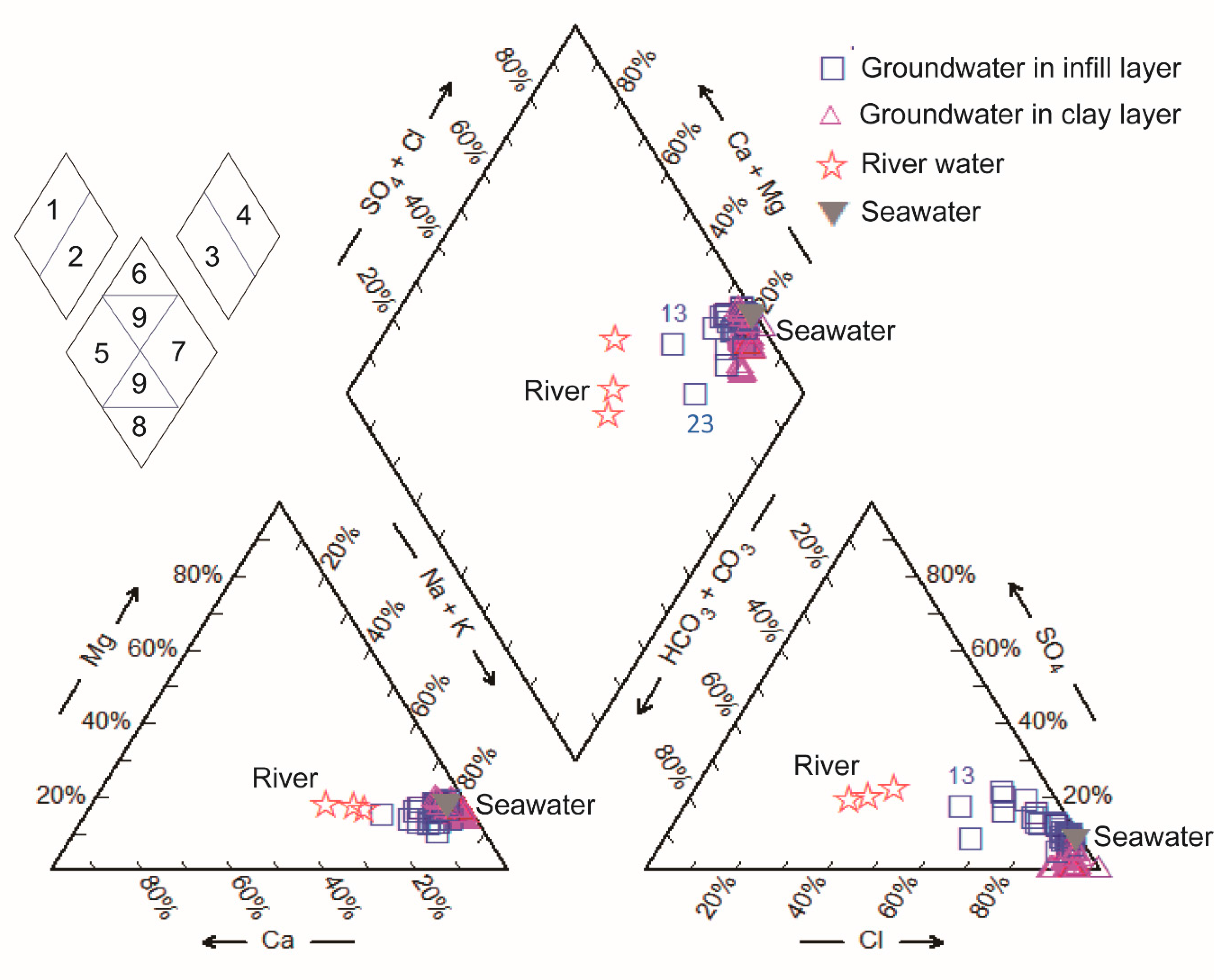
3.2.1. Hydrogeochemical Facies
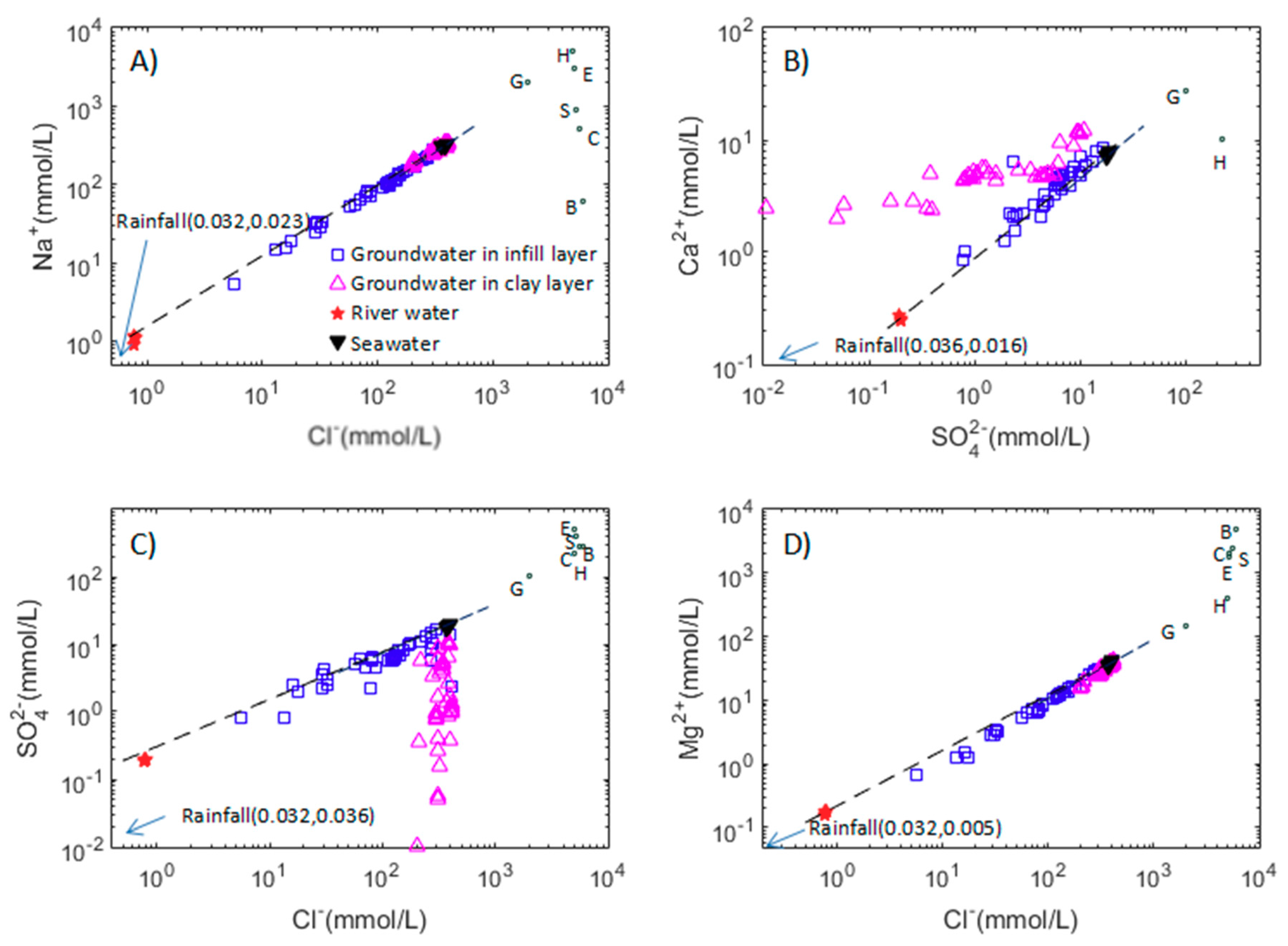
3.2.2. Major Ion Trends
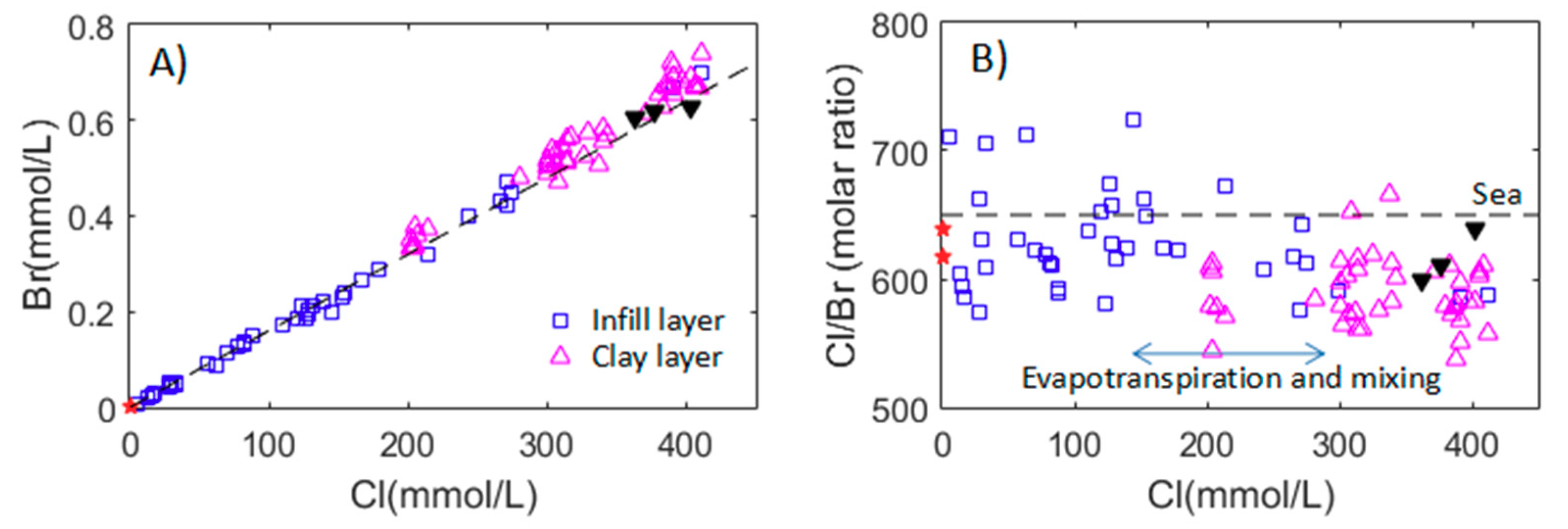
3.2.3. Cl/Br Ratios in the Groundwater
3.3. Nitrogen
3.4. Heavy Metal
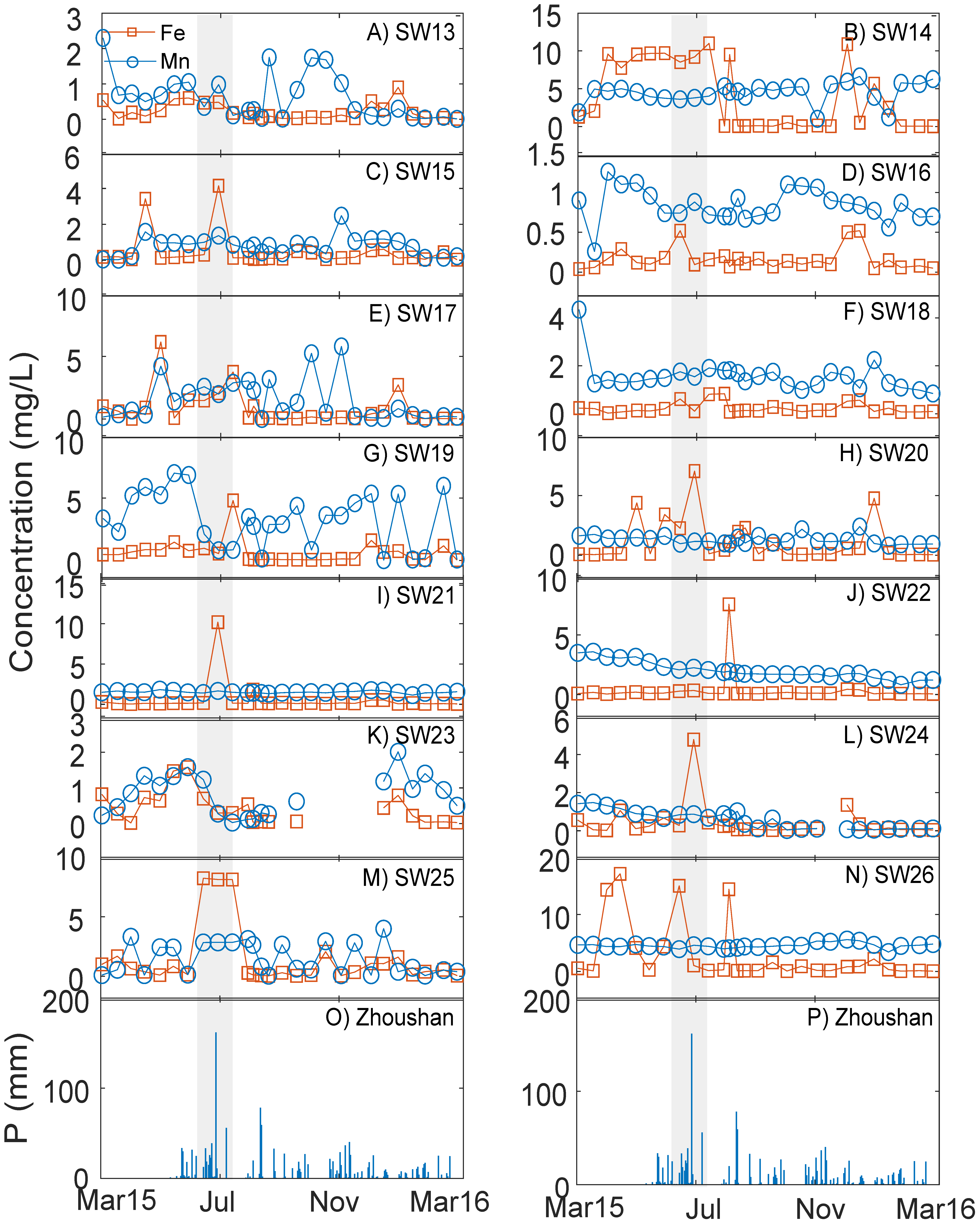
3.4.1. Fe and Mn
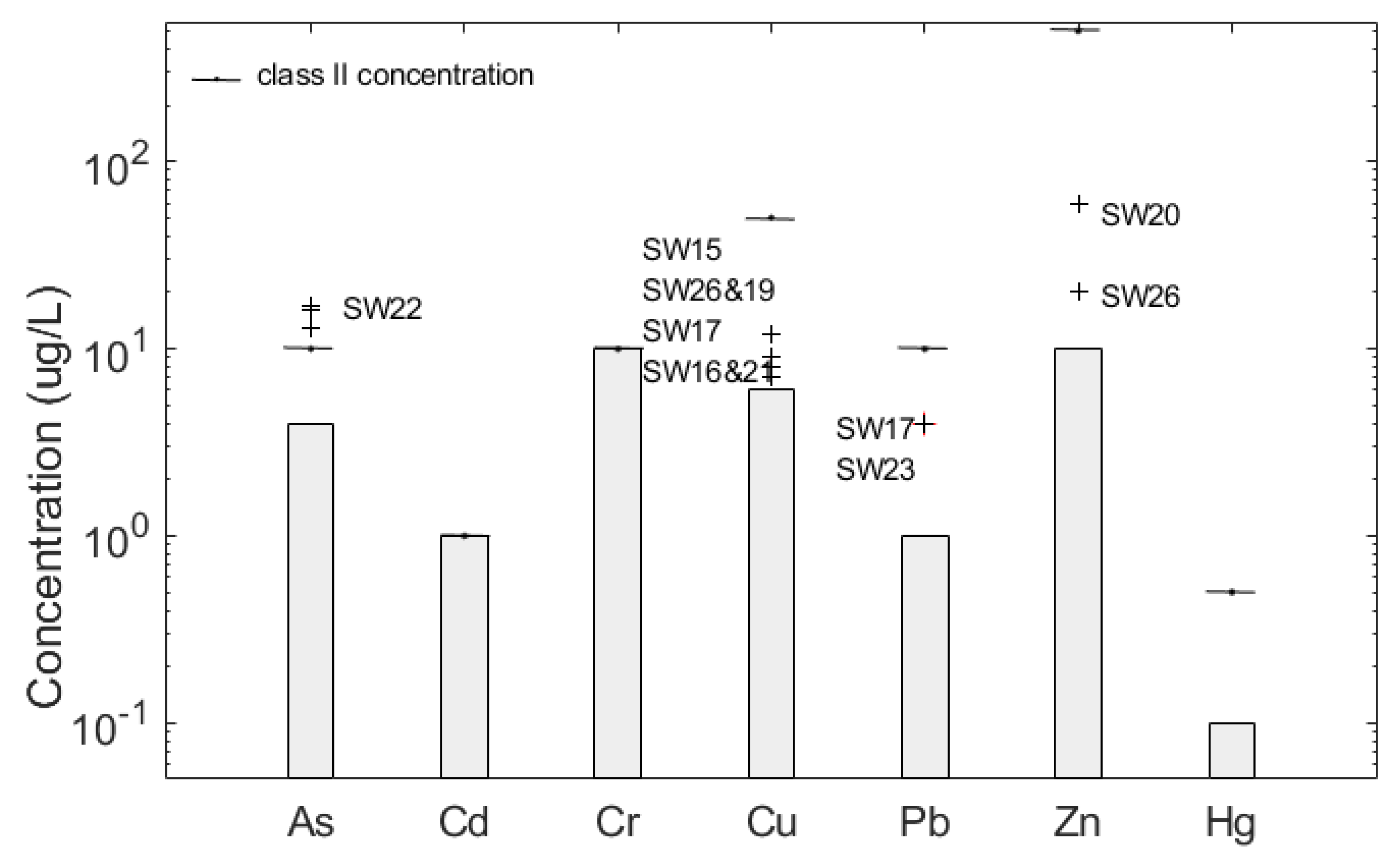
3.4.2. As, Cd, Cr, Cu, Pb, Zn and Hg
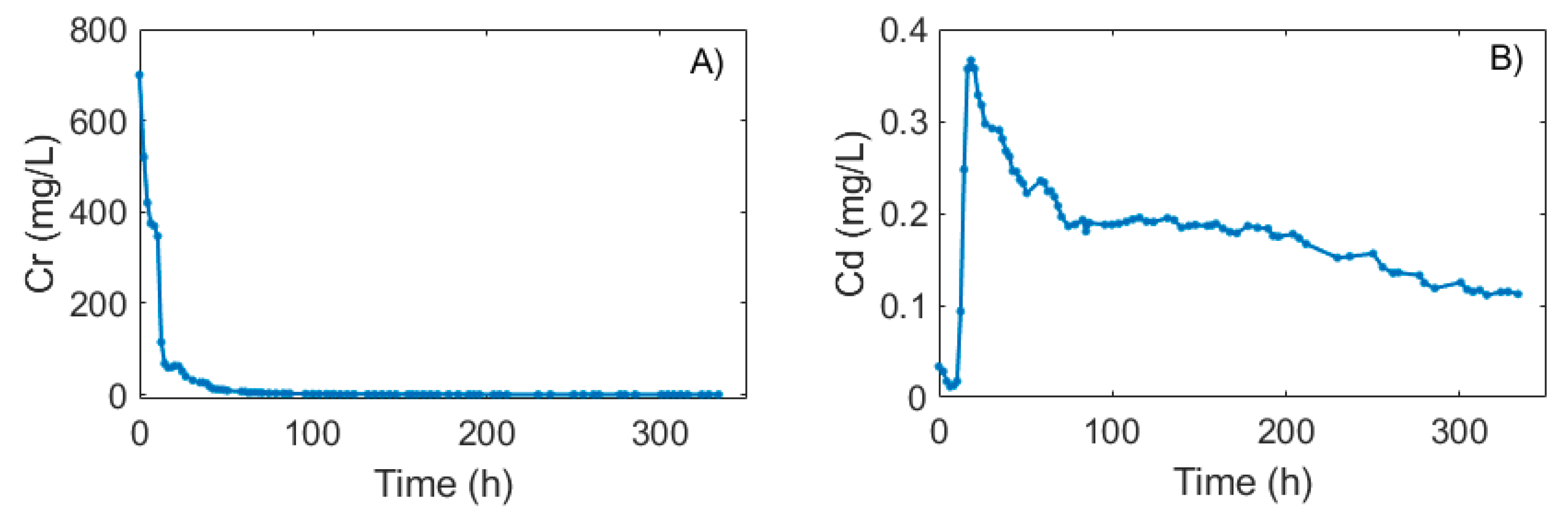
3.5. Column Experiment and Simulation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Montenegro, L.O. Environmental Trade-Offs from Coastal Reclamation: The Case of Cebu, Philippines. In Marine and Coastal Ecosystem Valuation, Institutions, and Policy in Southeast Asia; Springer: Singapore, 2016; pp. 225–238. [Google Scholar]
- Tian, B.; Wu, W.; Yang, Z.; Zhou, Y. Drivers, trends, and potential impacts of long-term coastal reclamation in China from 1985 to 2010. Estuar. Coast. Shelf Sci. 2016, 170, 83–90. [Google Scholar] [CrossRef]
- Goh, B.P.L.; Chou, L.M. Heavy metal levels in marine sediments of Singapore. Environ. Monit. Assess. 1997, 44, 67–80. [Google Scholar] [CrossRef]
- Jickells, T.D.; Andrews, J.E.; Parkes, D.J. Direct and indirect effects of estuarine reclamation on nutrient and metal fluxes in the global coastal zone. Aquat. Geochem. 2016, 22, 337–348. [Google Scholar] [CrossRef]
- Kim, R.; Kim, J.; Ryu, J.; Chang, H.W. Salinization properties of a shallow groundwater in a coastal reclaimed area, Yeonggwang, Korea. Environ. Geol. 2006, 49, 1180–1194. [Google Scholar] [CrossRef]
- Jiao, J.J.; Wang, X.; Nandy, S. Preliminary assessment of the impacts of deep foundations and land reclamation on groundwater flow in a coastal area in Hong Kong, China. Hydrogeol. J. 2006, 14, 100–114. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, H.; Huang, Q.; Zhang, Y.; Hu, M.; Niu, Y.; Zhu, J. Characterization and environmental impact analysis of sea land reclamation activities in China. Ocean Coast. Manag. 2016, 130, 128–137. [Google Scholar] [CrossRef]
- SOA (State Oceanic Administration, People’s Republic of China). Bulletin of China’s Marine Environment Status in 2015; SOA: Beijing, China, 2015. [Google Scholar]
- Wang, W.; Liu, H.; Li, Y.; Su, J. Development and management of land reclamation in China. Ocean Coast. Manag. 2014, 102, 415–425. [Google Scholar] [CrossRef]
- Chen, K.P.; Jiao, J.J. Metal concentrations and mobility in marine sediment and groundwater in coastal reclamation areas: A case study in Shenzhen. China Environ. Pollut. 2008, 151, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Economic and geographic backgrounds of land reclamation in Japanese. Mar. Pollut. Bull. 2003, 47, 226–229. [Google Scholar] [CrossRef]
- Bao, K.; Shen, J.; Sapkota, A. High-resolution enrichment of trace metals in a west coastal wetland of the southern Yellow Sea over the last 150 years. J. Geochem. Explor. 2017, 176, 136–145. [Google Scholar] [CrossRef]
- Barbier, E.B.; Koch, E.W.; Silliman, B.R.; Hacker, S.D.; Wolanski, E.; Primavera, J.; Granek, E.F.; Polasky, S.; Aswani, S.; Cramer, L.A.; et al. Coastal ecosystem-based management with nonlinear ecological functions and values. Science 2008, 319, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Kalnay, E.; Cai, M. Impact of urbanization and land-use change on climate. Nature 2003, 423, 528–531. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- POC (Pew Oceans Commission). America’s Living Oceans: Charting a Course for Sea Change; A Report to the Nation—Recommendations for a New Ocean Policy; POC: Arlington, VA, USA, 2003. [Google Scholar]
- U.S. Commission on Ocean Policy. (USCOP). Preliminary Report of the U.S. Commission on Ocean Policy; Governor’s Draft; USCOP: Washington, DC, USA, 2004. [Google Scholar]
- Boruvka, L.; Kozak, J. Geostatistical investigation of a reclaimed dumpsite soil with emphasis on aluminum. Soil Tillage Res. 2001, 59, 115–126. [Google Scholar] [CrossRef]
- Huang, J.; Huang, R.; Jiao, J.J.; Chen, K. Speciation and mobility of heavy metals in mud in coastal reclamation areas in Shenzhen, China. Environ. Geol. 2007, 53, 221–228. [Google Scholar] [CrossRef]
- Yang, J.; Pei, Y.; Tian, L.; Yuan, H. The influence of land reclamation in Tianjin Binhai new area on the environment of shallow groundwater in coastal low land. Geol. Bull. China 2016, 35, 1653–1660, (In Chinese with English Abstract). [Google Scholar]
- Hosono, T.; Su, C.C.; Okamura, K.; Taniguchi, M. Historical record of heavy metal pollution deduced by lead isotope ratios in core sediments from the Osaka Bay, Japan. J. Geochem. Explor. 2010, 107, 1–8. [Google Scholar] [CrossRef]
- Albanese, S.; De Vivo, B.; Lima, A.; Cicchella, D.; Civitillo, D.; Cosenza, A. Geochemical baselines and risk assessment of the Bagnoli brownfield site coastal sea sediments (Naples, Italy). J. Geochem. Explor. 2010, 105, 19–33. [Google Scholar] [CrossRef]
- Huang, Z.; Pan, X.D.; Wu, P.G.; Han, J.L.; Chen, Q. Health risk assessment of heavy metals in rice to the population in Zhejiang, China. PLoS ONE 2013, 8, e75007. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.D.; Wu, P.G.; Jiang, X. Levels and potential health risk of heavy metals in marketed vegetables in Zhejiang, China. Sci. Rep. 2016, 6, 20317. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Imai, N.; Tachibana, Y.; Ikehara, K. Statistical Analysis of the Spatial Distribution of Multi-Elements in an Island Arc Region: Complicating Factors and Transfer by Water Currents. Water 2017, 9, 37. [Google Scholar] [CrossRef]
- Bi, X.; Liu, F.; Pan, X. Coastal projects in China: From reclamation to restoration. Environ. Sci. Technol. 2012, 46, 4691–4692. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Zhang, Z.; Feng, G.; Huang, M.; Shi, X. Experimental Study on the Potential Use of Bundled Crop Straws as Subsurface Drainage Material in the Newly Reclaimed Coastal Land in Eastern China. Water 2018, 10, 31. [Google Scholar] [CrossRef]
- Hiscock, K.M. Hydrogeology: Principles and Practice; Blackwell Publishing: Malden, MA, USA, 2005. [Google Scholar]
- Xu, C.; Yu, Y. Review: Enclose tideland development in Zhejiang province. Zhejiang Hydrotechnol. 2003, 1, 8–10. [Google Scholar]
- Lin, F. The Public Climate Report of Zhoushan in May, 2015; Zhoushan Meteorological Administration: Zhejiang, China, 2016. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Elkhatib, E.A.; Hern, J.L. Kinetics of phosphorus desorption from Appalachian soils. Soil Sci. 1988, 145, 222–229. [Google Scholar] [CrossRef]
- Kuo, S.; Lotse, E.G. Kinetics of phosphate adsorption and desorption by hematite and gibbsite1. Soil Sci. 1973, 116, 400–406. [Google Scholar] [CrossRef]
- Khan, T.A.; Chaudhry, S.A.; Ali, I. Equilibrium uptake, isotherm and kinetic studies of Cd (II) adsorption onto iron oxide activated red mud from aqueous solution. J. Mol. Liq. 2015, 202, 165–175. [Google Scholar] [CrossRef]
- Kandpal, G.; Srivastava, P.C.; Ram, B. Kinetics of desorption of heavy metals from polluted soils: Influence of soil type and metal source. Water Air Soil Pollut. 2005, 161, 353–363. [Google Scholar] [CrossRef]
- Rahman, M.; Sathasivam, K.V. Heavy metal adsorption onto Kappaphycus sp. from aqueous solutions: The use of error functions for validation of isotherm and kinetics models. BioMed Res. Int. 2015, 2015, 126298. [Google Scholar] [CrossRef] [PubMed]
- Reyhanitabar, A.; Karimian, N. Kinetics of copper desorption of selected calcareous soils from Iran. Am Eur. J. Agric. Environ. Sci. 2008, 4, 287–293. [Google Scholar]
- Dang, Y.P.; Dalal, R.C.; Edwards, D.G.; Tiller, K.G. Kinetics of Zinc desorption from vertisols. Soil Sci. Soc. Am. J. 1994, 58, 1392–1399. [Google Scholar] [CrossRef]
- Winslow, A.G.; Kister, L.R. Saline-Water Resources of Texas (No. 1365); US Government Printing Office: Washington, DC, USA, 1956. [Google Scholar]
- Cartwright, I.; Weaver, T.R.; Fifield, L.K. Cl/Br ratios and environmental isotopes as indicators of recharge variability and groundwater flow: An example from the southeast Murray Basin, Australia. Chem. Geol. 2006, 231, 38–56. [Google Scholar] [CrossRef]
- Alcalá, F.J.; Custodio, E. Using the Cl/Br ratio as a tracer to identify the origin of salinity in aquifers in Spain and Portugal. J. Hydrol. 2008, 359, 189–207. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Freeman, J.T. The use of bromide and chloride mass ratios to differentiate salt-dissolution and formation brines in shallow groundwaters of the Western Canadian Sedimentary Basin. Hydrogeol. J. 2007, 15, 1377–1385. [Google Scholar] [CrossRef]
- Berner, E.K.; Berner, R.A. Global Water Cycle: Geochemistry and Environment; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1987; p. 397. [Google Scholar]
- Lin, Y.; Liu, S.; Lei, J.; Zhang, G.; Mao, X.; Zhang, J. Characteristic Analysis on the Anions and pH Profiles of Rainwater of Zhoushan Archipelago. Environ. Sci. 2005, 26, 49–54. (In Chinese) [Google Scholar]
- Lin, Y.; Liu, S.; Lei, J.; Bi, Y.; Zhang, J. Linear discriminate analysis on annual and seasonal characteristics of major cations’ profiles of rainwater at Zhoushan rain-sampling spot. Environ. Sci. 2006, 27, 1992–1997. (In Chinese) [Google Scholar]
- Muyzer, G.; Stams, A.J. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Siemann, M.G. Extensive and rapid changes in seawater chemistry during the Phanerozoic: Evidence from Br contents in basal halite. Terra Nova 2003, 15, 243–248. [Google Scholar] [CrossRef]
- Leybourne, M.I.; Goodfellow, W.D. Br/Cl ratios and O, H, C, and B isotopic constraints on the origin of saline waters from eastern Canada. Geochim. Cosmochim. Acta 2007, 71, 2209–2223. [Google Scholar] [CrossRef]
- Edmunds, W.M.; Ma, J.Z.; Aeschbach-Hertig, W.; Kipfer, R.; Darbyshire, D.P. Groundwater recharge history and hydrogeochemical evolution in the Minqin Basin, North West China. Appl. Geochem. 2006, 21, 2148–2170. [Google Scholar] [CrossRef]
- Davis, S.N.; Whittemore, D.O.; Fabryka-Martin, J. Uses of chloride/bromide ratios in studies of potable water. Groundwater 1998, 36, 338–350. [Google Scholar] [CrossRef]
- Custodio, E.; Herrera, C. Utilización de la relación Cl/Br como trazador hidroquímico en hidrología subterránea. Bol. Geol. Min. 2000, 111, 49–68. [Google Scholar]
- Edmunds, W.M.; Milne, C. (Eds.) Paleowaters in Coastal Europe: Evolution of Groundwater since the Late Pleistocene; Special Publications 189; Geological Society: London, UK, 2001; pp. 1–16. [Google Scholar]
- Fuge, R.; Power, G.M. Chlorine and fluorine in granitic rocks from SW England. Geochim. Cosmochim. Acta 1969, 33, 888–893. [Google Scholar] [CrossRef]
- Nissenbaum, A.; Magaritz, M. Bromine-rich groundwater in the Hula Valley, Israel. Naturwissenschaften 1991, 78, 217–218. [Google Scholar] [CrossRef]
- Bouchard, D.C.; Williams, M.K.; Surampallu, R.Y. Nitrate contamination of groundwater: Sources and potential health effects. J. Am. Water Works Assoc. 1992, 84, 85–90. [Google Scholar] [CrossRef]
- O’Connor, J.T. Iron and Manganese. In Water Quality and Treatment—A Handbook of Public Water Supplies; McGraw Hill Book Company: New York, NY, USA, 1971; Chapter 11; pp. 378–396. [Google Scholar]
- USEPA (United States Environmental Protection Agency). Prediction of Sediment Toxicity Using Consensus-Based Freshwater Sediment Quality Guidelines; EPA 905/R-00/007; Great Lakes National Program Office: Chicago, IL, USA, 2000.
- Morillo, J.; Usero, J.; Gracia, I. Heavy metal distribution in marine sediments from Southwest coast of Spain. Chemosphere 2004, 55, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Rainbow, P.S. Biomonitoring of heavy metal availabity in the marine environment. Mar. Pollut. Bull. 1995, 31, 183–192. [Google Scholar] [CrossRef]
- Yu, K.T.; Lam, M.H.W.; Yen, Y.F.; Leung, A.P. Behavior of trace metals in the sediment pore waters of intertidal mudflats of a tropical wetlands. Environ. Toxicol. Chem. 2000, 19, 535–542. [Google Scholar] [CrossRef]
- Li, X.; Wai, O.W.H.; Li, Y.S.; Coles, B.J.; Ramsey, M.H.; Thornton, I. Heavy metal distribution in sediment profiles of the Pearl River estuary, South China Sea. Appl. Geochem. 2000, 15, 567–581. [Google Scholar] [CrossRef]
- Libes, S.M. Introduction to Marine Biogeochemistry; John Wiley and Sons: New York, NY, USA, 1992. [Google Scholar]
- Nduka, J.K.; Orisakwe, O.E. Water-quality issues in the Niger Delta of Nigeria: A look at heavy metal levels and some physicochemical properties. Environ. Sci. Pollut. Res. 2011, 18, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Abrahim, G.M.; Parker, R.J. Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Yamaki Estuary, Auckland, New Zealand. Environ. Monit. Assess. 2008, 136, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.A.; Jansen, B.; Kalbitz, K.; Faz, A. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Coles, N.A.; Wu, C.; Wu, J. Spatial variability of heavy metals in the coastal soils under long-term reclamation. Estuar. Coast. Shelf Sci. 2014, 151, 310–317. [Google Scholar] [CrossRef]
- MLR (Ministry of Land and Resources of the People’s Republic of China). Quality Standard for Ground Water; GB/T 14848-2017; MLR: Beijing, China, 2017.
- AQSIQ (General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China). Marine Sediment Quality; GB 18668-2002; AQSIQ: Beijing, China, 2002.
- Krishnamurti, G.S.R.; Huang, P.M.; Kozak, L.M. Sorption and desorption kinetics of cadmium from soils: Influence of phosphate. Soil Sci. 1999, 164, 888–898. [Google Scholar] [CrossRef]
- Ogwada, R.A.; Sparks, D.L. A critical evaluation on the use of kinetics for determining thermodynamics of ion exchange in soils. Soil Sci. Soc. Am. J. 1986, 50, 300–305. [Google Scholar] [CrossRef]
- Toor, G.S.; Bahl, G.S. Kinetics of phosphate desorption from different soils as influenced by application of poultry manure and fertilizer phosphorus and its uptake by soybean. Bioresour. Technol. 1999, 69, 117–121. [Google Scholar] [CrossRef]

| Grain Size | 2–0.25 mm | 0.25–0.05 mm | 0.05–0.01 mm | 0.01–0.005 mm | 0.005–0.001 mm | <0.001 mm |
|---|---|---|---|---|---|---|
| Percentage (%) | 48.47 | 25.6 | 8.4 | 6.21 | 7.03 | 4.3 |
| Parameter | Statistics | SW13 | SW14 | SW15 | SW16 | SW17 | SW18 | SW19 | SW20 | SW21 | SW22 | SW23 | SW24 | SW25 | SW26 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | Max | 7.89 | 7.70 | 8.00 | 8.01 | 7.89 | 7.90 | 7.82 | 7.96 | 7.79 | 7.92 | 7.93 | 8.31 | 8.08 | 7.69 |
| Min | 7.37 | 6.95 | 7.35 | 7.66 | 7.51 | 7.60 | 7.52 | 7.50 | 7.51 | 7.70 | 7.55 | 7.63 | 7.37 | 7.21 | |
| Mean | 7.66 | 7.18 | 7.67 | 7.79 | 7.68 | 7.72 | 7.70 | 7.76 | 7.69 | 7.82 | 7.71 | 8.11 | 7.78 | 7.52 | |
| s.d. | 0.24 | 0.57 | 0.28 | 0.16 | 0.17 | 0.12 | 0.09 | 0.22 | 0.08 | 0.06 | 0.17 | 0.49 | 0.36 | 0.26 | |
| TDS | Max | 14.10 | 10.54 | 25.17 | 24.53 | 13.80 | 24.08 | 19.92 | 18.10 | 21.51 | 16.70 | 10.36 | 18.80 | 24.10 | 24.75 |
| Min | 2.58 | 5.96 | 5.88 | 2.08 | 3.99 | 13.38 | 7.35 | 17.20 | 11.55 | 11.87 | 2.57 | 12.05 | 1.37 | 22.90 | |
| Mean | 6.20 | 7.43 | 17.54 | 22.19 | 8.92 | 21.87 | 15.72 | 17.69 | 19.75 | 13.04 | 5.29 | 17.11 | 10.90 | 23.90 | |
| s.d. | 142.04 | 18.11 | 590.64 | 443.68 | 146.29 | 88.11 | 137.28 | 0.78 | 90.80 | 17.86 | 38.43 | 56.20 | 612.19 | 3.37 | |
| Conductivity | Max | 24.30 | 18.20 | 43.40 | 42.80 | 24.20 | 40.50 | 33.70 | 31.23 | 37.03 | 28.75 | 17.86 | 32.40 | 44.30 | 42.60 |
| Min | 4.47 | 8.31 | 9.71 | 40.85 | 6.88 | 35.70 | 12.69 | 29.15 | 28.90 | 20.50 | 4.44 | 21.00 | 2.28 | 39.50 | |
| Mean | 12.67 | 12.79 | 32.28 | 41.69 | 16.19 | 38.84 | 26.50 | 30.32 | 35.06 | 22.16 | 8.97 | 29.83 | 20.93 | 41.02 | |
| s.d. | 430.78 | 64.49 | 1439.77 | 3.51 | 430.37 | 20.33 | 337.03 | 3.55 | 46.53 | 50.40 | 113.76 | 134.05 | 2148.07 | 8.48 |
| Well Label | Ca2+ (mg/L) | Mg2+ (mg/L) | Na+ (mg/L) | K+ (mg/L) | HCO3− (mg/L) | SO42− (mg/L) | Cl− (mg/L) |
|---|---|---|---|---|---|---|---|
| SW13 | 89 | 75 | 756 | 34 | 196 | 280 | 1139 |
| 86 | 81 | 646 | 31 | 162 | 240 | 1130 | |
| 84 | 69 | 571 | 33 | 165 | 219 | 1009 | |
| 34 | 16 | 124 | 11 | 127 | 76 | 196 | |
| SW14 | 132 | 202 | 1861 | 61 | 144 | 432 | 3092 |
| 157 | 256 | 2080 | 66 | 165 | 550 | 3852 | |
| 180 | 299 | 2305 | 86 | 168 | 625 | 4497 | |
| 177 | 281 | 2235 | 82 | 184 | 592 | 4482 | |
| SW15 | 158 | 173 | 1812 | 68 | 184 | 605 | 2865 |
| 260 | 592 | 4740 | 176 | 186 | 1255 | 8502 | |
| 261 | 986 | 7523 | 301 | 1601 | 220 | 14,400 | |
| 144 | 320 | 2617 | 103 | 324 | 645 | 5065 | |
| SW16 | 246 | 768 | 6900 | 238 | 998 | 598 | 10,988 |
| 202 | 946 | 7720 | 268 | 1587 | 100 | 14,100 | |
| 211 | 974 | 7439 | 280 | 1544 | 95 | 14,300 | |
| 201 | 936 | 7436 | 282 | 1505 | 101 | 14,200 | |
| SW17 | 180 | 176 | 1845 | 65 | 187 | 580 | 2883 |
| 208 | 328 | 2700 | 95 | 276 | 768 | 4860 | |
| 154 | 156 | 1236 | 55 | 288 | 584 | 2202 | |
| 112 | 129 | 1182 | 52 | 284 | 479 | 1984 | |
| SW18 | 352 | 720 | 6540 | 212 | 714 | 830 | 10,735 |
| 218 | 878 | 7550 | 248 | 1475 | 248 | 13,500 | |
| 204 | 909 | 7208 | 269 | 1464 | 155 | 13,700 | |
| 194 | 841 | 7051 | 261 | 1607 | 83 | 13,300 | |
| SW19 | 343 | 733 | 6350 | 221 | 171 | 1590 | 10,499 |
| 210 | 392 | 3300 | 120 | 207 | 938 | 5848 | |
| 315 | 663 | 4906 | 191 | 194 | 1375 | 9485 | |
| 238 | 496 | 3928 | 154 | 191 | 1070 | 7504 | |
| SW20 | 216 | 578 | 5900 | 196 | 835 | 328 | 9828 |
| 173 | 608 | 5680 | 194 | 808 | 154 | 10,700 | |
| 181 | 610 | 5431 | 208 | 877 | 93 | 10,500 | |
| 175 | 604 | 5660 | 214 | 905 | 74 | 10,500 | |
| SW21 | 194 | 708 | 7060 | 232 | 1305 | 446 | 11,532 |
| 206 | 766 | 6510 | 230 | 1328 | 525 | 12,000 | |
| 201 | 748 | 6200 | 229 | 1261 | 475 | 11,900 | |
| 193 | 736 | 6442 | 238 | 1353 | 412 | 11,800 | |
| SW22 | 99 | 390 | 4620 | 153 | 1429 | 34 | 7253 |
| 98 | 383 | 4195 | 147 | 1432 | <1 | 7075 | |
| 98 | 380 | 3900 | 151 | 1384 | <1 | 7086 | |
| 97 | 390 | 4114 | 156 | 1438 | <1 | 7160 | |
| SW23 * | 175 | 168 | 1878 | 60 | 190 | 560 | 2865 |
| 41 | 30 | 333 | 24 | 299 | 79 | 463 | |
| SW24 | 193 | 472 | 4550 | 178 | 617 | 554 | 7489 |
| 105 | 640 | 6150 | 220 | 10 | 6 | 10,900 | |
| 80 | 628 | 5816 | 222 | 953 | 5 | 11,000 | |
| 115 | 620 | 5855 | 223 | 1023 | 25 | 10,800 | |
| SW25 | 107 | 68 | 705 | 36 | 154 | 346 | 1008 |
| 314 | 972 | 7500 | 264 | 667 | 1355 | 13,700 | |
| 63 | 37 | 349 | 25 | 149 | 235 | 556 | |
| 106 | 205 | 1634 | 67 | 248 | 434 | 3085 | |
| SW26 | 482 | 930 | 7740 | 212 | 726 | 1030 | 13,491 |
| 458 | 950 | 7290 | 210 | 631 | 965 | 13,600 | |
| 461 | 942 | 6701 | 214 | 667 | 938 | 13,900 | |
| 472 | 951 | 7030 | 219 | 680 | 910 | 13,600 | |
| River * | 10 | 4 | 25 | 5 | 54 | 19 | 28 |
| 11 | 4 | 20 | 2 | 41 | 18 | 27 | |
| Seawater * | 298 | 902 | 7270 | 282 | 112 | 1785 | 13,200 |
| 318 | 983 | 7528 | 326 | 133 | 1865 | 14,100 |
| Area | Chemical Composition | Characteristics of Subdivision of Diamond Shaped Fields | % Sample |
|---|---|---|---|
| 1 | Ca + Mg > Na + K | Alkaline earths exceeds alkalies | - |
| 2 | Na + K > Ca + Mg | Alkalies exceed alkaline earths | 100 |
| 3 | CO3 + HCO3 > SO4 + Cl | Weak acids exceed strong acids | - |
| 4 | CO3 + HCO3 < SO4 + Cl | Strong acids exceed weak acids | 100 |
| 5 | Ca - Mg - HCO3 | Alkaline earths and weak acids are dominated over the alkalies and strong acids. Carbonate hardness exceeds 50% | - |
| 6 | Ca - Mg - SO4 | Non-carbonate hardness exceeds 50% | - |
| 7 | Na - Cl | Non-carbonate alkali exceeds 50% | 100 |
| 8 | Na - HCO3 | Carbonate alkali exceeds 50% | - |
| 9 | Mixed | None of the cation and anion pairs exceed 50% | - |
| Metal Concentrations (mg/kg) | D01 | D03 | D08 | D10 | D14 | D16 | D24 | D26 | D37 |
|---|---|---|---|---|---|---|---|---|---|
| As | 5.09(I) | 5.39(I) | 5.8(I) | 5.3(I) | 5.52(I) | 6.17(I) | 6.87(I) | 4.78(I) | 6.02(I) |
| Cd | 0.79(II) | 2.56(III) | 1.68(III) | 0.48(I) | 3.08(III) | 1.64(III) | 0.25(I) | 0.28(I) | 2.36(III) |
| Cr | 132(II) | 140(II) | 130(II) | 129(II) | 140(II) | 143(II) | 144(II) | 122(II) | 130(II) |
| Cu | 16(I) | 13(I) | 18(I) | 18(I) | 19(I) | 18(I) | 20(I) | 12(I) | 20(I) |
| Pb | 33(I) | 23(I) | 34(I) | 31(I) | 39(I) | 43(I) | 24(I) | 31(I) | 26(I) |
| Zn | 53(I) | 57(I) | 34(I) | 71(I) | 62(I) | 57(I) | 270(II) | 61(I) | 63(I) |
| Hg | 0.002(I) | 0.055(I) | 0.188(I) | 0.201(I) | 0.002(I) | 0.002(I) | 5.25(III) | 0.002(I) | 1.28(III) |
| Metal Concentrations (mg/kg) | SW16 | SW18 | SW20 | SW22 | SW24 | SW26 |
|---|---|---|---|---|---|---|
| Moisture content (%) | 27.2 | 26.6 | 30 | 31.7 | 28.6 | 23.8 |
| As | 8.0(I) | 9.0(I) | 14.0(I) | 17.0(I) | 10.0(I) | 10.0(I) |
| Cd | <0.3(I) | <0.3(I) | 0.3(I) | 0.4(I) | 0.4(I) | <0.3(I) |
| Cr | 83.1(II) | 80.3(II) | 89.4(II) | 92.6(II) | 92.4(II) | 65.7(I) |
| Cu | 22.2(I) | 21.6(I) | 30.6(I) | 30.0(I) | 30.5(I) | 26.3(I) |
| Pb | 23.1(I) | 24.6(I) | 28.5(I) | 28.8(I) | 32.0(I) | 32.5(I) |
| Zn | 91.9(I) | 93.4(I) | 102.0(I) | 111.0(I) | 110.0(I) | 102.0(I) |
| Hg | <0.05(I) | <0.05(I) | 0.05(I) | <0.05(I) | 0.05(I) | <0.05(I) |
| Layer | Cr (mg/kg) | Cd (mg/kg) |
|---|---|---|
| Upper (0–5 cm) | 109 | 14.6 |
| Middle (10–15 cm) | 90 | 13.8 |
| Lower (21–26 cm) | 92.8 | 6.01 |
| Metal | Peseudo Second Order | Evolch | Two Constant Rate | |||
|---|---|---|---|---|---|---|
| R2 | SE | R2 | SE | R2 | SE | |
| Cr | 0.85 | 0.11 | 0.59 | 0.06 | 0.89 | 0.03 |
| Cd | 0.95 | 6.30 × 10−3 | 0.91 | 1.30 × 10−4 | 0.98 | 3.18 × 10−5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Hu, B.X.; Wang, P.; Chen, J.; Yang, L.; Xiao, K.; Zhang, X. Hydrogeochemical Evolution and Heavy Metal Contamination in Groundwater of a Reclaimed Land on Zhoushan Island. Water 2018, 10, 316. https://doi.org/10.3390/w10030316
Zhang X, Hu BX, Wang P, Chen J, Yang L, Xiao K, Zhang X. Hydrogeochemical Evolution and Heavy Metal Contamination in Groundwater of a Reclaimed Land on Zhoushan Island. Water. 2018; 10(3):316. https://doi.org/10.3390/w10030316
Chicago/Turabian StyleZhang, Xiaoying, Bill X. Hu, Peng Wang, Junbing Chen, Lei Yang, Kai Xiao, and Xiaowei Zhang. 2018. "Hydrogeochemical Evolution and Heavy Metal Contamination in Groundwater of a Reclaimed Land on Zhoushan Island" Water 10, no. 3: 316. https://doi.org/10.3390/w10030316
APA StyleZhang, X., Hu, B. X., Wang, P., Chen, J., Yang, L., Xiao, K., & Zhang, X. (2018). Hydrogeochemical Evolution and Heavy Metal Contamination in Groundwater of a Reclaimed Land on Zhoushan Island. Water, 10(3), 316. https://doi.org/10.3390/w10030316





