Characterization of the Adsorption of Cu (II) from Aqueous Solutions onto Pyrolytic Sludge-Derived Adsorbents
Abstract
1. Introduction
2. Materials and Methods
2.1. Sludge Collection and Preparation
2.2. Batch Adsorption Experiments
2.3. Analytical Methods and Characterization
3. Results and Analysis
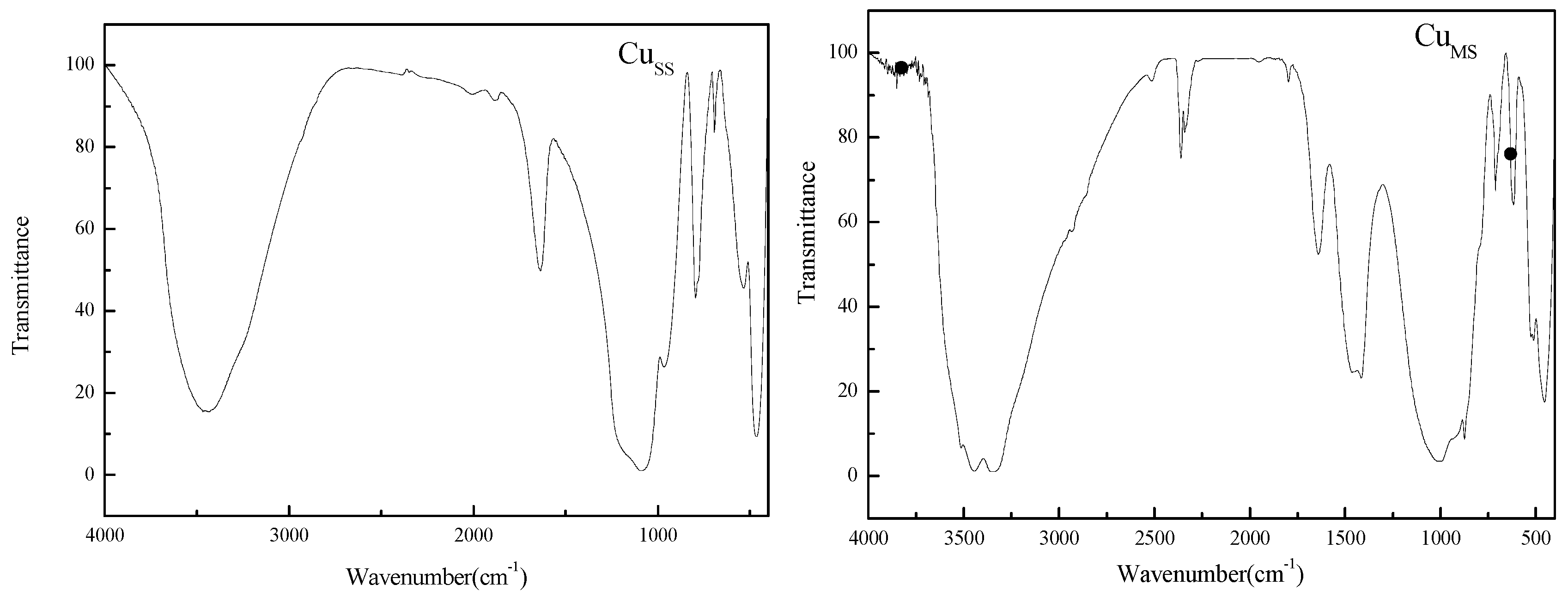
3.1. Physical and Chemical Properties and Leaching Toxicity Characteristics of Adsorbents
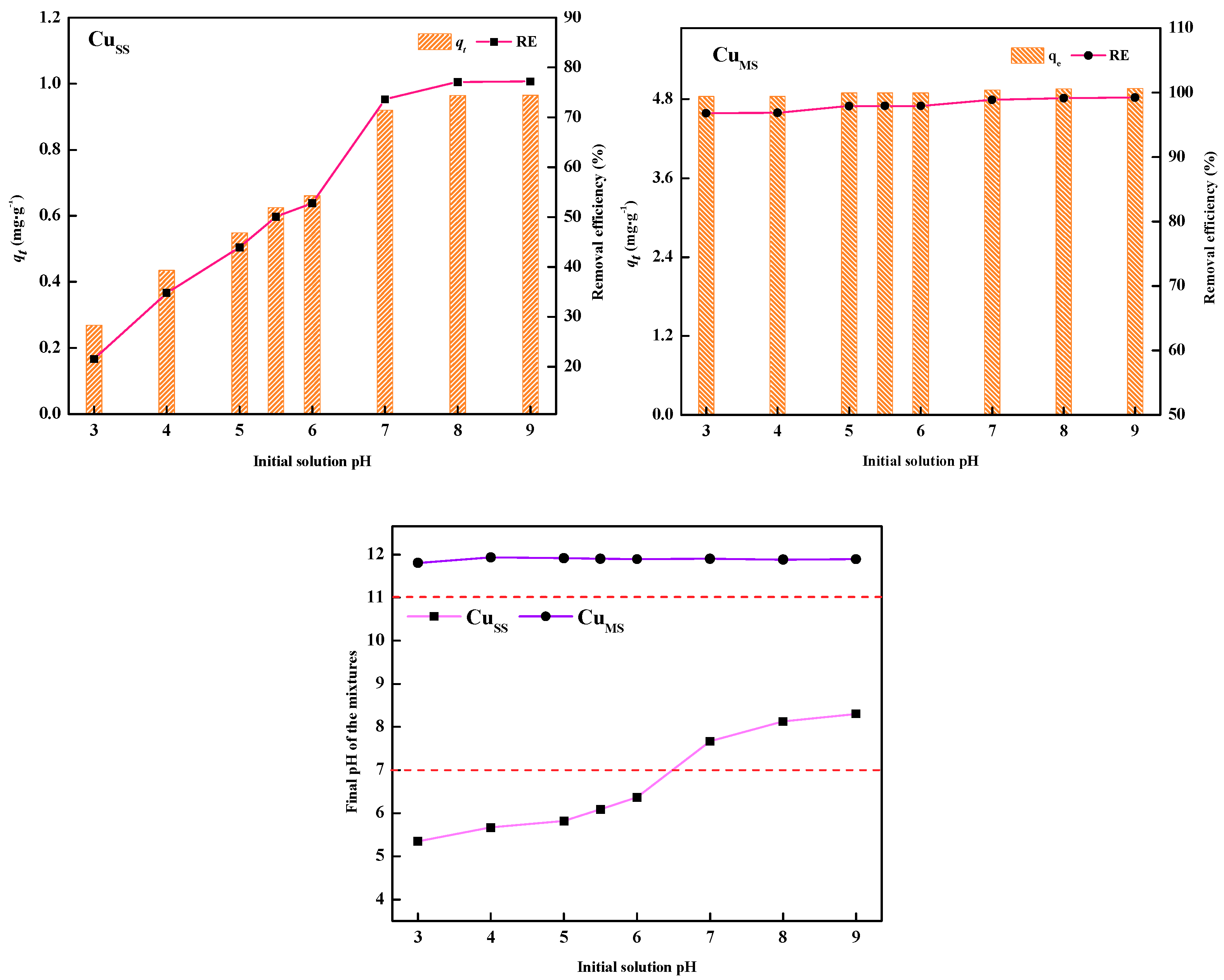
3.2. Effect of pH on Adsorption
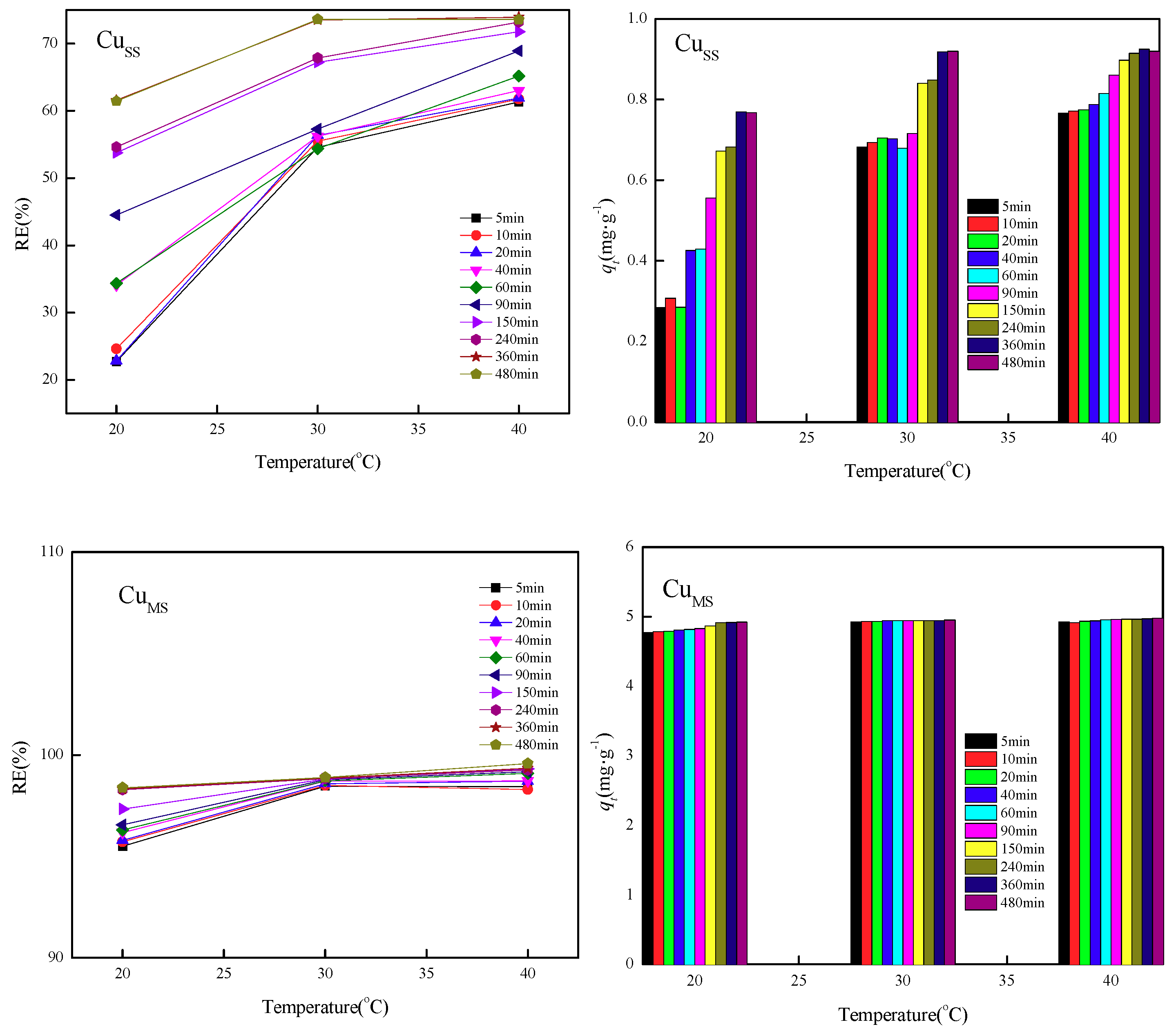
3.3. Effect of Constant Temperature on Adsorption
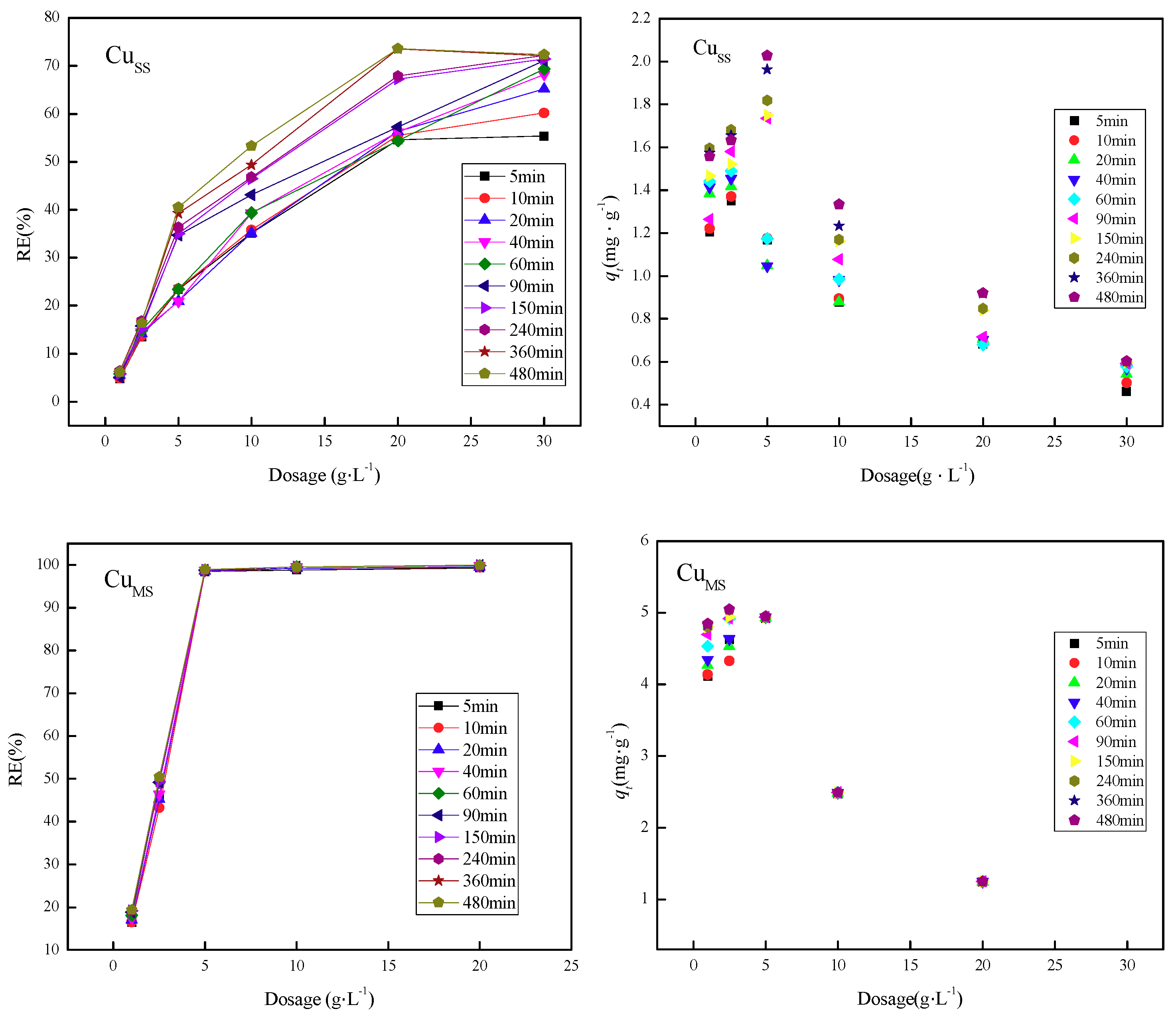
3.4. Effect of Adsorption Dosage on Adsorption
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Björklund, K.; Li, L.Y. Adsorption of organic stormwater pollutants onto activated carbon from sewage sludge. J. Environ Manag. 2017, 197, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Monárrez-Cordero, B.E.; Amézaga-Madrid, P.; Fuentes-Cobas, L.; Montero-Cabrera, M.E.; Miki-Yoshida, M. High and fast adsorption efficiency of simultaneous As+3, As+5, and F-, by Al-doped magnetite synthesized via AACVD. J. Alloys Compd. 2017, 718, 414–424. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Z.; Wang, J.; Yue, T.; Li, R.; Li, Z. Adsorption of Cd (II) and Pb (II) by in situ, oxidized Fe3O4, membrane grafted on 316L porous stainless steel filter tube and its potential application for drinking water treatment. J. Environ Manag. 2017, 196, 127–136. [Google Scholar] [CrossRef]
- Nielsen, L.; Zhang, P.; Bandosz, T.J. Adsorption of carbamazepine on sludge/fish waste derived adsorbents: Effect of surface chemistry and texture. Biochem. Eng. J. 2015, 267, 170–181. [Google Scholar] [CrossRef]
- Podstawczyk, D.; Witek-Krowiak, A.; Dawiec-Liśniewska, A.; Chrobot, P.; Skrzypczak, D. Removal of ammonium and orthophosphates from reject water generated during dewatering of digested sewage sludge in municipal wastewater treatment plant using adsorption and membrane contactor system. J. Clean. Prod. 2017, 161, 277–287. [Google Scholar] [CrossRef]
- Sanganyado, E.; Fu, Q.; Gan, J. Enantiomeric selectivity in adsorption of chiral β-blockers on sludge. Environ. Pollut. 2016, 214, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.M.; Zhou, F.P.; Bi, X.L.; Chen, D.D.; Li, J.; Sun, S.Y.; Chen, X.Q. Accelerated crystallization of magnetic 4A-zeolite synthesized from red mud for application in removal of mixed heavy metal ions. J. Hazard. Mater. 2018, 358, 441–449. [Google Scholar] [CrossRef]
- Wei, L.; Yang, L.; Noguera, D.R.; Zhao, N.; Yue, S.; Jing, D.; Zhao, Q.; Cui, F. Adsorption of Cu2+, and Zn2+, by extracellular polymeric substances (EPS) in different sludges: Effect of EPS fractional polarity on binding mechanism. J. Hazard. Mater. 2017, 321, 473–483. [Google Scholar] [CrossRef]
- Liu, J.Y.; Ning, X.N.; Yang, Z.Y. Study of adsorbent derived from sewage sludge incineration residue for the removal of Ni2+ in wastewater. Key Eng. Mater. 2011, 474–476, 158–161. [Google Scholar] [CrossRef]
- Pei, Y.Y.; Liu, J.Y. Adsorption of Pb2+ in wastewater using adsorbent derived from grapefruit peel. Adv. Compos. Mater. Res. 2011, 391–392, 968–972. [Google Scholar] [CrossRef]
- Ferreira, C.I.; Calisto, V.; Otero, M.; Nadais, H.; Esteves, V.I. Removal of tricaine methanesulfonate from aquaculture wastewater by adsorption onto pyrolysed paper mill sludge. Chemosphere 2017, 168, 139–146. [Google Scholar] [CrossRef]
- Rashed, M.N.; El-Daim, M.A.; Taher, E.; Fadlalla, S.M.M. Adsorption of methylene blue using modified adsorbents from drinking water treatment sludge. Water Sci. Technol. 2016, 74, 1885–1898. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, G.; Cavasotto, J.; Jr, M.F.; Colpani, G.L.; Magro, J.D.; Dalcanton, F.; Mello, J.M.; Fiori, M.A. An adsorbent with a high adsorption capacity obtained from the cellulose sludge of industrial residues. Chemosphere 2017, 169, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.L.; Ronix, A.; Pezoti, O.; Souza, L.S.; Leandro, P.K.T.; Bedin, K.C.; Beltrame, K.K.; Cazetta, A.L.; Almeida, V.C. Mesoporous activated carbon from industrial laundry sewage sludge: Adsorption studies of reactive dye Remazol Brilliant Blue R. Chem. Eng. J. 2016, 303, 467–476. [Google Scholar] [CrossRef]
- Jaria, G.; Calisto, V.; Gil, M.V.; Otero, M.; Esteves, V.I. Removal of fluoxetine from water by adsorbent materials produced from paper mill sludge. J. Colloid Interface Sci. 2015, 448, 32–40. [Google Scholar] [CrossRef]
- Zielińska, A.; Oleszczuk, P. Evaluation of sewage sludge and slow pyrolyzed sewage sludge-derived biochar for adsorption of phenanthrene and pyrene. Bioresour. Technol. 2015, 192, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Nelson, P.F. Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum). Chemosphere 2010, 78, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Saroha, A.K. Simultaneous adsorption and dechlorination of pentachlorophenol from effluent by Ni-ZVI magnetic biochar composites synthesized from paper mill sludge. Chem. Eng. J. 2015, 271, 195–203. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.J. Synthesis of magnetically separable and recyclable magnetic nanoparticles decorated with β-cyclodextrin functionalized graphene oxide an excellent adsorption of As (V)/(III). J. Mol. Liq. 2017, 237, 387–401. [Google Scholar] [CrossRef]
- Chaudhry, S.A.; Zaidi, Z.; Siddiqui, S.I. Isotherm, kinetic and thermodynamics of arsenic adsorption onto Iron-Zirconium Binary Oxide-Coated Sand (IZBOCS): Modelling and process optimization. J. Mol. Liq. 2017, 229, 230–240. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, P.; Li, X.; Zhang, G. Efficient adsorption of radioactive iodide ion from simulated wastewater by nano Cu2O/Cu modified activated carbon. Chem. Eng. J. 2017, 322, 129–139. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, Y.; Shukla, A.; Shukla, S.S.; Dorris, K.L. The removal of heavy metal from aqueous solutions by sawdust adsorption—removal of copper. J. Hazard. Mater. 2000, 80, 33–42. [Google Scholar] [CrossRef]
- Karabulut, S.; Karabakan, A.; Denizli, A.; Yürüm, Y. Batch removal of copper(II) and zinc(II) from aqueous solutions with low-rank Turkish coals. Sep. Purif. Technol. 2000, 18, 177–184. [Google Scholar] [CrossRef]
- Haoutia, R.E.; Anfara, Z.; Chennaha, A.; Amaterz, E.; Zbair, M.; Alem, N.E.; Benlhachemi, A.; Ezahria, M. Synthesis of sustainable mesoporous treated fish waste as adsorbent for copper removal. Groundwater Sustain. Dev. 2019, 8, 1–9. [Google Scholar] [CrossRef]
- Shim, J.; Shea, P.J.; Oh, B.T. Stabilization of heavy metals in mining site soil with silica extracted from corn cob. Water Air Soil Pollut. 2014, 225, 1–12. [Google Scholar] [CrossRef]
- Gyollai, I.; Krebsz, M.; Kereszturi, Á.; Bérczi, S.; Gucsik, A. FTIR-ATR spectroscopy of shock vein in mócs L6 chondrite. J. Geophys. Res. 2010, 116, 27–36. [Google Scholar]
- Lei, C.; Yan, B.; Chen, T.; Xiao, XM. Preparation and adsorption characteristics for heavy metals of active silicon adsorbent from leaching residue of lead-zinc tailings. Environ. Sci. Pollut. Res. 2018, 25, 21233–21242. [Google Scholar] [CrossRef]

| Chemical Composition | Al2O3 | CaO | Fe2O3 | K2O | MgO | MnO | Na2O | P2O5 | SiO2 | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| CuSS (wt %) | 18.41 | 3.59 | 7.97 | 3.93 | 2.72 | 0.06 | 0.78 | 10.42 | 51.22 | 0.92 |
| CuMS (wt %) | 15.95 | 50.34 | 0.84 | 0.35 | 2.17 | 0.03 | 0.42 | 0.96 | 28.41 | 1.03 |
| Materials | Heavy Metals | ||||||
|---|---|---|---|---|---|---|---|
| Cr | Ni | Cu | Pb | Mn | Zn | ||
| CuSS | total contents (mg kg−1) | 49.6 | 74.0 | 190 | 82.4 | 214 | 909 |
| leaching concentration (mg L−1) | 8.19 | 4.10 | 5.69 | ND 1 | 10.1 | 1.27 | |
| CuMS | total contents (mg kg−1) | 19.4 | 103 | 175 | ND | 161 | 717 |
| leaching concentration (mg L−1) | 1.32 | 3.30 | 6.41 | ND | 7.40 | 3.89 | |
| Threshold values for total contents 2 | pH ≥ 6.5 (mg kg−1) | 1000 | 200 | 500 | 1000 | NA | 1000 |
| pH < 6.5 (mg kg−1) | 600 | 100 | 250 | 300 | NA | 500 | |
| Permissible limits for leaching toxicity 3 (mg kg−1) | 15.0 | 5.00 | 100 | 5.00 | NA | 100 | |
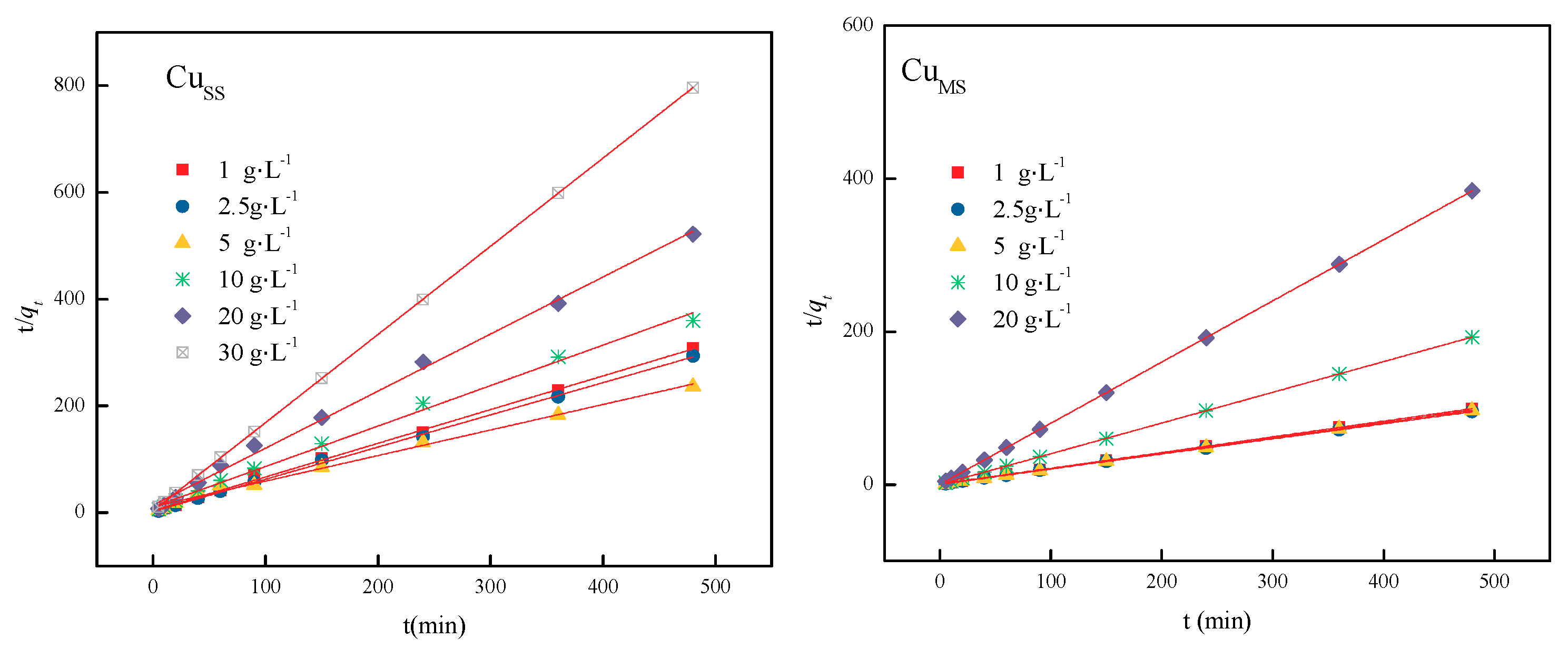
| Dosage | Temperature | Pseudo-Second-Order Model | R2 | 1/qe | qe | 1/(k2 × qe2) | k2 | |
|---|---|---|---|---|---|---|---|---|
| CuSS | 1 g·L−1 | 30 °C | y = 0.6267x + 4.2587 | 0.9964 | 0.6032 | 1.6578 | 2.4939 | 0.1459 |
| 2.5 g·L−1 | 30 °C | y = 0.6032x + 2.4939 | 0.9992 | 0.6032 | 1.6578 | 2.4939 | 0.1459 | |
| 5 g·L−1 | 30 °C | Y = 0.4787x + 11.448 | 0.9929 | 0.4787 | 2.0890 | 11.4480 | 0.0200 | |
| 10 g·L−1 | 30 °C | Y = 0.7584x + 10.749 | 0.9954 | 0.7584 | 1.3186 | 10.7490 | 0.0535 | |
| 20 g·L−1 | 30 °C | y = 1.0694x + 14.01 | 0.9968 | 1.0694 | 0.9351 | 14.0100 | 0.0816 | |
| 30 g·L−1 | 30 °C | y = 1.6519x + 3.6656 | 1.0000 | 1.6519 | 0.6054 | 3.6656 | 0.7444 | |
| CuMS | 1 g·L−1 | 30 °C | y = 0.2054x + 0.6007 | 0.9999 | 0.2054 | 4.8685 | 0.6007 | 0.0702 |
| 2.5 g·L−1 | 30 °C | y = 0.1973x + 0.4399 | 1.0000 | 0.1973 | 5.0684 | 0.4399 | 0.0885 | |
| 5 g·L−1 | 30 °C | y = 0.2021x + 0.0228 | 1.0000 | 0.2021 | 4.9480 | 0.0228 | 1.7914 | |
| 10 g·L−1 | 30 °C | y = 0.4016x + 0.0116 | 1.0000 | 0.4016 | 2.4900 | 0.0116 | 13.9037 | |
| 20 g·L−1 | 30 °C | y = 0.7999x + 0.0403 | 1.0000 | 0.7999 | 1.2502 | 0.0403 | 15.8769 |
| Adsorbents | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| R2 | qmax | KL | R2 | n | KF | |
| CuSS | 0.4796 | 3.179 | 0.052 | 0.6750 | 1.479 | 0.23 |
| CuMS | 0.9994 | 4.901 | 111.93 | 0.6749 | 5.963 | 3.48 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Chen, T.; Yan, B.; Li, L.; Xu, D.; Xiao, X. Characterization of the Adsorption of Cu (II) from Aqueous Solutions onto Pyrolytic Sludge-Derived Adsorbents. Water 2018, 10, 1816. https://doi.org/10.3390/w10121816
Wang M, Chen T, Yan B, Li L, Xu D, Xiao X. Characterization of the Adsorption of Cu (II) from Aqueous Solutions onto Pyrolytic Sludge-Derived Adsorbents. Water. 2018; 10(12):1816. https://doi.org/10.3390/w10121816
Chicago/Turabian StyleWang, Minghui, Tao Chen, Bo Yan, Lili Li, Damao Xu, and Xianming Xiao. 2018. "Characterization of the Adsorption of Cu (II) from Aqueous Solutions onto Pyrolytic Sludge-Derived Adsorbents" Water 10, no. 12: 1816. https://doi.org/10.3390/w10121816
APA StyleWang, M., Chen, T., Yan, B., Li, L., Xu, D., & Xiao, X. (2018). Characterization of the Adsorption of Cu (II) from Aqueous Solutions onto Pyrolytic Sludge-Derived Adsorbents. Water, 10(12), 1816. https://doi.org/10.3390/w10121816






