Karyotype Characterization of Nine Periwinkle Species (Gastropoda, Littorinidae)
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reid, D.G.; Dyal, P.; Williams, S.T. A global molecular phylogeny of 147 periwinkle species (Gastropoda, Littorininae). Zool. Scr. 2012, 41, 125–136. [Google Scholar] [CrossRef]
- Galindo, J.; Grahame, J.W. Ecological speciation and the intertidal snail Littorina saxatilis. Adv. Ecol. 2014, 2014, 239251. [Google Scholar] [CrossRef]
- Panova, M.; Johansson, T.; Canbäck, B.; Bentzer, J.; Rosenblad, M.A.; Johannesson, K.; Tunlid, A.; André, C. Species and gene divergence in Littorina snails detected by array comparative genomic hybridization. BMC Genom. 2014, 15, 687. [Google Scholar] [CrossRef] [PubMed]
- Rolán-Álvarez, E.; Austin, C.J.; Boulding, E.G. The contribution of the genus Littorina to the field of evolutionary ecology. Oceanogr. Mar. Biol. 2015, 53, 157–214. [Google Scholar] [CrossRef]
- Nishikawa, S. A comparative study of the chromosomes in marine gastropods, with some remarks on cytotaxonomy and phylogeny. J. Shimonoseki Coll. Fish. 1962, 11, 149–186. [Google Scholar]
- Thiriot-Quiévreux, C.; Ayraud, N. Les caryotypes de quelques espéces de bivalves et de gastéropodes marins. Mar. Biol. 1982, 70, 165–172. [Google Scholar] [CrossRef]
- Janson, K. Chromosome number in two phenotypically distinct populations of Littorina saxatilis Olivi from the Swedish west coast. J. Mollus. Stud. 1983, 49, 224–227. [Google Scholar] [CrossRef]
- Vitturi, R.; Catalano, E. Macaluso, M. Chromosome studies in three species of the gastropod superfamily Littorinoidea. Malacol. Rev. 1986, 19, 53–60. [Google Scholar]
- Vitturi, R.; Catalano, E.; Macaluso, M.; Zava, B. The karyology of Littorina neritoides (Linnaeus, 1758) (Mollusca, Prosobranchia). Malacologia 1988, 29, 319–324. [Google Scholar]
- Birstein, V.J.; Mikhailova, N.A. On the karyology of trematodes of the genus Microphallus and their intermediate gastropod host, Littorina saxatilis II. Karyological study of Littorina saxatilis (Gastropoda: Prosobranchia). Genetica 1990, 80, 167–170. [Google Scholar] [CrossRef]
- Vitturi, R.; Libertini, A.; Panozzo, M.; Mezzapelle, G. Karyotype analysis and genome size in three Mediterranean species of periwinkles (Prosobranchia: Mesogastropoda). Malacologia 1995, 37, 123–132. [Google Scholar]
- Rolán-Alvarez, E.; Buño, I.; Gonsalvez, J. Sex is determinated by sex chromosomes in Littorina saxatilis (Olivi) (Gastropoda, Prosobranchia). Hereditas 1996, 124, 261–267. [Google Scholar] [CrossRef]
- Ebied, A.M.; Hassan, H.A.; Abu-Almaaty, A.H.; Yaseen, A.E. Cytogenetic studies on metaphase chromosomes of eight gastropod species of orders Mesogastropoda and Neogastropoda from the Red Sea (Prosobranchia–Mollusca). J. Egypt. Ger. Soc. Zool. 2000, 33, 317–336. [Google Scholar]
- Colomba, M.S.; Vitturi, R.; Castriota, L.; Bertoni, R.; Libertini, A. FISH mapping of 18S–28S and 5S ribosomal DNA, periwinkle Melarhaphe neritoides (Prosobranchia, Gastropoda, Cenogastropoda). Heredity 2002, 88, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Libertini, A.; Trisolini, R.; Edmands, S. A cytogenetic study of the periwinkle Littorina keenae Rosewater, 1978 (Gastropoda: Littorinidae). J. Mollus. Stud. 2004, 70, 299–301. [Google Scholar] [CrossRef]
- World Register of Marine Species. Available online: http://www.marinespecies.org/ (accessed on 18 July 2018).
- Rolán, E.; Templado, J. Consideraciones sobre el complejo Littorina obtusata-mariae (Mollusca, Gastropoda, Littorinidae) en el Noroeste de la península Ibérica. Thalassas 1987, 5, 71–85. [Google Scholar]
- Méndez, J.; Pasantes, J.J.; Martínez-Expósito, M.J. Banding pattern of mussel (Mytilus galloprovincialis) chromosomes induced by 2× SSC/Giemsa-stain treatment. Mar. Biol. 1990, 106, 375–377. [Google Scholar] [CrossRef]
- Dixon, D.R.; Pascoe, P.L.; Gibbs, P.E.; Pasantes, J. The nature of Robertsonian chromosomal polymorphism in Nucella lapillus: A re-examination. In Genetics and Evolution of Aquatic Organisms; Beaumont, A.R., Ed.; Chapman and Hall: London, UK, 1994; pp. 389–399. ISBN 9780412493706. [Google Scholar]
- Martínez-Expósito, M.J.; Pasantes, J.J.; Méndez, J. Proliferation kinetics of mussel (Mytilus galloprovincialis) gill cells. Mar. Biol. 1994, 120, 41–45. [Google Scholar] [CrossRef]
- Pérez-García, C.; Cambeiro, J.M.; Morán, P.; Pasantes, J.J. Chromosomal mapping of rDNAs, core histone genes and telomeric sequences in Perumytilus purpuratus (Bivalvia: Mytilidae). J. Exp. Mar. Biol. Ecol. 2010, 395, 199–205. [Google Scholar] [CrossRef]
- Pérez-García, C.; Guerra-Varela, J.; Morán, P.; Pasantes, J.J. Chromosomal mapping of rRNA genes, core histone genes and telomeric sequences in Brachidontes puniceus and Brachidontes rodriguezi (Bivalvia: Mytilidae). BMC Genet. 2010, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, C.; Morán, P.; Pasantes, J.J. Cytogenetic characterization of the invasive mussel species Xenostrobus securis Lmk. (Bivalvia: Mytilidae). Genome 2011, 54, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, C.; Morán, P.; Pasantes, J.J. Karyotypic diversification in Mytilus mussels (Bivalvia: Mytilidae) inferred from chromosomal mapping of rRNA and histone gene clusters. BMC Genet. 2014, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- García-Souto, D.; Pérez-García, C.; Morán, P.; Pasantes, J.J. Divergent evolutionary behavior of H3 histone gene and rDNA clusters in venerid clams. Mol. Cytogenet. 2015, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- García-Souto, D.; Pérez-García, C.; Kendall, J.; Pasantes, J.J. Molecular cytogenetics in trough shells (Mactridae, Bivalvia): Divergent GC-rich heterochromatin content. Genes 2016, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- García-Souto, D.; Pérez-García, C.; Pasantes, J.J. Are pericentric inversions reorganizing wedge shell genomes? Genes 2017, 8, 370. [Google Scholar] [CrossRef] [PubMed]
- García-Souto, D.; Pasantes, J.J. Cytogenetics in Arctica islandica (Bivalvia, Arctiidae): The longest lived non-colonial metazoan. Genes 2018, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Thiriot-Quiévreux, C. Advances in chromosomal studies of gastropod Mollusc. J. Mollus. Stud. 2002, 69, 187–201. [Google Scholar] [CrossRef]
- García-Souto, D.; Pasantes, J.J. Molecular cytogenetics in digenean parasites: Linked and unlinked Major and 5S rDNAs, B chromosomes and karyotype diversification. Cytogenet. Genome Res. 2015, 147, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Westram, A.M.; Rafajlovic, M.; Chaube, P.; Faria, R.; Larsson, T.; Panova, M.; Ravinet, M.; Blomberg, A.; Mehlig, B.; Johannesson, K.; et al. Clines on the seashore: The genomic architecture underlying rapid divergence in the face of gene flow. Evol. Lett. 2018, 2, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Westram, A.M. (University of Sheffield, Sheffield, UK); Faria, R. (University of Sheffield, Sheffield, UK); Butlin, R.K. (University of Sheffield, Sheffield, UK); Johannesson, K. (University of Gothenburg, Gothenburg, Sweden). Personal communication, 2018.
- Coyne, J.A.; Orr, H.A. Speciation; Sinauer Associates, Inc.: Sunderland, MA, USA, 2004; pp. 284–311. ISBN 9780878930890. [Google Scholar]
- Sá-Pinto, A.; Martínez-Fernández, M.; López-Fernández, C.; Ferreira, Z.; Pereira, R.; Gosálvez, J.; Rolán-Alvarez, E. Incipient post-zygotic barrier in a model system of ecological speciation with gene flow. J. Evol. Biol. 2013, 26, 2750–2756. [Google Scholar] [CrossRef] [PubMed]
- Hull, S.L.; Grahame, J.; Mill, P.J. Morphological divergence and evidence for reproductive isolation in Littorina saxatilis (Olivi) in northeast England. J. Mollus. Stud. 1996, 62, 89–99. [Google Scholar] [CrossRef]
- Stankiewicz, P.; Lupski, J.R. Genome architecture, rearrangements and genomic disorders. Trends Genet. 2002, 18, 74–81. [Google Scholar] [CrossRef]
- Torres, G.A.; Gong, Z.; Iovene, M.; Hirsh, C.D.; Buell, C.R.; Bryan, G.J.; Novák, P.; Macas, J.; Jiang, J. Organization and evolution of subtelomeric satellite repeats in the potato genome. G3 (Bethesda) 2011, 1, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Linardopoulou, E.V.; Williams, E.M.; Fan, Y.X.; Friedman, C.; Young, J.M.; Trask, B.J. Human subtelomeres are hot spots of interchromosomal recombination and segmental duplication. Nature 2005, 437, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Cabrero, J.; López-León, M.D.; Teruel, M.; Camacho, J.P.M. Chromosome mapping of H3 and H4 histone gene clusters in 35 species of acridid grasshoppers. Chromosome Res. 2009, 17, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bao, Z.; Wang, S.; Huang, X.; Hu, J. Chromosome rearrangements in Pectinidae (Bivalvia; Pteriomorphia) implied based on chromosomal localization of histone H3 gene in four scallops. Genetica 2007, 130, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, J.; Pérez-García, C.; Leitão, A.; Malheiro, I.; Pasantes, J.J. Cytogenetic characterization and mapping of rDNAs, core histone genes and telomeric sequences in Venerupis aurea and Tapes rhomboides (Bivalvia: Veneridae). Genetica 2011, 139, 823–830. [Google Scholar] [CrossRef] [PubMed]
- García-Souto, D.; Sumner-Hempel, A.; Fervenza, S.; Pérez-García, C.; Torreiro, A.; González-Romero, R.; Eirín-López, J.M.; Morán, P.; Pasantes, J.J. Detection of invasive and cryptic species in marine mussels (Bivalvia, Mytilidae): A chromosomal perspective. J. Nat. Conserv. 2017, 39, 58–67. [Google Scholar] [CrossRef]
- García-Souto, D.; Rios, G.; Pasantes, J.J. Karyotype differentiation in tellin shells (Bivalvia: Tellinidae). BMC Genet. 2017, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Coghlan, A.; Eichler, E.E.; Oliver, S.G.; Paterson, A.H.; Stein, L. Chromosome evolution in eukaryotes: A multi-kingdom perspective. Trends Genet. 2005, 21, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Peichel, C.L. Chromosome evolution: Molecular mechanisms and evolutionary consequences. J. Hered. 2017, 108, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Faria, R.; Chaube, P.; Morales, H.; Larsson, T.; Lemmon, A.R.; Lemmon, E.M.; Rafajlović, M.; Panova, M.; Ravinet, M.; Johannesson, K.; et al. Multiple chromosomal rearrangements in a hybrid zone between Littorina saxatilis ecotypes. Mol. Ecol. 2018, in press. [Google Scholar]
| Species | n | 2n | Karyotype | Reference |
|---|---|---|---|---|
| Echinolittorina hawaiensis (Rosewater & Kadolsky, 1981) | 15 | 30 | [5] | |
| Echinolittorina miliaris (Quoy & Gaimard, 1833) | 18 | [5] | ||
| Echinolittorina punctata (Gmelin, 1791) | 16 | [8] | ||
| 17 | 34 | [11] | ||
| Echinolittorina radiata (Souleyet, 1852) | 18 | [5] | ||
| Echinolittorina subnodosa (Molina, 1782) | 8 | 16 | 8 m | [13] |
| Littoraria strigata (Philippi, 1846) | 17 | [5] | ||
| Littorina brevicula (Philippi, 1844) | 17 | [5] | ||
| Littorina keena Rosewater, 1978 | 34 | 10 m/sm, 7 st/t | [15] | |
| Littorina obtusata (Linnaeus, 1758) | 34 | [7] | ||
| Littorina saxatilis (Olivi, 1792) | 34 | 8 m/sm, 9 st/t | [7] | |
| 34 | 10 m/sm, 7 st/t | [10] | ||
| 34 | 6 m, 9 sm, 2 st | [11] | ||
| 34 XX ♀/34 XY ♂ | 10 m/sm, 7 st | [12] | ||
| Melarhaphe neritoides (Linnaeus, 1758) | 17 | 34 | 8 m, 2 sm, 7 t | [6] |
| 17 | 34 XX ♀/33 X0 ♂ | 10 m, 3 sm, 3 st, 1 t | [9] | |
| 34 XX ♀/33 X0 ♂ | 10 m, 3 sm, 3 st, 1 t | [11] |
| Species | Locality | Coordinates |
|---|---|---|
| Littorina arcana | Holy Island, UK | 53.299781, −4.679846 |
| Littorina saxatilis | Cabo Estai, Ría de Vigo, Spain | 42.182181, −8.813204 |
| Cabo Silleiro, Spain | 42.104987, −8.898929 | |
| Littorina compressa | Holy Island, UK | 53.299781, −4.679846 |
| Littorina obtusata | Rande, Ría de Vigo, Spain | 42.284311, −8.658661 |
| Littorina fabalis | Santo André, Portugal | 41.412417, −8.787778 |
| Praia da Borna, Ría de Vigo, Spain | 42.280867, −8.697019 | |
| O Grove, Ría de Arousa, Spain | 42.460500, −8.872028 | |
| Littorina littorea | Marín, Ría de Pontevedra, Spain | 42.396351, −8.695599 |
| Littoraria angulifera | Miami, FL, USA | 25.810107, −80.164063 |
| Cenchritis muricatus | Miami, FL, USA | 25.889330, −80.150129 |
| Melarhaphe neritoides | Cabo Udra, Ría de Aldán, Spain | 42.333599, −8.827797 |
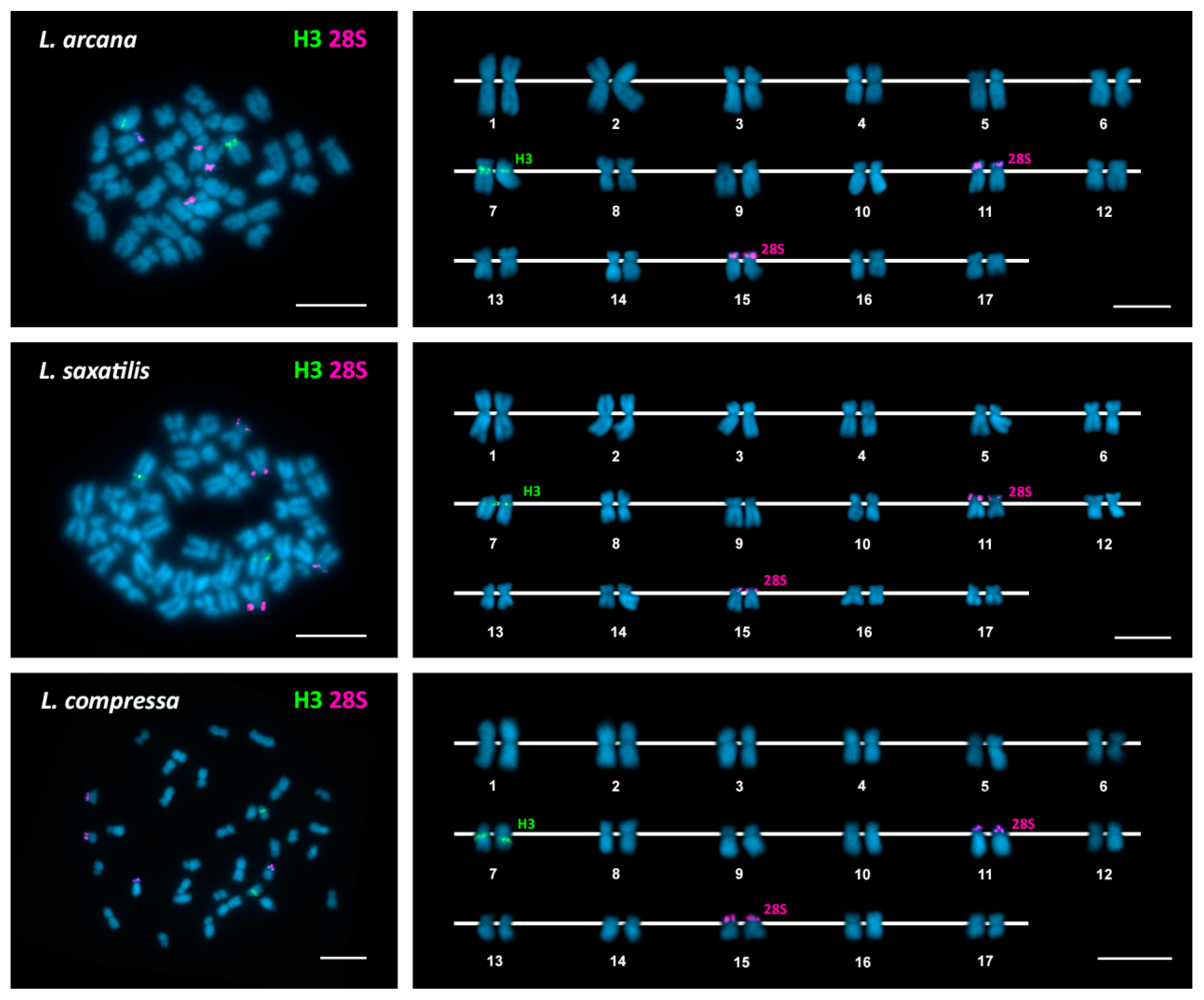
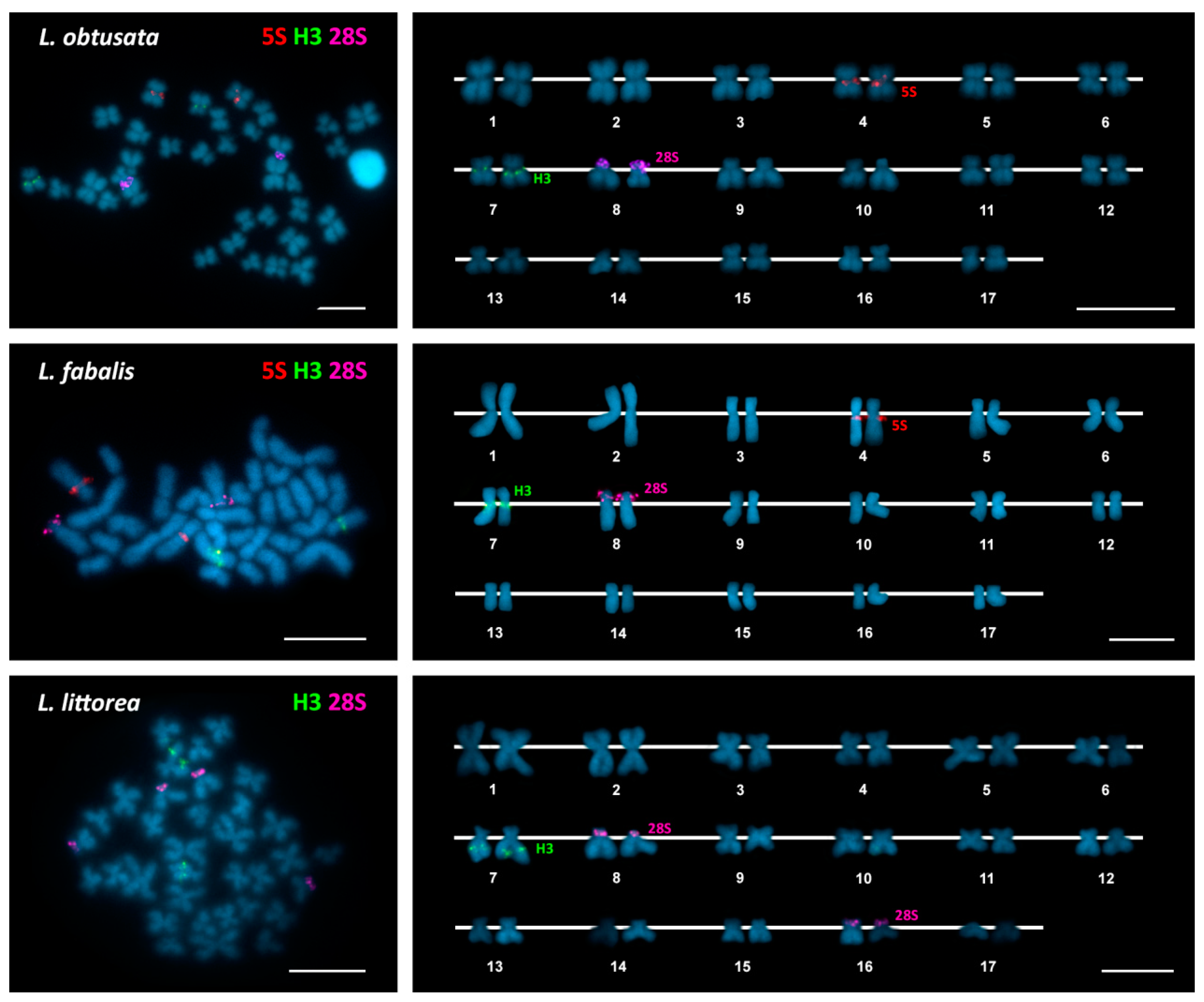
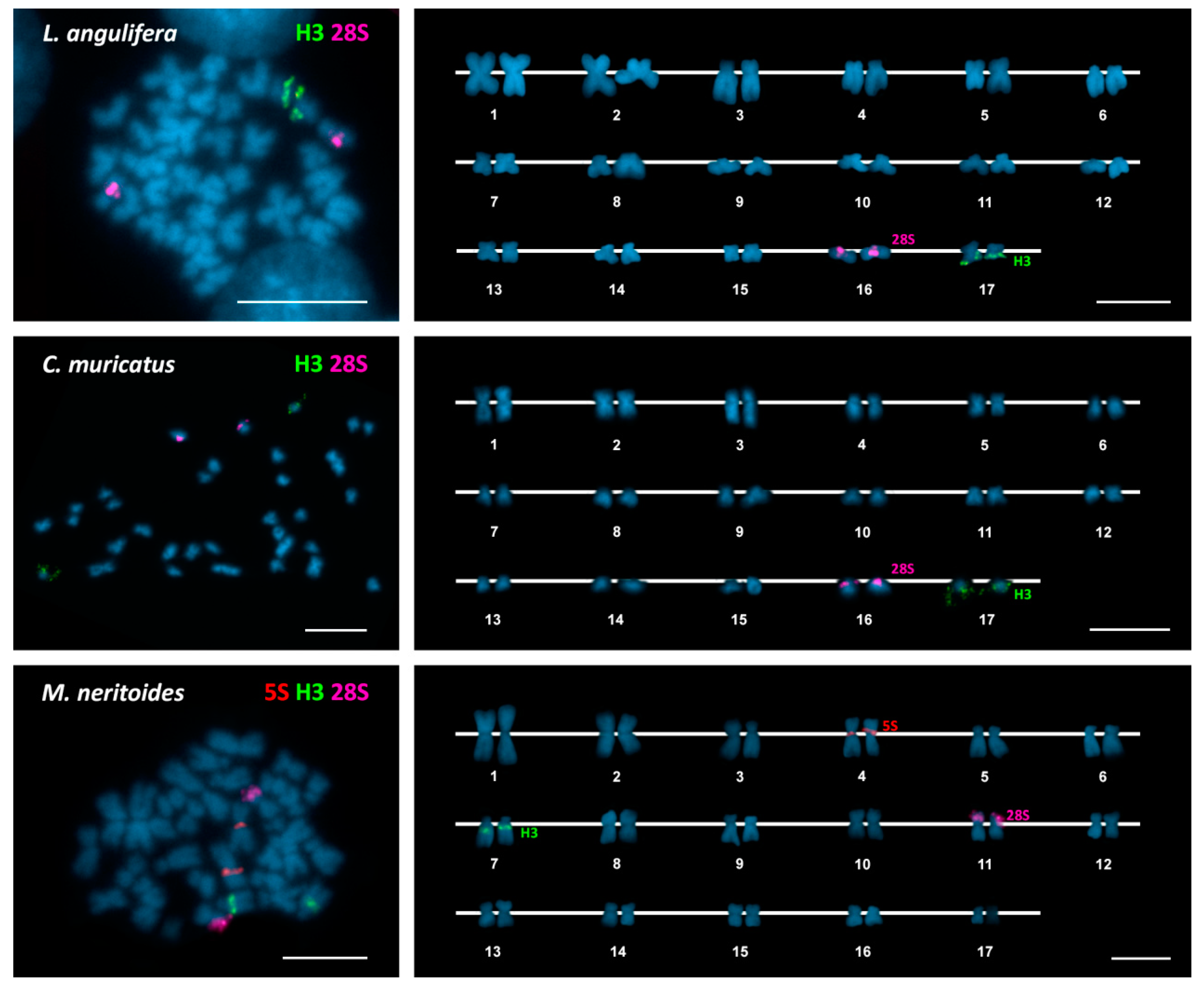
| Species | 2n | 45S rDNA | 5S rDNA | H3 Histone Genes | Reference |
|---|---|---|---|---|---|
| Littorina arcana | 34 | 11p ter (st) | 7q cen (sm) | This work | |
| 15p ter (sm) | |||||
| Littorina saxatilis | 34 | 11p ter (sm) | 7q cen (sm) | This work | |
| 15p ter (sm) | |||||
| Littorina compressa | 34 | 11p ter (sm) | 7q cen (sm) | This work | |
| 15p ter (sm) | |||||
| Littorina obtusata | 34 | 8p ter (sm) | 4q cen (m) | 7q cen (m) | This work |
| Littorina fabalis | 34 | 8p ter (sm) | 4q cen (sm) | 7q cen (sm) | This work |
| Littorina littorea | 34 | 8p ter (m) | 7q ic (sm) | This work | |
| 16p ter (sm) | |||||
| Littoraria angulifera | 34 | 16p ter (st) | 17q ter (m) | This work | |
| Cenchritis muricatus | 34 | 16p ter (st) | 17q ter (st) | This work | |
| Melarhaphe neritoides | 34 | 11p ter (m) | 4p cen (m) | 7q ic (st) | This work |
| 34/33 | p ter (m) * | [14] | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Souto, D.; Alonso-Rubido, S.; Costa, D.; Eirín-López, J.M.; Rolán-Álvarez, E.; Faria, R.; Galindo, J.; Pasantes, J.J. Karyotype Characterization of Nine Periwinkle Species (Gastropoda, Littorinidae). Genes 2018, 9, 517. https://doi.org/10.3390/genes9110517
García-Souto D, Alonso-Rubido S, Costa D, Eirín-López JM, Rolán-Álvarez E, Faria R, Galindo J, Pasantes JJ. Karyotype Characterization of Nine Periwinkle Species (Gastropoda, Littorinidae). Genes. 2018; 9(11):517. https://doi.org/10.3390/genes9110517
Chicago/Turabian StyleGarcía-Souto, Daniel, Sandra Alonso-Rubido, Diana Costa, José M. Eirín-López, Emilio Rolán-Álvarez, Rui Faria, Juan Galindo, and Juan J. Pasantes. 2018. "Karyotype Characterization of Nine Periwinkle Species (Gastropoda, Littorinidae)" Genes 9, no. 11: 517. https://doi.org/10.3390/genes9110517
APA StyleGarcía-Souto, D., Alonso-Rubido, S., Costa, D., Eirín-López, J. M., Rolán-Álvarez, E., Faria, R., Galindo, J., & Pasantes, J. J. (2018). Karyotype Characterization of Nine Periwinkle Species (Gastropoda, Littorinidae). Genes, 9(11), 517. https://doi.org/10.3390/genes9110517







