DMRTC2, PAX7, BRACHYURY/T and TERT Are Implicated in Male Germ Cell Development Following Curative Hormone Treatment for Cryptorchidism-Induced Infertility
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Biopsy Sample Collection
2.2. Histological Analyses
2.3. RNA Preparation, Sequencing, Data Analyses, and RNA Expression Levels
2.4. Data and Differential Gene Expression Analyses
2.5. Protein Interaction Network
2.6. Ethics Statement
3. Results
3.1. Decreased Expression of Gonocyte and Spermatogonial Marker Genes in Testes with Altered Mini-Puberty
3.2. Gonocyte and Spermatogonial Marker Genes Respond to GnRHa Treatment
4. Discussion
4.1. Luteinizing Hormone and Testosterone Deprivation Decreases Gonocyte and Spermatogonial Marker Gene Expression
4.2. Genes with Augmented Levels after GnRH Treatment
4.3. Combining Classical Physiological Information and Cutting-Edge Genomics Data into a Complete Picture
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Forest, M.G.; Sizonenko, P.C.; Cathiard, A.M.; Bertrand, J. Hypophyso-gonadal function in humans during the first year of life. Evidence for testicular activity in early infancy. J. Clin. Investig. 1974, 53, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.S.D.; Hughes, I.A.; Reyes, F.I.; Faiman, C. Pituitary gonadal relations in infancy: II. Patterns of serum gonadal steroid concentrations in man from birth to two years of age. J. Clin. Endocrinol. Metab. 1976, 42, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Corbier, P.; Edwards, D.A.; Roffi, J. The neonatal testosterone surge: A comparative study. Arch. Int. Physiol. Biochim. Biophys. 1992, 100, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Hadziselimovic, F.; Thommen, L.; Girard, J.; Herzog, B. The significance of postnatal gonadotropin surge for testicular development in normal and cryptorchid testes. J. Urol. 1986, 136, 274–276. [Google Scholar] [CrossRef]
- Hadziselimovic, F.; Emmons, L.R.; Buser, M.W. A diminished postnatal surge of Ad spermatogonia in cryptorchid infants is additional evidence for hypogonadotropic hypogonadism. Swiss Med. Wkly. 2004, 134, 381–384. [Google Scholar] [PubMed]
- Hadziselimovic, F.; Zivkovic, D.; Bica, D.T.G.; Emmons, L.R. The importance of mini-puberty for fertility in cryptorchidism. J. Urol. 2005, 174, 1536–1539. [Google Scholar] [CrossRef] [PubMed]
- Hadziselimovic, F.; Herzog, B. The importance of both an early orchidopexy and germ cell maturation for fertility. Lancet 2001, 358, 1156–1157. [Google Scholar] [CrossRef]
- Hadziselimovic, F.; Höcht, B.; Herzog, B.; Buser, M.W. Infertility in cryptorchidism is linked to the stage of germ cell development at orchidopexy. Horm. Res. 2007, 68, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Hadziselimovic, F.; Höcht, B. Testicular histology related to fertility outcome and postpubertal hormone status in cryptorchidism. Klin. Padiatr. 2008, 220, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Hadziselimovic, F.; Herzog, B.; Barthold, J.S. Treatment with a luteinizing hormone-releasing hormone analogue after successful orchiopexy markedly improves the chance of fertility later in life. J. Urol. 1997, 158, 1193–1195. [Google Scholar] [CrossRef]
- Hadziselimovic, F.; Höcht, B.; Herzog, B.; Girard, J. Does Long Term Treatment with Buserelin Improve the Fertility Chances of Cryptorchid Testes? In LH-RH and Its Analogues; Labrie, F., Belanger, A., Dupont, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1984; p. 457. [Google Scholar]
- Hadziselimovic, F.; Huff, D.; Duckett, J.; Herzog, B.; Elder, J.; Snyder, H.; Buser, M. Treatment of cryptorchidism with low doses of buserelin over a 6-months period. Eur. J. Pediatr. 1987, 146, S56–S58. [Google Scholar] [CrossRef] [PubMed]
- Hadziselimovic, F. Successful treatment of unilateral cryptorchid boys risking infertility with LH-RH analogue. Int. Braz. J. Urol. 2008, 34, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Dym, M.; Kokkinaki, M.; He, Z. Spermatogonial stem cells: Mouse and human comparisons. Birth Defects Res. Part C Embryo Today Rev. 2009, 87, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Hermann, B.P.; Sukhwani, M.; Hansel, M.C.; Orwig, K.E. Spermatogonial stem cells in higher primates: Are there differences from those in rodents? Reproduction 2010, 139, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Kersemaekers, A.M.F.; Honecker, F.; Stoop, H.; Cools, M.; Molier, M.; Wolffenbuttel, K.; Bokemeyer, C.; Li, Y.; Lau, Y.F.C.; Oosterhuis, J.W.; et al. Identification of germ cells at risk for neoplastic transformation in gonadoblastoma: An immunohistochemical study for OCT3/4 and TSPY. Hum. Pathol. 2005, 36, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Rajpert-De Meyts, E. Developmental model for the pathogenesis of testicular carcinoma in situ: Genetic and environmental aspects. Hum. Reprod. Update 2006, 12, 303–323. [Google Scholar] [CrossRef] [PubMed]
- Pesce, M.; Wang, X.; Wolgemuth, D.J.; Scholer, H. Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech. Dev. 1998, 71, 89–98. [Google Scholar] [CrossRef]
- Robinson, L.L.; Gaskell, T.L.; Saunders, P.T.; Anderson, R.A. Germ cell specific expression of c-kit in the human fetal gonad. Mol. Hum. Reprod. 2001, 7, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Pauls, K.; Schorle, H.; Jeske, W.; Brehm, R.; Steger, K.; Wernert, N.; Buttner, R.; Zhou, H. Spatial expression of germ cell markers during maturation of human fetal male gonads: An immunohistochemical study. Hum. Reprod 2006, 21, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Gkountela, S.; Li, Z.; Vincent, J.J.; Zhang, K.X.; Chen, A.; Pellegrini, M.; Clark, A.T. The ontogeny of cKIT+ human primordial germ cells proves to be a resource for human germ line reprogramming, imprint erasure and in vitro differentiation. Nat. Cell Biol. 2012, 15, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Hadziselimovic, F.; Gegenschatz-Schmid, K.; Verkauskas, G.; Docampo-Garcia, M.J.; Demougin, P.; Bilius, V.; Malcius, D.; Dasevicius, D.; Stadtler, M.B. Gene expression changes underlying idiopathic central hypogonadism in cryptorchidism with defective mini-puberty. Sex. Dev. 2016, 10, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Hadziselimovic, F.; Gegenschatz-Schmid, K.; Verkauskas, G.; Demougin, P.; Bilius, V.; Dasevicius, D.; Stadler, M.B. GnRHa Treatment of Cryptorchid Boys Affects Genes Involved in Hormonal Control of the HPG Axis and Fertility. Sex. Dev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Seguchi, H.; Hadziselimovic, F. Ultramicroscopic studies on the seminiferous tubule in children from birth to puberty. I. Spermatogonia development. Verh. Anat. Ges. 1974, 68, 133–148. [Google Scholar] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Hadziselimovic, F.; Herzog, B. Hodenerkrankungen im Kindesalter; Daum, R., Mildenberger, H., Rehbein, F., Eds.; Hippokrates Verlag Stuttgart (Bibliothek für Kinderchirurgie): Sttutgart, Germany, 1990; ISBN 377730929X. [Google Scholar]
- Hadziselimovic, F.; Hadziselimovic, N.O.; Demougin, P.; Krey, G.; Höcht, B.; Oakeley, E.J. EGR4 is a master gene responsible for fertility in cryptorchidism. Sex. Dev. 2009, 3, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Hadziselimovic, F.; Hadziselimovic, N.O.; Demougin, P.; Oakeley, E.J. Testicular gene expression in cryptorchid boys at risk of azoospermia. Sex. Dev. 2011, 5, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Marikawa, Y.; Tamashiro, D.A.A.; Fujita, T.C.; Alarcon, V.B. Dual Roles of Oct4 in the Maintenance of Mouse P19 Embryonal Carcinoma Cells: As Negative Regulator of Wnt/β-Catenin Signaling and Competence Provider for Brachyury Induction. Stem Cells Dev. 2011, 20, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Goodyear, S.M.; Tobias, J.W.; Avarbock, M.R.; Brinster, R.L. Spermatogonial Stem Cell Self-Renewal Requires ETV5-Mediated Downstream Activation of Brachyury in Mice1. Biol. Reprod. 2011, 85, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Rajpert-De Meyts, E.; Hanstein, R.; Jørgensen, N.; Græm, N.; Vogt, P.H.; Skakkebæk, N.E. Developmental expression of POU5F1 (OCT-3/4) in normal and dysgenetic human gonads. Hum. Reprod. 2004, 19, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Pauls, K.; Jäger, R.; Weber, S.; Wardelmann, E.; Koch, A.; Büttner, R.; Schorle, H. Transcription factor AP-2γ, a novel marker of gonocytes and seminomatous germ cell tumors. Int. J. Cancer 2005, 115, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, G.; Carnes, K.; Morrow, C.; Kostereva, N.V.; Ekman, G.C.; Meling, D.D.; Hostetler, C.; Griswold, M.; Murphy, K.M.; Hess, R.A.; et al. Loss of Etv5 Decreases Proliferation and RET Levels in Neonatal Mouse Testicular Germ Cells and Causes an Abnormal First Wave of Spermatogenesis. Biol. Reprod. 2009, 81, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ouyang, W.; Grigura, V.; Zhou, Q.; Carnes, K.; Lim, H.; Zhao, G.-Q.; Arber, S.; Kurpios, N.; Murphy, T.L.; et al. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature 2005, 436, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, A.S.; Faial, T.; Gardner, L.; Niakan, K.K.; Ortmann, D.; Senner, C.E.; Callery, E.M.; Trotter, M.W.; Hemberger, M.; Smith, J.C.; et al. BRACHYURY and CDX2 Mediate BMP-Induced Differentiation of Human and Mouse Pluripotent Stem Cells into Embryonic and Extraembryonic Lineages. Cell Stem Cell 2011, 9, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Aramaki, S.; Hayashi, K.; Kurimoto, K.; Ohta, H.; Yabuta, Y.; Iwanari, H.; Mochizuki, Y.; Hamakubo, T.; Kato, Y.; Shirahige, K.; et al. A Mesodermal Factor, T, Specifies Mouse Germ Cell Fate by Directly Activating Germline Determinants. Dev. Cell 2013, 27, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Eildermann, K.; Aeckerle, N.; Debowski, K.; Godmann, M.; Christiansen, H.; Heistermann, M.; Schweyer, S.; Bergmann, M.; Kliesch, S.; Gromoll, J.; et al. Developmental expression of the pluripotency factor sal-like protein 4 in the monkey, human and mouse testis: Restriction to premeiotic germ cells. Cells Tissues Organs 2012, 196, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Gassei, K.; Orwig, K.E. SALL4 Expression in Gonocytes and Spermatogonial Clones of Postnatal Mouse Testes. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, D.L.; Gao, Z.; Mutoji, K.; Song, Y.C.; Ruan, J.; Hermann, B.P. The regulatory repertoire of PLZF and SALL4 in undifferentiated spermatogonia. Development 2016, 143, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Sasaoka, Y.; Kiso, M.; Abe, K.; Haraguchi, S.; Kobayashi, S.; Saga, Y. Conserved role of nanos proteins in germ cell development. Science 2003, 301, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Saga, Y. Nanos2 suppresses meiosis and promotes male germ cell differentiation. Genes Dev. 2008, 22, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Barrios, F.; Filipponi, D.; Pellegrini, M.; Paronetto, M.P.; Di Siena, S.; Geremia, R.; Rossi, P.; De Felici, M.; Jannini, E.A.; Dolci, S. Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J. Cell Sci. 2010, 123, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Kiso, M.; Saga, Y. Implication of nanos2-3′UTR in the expression and function of nanos2. Mech. Dev. 2006, 123, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Kusz, K.M.; Tomczyk, L.; Sajek, M.; Spik, A.; Latos-Bielenska, A.; Jedrzejczak, P.; Pawelczyk, L.; Jaruzelska, J. The highly conserved NANOS2 protein: Testis-specific expression and significance for the human male reproduction. Mol. Hum. Reprod. 2009, 15, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Murphy, M.W.; Gearhart, M.D.; Bardwell, V.J.; Zarkower, D. The mammalian Doublesex homolog DMRT6 coordinates the transition between mitotic and meiotic developmental programs during spermatogenesis. Development 2014, 141, 3662–3671. [Google Scholar] [CrossRef] [PubMed]
- Desimio, M.G.; Campolo, F.; Dolci, S.; De Felici, M.; Farini, D. SOHLH1 and SOHLH2 directly down-regulate stimulated by retinoic acid 8 (STRA8) expression. Cell Cycle 2015, 14, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.; Bhasin, S.; Jasuja, R.; Pervin, S.; Singh, R. Testosterone inhibits transforming growth factor-β signaling during myogenic differentiation and proliferation of mouse satellite cells: Potential role of follistatin in mediating testosterone action. Mol. Cell. Endocrinol. 2012, 350, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Goriely, A.; Turner, G.D.; Ewen, K.A.; Jacobsen, G.K.; Graem, N.; Wilkie, A.O.; Rajpert-De Meyts, E. OCT2, SSX and SAGE1 reveal the phenotypic heterogeneity of spermatocytic seminoma reflecting distinct subpopulations of spermatogonia. J. Pathol. 2011, 224, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Pech, M.F.; Garbuzov, A.; Hasegawa, K.; Sukhwani, M.; Zhang, R.J.; Benayoun, B.A.; Brockman, S.A.; Lin, S.; Brunet, A.; Orwig, K.E.; et al. High telomerase is a hallmark of undifferentiated spermatogonia and is required for maintenance of male germline stem cells. Genes Dev. 2015, 29, 2420–2434. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Zhang, B.; Tang, Z.-W.; Hou, L. Expression of a telomerase-associated gene in normal, atrophic, and tumorous testes. Chin. Med. Sci. J. 2005, 20, 217–220. [Google Scholar]
- Schrader, M.; Müller, M.; Schulze, W.; Heicappell, R.; Krause, H.; Straub, B.; Miller, K. Quantification of telomerase activity, porphobilinogen deaminase and human telomerase reverse transcriptase mRNA in testicular tissue—New parameters for a molecular diagnostic classification of spermatogenesis disorders. Int. J. Androl. 2002, 25, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Schrader, M.; Müller, M.; Schulze, W.; Heicappell, R.; Krause, H.; Straub, B.; Miller, K. Quantification of the expression level of the gene encoding the catalytic subunit of telomerase in testicular tissue specimens predicts successful sperm recovery. Hum. Reprod. 2002, 17, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Calado, R.T.; Yewdell, W.T.; Wilkerson, K.L.; Regal, J.A.; Kajigaya, S.; Stratakis, C.A.; Young, N.S. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood 2009, 114, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Pinto, F.; Pértega-Gomes, N.; Vizcaíno, J.R.; Andrade, R.P.; Cárcano, F.M.; Reis, R.M. Brachyury as a potential modulator of androgen receptor activity and a key player in therapy resistance in prostate cancer. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Hamra, K.F.; Schultz, N.; Chapman, K.M.; Grellhesl, D.M.; Cronkhite, J.T.; Hammer, R.E.; Garbers, D.L. Defining the spermatogonial stem cell. Dev. Biol. 2004, 269, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Lolicato, F.; Grimaldi, P.; Dolci, S.; Di Sauro, A.; Filipponi, D.; Geremia, R. Transcriptome analysis of differentiating spermatogonia stimulated with kit ligand. Gene Expr. Patterns 2008, 8, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Eto, K.; Honmyou, A.; Nakao, K.; Kiyonari, H.; Abé, S. Neuregulins are essential for spermatogonial proliferation and meiotic initiation in neonatal mouse testis. Development 2011, 138, 3159–3168. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.; Medrano, G.; Chaudhary, J.; Hamra, F. NRG1 and KITL signal downstream of retinoic acid in the germline to support soma-free syncytial growth of differentiating spermatogonia. Cell Death Discov. 2015, 1, 15018. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.T.K.; Turner, J.M.A.; Ruggiu, M.; Taggart, M.; Burgoyne, P.S.; Elliott, D.; Cooke, H.J. Absence of mDazl produces a final block on germ cell development at meiosis. Reproduction 2003, 126, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hong, J.; Kim, E.; Kim, K.; Kim, S.W.; Krishnamurthy, H.; Chung, S.S.W.; Wolgemuth, D.J.; Rhee, K. Developmental stage-specific expression of Rbm suggests its involvement in early phases of spermatogenesis. Mol. Hum. Reprod. 2004, 10, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.J.; Millar, M.R.; Oghene, K.; Ross, A.; Kiesewetter, F.; Pryor, J.; McIntyre, M.; Hargreave, T.B.; Saunders, P.T.; Vogt, P.H.; et al. Expression of RBM in the nuclei of human germ cells is dependent on a critical region of the Y chromosome long arm. Proc. Natl. Acad. Sci. USA 1997, 94, 3848–3853. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.J.; Oghene, K.; Makarov, G.; Makarova, O.; Hargreave, T.B.; Chandley, A.C.; Eperon, I.C.; Cooke, H.J. Dynamic changes in the subnuclear organisation of pre-mRNA splicing proteins and RBM during human germ cell development. J. Cell Sci. 1998, 111 (Pt 9), 1255–1265. [Google Scholar] [PubMed]
- Kawamata, M.; Nishimori, K. Mice deficient in Dmrt7 show infertility with spermatogenic arrest at pachytene stage. FEBS Lett. 2006, 580, 6442–6446. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Namekawa, S.H.; Niswander, L.M.; Ward, J.O.; Lee, J.T.; Bardwell, V.J.; Zarkower, D. A Mammal-Specific Doublesex Homolog Associates with Male Sex Chromatin and Is Required for Male Meiosis. PLoS Genet. 2007, 3, e62. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.J.; Qiu, Y.; Kido, T.; Lau, Y.-F.C. The Y-located proto-oncogene TSPY exacerbates and its X-homologue TSPX inhibits transactivation functions of androgen receptor and its constitutively active variants. Hum. Mol. Genet. 2017, 26, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Oatley, J.M.; Avarbock, M.R.; Telaranta, A.I.; Fearon, D.T.; Brinster, R.L. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc. Natl. Acad. Sci. USA 2006, 103, 9524–9529. [Google Scholar] [CrossRef] [PubMed]
- Oatley, M.J.; Kaucher, A.V.; Racicot, K.E.; Oatley, J.M. Inhibitor of DNA Binding 4 Is Expressed Selectively by Single Spermatogonia in the Male Germline and Regulates the Self-Renewal of Spermatogonial Stem Cells in Mice1. Biol. Reprod. 2011, 85, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Jin, L.; Yang, G.; Ji, L.; Wang, F.; Lu, Z. Post-transcriptional repression of FOXO1 by QKI results in low levels of FOXO1 expression in breast cancer cells. Oncol. Rep. 2014, 31, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Koli, S.; Mukherjee, A.; Reddy, K.V.R. Retinoic acid triggers c-kit gene expression in spermatogonial stem cells through an enhanceosome constituted between transcription factor binding sites for retinoic acid response element (RARE), spleen focus forming virus proviral integration oncogene (SPFI1) (PU.1) and E26 transformation-specific (ETS). Reprod. Fertil. Dev. 2017, 29, 521. [Google Scholar] [CrossRef] [PubMed]
- Colleoni, S.; Galli, C.; Gaspar, J.A.; Meganathan, K.; Jagtap, S.; Hescheler, J.; Sachinidis, A.; Lazzari, G. Development of a Neural Teratogenicity Test Based on Human Embryonic Stem Cells: Response to Retinoic Acid Exposure. Toxicol. Sci. 2011, 124, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Willis, W.D.; Eddy, E.M. Targeting the Gdnf Gene in peritubular myoid cells disrupts undifferentiated spermatogonial cell development. Proc. Natl. Acad. Sci. USA 2016, 113, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kanatsu-Shinohara, M.; Lei, Z.; Rao, C.V.; Shinohara, T. The Luteinizing Hormone-Testosterone Pathway Regulates Mouse Spermatogonial Stem Cell Self-Renewal by Suppressing WNT5A Expression in Sertoli Cells. Stem Cell Rep. 2016, 7, 279–291. [Google Scholar] [CrossRef] [PubMed]
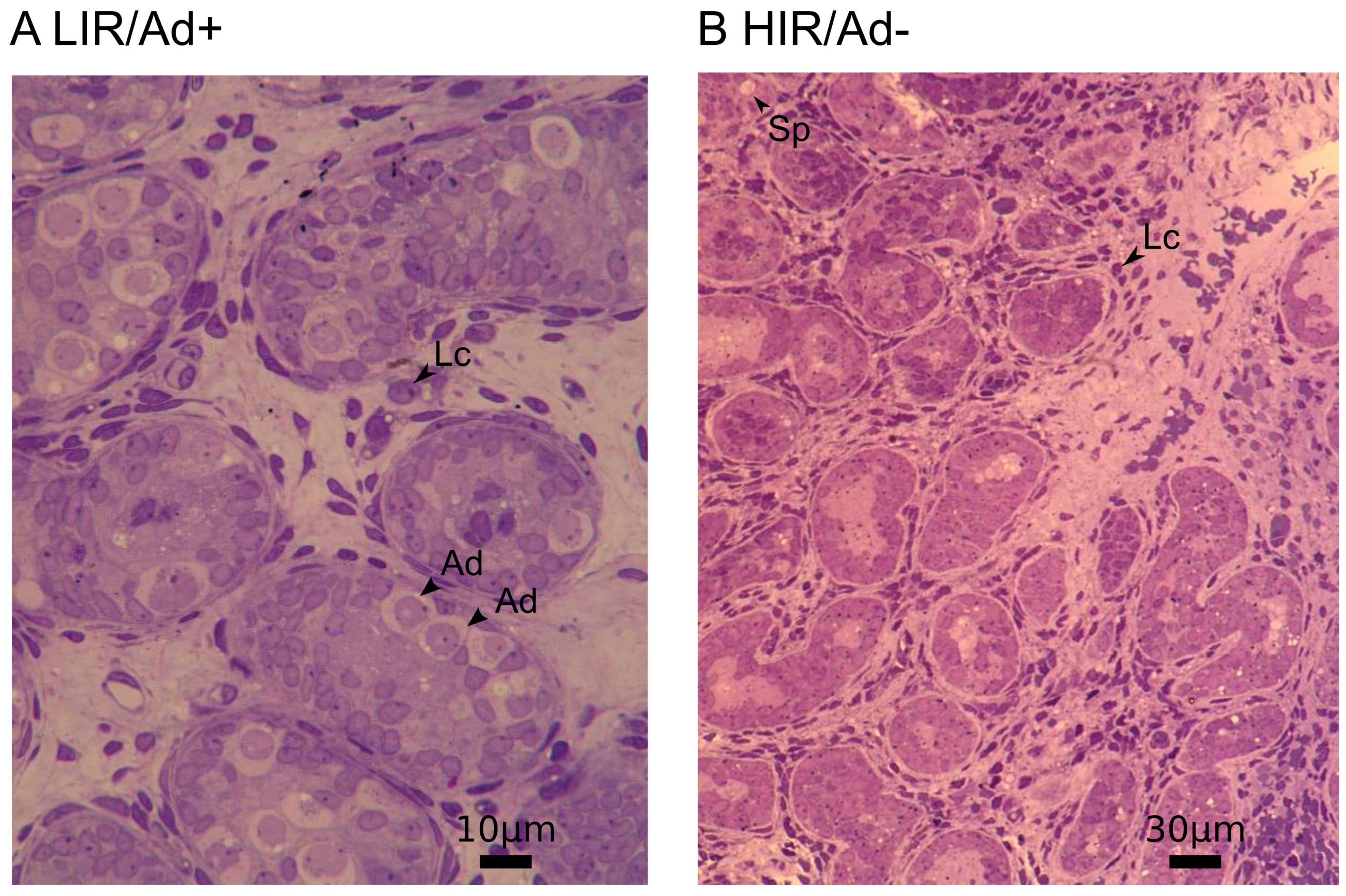
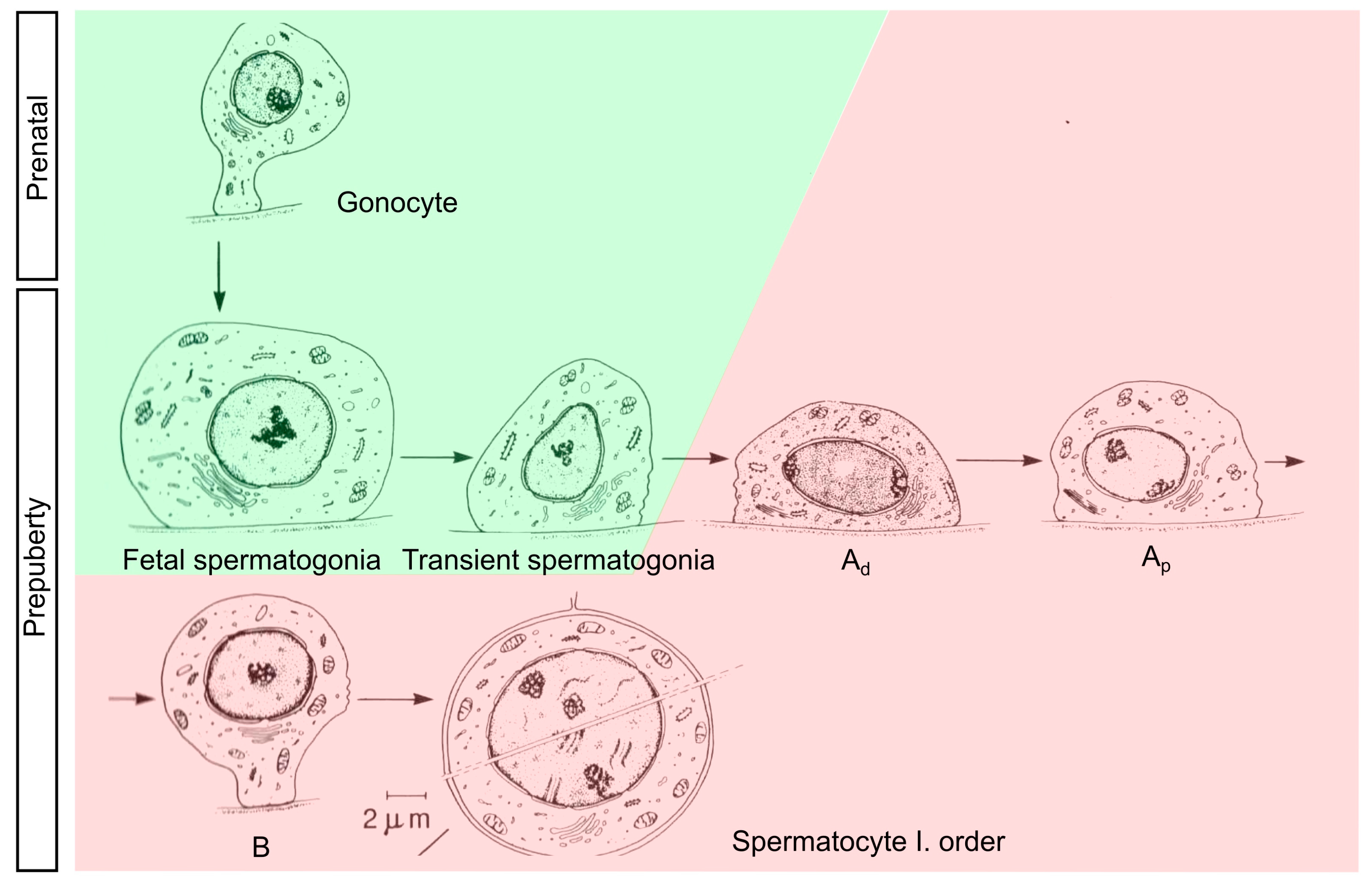
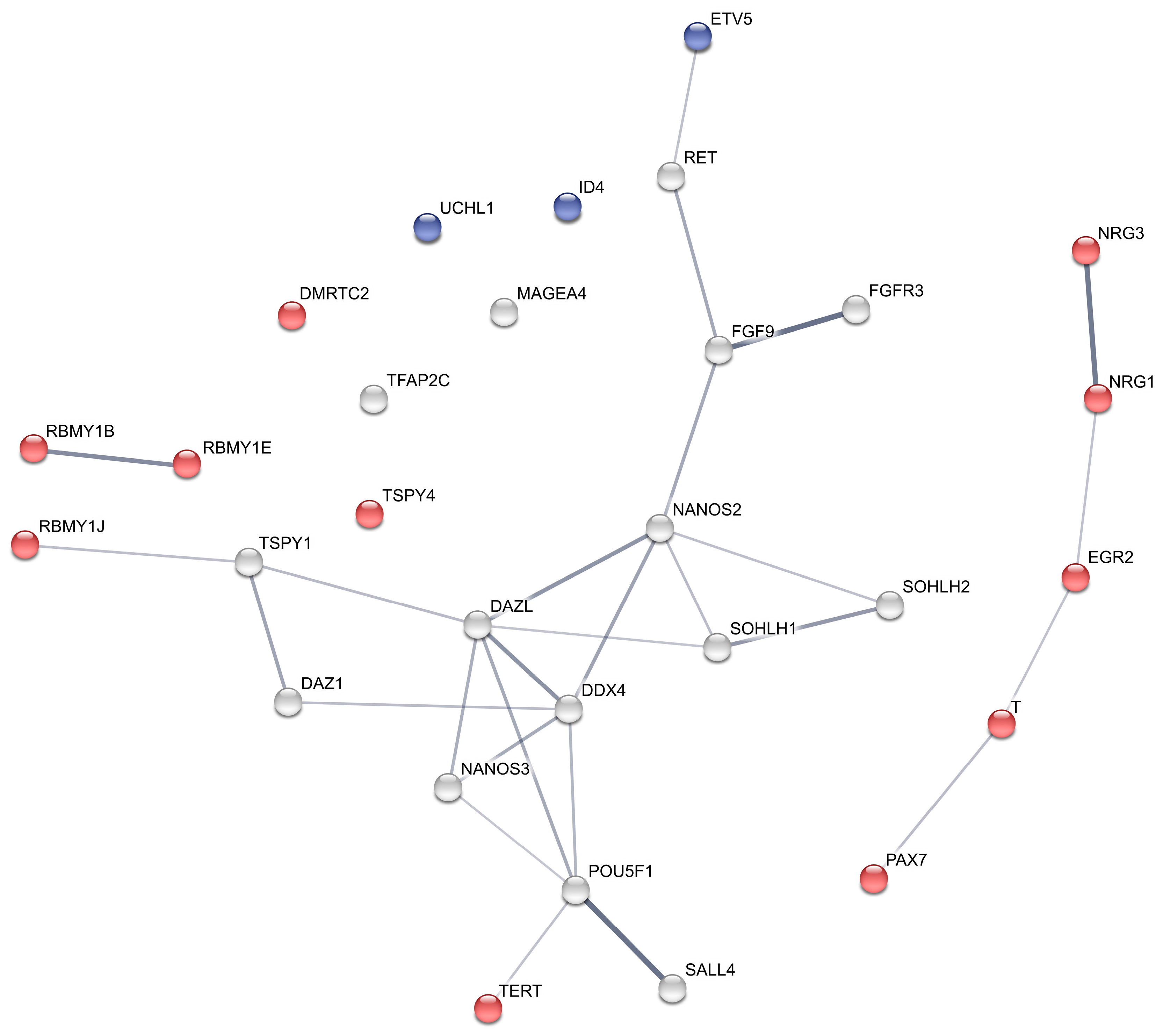
| Gene ID | Name | Cell Marker | logFC Ad−/Ad+ | FDR Ad−/Ad+ | logFC GnRHa | FDR GnRHa |
|---|---|---|---|---|---|---|
| ALPP/PLAP | Alkaline phosphatase, placental | gonocytes | n.d. | n.d. | n.s. | n.s. |
| EPS8 | Epidermal growth factor receptor pathway substrate 8 | gonocytes | n.s. | n.s. | −0.6181 | 0.0091 |
| KIT/c-KIT | KIT proto-oncogene receptor tyrosine kinase | gonocytes | n.s. | n.s. | −0.6307 | 0.0086 |
| NANOG | Nanog homeobox | gonocytes | n.s. | n.s. | n.s. | n.s. |
| POU5F1/OCT4 | POU class 5 homeobox 1 | gonocytes | −3.0537 | 0.0059 | n.s. | n.s. |
| TFAP2C/AP2γ | Transcription factor AP-2 gamma/activating enhancer binding protein 2 gamma | gonocytes | −2.3598 | 0.0041 | n.s. | n.s. |
| ADGRA3/GPR125 | Adhesion G protein-coupled receptor A3/G-protein coupled receptor 125 | undifferentiated spermatogonia | n.d. | n.d. | −0.6740 | 0.0047 |
| BCL6B | B-cell CLL/lymphoma 6B | undifferentiated spermatogonia | n.s. | n.s. | n.s. | n.s. |
| CDH1 | Cadherin 1 | undifferentiated spermatogonia | n.s. | n.s. | n.s. | n.s. |
| CHD1L | Chromodomain helicase DNA binding protein 1-like | undifferentiated spermatogonia | n.s. | n.s. | −0.6220 | 0.0068 |
| DAZ1 | Deleted in azoospermia 1 | undifferentiated spermatogonia | −2.0896 | 0.0038 | n.s. | n.s. |
| DAZL | Deleted in azoospermia-like | undifferentiated spermatogonia | −1.3031 | 0.0073 | n.s. | n.s. |
| DDX4/VASA | DEAD (Asp-Glu-Ala-Asp) box polypeptide 4 | undifferentiated spermatogonia | −2.8616 | 0.0002 | n.s. | n.s. |
| DMRT1 | Doublesex and mab-3 related transcription factor 1 | undifferentiated spermatogonia | n.s. | n.s. | −0.7838 | 0.0010 |
| ETV5 | ETS variant gene 5 | undifferentiated spermatogonia | −1.0118 | 0.0037 | −0.4611 | 0.0490 |
| FGF9 | Fibroblast growth factor 9 | undifferentiated spermatogonia | −1.0605 | 0.0016 | n.s. | n.s. |
| FGFR3 | Fibroblast growth factor receptor 3 | undifferentiated spermatogonia | −3.3279 | 0.0002 | n.s. | n.s. |
| FOXO1 | Forkhead box O1 | undifferentiated spermatogonia | n.s. | n.s. | −0.6078 | 0.0097 |
| GFRA1 | GDNF family receptor alpha 1 | undifferentiated spermatogonia | n.s. | n.s. | n.s. | n.s. |
| ID4 | Inhibitor of DNA binding 4 | undifferentiated spermatogonia | −1.5342 | 0.0011 | −0.5512 | 0.0322 |
| MAGEA4 | Melanoma antigen family A4 | undifferentiated spermatogonia | −2.6591 | 0.0002 | n.s. | n.s. |
| NANOS2 | Nanos C2HC-type zinc finger 2 | undifferentiated spermatogonia | −4.0281 | 0.0003 | n.s. | n.s. |
| NANOS3 | Nanos C2HC-type zinc finger 3 | undifferentiated spermatogonia | −2.6621 | 0.0043 | n.d. | n.d. |
| NEUROG3 | Neurogenin 3 | undifferentiated spermatogonia | n.d. | n.d. | n.d. | n.d. |
| PAX7 | Paired box 7 | undifferentiated spermatogonia | −1.2949 | 0.0318 | 1.8592 | 0.0005 |
| PHF13/SPOC1 | PHD finger protein 13 | undifferentiated spermatogonia | n.s. | n.s. | n.s. | n.s. |
| POU2F2/OCT2 | POU class 2 homeobox 2 | undifferentiated spermatogonia | n.s. | n.s. | 0.9912 | 0.0166 |
| RET | Ret proto-oncogene | undifferentiated spermatogonia | −2.1556 | 0.0002 | n.s. | n.s. |
| SALL4 | Spalt-like transcription factor 4 | undifferentiated spermatogonia | −1.2253 | 0.0087 | n.s. | n.s. |
| SOHLH1 | Spermatogenesis and oogenesis specific basic helix-loop-helix 1 | undifferentiated spermatogonia | −2.9639 | 0.0002 | n.s. | n.s. |
| SOHLH2 | Spermatogenesis and oogenesis specific basic helix-loop-helix 2 | undifferentiated spermatogonia | −1.3457 | 0.0105 | n.s. | n.s. |
| T | T brachyury transcription factor | undifferentiated spermatogonia | −2.4149 | 0.0146 | 1.9341 | 0.0221 |
| TAF4B | TATA box binding protein (TBP)-associated factor 4B | undifferentiated spermatogonia | n.s. | n.s. | −0.8142 | 0.0008 |
| TERT | Telomerase reverse transcriptase | undifferentiated spermatogonia | −2.2152 | 0.0006 | 1.5623 | 0.0155 |
| THY1 | Thy-1 cell surface antigen | undifferentiated spermatogonia | n.s. | n.s. | −0.9577 | 0.0011 |
| TSPAN8 | Tetraspanin 8 | undifferentiated spermatogonia | n.s. | n.s. | 1.1760 | 0.0154 |
| TSPY1 | Testis specific protein, Y-linked 1 | undifferentiated spermatogonia | −2.4939 | 0.0003 | n.s. | n.s. |
| UCHL1 | Ubiquitin C-terminal hydrolase L1 | undifferentiated spermatogonia | −1.1036 | 0.0064 | −1.0168 | 0.0003 |
| UTF1 | Undifferentiated embryonic cell transcription factor 1 | undifferentiated spermatogonia | n.d. | n.d. | n.d. | n.d. |
| ZBTB16/PLZF | Zinc finger and BTB domain containing 16 | undifferentiated spermatogonia | n.s. | n.s. | n.s. | n.s. |
| DMRTC2/DMRT7 | DMRT-like family C2 | spermatogonia? | −1.6666 | 0.0004 | 1.0740 | 0.0199 |
| EGR2 | Early growth response 2 | spermatogonia? | −1.1786 | 0.0013 | 1.2310 | 0.0022 |
| NRG1 | Neuregulin 1 | spermatogonia? | −0.9213 | 0.0136 | 0.7797 | 0.0099 |
| NRG3 | Neuregulin 3 | spermatogonia? | −0.8806 | 0.0160 | 0.7177 | 0.0291 |
| RBMY1B | RNA binding motif protein, Y-linked, family 1, member B | spermatogonia? | −1.9326 | 0.0004 | 1.1699 | 0.0023 |
| RBMY1E | RNA binding motif protein, Y-linked, family 1, member E | spermatogonia? | −1.9032 | 0.0020 | 1.3151 | 0.0010 |
| RBMY1J | RNA binding motif protein, Y-linked, family 1, member J | spermatogonia? | −1.9522 | 0.0007 | 0.8343 | 0.0158 |
| TSPY4 | Testis specific protein, Y-linked 4 | spermatogonia? | −1.9952 | 0.0004 | 1.0862 | 0.0325 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gegenschatz-Schmid, K.; Verkauskas, G.; Demougin, P.; Bilius, V.; Dasevicius, D.; Stadler, M.B.; Hadziselimovic, F. DMRTC2, PAX7, BRACHYURY/T and TERT Are Implicated in Male Germ Cell Development Following Curative Hormone Treatment for Cryptorchidism-Induced Infertility. Genes 2017, 8, 267. https://doi.org/10.3390/genes8100267
Gegenschatz-Schmid K, Verkauskas G, Demougin P, Bilius V, Dasevicius D, Stadler MB, Hadziselimovic F. DMRTC2, PAX7, BRACHYURY/T and TERT Are Implicated in Male Germ Cell Development Following Curative Hormone Treatment for Cryptorchidism-Induced Infertility. Genes. 2017; 8(10):267. https://doi.org/10.3390/genes8100267
Chicago/Turabian StyleGegenschatz-Schmid, Katharina, Gilvydas Verkauskas, Philippe Demougin, Vytautas Bilius, Darius Dasevicius, Michael B. Stadler, and Faruk Hadziselimovic. 2017. "DMRTC2, PAX7, BRACHYURY/T and TERT Are Implicated in Male Germ Cell Development Following Curative Hormone Treatment for Cryptorchidism-Induced Infertility" Genes 8, no. 10: 267. https://doi.org/10.3390/genes8100267
APA StyleGegenschatz-Schmid, K., Verkauskas, G., Demougin, P., Bilius, V., Dasevicius, D., Stadler, M. B., & Hadziselimovic, F. (2017). DMRTC2, PAX7, BRACHYURY/T and TERT Are Implicated in Male Germ Cell Development Following Curative Hormone Treatment for Cryptorchidism-Induced Infertility. Genes, 8(10), 267. https://doi.org/10.3390/genes8100267





